Abstract
Introduction
The novel coronavirus pandemic is an ongoing challenge faced by the public and health care systems around the globe. Majority of information and evidence gathered so far regarding COVID-19 has been derived from data and studies in adult populations. Crucial information regarding the characterization, clinical symptomatology, sequelae, and overall outcomes in the pediatric population is lacking. As such, we aimed to conduct a comprehensive meta-analysis and systematic review to collect and analyze current evidence about COVID-19 in the pediatric population.
Method
A systematic search and review of scientific literatures was conducted following the PRISMA guidelines using PubMed, Embase, Scopus, Medline, and Google Scholar databases. All relevant studies until June 16, 2020 were included. Studies were reviewed for methodological quality, and random-effects model was used to conduct the primary meta-analysis. I2 value and Egger’s test was used to estimate heterogeneity and publication bias respectively.
Results
We reviewed 20 eligible studies that included 1810 pediatric patient population (<21 yo) with PCR tested COVID-19 positivity. In pooled data, majority (25 % [CI 18–32], I2 59 %) of overall COVID-19 positive patients fell in the 6−10 yr age group. 13 % ([CI 11–14], I2 78 %) of the patients were asymptomatic, with headache (67 % [CI 60–74], I2 46 %), fever (55 % [CI 52–58], I2 61 %), and cough (45 % [CI 42–49], I2 79 %) accounting for the most prevalent physical signs seen in symptomatic patients. Leukopenia (12 % [CI 9–15], I250 %) and lymphopenia (15 % [CI 13–19], I2 85 %) was common. Elevated Ferritin (26 % [CI 16–40], I2 73 %), Procal (25 % [CI 21–29 %], I2 83 %), and CRP (19 % [CI 16–22 %], I2 74 %) were other laboratory abnormalities commonly observed. Common radiological features were ground-glass opacities (36 % [CI 32–39 %], I2 92 %), normal finding (33 % [CI 30–36 %], I2 81 %), and consolidation. 29 % ([CI 26–33], I2 85 %) of the patient cases was non-severe, whereas only 5 % ([CI 1–8], I2 87 %) was severe. Mortality was observed in 0.3 % ([CI 0.1−0.4], I2 0%) of the overall cases.
Conclusion
COVID-19 is prevalent across all pediatric age-groups and presents with varying degree of symptomology. However, children have a milder course of the disease with extremely favorable prognosis. Laboratory and radiological features are inconsistent and require further investigations. Additional studies are needed on this topic to corroborate findings and establish evidence-based and consistent characterization of COVID-19 in the pediatric population.
Keywords: Pediatric, COVID-19, Clinical characteristics, Outcomes, Meta-analysis, Systematic review
1. Background
In late 2019, an outbreak of pneumonia cases of unknown origin appeared in Wuhan, China. In the span of a few months the coronavirus disease (COVID-19), the source of that outbreak, was declared a global pandemic by the World Health Organization (WHO) [1]. The novel COVID-19 caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has since become a growing public health emergency of international concern. As of November 19, 2020, it has affected more than 56 million individuals with 1,355,143 reported deaths in 191 countries [2]. Initially, the majority of infected patients were correlated to the Wuhan Sea-food market, implying primarily and predominantly zoonotic transmission. However, no clear animal source has been identified to date and human to human transmission is now the predominant mode of transmission of new infections [3].
Studies have demonstrated that people of all ages are vulnerable to SARS-CoV-2, particularly with higher severity and mortality in older age populations and in individuals with underlying comorbidities [4]. Children, especially infants, are usually more susceptible to certain infectious diseases as compared to adults, due to their developing immune system [5]. However based on current observations, in children the susceptibility to COVID-19 seems to be much lower, with less frequency of severe cases and only rare cases of fatality [6]. Also, in pediatric COVID-19 infections the spectrum of clinical characteristics and outcomes seem to vary considerably in the limited number of studies currently available [7]. Although children typically experience a milder form of the disease, they can still be a major source of transmission, possibly more so than adults, given the lack of and varying degree of signs and symptoms. With scarce data and lack of large-scale studies, the characterization of COVID-19 in children remains an active and necessary area of study.
In this systematic review and meta-analysis, we aim to collect updated evidence regarding COVID-19 in children and investigate prevalence of clinical symptoms, laboratory and radiological findings, and clinical outcomes. Further understanding and characterization of COVID-19 in children can better inform the ongoing efforts to control this global pandemic.
2. Methods
2.1. Search strategy and study selection criteria
Our systematic review and meta-analysis was conducted according to the PRISMA guidelines [8]. A comprehensive search spanning 2019 – June 16, 2020 was conducted in PubMed, Embase, Scopus, Medline, and Google Scholar using various combinations of keywords: “coronavirus or COVID-19 or 2019-nCoV or SARS-CoV-2” and “pediatric or neonate or newborn or infant or children or adolescence” (Appendix 1). A total of 1154 publications were identified in various databases. We included studies that offered epidemiological, clinical information and one or more outcome data on pediatric (<21 years) COVID-19 cases diagnosed with PCR. Excluded articles included those that did not contain relevant and adequate data concerning prevalence, clinical features, and outcomes; exclusively neonatal or specific age group studies; comorbidity specific studies; and non-English publications (Fig. 1 ).
Fig. 1.
PRISMA flow chart of the systematic literature review and article identification process.
2.2. Data extraction and quality assessment
Two independent reviewers extracted all relevant data following full-text screening for eligibility. The following items were extracted from each study: author, publication date, study design, participant demographics, timeline, and if available, clinical symptoms, laboratory markers, radiological features, mortality/severity outcomes, length of hospital stay and clearance of disease. Any variations between authors were resolved through a consensus-based detailed discussion. The quality of included studies was assessed independently by two authors using the Newcastle-Ottawa Scale (NOS) [9].
2.3. Data synthesis and analysis
The primary outcomes of the study were prevalence of - COVID-19 in various age groups; clinical symptoms, laboratory markers, radiological features; and severe/mortal outcomes. Length of hospital stay (LOHS) and clearance of virus outcomes was also analyzed from available reports. Disease severity was categorized into four types: mild, moderate, severe, and critical according to published guidelines [10]. Severity outcomes were further classified into non-severe and severe groups consisting of mild/moderate cases and severe/critical cases respectively for pooled analysis. Random effects model was used to calculate pooled estimates of prevalence with 95 % confidence intervals. Freeman-Tukey double arcsine transformation was used to stabilize the variance of specific prevalence rates. I2 index was used to assess heterogeneity with values 25 %, 50 %, and 75 % representing low, moderate, and high heterogeneity respectively [11]. If analysis included more than three studies, egger precision weighted linear regression tests and funnel plots were used to test potential publication bias, with p-value <0.05 considered significant for publication bias. If publication bias was present, the trim and fill method was performed to evaluate the effect. Meta-analysis was performed using metapackages in R (version 3.6.0).
3. Results
3.1. Study selection, quality, and epidemiological characteristics
A total of 1154 articles were identified in the initial search. After removing duplicates and performing detailed selection process, a total of 20 retrospective studies were finalized (Fig. 1) [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]]. The quality of study assessed using the NOS ranged from good to high quality (Table 1 ; Supp. Table A). The total number of study population was 1810, with majority of cases coming from China (N = 1286). The general timeline of these studies ranged from December 2019 to April 2020. The median age of the patient population was approximately 8 yr [6–10], with male to female ratio of 1.34 (Table 1).
Table 1.
Main characteristics and quality of reviewed studies.
| Study (n = 20) | Timeline (mm.dd) | Location | N = 1810 | Age (yr) a | Gender (M/F) | Quality ⍭ |
|---|---|---|---|---|---|---|
| Dong, Y. et al. | Jan 06 - Feb 08 | China | 728 | 10 [4–15] | 418/310 | ********* |
| Peng, H. et al. | Dec 08 - Feb 29 | Wuhan, China | 75 | 6.06 ± 4.78 | 44/31 | ******** |
| Bai. K. et al. | Jan 19 - Mar 12 | Chongqing, China | 25 | 11 [6.3−14.5] | 14/11 | ******** |
| Du, H. et al. | Jan 28 - Feb 28 | Wuhan, China | 182 | 6 [0.01−15] | 120/62 | ********* |
| Qiu, H. et al. | Jan 17 - Mar 01 | Zhejiang, China | 36 | 8.3 ± 3.5 | 23/13 | ******** |
| Shen, Q. et al. | Jan 08 - Feb 26 | China | 9 | 8 [1–12] | 3/6 | ******* |
| Song, W. et al. | Jan 01 - Mar 17 | Hubei, China | 16 | 8.5 [11.5−14] | 10/6 | ******* |
| Tan, Y.P. et al. | Jan 27 -Mar 10 | Changsha, China | 10 | 7 [1–12] | 3/7 | ******* |
| Zheng, F. et al. | Feb 01- Feb 10 | Hubei, China | 25 | 3 [2–9] | 14/11 | ******** |
| Zhu, L. et al. | Jan 24 - Feb 22 | China | 10 | 9.5 [6.25−11.75] | 5/5 | ****** |
| Cai, J. et al. | Jan 19 - Feb 03 | China | 10 | 6.5 [4.25−8.75] | 4/6 | ****** |
| Lu, Y. et al. | Jan 30 - Mar 10 | Wuhan, China | 110 | 6 [2–9] | 59/51 | ********* |
| Ma, H. et al. | Jan 21 - Feb 14 | Wuhan, China | 50 | 2.5 [0.9–7.0] | 28/22 | ******** |
| Garazzino, S. et al. | Mar 25 - Apr 10 | Italy | 168 | 2.3 [0.3–9.6] | 94/74 | ********* |
| Parri, N. et al. | Mar 03-Mar 26 | Italy | 130 | 6 (0–11) | 73/57 | ********* |
| Garcia-Salido, A. et al. † | Mar 01 - Apr 15 | Spain | 7 | 8.35 [4.46−11.11] | 4/3 | ****** |
| Foster, C.E. et al. | Mar 10 - Apr 18 | Texas | 57 | 10.7 [0.1–20.2] | 32/25 | ********* |
| Zachariah, P. et al. | Mar 01 - Apr 15 | New York City | 50 | 9 [0.5−21]; 14 [8–19] b | 27/23 | ******** |
| Mannheim, J. et al. | Mar 05 - Apr 08 | Chicago, Illinois | 64 | 11 [7–16] | 36/28 | ********* |
| Shekerdemian, L. S. † | Mar 14 - Apr 10 | USA/Canada | 48 | 13 [4.2−16.6] | 25/23 | ******* |
Data from PICU cases only.
Newcastle-Ottawa Scale (NOS) quality assessment rating.
Mean ± SD or Median [IQR].
Age reported separately as Median [IQR] for severe and non-severe cases respectively.
3.2. Meta-analysis results
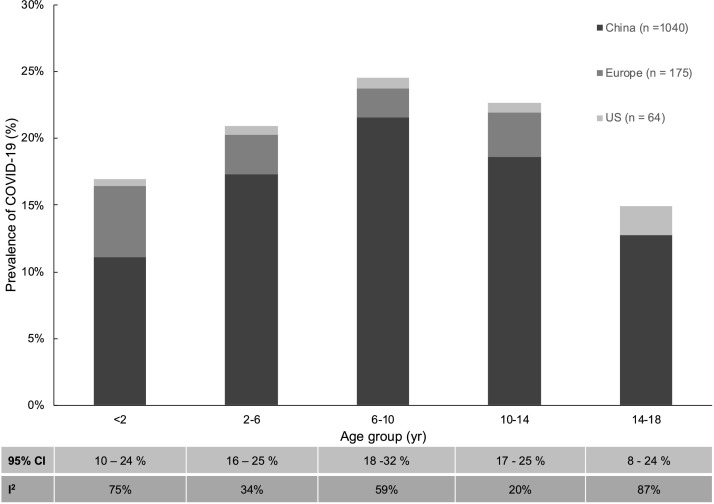
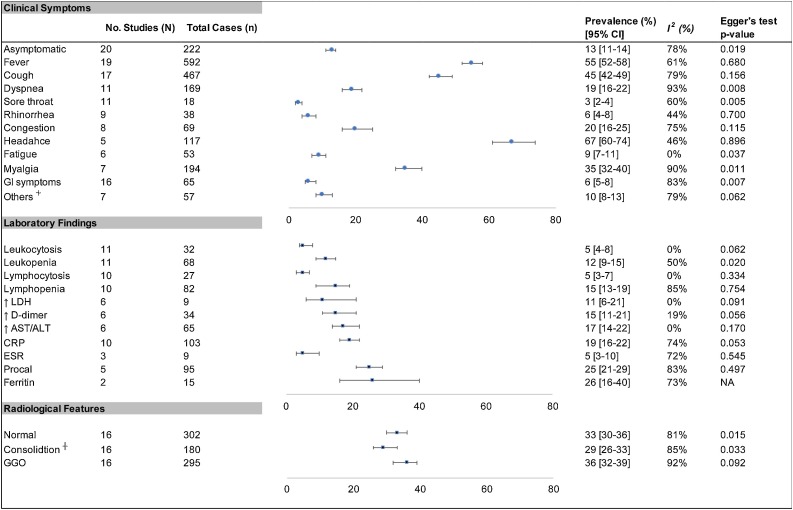
Eleven of the eligible studies included data on COVID-19 positive population categorized across various pediatric age groups. These data were structured into age ranges in four years interval and analysis was performed to determine prevalence of the disease (Fig. 2 ). Overall, prevalence of COVID-19 across various age groups were, 17 % in <2 yr (CI 10–24); 21 % in 2−6yr (CI 16–25); 25 % in 6−10yr (CI 18–32); 23 % in 10−14yr (CI 17–25); 15 % in 8−24yr (CI 8–24). Further meta-analysis was conducted on 12 clinical symptoms, 11 laboratory markers, and 3 radiological features (Fig. 3 ). Overall, 13 % (CI 11–14) of the patient population were asymptomatic, with headache (67 % [60–74]), fever (55 % [52–58]), and cough (45 % [42–49]), being the most common symptoms. The remaining clinical symptoms had an overall estimated prevalence of <20 %. Among commonly reported laboratory findings, elevated inflammatory markers including ferritin (26 % [16–40]), Procal (25 % [21–29]), and CRP (19 % [16–22]) were the most common. Decreased lymphocytes (15 % [13–19]) and leukocytes (12 % [9–15]) were also more common than elevated counts of these immunological markers. Regarding radiological features, only small differences in prevalence was found between normal (33 % [30–36]), consolidation (29 % [26–33]), and ground glass opacification (GGO) (36 % [32–39]) findings. In analysis of the clinical outcomes including mortality and severity, prevalence of mortality was 0.3 % (CI 01.−0.4), while severe and non-severe groups had 5% (1–8) and 84 % (92–97) estimated prevalence respectively (Table 2 ). Average LOH from available reports was 10.3 days and clearance of virus occurred at an average of 9.4 days.
Fig. 2.
Prevalence (%) of COVID-19 across various pediatric age groups. Pooled studies (N = 11) included data from China, Europe and US with varying total number of cases (n) for each age group as demonstrated. (Estimated p-values <0.05 for each age group).
Fig. 3.
Meta-analysis of the prevalence of clinical symptoms, laboratory findings and radiological features in pediatric COVID-19 patients.
┼ Includes Unilateral and/or bilateral findings; ⏆ Includes other Neurological, Hematological, and Musculoskeletal symptoms. Abbreviations: GI: Gastrointestinal; GGO: Ground Glass Opacity; LDH: Lactate Dehydrogenase; AST: Aspartate transaminase; ALT: Alanine transaminase; ESR: Erythrocyte Sedimentation Rate; Procal: Procalcitonin.
Table 2.
Summary of clinical outcomes of reviewed studies.
| Study (n = 20) | N | Mortality | Severity n (%) |
LOHS (days) | Clearance (days) ⍭ | |||
|---|---|---|---|---|---|---|---|---|
| Mild | Mod. | Severe | Critical | |||||
| Dong, Y. et al. | 728 | 1a | 409 (56) | 298 (41) | 18 (2) | 3 (0.4) | ⏤ | ⏤ |
| Peng, H. et al. | 75 | 0 | 22 (29) | 51 (68) | 1 (1) | 1 (1) | 10.6 | 6.4 |
| Bai. K. et al. | 25 | 0 | 25 (100) | 0 | 0 | 0 | 15.2 | 15.2 |
| Du, H. et al. | 182 | 1b | 178 (98) | 0 | 4 (2) | 0 | 12 | 7 |
| Qiu, H. et al. | 36 | 0 | 17 (47) | 19 (53) | 0 | 0 | 13 | 9 |
| Shen, Q. et al. | 9 | 0 | 9 (100) | 0 | 0 | 0 | 15 | ⏤ |
| Song, W. et al. | 16 | 0 | 16 (100) | 0 | 0 | 0 | 14 | ⏤ |
| Tan, Y.P. et al. | 10 | 0 | 10 (100) | 0 | 0 | 0 | 17.2 | ⏤ |
| Zheng, F. et al. | 25 | 0 | 23 92) | 0 | 0 | 2 (8) | ⏤ | ⏤ |
| Zhu, L. et al. | 10 | 0 | 10 (100) | 0 | 0 | 0 | ⏤ | ⏤ |
| Cai, J. et al. | 10 | 0 | 10 (100) | 0 | 0 | 0 | ⏤ | ⏤ |
| Lu, Y. et al. | 110 | 0 | 110 (100) | 0 | 0 | 0 | 10 | ⏤ |
| Ma, H. et al. | 50 | 0 | 48 (96) | 0 | 0 | 2 (4) | ⏤ | ⏤ |
| Garazzino, S. et al. | 168 | 0 | 152 (90) | 0 | 14 (8) | 2 (1) | ⏤ | ⏤ |
| Parri, N. et al. | 130 | 0 | 99 (76) | 11 (8) | 11 (8) | 9 (7) | ⏤ | ⏤ |
| Garcia-Salido, A. et al. | 7 | 0 | 0 | 0 | 0 | 7 (100) | ⏤ | ⏤ |
| Foster, C.E. et al. | 57 | 0 | 57 (100) | 0 | 0 | 0 | 2 | ⏤ |
| Zachariah, P. et al. | 50 | 1c | 34 (68) | 0 | 16 (32) | 0 | 3 | ⏤ |
| Mannheim, J. et al. | 64 | 0 | 54 (84) | 0 | 0 | 10 (16) | 4 | ⏤ |
| Shekerdemian, L. S. | 48 | 2d | 14 (29) | 1 (2) | 16 (33) | 17 (35) | 7 | ⏤ |
| Total (%) | 1810 | 5 (0.23) | 1297 (72) | 380 (21) | 80 (4) | 53 (3) | 10.3 | 9.4 |
| Prevalence [95 % CI] ☨ | 0.3 [0.1−0.4] | Non-Severe: 84 [72−97] | Severe: 5 [1–8] | ⏤ | ⏤ | |||
| I2 ☨ | 0% | 91 % | 87 % | ⏤ | ⏤ | |||
Mortality Cases: aUnknown Complications; b10 mo F with intestinal necrosis, septic shock, multiorgan failure; cSudden Cardiac Arrest; dUnknown Complications, ⍭ Days from onset to RNA turning negative; ☨ Pooled estimates of study results; Non-severe: mild/moderate cases, Severe: Severe/critical cases.
3.3. Sensitivity and publication bias
Substantial heterogeneity was found among most sub-groups (Fig. 2, Fig. 3, Table 2). In sensitivity analysis performed to assess stability of pooled results, no significant changes in overall results were found after sequential omission of studies. The Egger’s regression test showed significant symmetry in certain results signifying publication bias (p < 0.05) (Fig. 3). In these cases, non-parametric trim and fill analysis was performed, which largely indicated little impact of publication bias (data not shown) [32].
4. Discussion
Following the COVID-19 outbreak and its subsequent global spread, the epidemiology, clinical characteristics, and patient outcomes in affected individuals has been an urgent and active area of study. Gradually, information and evidence regarding SARS-Cov-2 pathogenesis, effective diagnostic measures, and treatment options have come into light. However, current knowledge and evidence regarding COVID-19 is primarily based on studies derived from adult cases. The high prevalence of this disease in adult and elderly populations has indeed played a major role in formulating our understanding of this disease [33]. Further, lack of available data and sporadic literature reports from major worldwide epicenters regarding pediatric COVID-19 cases continues to contribute to unclear characterization of this disease in children [[34], [35], [36]]. More reporting of pediatric data in combination with high powered and multi-national studies are needed, in this regard, to improve our knowledge of COVID-19 in children. Our systematic review and meta-analysis of 20 studies involves 1810 pediatric patient population from multiple global epicenters, and provides an overview of epidemiological characteristics, clinical symptoms, laboratory findings, radiological features, and outcomes of COVID-19 (Table 1).
COVID-19 is present across all age ranges. Based on current evidence, there does not appear to be any age restriction for COVID-19 susceptibility [37]. However, substantial evidence has established that compared to adults, children only account for a small number of COVID-19 case [33,38]. In our meta-analysis of COVID-19 prevalence across various pediatric age groups the distribution of cases varied but was highly frequent (21–25 %) in the 6–14 year-old age range (Fig. 2). Similar observations were made by Götzinger et al. in a large European study [39]. Similarly, in regard to common clinical indicators, fever, cough, and headache accounted for the most dominant symptoms while sore throat, rhinorrhea, and GI symptoms were rare. This strongly substantiates major results seen in other studies, while adding further information regarding prevalence of several other clinical signs and symptoms (Fig. 3) [37,38]. The most frequently evaluated laboratory markers for COVID-19 in adults and children include various immunological and inflammatory factors (Fig. 3). The primary mechanism of SARS-CoV2 pathogenesis is thought to involve its interaction with human ACE-2 receptors leading to T-cell activation and subsequent inflammatory response [40]. This pro-inflammatory state with further vascular and multi-organ injury likely contributes to the variety of laboratory abnormalities commonly seen in infected patients. Previous studies including infections caused by other novel coronaviruses have reported typically normal or reduced immunological markers with normal to elevated LDH and D-dimer levels [7]. Our findings were consistent with higher prevalence of leukopenia and lymphopenia as well as elevated levels of D-dimer and LDH. However, the most common laboratory markers seen in our study were elevated Procalcitonin and Ferritin levels consistent with a highly inflammatory state. These clinical markers alone however are non-specific, thus limiting their clinical utility in absence of a strong clinical correlation to the patient’s history and physical symptoms. In this regard, radiological features can provide helpful evidence in diagnosis of a primary respiratory process, commonly seen in coronavirus infections.7 Radiological findings of bilateral patchy infiltrates and GGO have been observed on imaging of COVID-19 patients [7]. Our meta-analysis is consistent with these findings, however unspecific to a predominant imaging finding as the overall prevalence of abnormal findings was similar to that of a normal radiological evaluation (Fig. 3). Overall, these clinical symptoms, laboratory markers, and radiological features are highly non-specific and provide little evidence of COVID-19 infection when assessed individually. Clinicians should not diagnose or rule out COVID-19 infection solely based on any one of these findings, but synthesize information based on prevalence of common features as evidenced in ours and other studies.
Outcomes of COVID-19 in children are generally favorable with rare reported cases of fatality [12,15,29,31]. This is consistent with typically milder form of the disease seen in children as evidenced in several studies [37,38]. In our study, only 5% of cases were found to be severe with majority of cases falling in the non-severe (84 %) category. A total of five deaths observed in various studies accounted for an estimated case fatality rate (CFR) of 0.3 % (Table 2). Apart from rare cases of severe disease, usually seen in children with prior health conditions, and in some newer cases of hyperinflammatory syndrome, majority of pediatric cases seem to have a milder course as compared to adults [41]. Based on our current understanding of the clinicopathogenesis of COVID-19, several reasons could account for the low severity and mortality of COVID-19 in children. Some studies have suggested that the expression of ACE-2 receptor, primary binding site for SARS-CoV2, may be less developed in children [5,40]. Reversely, long-term exposure to air pollutants and smoking in adults might contribute to higher expression of ACE-2 in the lung epithelium leading to increased disease complications [42]. Additionally, higher prevalence of comorbidities in adults as compared to children likely contributes to poorer prognosis. Overall, our findings were consistent with other studies in the observation of milder clinical course of disease in children as well as rare mortality outcome. However, the pathogenesis of COVID-19 in children as well as adults still remains unclear, and more detailed and powerful studies are needed to elucidate the marked differences seen in terms of presentation, clinical course and outcome of COVID-19 in these population groups.
Given the milder presentation of disease with inconsistent clinical markers usually seen in children, serious thought must be given in terms of precautionary and preventative measures regarding children in fighting this pandemic. Studies show similar level of susceptibility across all age groups, however, with mild to none symptoms, children can be a major source of SARS-CoV2 viral transmission [37]. As academic institutions reopen, extensively planned strategies might be needed to limit the spread of COVID-19. In communities with widespread transmission experts advocate for the cancellation of gatherings including school closures, playground limitations, and alternate education strategies [43]. Further, clinicians and policymakers should consider working on limiting non-urgent children visits to the clinic, while developing appropriate plans to allow for newborn care and wellness visits. COVID-19 is an ongoing and evolving pandemic and lack of appropriate measures in any sector of community or age group can easily lead to an uprising in new cases. Preventative measures for public locations, workplace, and academic institutions must be applied universally and diligently in order to reduce the spread of this disease.
This study has several limitations and strengths. It was conducted during a 3-month period on an ongoing pandemic. One principal limitation was that the majority of data was derived from China, and sample sizes varied considerably across studies. Exclusion of non-English sources might have limited addition of certain pediatric cohorts. Also, data on multisystem inflammatory syndrome (MIS-C) and coagulation disorders in children were lacking, which resulted in these topics being unexplored. We found substantial heterogeneity between studies and significant publication bias in several pooled analysis. Therefore, interpretation of data must be done with caution and in this context. This study includes latest scientific publications and a relatively large composite sample size. To our knowledge our study is the first to include data with detailed analysis from multiple COVID-19 epicenters outside of China, including European countries and cities within the USA. This allowed for patients of diverse socio-cultural, and ethnic backgrounds to be included in the study, which allows our findings to be generalized.
5. Conclusions
In conclusion, our study shows that all pediatric age groups are prone to COVID-19 infection with the disease usually having a mild clinical presentation and sequelae. Critical illness and death are extremely rare. Fever, cough, and headache are the most frequently observed symptoms, while laboratory and radiological markers are inconsistent and unlikely predictive of disease. RNA PCR remains the diagnostic gold standard, especially given the largely variable presentation in children. Overall, prognosis of COVID-19 in the pediatric age group is favorable. However, these disparate features of COVID-19 in children have implications for unchecked viral transmission and infection control. Therefore, appropriate guidelines for testing and quarantine in children are needed. Effective strategies to ensure contact precautions and maintenance of access to routine preventative care is highly recommended for appropriate management of this pandemic in the pediatric population.
Author contributions
The concept of the study was developed together and in consensus by all the listed authors. SB and BBB designed the initial protocol, searched the database, and performed the quality assessment. Data extraction was carried out by all included authors. SB conducted the statistical analysis and drafted the results section. All the authors equally contributed in developing the initial manuscript draft. MJS provided guidance in refining the concept and assisted with edit and revision of the final manuscript. All the authors were involved in editing and finalizing the complete version of the manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
Software and technology used in this research including Endnote X9.0 reference manager, Excel (16.46), and access to certain scientific databases was made available through the University of Minnesota -Biomedical Research Library and IT services. R software used for the meta-analysis was available from the free online R-project website. Ada Moreno, University of Minnesota, Humphrey School of Public Affairs provided assistance with use of the softwares and formatting of the tables and figures. None of the acknowledged parties had any role in the design and conduct of the study, or final approval of the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104715.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Harapan H., Itoh N., Yufika A., et al. Coronavirus disease 2019 (COVID-19): a literature review. J. Infect. Publ. Health. 2020;13(May (5)):667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2020. COVID-19 World Map and Mortality Analysis; p. 1.https://coronavirus.jhu.edu/data/mortality COVID-19. May 22, 2020. [Google Scholar]
- 3.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajgain K.T., Badal S., Bajgain B.B., Santana M.J. Prevalence of comorbidities among individuals with COVID-19: a rapid review of current literature. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.06.213. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon A.K., Hollander G.A., McMichael A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015;282(December (1821)):20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr. Infect. Dis. J. 2020;39(5):355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(July (7)):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margulis A.V., Pladevall M., Riera-Guardia N., et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin. Epidemiol. 2014;6:359–368. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen K., Yang Y., Wang T., et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J. Pediatr. 2020;16(3):223–231. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 12.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 13.Peng H., Gao P., Xu Q., et al. Coronavirus disease 2019 in children: characteristics, antimicrobial treatment, and outcomes. J. Clin. Virol. 2020;128:104425. doi: 10.1016/j.jcv.2020.104425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai K., Liu W., Liu C., et al. Clinical analysis of 25 COVID-19 infections in children. Pediatr. Infect. Dis. J. 2020;39(7):e100–e103. doi: 10.1097/INF.0000000000002740. [DOI] [PubMed] [Google Scholar]
- 15.Du H., Dong X., Zhang J.J., et al. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy. 2020;(June) doi: 10.1111/all.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 2020;20(6):689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Q., Guo W., Guo T., et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr. Pulmonol. 2020;55(6):1424–1429. doi: 10.1002/ppul.24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song W., Li J., Zou N., Guan W., Pan J., Xu W. Clinical features of pediatric patients with coronavirus disease (COVID-19) J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan Y.P., Tan B.Y., Pan J., Wu J., Zeng S.Z., Wei H.Y. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J. Clin. Virol. 2020;127:104353. doi: 10.1016/j.jcv.2020.104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng F., Liao C., Fan Q.H., et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr. Med. Sci. 2020;40(April (2)):275–280. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu L., Wang J., Huang R., et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr. Pulmonol. 2020;55(6):1430–1432. doi: 10.1002/ppul.24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai J., Xu J., Lin D., et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis. 2020;(February) doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma H., Hu J., Tian J., et al. A single-center, retrospective study of COVID-19 features in children: a descriptive investigation. BMC Med. 2020;18(1):123. doi: 10.1186/s12916-020-01596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X., Zhang L., Du H., et al. SARS-CoV-2 infection in children. N. Engl. J. Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garazzino S., Montagnani C., Donà D., et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020;25(May (18)) doi: 10.2807/1560-7917.es.2020.25.18.2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parri N., Magistà A.M., Marchetti F., et al. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur. J. Pediatr. 2020;179(8):1315–1323. doi: 10.1007/s00431-020-03683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Salido A., Leoz-Gordillo I., Martínez de Azagra-Garde A., et al. Children in critical care due to severe acute respiratory syndrome coronavirus 2 infection: experience in a Spanish hospital. Pediatr. Crit. Care Med. 2020;(May) doi: 10.1097/PCC.0000000000002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster C.E., Moulton E.A., Munoz F.M., et al. Coronavirus disease 2019 in children cared for at Texas children’s hospital: initial clinical characteristics and outcomes. J. Pediatric Infect. Dis. Soc. 2020;9(July (3)):373–377. doi: 10.1093/jpids/piaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zachariah P., Johnson C.L., Halabi K.C., et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York City, New York. JAMA Pediatr. 2020:e202430. doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannheim J., Gretsch S., Layden J.E., Fricchione M.J. Characteristics of hospitalized pediatric COVID-19 cases - Chicago, Illinois, March – April 2020. J. Pediatr. Infect. Dis. Soc. 2020;(June) doi: 10.1093/jpids/piaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 2020;(May) doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duval S., Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000;95(449):89–98. doi: 10.1080/01621459.2000.10473905. 2000/03/01. [DOI] [Google Scholar]
- 33.CDC: CDC; 2020. COVID-19 -Information for Pediatric Healthcare Providers. [Google Scholar]
- 34.Antúnez-Montes O.Y., Escamilla M.I., Figueroa-Uribe A.F., et al. COVID-19 in South American children: a call for action. Pediatr. Infect. Dis. J. 2020;39(10):e332–e334. doi: 10.1097/INF.0000000000002851. [DOI] [PubMed] [Google Scholar]
- 35.WHO; 2020. PMNCH Call to Action on COVID-19; p. 2. [Google Scholar]
- 36.Antúnez-Montes O.Y., Escamilla M.I., Figueroa-Uribe A.F., et al. COVID-19 and multisystem inflammatory syndrome in latin American children: a multinational study. Pediatr. Infect. Dis. J. 2020;(October) doi: 10.1097/INF.0000000000002949. [DOI] [PubMed] [Google Scholar]
- 37.Castagnoli R., Votto M., Licari A., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;(April) doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 38.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Götzinger F., Santiago-García B., Noguera-Julián A., et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc. Health. 2020;(June) doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandit K., Gupta S., Sharma A.G. Clinico-pathogenesis of COVID-19 in children. IJBB. 2020;57(3) [Google Scholar]
- 41.2020. Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. [Google Scholar]
- 42.Hung Y.H., Hsieh W.Y., Hsieh J.S., et al. Alternative roles of STAT3 and MAPK signaling pathways in the MMPs activation and progression of lung injury induced by cigarette smoke exposure in ACE2 knockout mice. Int. J. Biol. Sci. 2016;12(4):454–465. doi: 10.7150/ijbs.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasmussen S.A., Thompson L.A. Coronavirus disease 2019 and children: what pediatric health care clinicians need to know. JAMA Pediatr. 2020;(April) doi: 10.1001/jamapediatrics.2020.1224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.