Abstract
Background
The objective of this study was to determine if there is an impact of surgical delay on 5-year overall survival (OS) from early stage colon cancer, and if so, to define how long surgery can safely be postponed.
Methods
Using the NCDB, we compared early (14–30 days) and delayed surgery (31–90 days) in patients with Stage I/II colon cancer. Outcomes included OS at five years and odds of death.
Results
Delayed resection conferred a decreased 5-year OS of 73.0% (95% CI, 72.6–73.4), compared to early resection 78.3% (95% CI, 77.9–78.8). When time to surgery was divided into one-week intervals, there was no difference in the odds of death with delay up to 35–41 days (6 weeks), but odds of death increased by 9% per week thereafter.
Conclusions
These data support that definitive resection for early stage colon cancer may be safely delayed up to 6 weeks.
Keywords: Colon cancer, Cancer outcomes, Surgical delay, Overall survival
Introduction
The surgical community has been compelled to make significant changes to how we treat and counsel patients during the COVID-19 pandemic.1 Quarantine and social distancing recommendations have resulted in significant delays of elective surgeries including selective cancer operations. Alarmingly, patients with asymptomatic COVID-19 undergoing surgery may have a perioperative mortality rate up to 20%.2 Under these circumstances performing surgery in a patient who otherwise does not need urgent surgery may present a higher risk than previously believed. While there is some guidance from the American College of Surgeons (ACS),3 Society of Surgical Oncology,4 and National Comprehensive Cancer Network5 regarding how to triage elective cancer operations, there remain no discrete recommendations for how long of a delay is expected or safe. This uncertainty is even more pronounced in cancer patients who are felt to have early stage disease and have no urgent or emergent indication for surgery.
Irrespective of the COVID-19 pandemic, there are no official recommendations for how quickly treatment should be initiated for patients with colon cancer, and it is unclear to what degree delay can adversely effect survival.6, 7, 8, 9, 10 The current available data has either investigated a heterogeneous group of colon and rectal cancer patients,6 , 10, 11, 12 early and late stage colon cancer patients,9 , 13 , 14 patients receiving neoadjuvant therapy,9 , 11 , 13 or included patients with delays longer than three months13 , 14 when considering this risk. To date, no study has exclusively evaluated the impact of delay in surgery for early stage colon cancer, for which surgery is widely accepted as the most appropriate first treatment.
The objective of this study was to determine if there is an impact on 5-year overall survival (OS) from colon cancer with delays of surgery less than 90 days, and if so, to define how long surgery can safely be delayed in Stage I and II colon cancer without negatively impacting OS.
Methods
Patient population
The National Cancer Database (NCDB) is a cancer registry jointly maintained by the ACS and the American Cancer Society that includes data from patients treated at Commission on Cancer-accredited centers, comprising approximately 70% of newly diagnosed cancer cases from more than 1500 hospitals in the United States.15 De-identified information for colon cancer patients was abstracted from the NCDB Participant User File. Patients with early stage (NCDB analytic stage I and II) colon cancer that underwent primary definitive surgical resection from January 1, 2004 to December 31, 2015 were included. Patients were excluded for uncommon histology such as lymphoma, neuroendocrine cancer, and GI stromal tumors, to limit the study to adenocarcinoma. In order to capture an early stage cohort, patients undergoing surgery less than 14 days after diagnosis and those with T4 tumors were excluded. Those who received an operation greater than 90 days from diagnosis were excluded as well. Patients undergoing an operation within 14 days were excluded to ensure the patient population included only elective operations. Delay up to 90 days from diagnosis was included because initial ACS COVID-19 Guidelines for Triage of Colorectal Cancer Patients raised the possibility of delaying certain cancer operations up to 90 days.3
Study variables and endpoints
The number of days from either radiologic or histologic diagnosis of colon cancer to definitive surgical resection was defined as either early (14–30 days) or delayed (31–90 days). Twelve patient variables were abstracted and included age, sex, race, Hispanic ethnicity, zip code-level, estimates of income and education, facility type where surgery was performed (Community Cancer Center, Comprehensive Cancer Center, Academic/Research Cancer Center, Integrated Network Cancer Program), distance to treatment facility, insurance status, Charlson/Deyo comorbidity score, NCDB analytic stage, and receipt of adjuvant chemotherapy. For estimates of zip-code level income, quartiles are based on the following median household income ranges from 2016 survey data (1) < $40,227; (2) $40,227 – $50,353; (3) 50,354 – $63,332; and (4) >$63,332. For estimates of zip-code level of educational attainment, quartiles are based on the following percent of residents that did not graduate high school from 2016 survey data (1) 17.6%+; (2) 10.9%–17.5%; (3) 6.3%–10.8%; and (4) <6.3%. Facility type was based on Commission of Cancer accreditation. The primary outcome of interest was the time to death after colon cancer diagnosis. Patients with missing data of the above abstracted variables were excluded.
Statistical analysis
In the main patient-level analysis, inverse probability of treatment weighting based on the propensity score16 was used to balance baseline characteristics between the two cohorts of interest, early (14–30 days) versus delayed (31–90 days) definitive surgical resection. To calculate the inverse probability of treatment weights, the predicted probability of each patient to either receive early or delayed surgery was determined using multivariate binary logistic regression modeling that included the twelve predictor variables described above. These variables were chosen based on the possibility that they may confound the relationship between timing of surgery and survival. Patients who underwent delayed surgery were assigned a weight of 1/(predicted probability) and the patients who underwent early surgery a weight of 1/(1-predicted probability). To reduce variability, weights were stabilized.17 Once the cohort was weight-adjusted in this manner, Kaplan-Meier survival analysis and the log-rank test were used to compare OS between early and delayed surgery groups. Weight-adjusted logistic regression was performed with early and delayed groups, as well as 1-week intervals from diagnosis to surgery, as the predictor variable and death as the outcome in order to estimate a threshold for postponing surgery safely. Distributions of continuous variables with the independent samples t-test and distributions of categorical variables with the chi-square test were compared. All data analysis was performed with IBM SPSS Statistics, version 25.0.0.1 (IBM Corp., Armonk, N.Y., USA).
Results
Patient cohort
A total of 365,239 patients with Stage I or II colon cancer underwent surgery from January 1, 2004 to December 31, 2015. After excluding patients who underwent surgery either <14 or >90 days from diagnosis, received neoadjuvant chemotherapy or any radiotherapy, had missing data, clinically or pathologically stage T4 tumors, or uncommon histology [Supplemental Fig. 1], a total of 107,774 patients were included for analysis. The majority of patients (59%; n = 63,568) underwent surgery from 14 to 30 days and the remainder from 31 to 90 days (41%, n = 44,206). Select patient characteristics are summarized in Table 1 . Adjustment with inverse probability weighting improved similarity between early and delayed surgery groups.
Table 1.
Patient demographics in the unadjusted cohort and inverse probability weighted cohort.
| Unadjusted Cohort |
p | IPW Adjusted Cohort |
p | |||
|---|---|---|---|---|---|---|
| 14–30 days |
31–90 days |
14–30 days |
31–90 days |
|||
| N = 63,568 (59%) | N = 44,206 (41%) | Na = 63,607 (59%) | Na = 44,157 (41%) | |||
| Age, mean (SD) | 70.3 (11.6) | 71.4 (11.4) | <0.001 | 70.8 (11.4) | 70.8 (11.6) | 0.69 |
| Female sex | 52.4 | 51.3 | 0.001 | 51.9 | 51.9 | 0.97 |
| Race | <0.001 | 1.00 | ||||
| White | 87.9 | 84.2 | 86.4 | 86.4 | ||
| Black | 8.3 | 11.6 | 9.7 | 9.7 | ||
| Native American | 0.2 | 0.3 | 0.2 | 0.2 | ||
| East Asian or Polynesian | 2 | 2.2 | 2.1 | 2.1 | ||
| South Asian | 0.2 | 0.3 | 0.3 | 0.3 | ||
| Other or Unknown | 1.3 | 1.4 | 1.3 | 1.3 | ||
| Ethnicity | <0.001 | 0.99 | ||||
| Not Hispanic | 89.8 | 88.8 | 89.4 | 89.4 | ||
| Hispanic | 3.5 | 5 | 4.1 | 4.1 | ||
| Unknown | 6.7 | 6.2 | 6.5 | 6.4 | ||
| Median Income | <0.001 | 0.99 | ||||
| Quartile 1 | 15.9 | 17.5 | 16.5 | 16.6 | ||
| Quartile 2 | 24.2 | 23.3 | 23.7 | 23.7 | ||
| Quartile 3 | 27.3 | 27.1 | 27.2 | 27.2 | ||
| Quartile 4 | 32.7 | 32.2 | 32.5 | 32.5 | ||
| HS Diploma Attainment | <0.001 | 1.00 | ||||
| Quartile 1 | 14.3 | 16.8 | 15.3 | 15.3 | ||
| Quartile 2 | 25.2 | 26.1 | 25.5 | 25.5 | ||
| Quartile 3 | 34.7 | 34.1 | 34.5 | 34.5 | ||
| Quartile 4 | 25.9 | 23 | 24.7 | 24.7 | ||
| Charlson/Deyo Score | <0.001 | 1.00 | ||||
| 0 | 68.6 | 63.5 | 66.5 | 66.4 | ||
| 1 | 23.5 | 25.8 | 24.5 | 24.5 | ||
| 2 | 5.9 | 7.7 | 6.7 | 6.7 | ||
| 3+ | 1.9 | 3 | 2.4 | 2.4 | ||
| Distance to Facility | 0.001 | 0.88 | ||||
| <20 mi | 79.4 | 78.6 | 79.1 | 79.1 | ||
| >20 mi | 20.6 | 21.4 | 20.9 | 20.9 | ||
| Insurance type | <0.001 | 0.99 | ||||
| No Insurance | 1.4 | 1.7 | 1.5 | 1.5 | ||
| Private Insurance | 31.8 | 27.4 | 30 | 29.9 | ||
| Government Healthcare Plan | 65.5 | 69.3 | 67.1 | 67.2 | ||
| Unknown | 1.3 | 1.6 | 1.4 | 1.4 | ||
| NCDB Stage | <0.001 | 0.84 | ||||
| Stage I (T1/2, N0) | 43.8 | 49.9 | 46.3 | 46.4 | ||
| Stage II (T3, N0) | 56.2 | 50.1 | 53.7 | 53.6 | ||
| Facility type | <0.001 | 1.00 | ||||
| Community Cancer Center | 12.9 | 10.8 | 12 | 11.9 | ||
| Comprehensive Cancer Center | 51.2 | 44.9 | 48.7 | 48.7 | ||
| Academic/Research Cancer Center | 25.6 | 32.6 | 28.4 | 28.5 | ||
| Integrated Network Cancer Program | 10.3 | 11.7 | 10.9 | 10.9 | ||
| Adjuvant chemo | <0.001 | 0.94 | ||||
| No | 87.6 | 90.5 | 88.8 | 88.8 | ||
| Yes | 8.3 | 5.4 | 7.1 | 7.1 | ||
| Unknown | 4.1 | 4.1 | 4.1 | 4.1 | ||
Inverse probability of treatment weight-adjusted.
On multivariate analysis used to determine weights, patients who were older (OR = 1.01, 95% CI = 1.01–1.01), male (OR = 1.06, 95% CI = 1.04–1.09), black (OR = 1.44; 95% CI = 1.38–1.51), Hispanic ethnicity (OR = 1.42; 95% CI = 1.34–1.52), lived >20 miles from the treating hospital (OR = 1.05; 95% CI = 1.02–1.08), had an increasing Charlson/Deyo comorbidity score (OR = 1.60, 95% CI 1.48–1.74, for score of 3+), or those treated at an Academic or Research Cancer Center (OR = 1.53; 95% CI = 1.46–1.60), were significantly more likely to experience delays in surgery [Supplemental Table 1]. Patients were less likely to experience delays in surgery if they had private health insurance (OR = 0.79, 95% CI = 0.71–0.87) or were insured through a government healthcare program (OR = 0.85, 95% CI 0.77–0.95) as compared to no health care coverage. Additionally, patients were less likely to experience delays with increasing educational attainment (OR = 0.73, 95% CI 0.69–0.76, for highest quartile) [Supplemental Table 1].
Impact of delay in surgery
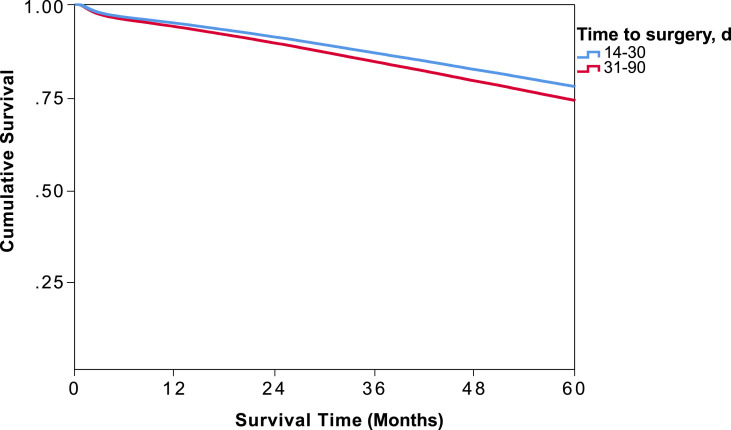
On Kaplan Meier survival analysis of weight-adjusted data, the 5-year OS survival for the entire cohort was 76.2% [Table 2 ]. Patients who experienced a delay in surgery from 31 to 90 days after diagnosis had a significantly increased odds of death (OR = 1.13; 95% CI = 1.10–1.16, p < 0.001). Five-year survival curves for patients who underwent early (14–30 days) versus delayed (31–90 days) surgery are shown in Fig. 1 . The survival probability for patients in the early surgery cohort was significantly higher at 78.3% (95% CI, 77.9–78.8), compared to 73.0% (95% CI, 72.6–73.4) [Table 2; p < 0.001].
Table 2.
Five-year overall survival probability of early- and delayed-groups and by 1-week interval delays in operation timing after diagnosis of early stage colon cancer.
| Estimate (%) | Std. Error | 95% C.I. |
||
|---|---|---|---|---|
| Lower | Upper | |||
| 14–30 days | 78.3 | 0.2 | 77.9 | 78.7 |
| 31–90 days | 73.0 | 0.2 | 72.6 | 73.4 |
| Overall | 76.2 | 0.1 | 74.2 | 76.4 |
| 14–20 days | 78.7 | 0.3 | 78.1 | 79.2 |
| 21–27 days | 78.2 | 0.3 | 77.6 | 78.8 |
| 28–34 days | 76.7 | 0.3 | 76.1 | 77.3 |
| 35–41 days | 76.1 | 0.4 | 75.3 | 76.9 |
| 42–48 days | 73.5 | 0.6 | 72.3 | 74.7 |
| 49–56 days | 71.3 | 0.7 | 69.9 | 72.7 |
| 57–62 days | 68.8 | 0.9 | 67.0 | 70.6 |
| 63–60 days | 66.5 | 1.1 | 64.3 | 68.7 |
| 70–76 days | 66.5 | 1.3 | 64.0 | 69.1 |
| 77–83 days | 63.2 | 1.6 | 60.1 | 66.3 |
| 84–90 days | 63.0 | 1.8 | 59.5 | 66.5 |
Figure 1.
Weight-adjusted Kaplan Meier 5-year overall survival analysis of 14–30 or 31–90 days cohorts, based on timing of operation after early stage colon cancer diagnosis.
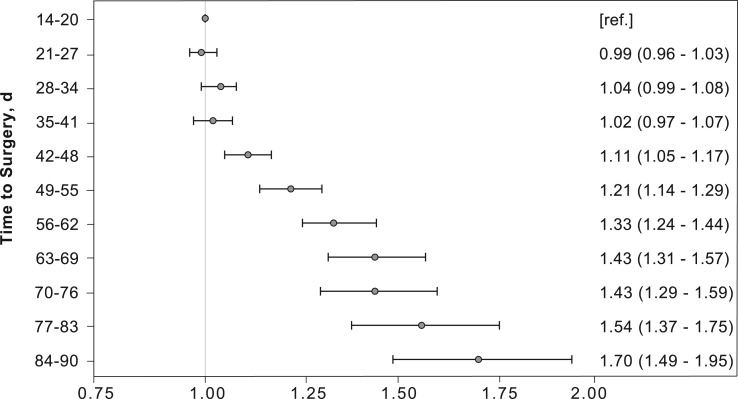
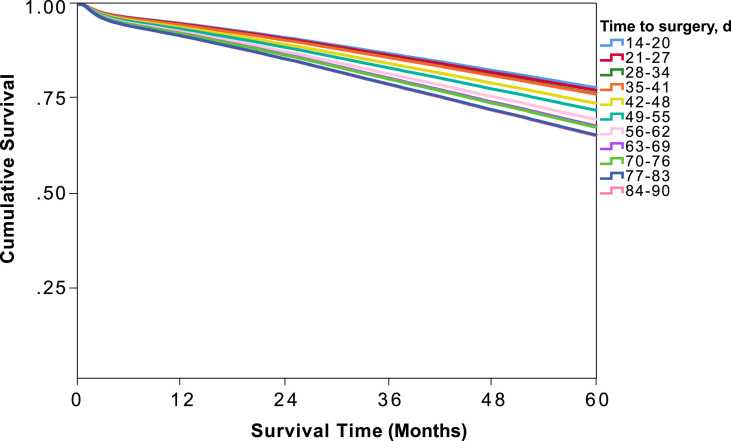
Upon further dividing the time from diagnosis to definitive surgery into 1-week intervals and using time to surgery of 14–20 days (3 weeks) after diagnosis as a reference, there was no difference in the odds of death with delay up to 35–41 days (6 weeks) [Fig. 2 ]. The odds of death increased by an average of 9% per week thereafter, with odds of death of 1.70 (95% CI 1.49–1.90) with a delay of 84–90 days [Fig. 2]. Survival probability at 6 weeks was 76.1% (95% CI, 75.3–76.9) but decreased by nearly 2% per week thereafter, with an overall survival of 63.0% (95% CI, 59.5–66.5) with delay of 84–90 days [Table 2]. Five-year overall survival curves for each interval are shown in Fig. 3 .
Figure 2.
Weight-adjusted odds ratio of death with each 1-week interval increase in delay of timing of the operation after early stage colon cancer diagnosis.
Fig. 3.
Weight-adjusted Kaplan Meier 5-year overall survival analysis comparing 1-week delay increments in the timing of the operation after early-stage colon cancer diagnosis.
Discussion
In this study using the NCDB, we found that surgery for patients with early stage colon cancer may be safely delayed as long as 35–41 days (or 6 weeks) after diagnosis. These results are timely in the era of COVID-19 to help clinicians prioritize which colon cancer operations may be safely delayed and for how long. Beyond COVID-19, these results can also be used to help create a timeline when considering those patients who need preoperative medical optimization prior to surgery.
According to the most recent guidelines from the ACS for how to triage surgery during the COVID-19 pandemic, asymptomatic colon cancers are considered “cases that need to be done as soon as feasible” during Acute Phase I (semi-urgent setting/preparation phase: no limitations on operating room capacity). Once institutions enter Acute Phase II (Urgent setting: limitations on operating room capacity) or Acute Phase III (no operating room capacity) however, elective colorectal procedures should be deferred per these guidelines. During these phases, alternative treatment approaches recommended in the guidelines include neoadjuvant therapy for colon cancer. Additionally, once hospitals enter the Recovery Phase, clinicians should identify the optimal window for patients to receive definitive surgical care to avoid jeopardizing the long-term outcome and achieve the best chance for cure.3
The patient population in this study was carefully selected to provide specific guidance for clinicians to define that optimal window for early stage cancers that may have been delayed during the Acute Phases. Other database studies have shown that patients with colon cancer who undergo immediate surgery have worse survival, likely representing urgent or emergent surgeries secondary to perforation, obstruction, or hemorrhage.6 To date, none of the other large database studies investigating delays to surgery have excluded patients undergoing immediate operations.6 , 7 , 9 , 14 We specifically chose time from diagnosis to surgery <14 days as the early exclusion threshold in order to capture only those cases performed on an elective basis and to minimize confounding associated with unplanned urgent/emergent surgery and overall survival. There is limited data in the literature defining an optimal time point to exclude these cases among stage I/II colon cancer patients, but for stage III colon cancer, using the NCDB, the optimal time from diagnosis to surgery for overall survival appears to be 14–28 days.18 Concordantly, we found that median survival decreased sharply for patients undergoing surgery within 0–5 days of diagnosis, but did not rebound and stabilize until 10+ days (Supplemental Fig. 2), supporting < 14 days as a conservative cut-off for excluding patients that present acutely. Patients with delay in surgery beyond 90 days were also excluded in this study because this was not felt to be an acceptable treatment approach.
This study is also unique because it excludes patients with T4 and Stage III disease. These patients have a sufficiently distinct treatment options and prognosis when compared with patients with Stage I and II colon cancer.19 Prior to the COVID-19 pandemic, there was growing interest in treating Stage III and high risk Stage II colon cancer patients with neoadjuvant chemotherapy.20 Therefore it is reasonable to consider that patients with clinical Stage III disease and T4 lesions appreciated on preoperative imaging may have a viable alternative treatment option in neoadjuvant chemotherapy.
Aside from the immediate concern with the COVID-19 pandemic, this study has relevance when considering the increasing popularity of prehabilitation programs. Several studies have investigated whether prehabilitation programs may reduce postoperative complications and loss of independence in geriatric and frail patients.21, 22, 23 One of the current limitations with these programs is finding the right balance between limited duration of the intervention that will actually enhance outcomes. For example, Carli et al. performed a randomized controlled trial assessing a multimodal prehabilitation program on postoperative complications for frail patients undergoing resection of colorectal cancer. The median duration of time between the baseline assessment and surgery was 40 days (interquartile range [IQR] 28–51) with an overall improvement in functional status.22 The results of our study suggest that a short delay to allow for optimization is acceptable but that longer delays may adversely impact survival.
Finally, several studies have demonstrated that the interval between diagnosis and initiation of treatment for colon cancer has been increasing.24, 25, 26 There are many explanations for this occurrence such as socioeconomic factors including access to care, education level, household income, and racial disparities.25 Our study adds to the existing published evidence raising concern that long delays should be avoided and offers novel insight that there is a negative impact on survival when delaying surgery for early stage colon cancer more than 6 weeks from diagnosis.
There are several limitations of this study. First, pathologic stage is often not known at the time of presentation. Therefore, accurately selecting only Stage I and II by using clinical factors may not be feasible when deciding how to triage colon cancer patients to operative delay or other therapeutics options. Second, there are inherent limitations in using large retrospective datasets to answer clinically meaningful questions. We are using specific inclusion/exclusion criteria to create an average patient with early stage colon cancer that may not be accurate with a particular individual patient. We also may not be accounting for all variables that influence whether a patient undergoes early versus delayed operation for colon cancer, including social, economic, and health disparities, but we did attempt to mitigate this selection bias by balancing the available patient demographics with inverse probability of treatment weighting. Additionally, we do not have data on the circumstances of the diagnosis (e.g., age-specific screening colonoscopy versus symptomatic presentation), the time from presentation to biopsy-proven diagnosis, or the details about preoperative medical optimization prior to surgery, each of which would directly inform the reasons for surgical delay. For example, preoperative cardiac and pulmonary evaluations may warrant longer delay than a patient with history of severe chronic kidney disease although they may both contribute to elevated Charlson/Deyo Score. Nevertheless, this score has been shown in some studies to be comparable to individual comorbidity data.27 We also do not have information on the quality of the operative resection, nor do we have cancer-specific outcomes such as disease-free survival, local recurrence, and distant recurrence. Nevertheless, this study aimed to determine the risk of delaying operations in the treatment of early stage colon cancer patients who we as surgical community are trying to successfully prioritize and effectively triage without sacrificing long-term survival.
Conclusions
Delaying operations up to 6 weeks for early stage (I/II) colon cancer does not impact overall survival. These data may be used by clinicians to guide decision-making when delaying operations during the COVID-19 pandemic and when considering medical optimization to reduce post-operative complications.
Acknowledgements
The authors thank Dr. Joel Vetter for support with statistical analysis. The final manuscript has been seen and approved of by all authors and all authors fulfill the Committee on Publication Ethics requirements for authorship. None of the authors have any personal or financial conflicts of interest to disclose. No outside sources of funding were received to support this research. In addition, no financial support from industry was received for this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjsurg.2020.11.048.
Funding
National Cancer Institute (NCI) grantsT32 CA 009621, United States (J.T.D.).
Financial Disclosures
None to report
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Prachand V.N., Milner R., Angelos P., et al. Medically necessary, time-sensitive procedures: scoring system to ethically and efficiently manage resource scarcity and provider risk during the COVID-19 pandemic. J Am Coll Surg. 2020;S1072–7515(20):30317. doi: 10.1016/j.jamcollsurg.2020.04.011. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei S., Jiang F., Su W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACS guidelines for triage and management of elective cancer surgery cases during the Acute and Recovery phases of coronavirus disease 2019 (COVID-19) pandemic. https://www.facs.org/-/media/files/covid19/acs_triage_and_management_elective_cancer_surgery_during_acute_and_recovery_phases.ashx Updated.
- 4.Bartlett D.L., Howe J.R., Chang G., et al. Management of cancer surgery cases during the COVID-19 pandemic: considerations. Ann Surg Oncol. 2020;27(6):1717–1720. doi: 10.1245/s10434-020-08461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Principles for Management of Colorectal Cancer Patients During the COVID-19 Pandemic. National Comprehensive Cancer Network. https://www.nccn.org/covid-19/pdf/Colorectal COVID-19.pdf. Updated May 1, 2020. Accessed.
- 6.Pruitt S.L., Harzke A.J., Davidson N.O., Schootman M. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Causes Control. 2013;24(5):961–977. doi: 10.1007/s10552-013-0172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neal R.D., Tharmanathan P., France B., et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112(Suppl 1):S92–S107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos M., Esteva M., Cabeza E., et al. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: a review. Eur J Cancer. 2007;43(17):2467–2478. doi: 10.1016/j.ejca.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Khorana A.A., Tullio K., Elson P., et al. Time to initial cancer treatment in the United States and association with survival over time: an observational study. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biagi J.J., Raphael M.J., Mackillop W.J., et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. J Am Med Assoc. 2011;305(22):2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 11.Zarcos-Pedrinaci I., Fernández-López A., Téllez T., et al. Factors that influence treatment delay in patients with colorectal cancer. Oncotarget. 2017;8(22):36728–36742. doi: 10.18632/oncotarget.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos M., Esteva M., Cabeza E., et al. Lack of association between diagnostic and therapeutic delay and stage of colorectal cancer. Eur J Cancer. 2008;44(4):510–521. doi: 10.1016/j.ejca.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y.H., Kung P.T., Wang Y.H., et al. Effect of length of time from diagnosis to treatment on colorectal cancer survival: a population-based study. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0210465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaltenmeier C., Shen C., Medich D.S., et al. Ann Surg; 2019. Time to Surgery and Colon Cancer Survival in the United States. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Boffa D.J., Rosen J.E., Mallin K., et al. Using the national cancer database for outcomes Research: a review. JAMA Oncol. 2017;3(12):1722–1728. doi: 10.1001/jamaoncol.2016.6905. [DOI] [PubMed] [Google Scholar]
- 16.Austin P.C. A tutorial and case study in propensity score analysis: an application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivariate Behav Res. 2011;46(1):119–151. doi: 10.1080/00273171.2011.540480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin P.C., Stuart E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pataroque D., Taira M.A., Aurit S.J., et al. Time to surgery and survival in patients with stage III colon cancer: an NCDB study. J Am Coll Surg. 2020;231(4):e111. 2020. [Google Scholar]
- 19.O’Connell J.B., Maggard M.A., Ko C.Y. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96(19):1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 20.Karoui M., Rullier A., Piessen G., et al. Perioperative FOLFOX 4 versus FOLFOX 4 plus cetuximab versus immediate surgery for high-risk stage II and III colon cancers: a phase II multicenter randomized controlled trial (PRODIGE 22) Ann Surg. 2020;271(4):637–645. doi: 10.1097/SLA.0000000000003454. [DOI] [PubMed] [Google Scholar]
- 21.Gearhart S.L., Do E.M., Owodunni O., et al. Loss of independence in older patients after operation for colorectal cancer. J Am Coll Surg. 2020;230(4):573–582. doi: 10.1016/j.jamcollsurg.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Carli F., Bousquet-Dion G., Awasthi R., et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg. 2020;155(3):233–242. doi: 10.1001/jamasurg.2019.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ven Fong Z., Chang D.C., Lillemoe K.D., et al. Contemporary opportunity for prehabilitation as part of an enhanced Recovery after surgery pathway in colorectal surgery. Clin Colon Rectal Surg. 2019;32(2):95–101. doi: 10.1055/s-0038-1676473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilimoria K.Y., Ko C.Y., Tomlinson J.S., et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253(4):779–785. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 25.Merkow R.P., Bilimoria K.Y., Sherman K.L., et al. Efficiency of colorectal cancer care among veterans: analysis of treatment wait times at Veterans Affairs Medical Centers. J Oncol Pract. 2013;9(4):e154–163. doi: 10.1200/JOP.2012.000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh H., De Coster C., Shu E., et al. Wait times from presentation to treatment for colorectal cancer: a population-based study. Can J Gastroenterol. 2010;24(1):33–39. doi: 10.1155/2010/692151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin S.R., Wong Y.N., Uzzo R.G., et al. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser score work. Med Care. 2015;53(9):e65–72. doi: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.