Abstract
The current study was performed to examine if heat treatment (HT) has beneficial effects on the exaggerated exercise pressor reflex in rats with peripheral artery disease (PAD). We further determined if the temperature-sensitive P2X receptor is involved in the effects of HT. The pressor response to static muscle contraction and α,β-methylene ATP (αβ-me ATP, a P2Xagonist) was examined. Western blot analysis was used to determine the protein levels of P2X3 in the dorsal root ganglion (DRG), and the whole cell patch clamp was used to examine the amplitude of P2X currents in the DRG neurons. The basal muscle temperature (Tm) was lower in PAD rats than in control rats. Tm was increased by ~1.5°C and this increase was maintained for 30 min. This HT protocol was performed tweice daily for three continuous days. A greater blood pressure (BP) response to contraction was observed in PAD rats. HT attenuated the amplification of the BP response in PAD rats. HT also attenuated the enhancement of the BP response induced by the arterial injection of αβ-me ATP in PAD rats. In addition, HT attenuated the upregulation of the P2X3 and increased P2X currents in the DRG neurons of PAD rats. In conclusion, previous heat exposure plays an inhibitory role in modifying the exaggeration of the exercise pressor reflex in PAD and a reduction of the activity of the P2X receptor pathway is probably a part of mechanisms leading to the beneficial effects of HT.
Keywords: blood pressure, exercise, heat treatment, muscle contraction, P2X, peripheral artery disease
Introduction
During exercise, increases in arterial blood pressure (BP), heart rate (HR), myocardial contractility and peripheral vasoconstriction occur in response to muscle contraction (Victor et al. 1988; Sinoway et al. 1989). Peripheral and central mechanisms are considered to contribute to these cardiovascular responses to exercise. For the peripheral mechanism, sympathetic nervous activity (SNA) plays a predominant role in increasing BP and HR responses to muscle contraction, termed the ‘exercise pressor reflex’ (Coote et al. 1971; McCloskey & Mitchell, 1972). Both metabolic and mechanical stimuli within the contracting muscles evoke the reflex. The afferent arm of the reflex consists of group III (mechanosensitive) and group IV (metabosensitive) fibres (Kaufman & Forster, 1996). Activation signals originating in the contracting muscles are projected to the primary sensory neurons in the dorsal root ganglion (DRG), spinal cord and then the cardiovascular nuclei in the brainstem (Mitchell et al. 1983; Kaufman & Forster, 1996). As a result, stimulation of the cardiovascular nuclei in the brainstem induces increases in SNA and the consequent amplification of cardiovascular activities. In terms of the central mechanism, central command suggests a parallel and simultaneous increase in SNA and alpha motor neuron discharge (Goodwin et al. 1972; Waldrop et al. 1996).
Peripheral artery disease (PAD) affects 236.62 million individuals aged 25 years and older worldwide (Song et al. 2019). Intermit claudication is one of the most common manifestations among PAD patients. Patients who experience intermit claudication are commonly observed to be exhausted from continuous physical activity (i.e. a 6 min walk), and frequently occurring fatigue can be improved by taking an interval break (McDermott et al. 2004). This symptom largely limits the daily exercise performance for PAD patients. It has been noted that during walking a greater increase in arterial BP is observed in PAD patients than in normal subjects (Baccelli et al. 1999; Bakke et al. 2007). It has also been suggested that the exercise pressor reflex contributes to the exaggerated BP response to walking in PAD patients (Baccelli et al. 1999).
In general, SNA plays a prominent role in cardiovascular disease progression and is inversely related to prognosis (Cohn et al. 1984; Brede et al. 2002). Specifically, an exaggerated SNA response to exercise in cardiovascular diseases lowers the ventricular fibrillation threshold, thus increasing the probability of fatal arrhythmias as a cardiovascular risk in patients (Collins & Billman, 1989; Vanoli & Schwartz, 1990). In addition, amplified SNA attenuates metabolite-induced vasodilatation in exercising muscles (Shoemaker et al. 1999; Hammond et al. 2001), which is, in turn, an important contributor to exercise intolerance (Wilson & Mancini, 1993). Furthermore, an exaggerated exercise pressor reflex contributes to poor clinical outcomes (Piepoli et al. 1999; Ponikowski et al. 2001). Overall, SNA affects blood flow directed to the exercising muscles and is an important factor affecting exercise performance in cardiovascular diseases. Thus, it is interesting to study SNA-regulated BP responses during exercise in PAD.
Femoral artery occlusion in rats has been used to study human PAD. Although this model does not fully exhibit all the clinical symptoms of PAD, it mimics one of the critical characteristics observed in PAD, namely intermittent claudication manifested by insufficient blood flow (Waters et al. 2004). In particular, in occluded rats, the blood flow limitation is observed during exercise while the resting blood flow is maintained (Waters et al. 2004). This makes it appropriate to investigate exercise physiology in PAD. Importantly, in a rat model of PAD induced by femoral artery occlusion, the SNA and BP responses are also augmented during muscle contraction and/or stimulation of muscle metabolic receptors (Li & Xing, 2012).
Using a PAD rat model induced by femoral artery occlusion, our previous studies have demonstrated that (1) 24–72 h of femoral artery occlusion induces highlighted expression of the purinergic P2X3 (a receptor subtype that senses the extracellular ATP) in the DRG, and (2) the BP response to the stimulation of P2X in the afferent nerves is amplified in PAD rats (Liu et al. 2011; Xing et al. 2013). This suggests a role for P2X receptors in the exaggeration of the exercise pressor reflex. Interestingly, the functions of P2X receptors have also been found to be temperature-dependent (Garcia-Villalon et al. 1997; Ziganshin et al. 2002; Kluess et al. 2005). In particular, with a basal muscle temperature (Tm) of 39°C, a lower increase of BP was observed after femoral artery injection of α,β-methylene ATP (αβ-me ATP, a P2X receptor agonist) to activate P2X receptors of muscle afferent nerves; by contrast, a Tm of 35°C led to a greater BP response with arterial injection of αβ-me ATP (Gao et al. 2006).
Accordingly, we hypothesized that HT attenuates the upregulation of the P2X3 receptor and the amplitude of DRG currents induced by αβ-me ATP, thereby attenuating the amplified BP response to muscle contraction and to stimulation of P2X3 in a rat model of PAD. Overall, it is anticipated that alterations in the P2X3 receptor pathway mediate the beneficial effects of HT on the exaggerated exercise pressor reflex in PAD.
Methods
Ethical approval
The animal experimental procedures were performed in compliance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State College of Medicine. Male Sprague–Dawley rats (200–300 g) were obtained from Charles River Laboratory (Wilmington, MA, USA), housed in individual cages with free access to food and water, and were kept in a temperature-controlled room (25°C) on a 12 h/12 h light/dark cycle.
Experimental animals and categorization
For the experiment using whole animals, the ligation and sham surgery procedures were performed in two sets of rats. Those that underwent right femoral artery occlusion served as ‘PAD rats’, whereas those that underwent sham surgeries on the right limb served as ‘control rats’. For Western blotting and patch clamp experiments, in the same animal the ligation was performed in one limb and the sham surgery was performed in the other limb. Three days after the surgery, heat treatment (HT) was performed in some of the control rats/limbs and PAD rats/occluded limbs. Thus, in the present study four groups of animals were included: control, control + HT, PAD and PAD + HT.
Femoral artery occlusion
After being anaesthetized with an isoflurane–oxygen mixture (2–5%isoflurane in100%oxygen), femoral artery ligation was performed as previously described (Lu et al. 2013; Xing et al. 2018). In brief, an ~1 cm surgical incision was made on the skin on the right side of the groin. After carefully cutting the fascia and removing the soft tissue around the veins, the femoral artery was exposed, dissected and ligated with a surgical suture ~3 mm distal to the inguinal ligament. For the sham surgery, the same procedures were performed except that a suture was placed below the femoral artery without ligating the artery. Buprenorphine hydrochloride (0.05 mg/kg, S.C.) was administered before the surgery for post-operative pain relief. Following surgery, the animals were kept in the surgery room for 2–3 h for observation, and then returned to the animal facility.
Tm monitoring and HT procedure
Rats were anaesthetized with an isoflurane–oxygen mixture (2–5% isoflurane in 100% oxygen) and placed on a surgery mat in the prone position. A temperature probe was carefully inserted into the gastrocnemius of the examined limb at approximately the mid-point, and Tm was continuously monitored. When the baseline Tm was stabilized, two heating pads were placed around the examined limb until Tm gradually increased by ~1.5°C. The heating pads were then removed to avoid overheating and placed around the limb again when Tm tended to decrease. Thus, during HT, Tm was maintained at ~1.5°C above baseline. The length of the treatment process was 30 min and the frequency was twice a day for three continuous days. The heating procedure was performed after the sham and ligation surgeries. During the treatment, body temperature was also monitored continuously.
Western blot analysis
Western blotting was performed to examine protein expression of the P2X3 receptor in the L4–6 DRG tissues of both control limbs and occluded limbs. The rats were anaesthetized with an isoflurane–oxygen mixture and then killed by cervical dislocation. After removal of the spinal cord, the L4–6 DRGs were dissected. For protein evaluation, the tissue samples were homogenized with ice-cold radioimmunoprecipitation assay (RIPA) buffer with proteinase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The samples were then centrifuged at 4°C at 13,000 rpm for 25 min. The supernatants were carefully collected and the total protein concentration was determined with a bicinchoninic acid (BCA) assay kit (Pierce Biotechnology, Rockford, IL, USA) (Liu et al. 2011). The tissue extracted was then divided into the tubes and stored at −80°C until analysis.
Supernatant samples with 25 μg of protein were then boiled at ~95°C for 5 min in SDS sample buffer and then loaded onto 4–20% Mini-Protean TGX Precast gels (Bio-Rad, Hercules, CA, USA) for gel electrophoresis. Following electrophoresis, the proteins were electrically transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was then blocked with 5% non-fat milk in 0.1% Tween-TBS buffer (TBST) for 1 h and incubated with a mouse anti-P2X3 primary antibody (1:500, Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C.
After overnight incubation with the primary antibody, the membrane was incubated with a HRP-conjugated anti-mouse secondary antibody (1:1000, Abcam, Cambridge, MA, USA) at room temperature for 1 h, and the immunoreactivity was visualized using an enhanced chemiluminescence system (Cell Signaling Technology, Inc., Danvers, MA, USA). The membrane was then stripped and incubated with an anti-β-actin antibody. The densities of the P2X3 receptor and β-actin bands were examined using the NIH Scion Image Software.
Electrophysiology
Rats were anaesthetized by inhalation of an isoflurane–oxygen mixture (2–5% isoflurane in 100% oxygen). The skin was incised and pulled away from the underlying muscle tissues, and the fluorescent retrograde tracer DiI (60 mg/ml) was injected into the white portion of the gastrocnemius muscle. An injection volume of 1 μl was administered, and the injection was repeated three times at different locations (Xing et al. 2013). The injection needle was left in the muscle for 5–10 min to prevent leakage of the tracer. The skin overlying the muscle was then sutured. The animals were returned to their cages for 4–5 days to allow the retrograde tracer to be transported to DRG neurons. At the end of each experiment, the gastrocnemius muscle was dissected, frozen and sectioned to confirm that DiI was located in the white portion of the gastrocnemius muscle.
The rats were anaesthetized with an isoflurane–oxygen mixture and then killed by cervical dislocation. The L4–6 DRGs were quickly removed and transferred immediately into Dulbecco’s modified Eagle’s Medium (DMEM). The DRGs were minced, and the ganglion fragments were processed to obtain dissociated DRG neurons. The cell suspension was centrifuged to remove the supernatant, and the cell pellet was resuspended in DMEM. The cells were then plated onto a 35 mm culture dish containing pre-coated coverslips.
Next, patch recordings were performed within 6 h after dissociation. Neurons were first visualized using a combination of epifluorescence illumination and differential interference contrast (DIC, 20–40×) optics on an inverted microscope (Nikon TE2000). Under DIC, images of Dil-positive neurons were displayed on a video monitor. The diameter of all neurons recorded was <40 μm. Neurons were patched in the whole-cell configuration and recorded at a holding potential of −70 mV using a MultiClamp 700B amplifier. Seals (1–10 GΩ) between the glass electrode (2–5 MΩ resistance) and the cell were established in modified Tyrode’s solution. After the whole-cell configuration was established, we electronically compensated for the cell membrane capacitance and series resistance.
Cells in the recording chamber were continuously bathed in Tyrode’s solution. αβ-me ATP stored in stock solution was diluted in extracellular solution immediately before being used and it was held in a syringe connected to a silica column for delivery to the cells (Xing et al. 2013). The distance from the column mouth to the examined cell was ~100 μm. P2X currents were acquired using pClamp 9.0 software and the data were analysed using Clampfit software. Neurons were considered P2X-sensitive if αβ-me ATP elicited an inward current with a peak amplitude >50 pA.
Examination of the BP response
The rats were anaesthetized with a mixture of 2–5% isoflurane and oxygen and ventilated as described previously (Xing et al. 2018). The jugular vein and common carotid artery were cannulated. Fluids were delivered via the jugular vein while a pressure transducer was connected to the common carotid artery for measurement of the arterial BP.HR was calculated beat to beat from the arterial pressure pulse. To examine P2X-mediated cardiovascular responses, a catheter (PE10) was inserted into the femoral artery for injection of αβ-me ATP. In the PAD rats, a small incision was carefully made in the femoral artery distal to the previously occluded site. The catheter was then inserted into the artery towards the distal end to deliver the drug into the ischaemic limb. During the experiments, baseline BP and fluid balance were maintained with a continuous infusion of saline and body temperature was also maintained at ~37°C.
Decerebration was performed to eliminate the effects of anaesthesia on the reflex pressor response. Before the procedure, dexamethasone (0.2 mg, I.V.) was injected to minimize brainstem oedema. After decerebration, anaesthesia was withdrawn from the rats, and the animals were switched to a ventilator.
A laminectomy procedure (Smith et al. 2001) was performed to expose the lower lumbar and upper sacral portions of the spinal cord, and the peripheral ends of the transected L4 and L5 ventral roots were placed on platinum bipolar stimulating electrodes. Experiments were performed 60 min later. Static muscle contractions were induced by electrical stimulation of the L4 and L5 ventral roots (30 s, 3× motor threshold with a period of 0.1 ms at 40 Hz). The reflex BP and HR responses to contraction were examined in control, control + HT, PAD and PAD + HT.
BP and HR responses to arterial injections of αβ-me ATP (0.125 mM) were also examined in the four groups of rats. The concentration of αβ-me ATP was selected on the basis of results of a previous study (Liu et al. 2011). The injection volume was ~ 0.1 ml according to the rat’s body weight. The duration of the injection was 1 min, and an interval of 20 min was allowed between injections. At the end of the experiments, the animals were killed by inhalation of an overdose of isoflurane followed by cardiac puncture.
Statistical analysis
Unless specified, the data in this study are presented as mean ± SD. SPSS for Windows version 26.0 was utilized for all statistical analyses. Two-way repeated measures ANOVA was applied to compare differences in Tm and core temperature (control rats vs. PAD rats over different time points), and one-way ANOVA was used to compare the differences in BP and HR, the expression of P2X3 and the amplitude of P2X currents among the groups (control, control + HT, PAD and PAD + HT). As appropriate, post hoc analysis with Tukey’s or least significant difference LSD test was applied to compare the differences between specific groups. A P value of <0.05 was considered statistically significant.
Results
Tm in control rats and PAD rats
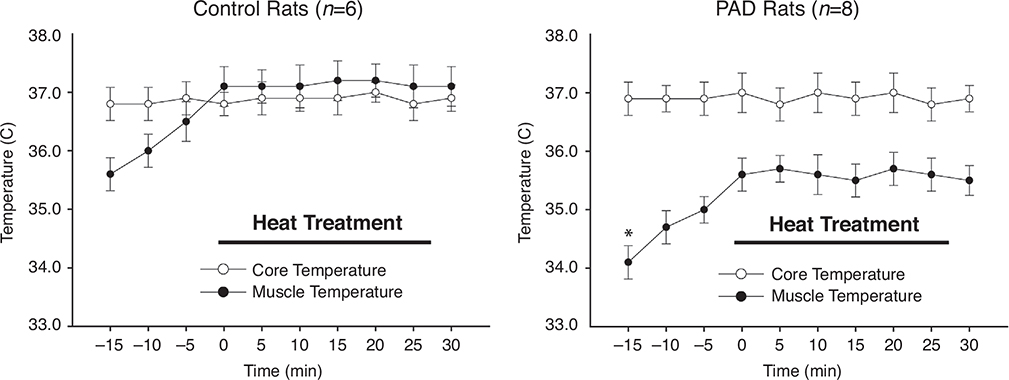
Figure 1 shows that the basal Tm in the occluded limbs of PAD rats was lower than that in the control rats during baseline evaluation (i.e. 34.1 ± 0.28°C in PAD rats/n = 8 vs. 35.6 ± 0.25°C in control rats/n = 6; P < 0.05 between groups), and that during HT, the Tm of PAD rats and control rats was continuously elevated by ~1.5°C above the baseline level and was maintained for 30 min (P > 0.05 at different time points during the 30-min period). Tm was higher in control rats than in PAD rats (P < 0.05), with the increase of ~1.5°C since the beginning of heating treatment. However, there was no significant difference in the core temperature of control rats and PAD rats (P > 0.05). In addition, compared with the baseline level, the core temperature was not altered in either group at all the time points of HT (P > 0.05).
Figure 1. Basal muscle temperature and heat treatment (HT).
Baseline muscle temperature was lower in PAD rats (n = 8) than in control rats (n = 6). Following HT, the muscle temperature was increased by ~1.5°C. The length of HT was 30 min. Core temperature was similar between control rats and PAD rats and it was not significantly altered during HT. *P < 0.05 for baseline muscle temperature between control rats and PAD rats.
Expression of P2X3 in DRG
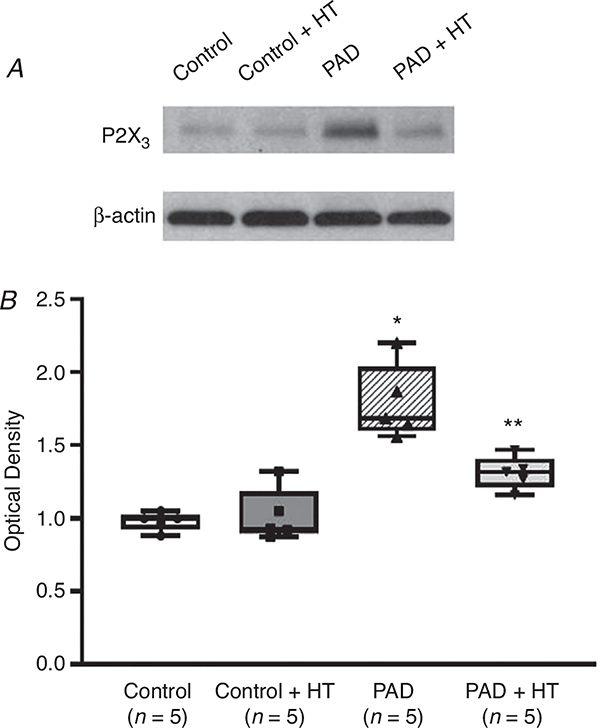
As shown in Fig. 2, in the Western blot experiment, protein expression of the P2X3 receptor in the DRGs of control, control + HT, PAD and PAD + HT animals was examined. Compared with that in controls, the density of the P2X3 signal was greater in the DRG tissues of PAD (optical density: 1.79 ± 0.25 in PAD vs. 0.98 ± 0.06 in control; P < 0.05, n = 5 in each group). Notably, compared with that in PAD rats not exposed to HT, P2X3 expression was decreased in PAD + HT (optical density: 1.31 ± 0.11; P < 0.05 vs. PAD). However, HT did not significantly decrease P2X3 expression in control rats (optical density: 1.02 ± 0.18; P > 0.05, control + HT vs. control, n = 5 in each group).
Figure 2. P2X3 protein expression in control, control + HT, PAD and PAD + HT rats.
The optical density of P2X3 receptor expression was normalized to that of the internal reference protein β-actin. Seventy-two hours of femoral artery ligation increased P2X3 protein expression in the L4–L6 DRGs of PAD rats compared with those of control rats (n = 5 in each group). Following HT, P2X3 protein expression was suppressed in PAD rats compared with PAD rats not exposed to HT (n = 5 in each group). Note that there was no significant difference in P2X3 receptor expression between control rats and control rats + HT (P > 0.05). *P < 0.05 vs. control rats; **P < 0.05 vs. PAD rats.
P2X currents in DRG neurons
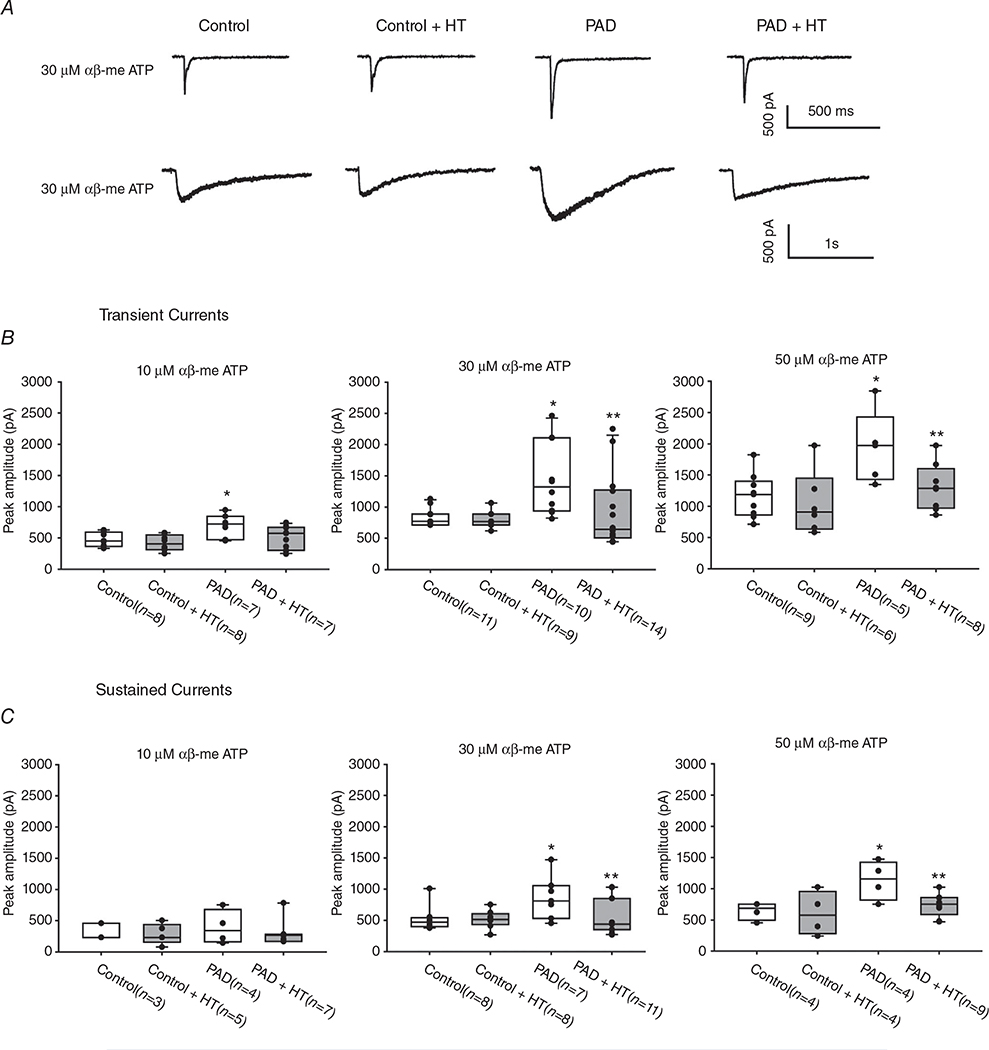
Inward currents were elicited in a dose-dependent manner when 10, 30 or 50 μM αβ-me ATP was applied to DRG neurons. Typical transient and sustained currents evoked by 30 μM αβ-me ATP are shown in Fig. 3A. The transient currents appeared to have a short rise time and a rapid inactivating time, whereas sustained inward currents reached a peak slowly and decayed slowly. DRG neurons from both control rats and PAD rats exhibited transient and sustained current responses. However, the peak amplitudes of inward currents responsive to αβ-me ATP were significantly greater in the DRG neurons of PAD than those of controls (Fig. 3B and C); that is, the peak amplitudes of transient P2X currents evoked by 30 μM αβ-me ATP were 842.1 ± 230.4 pA in control rats (n = 11) and 1448.9 ± 582.8 pA in PAD rats (n = 10; P < 0.05 between the groups). The peak amplitudes of sustained P2X currents evoked by 30 μM αβ-me ATP were 528.6 ± 191.2 pA in control rats (n = 8) and 863.8 ± 344.8 pA in PAD rats (n = 7; P < 0.05 between the groups). In addition, we examined the effects of HT on P2X currents from the DRG neurons of control rats and PAD rats (Fig. 3). Heating significantly attenuated the peak amplitudes of the transient and sustained P2X currents from the DRG neurons of PAD rats, but not from the DRG neurons of control animals; that is, the peak amplitudes of transient P2X currents evoked by 30 μM αβ-me ATP were 929.9 ± 595.2 pA in PAD rats exposed to HT (n = 14; P < 0.05 vs. PAD rats not exposed to HT), and the peak amplitudes of sustained P2X currents were 536.0 ± 289.9 pA in PAD rats exposed to HT (n = 11; P < 0.05 vs. PAD rats not exposed to HT).
Figure 3. Effect of heat treatment on P2X currents in rat DRG neurons.
A, representative P2X current traces induced by αβ me-ATP in the four different groups (top: transient currents; bottom: sustained currents). The peak amplitude of transient and sustained currents was amplified in the DRG neurons of PAD rats and HT attenuated the increase in P2X currents. Note that HT did not have significant effects on P2X currents in control rats. B and C, averaged data showing the effect of heat on the peak amplitude of transient and sustained P2X currents in the DRG neurons of the four groups. *P < 0.05 between control rats and PAD rats; **P < 0.05 between PAD not exposed to HT and PAD exposed to HT. The number of DRG cells in all groups is shown.
BP and HR responses to muscle contraction and P2X stimulation
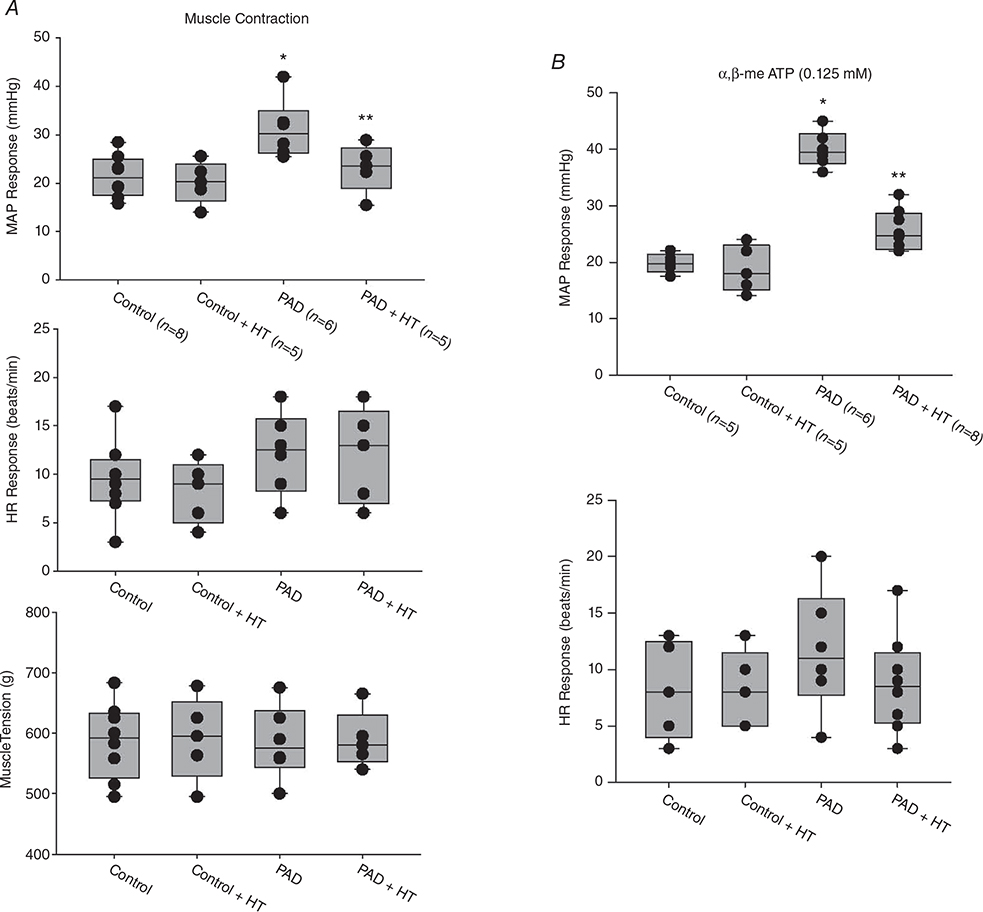
Before static muscle contraction, baseline values of mean arterial pressure (MAP)/HR were 95 ± 14 mmHg/379 ± 25 bpm in control rats (n = 8), 97 ± 18 mmHg/385 ± 29 bpm in control rats + HT (n = 5), 89 ± 19 mmHg/386 ± 37 bpm in PAD rats (n = 6) and 93 ± 13 mmHg/390 ± 36 bpm in PAD rats + HT (n = 5). No significant differences in basal MAP or HR were found among the groups (P > 0.05). As shown in Fig. 4A, a greater MAP response during static muscle contraction was observed in PAD rats (31 ± 12 mmHg in PAD rats/n = 6 vs. 22 ± 8 mmHg in control rats/n = 8; P < 0.05). HT attenuated the amplification of the BP response in PAD rats (MAP response: 23 ± 7mmHgin PAD rats + HT/n=5; P < 0.05 vs. PAD rats not exposed to HT). Note that HT did not significantly attenuate the MAP response to muscle contraction in control rats (P > 0.05; control + HT vs. control). There were insignificant differences in the HR response and developed muscle tension during static contraction among the four groups (P > 0.05).
Figure 4. MAP and HR responses evoked by static muscle contraction and arterial injection of αβ-me ATP.
A, static muscle contraction was evoked by electrical stimulation of the L4 and L5 ventral roots. Muscle tension induced by static muscle contraction was similar among the groups. Compared with that in control rats (n = 8), the MAP response was exaggerated in PAD rats (n = 6). HT significantly attenuated the amplification of the MAP response in PAD + HT rats (n = 5). *P < 0.05 vs. control rats; **P < 0.05 vs. PAD rats. No significant difference was found in the HR response among the groups. B, with administration of the same dosage of αβ-me ATP (0.125 nM), the enhancement in MAP was greater in PAD rats (n = 6) than in control rats (n = 5). *P < 0.05 vs. control rats. A significant attenuation of MAP enhancement was observed in PAD rats exposed to HT (n = 8). **P < 0.05 vs. PAD rats not exposed to HT.
In an additional group, before administration of αβ-me ATP, the baseline values of MAP/HR were 92 ± 18 mmHg/395 ± 27 bpm in control rats (n = 5), 103 ± 23 mmHg/391 ± 31 bpm in control rats + HT (n = 5), 102 ± 22 mmHg/389 ± 24 bpm in PAD rats (n = 6) and 97 ± 20 mmHg/383 ± 42 bpm in PAD rats + HT (n = 8; P > 0.05 among all groups). As shown in Fig. 4B, following αβ-me ATP injection, the MAP response in PAD rats was significantly higher than that in control rats (40 ± 7 mmHg in PAD rats/n = 6 vs. 20 ± 9 mmHg in control rats/n = 5; P < 0.05). Compared with that in PAD rats, the enhancement in the MAP response induced by αβ-me ATP injection in PAD rats + HT (26 ± 9 mmHg/n = 8; P < 0.05 vs. PAD not exposed to HT) was significantly lower. No significant difference in the HR response among the four groups (P > 0.05) was observed after injection of αβ-me ATP.
Discussion
In this study, we examined the effects of HT on the exercise pressor reflex in PAD rats following 72 h of femoral artery occlusion. We further determined the underlying mechanism in terms of the expression and function of P2X receptors following HT. The key findings were that: (1) in PAD rats, baseline Tm is lower, and the expression of P2X3 receptor and P2X currents in DRG neurons are increased following femoral artery occlusion; (2) following HT, the upregulated expression of P2X3 in the DRG and the amplification of P2X current response are suppressed in PAD; and (3) HT alleviates the amplified BP response induced by both static muscle contraction and arterial administration of αβ-me ATP in PAD rats.
Blood flow directed to the hind limb is vital for the maintenance of muscle metabolism and muscle temperature. Due to obstruction of blood flow, decreases in ankle pulse and skin temperature in the affected limb are commonly observed in PAD patients (Bailey et al. 2014). Skin temperature, in part, decreases along with the reduction in muscle temperature (Kenny et al. 2003). Triggered by muscle temperature alterations, the inner environmental metabolites that stimulate the muscle afferents are probably changed (Grey et al. 2006), and this may therefore induce subsequent aberrant neural activity during the exercise pressor reflex. It is well recognized that the exercise pressor reflex in PAD patients is exaggerated. As a result, patients commonly experience a greater blood pressure response to muscle movement than healthy subjects (Baccelli et al. 1999; Bakke et al. 2007; Muller et al. 2012). In recent years, HT has gained increasing attention and has been found to be one of the most economical and effective means to attenuate the BP response in PAD patients. It has been reported that compared with a 33°C water immersion control, thermotherapy by wearing a 48°C leg garment (by increasing the skin temperature to 39–40°C) for 90 min significantly reduces systolic BP by ~11 mmHg and diastolic BP by ~6 mmHg in PAD patients with intermittent claudication (Neff et al. 2016). HT with 42.1 ± 0.6°C of hot water immersion in the lower limb for 30 min also decreases arterial BP, and increases brachial artery blood flow and muscle oxyhaemoglobin in healthy elderly control and PAD patients (Thomas et al. 2017). In a long-term intervention study, 30 min of a 39°C heat spa with a frequency of 3–5 days/week for 12 weeks induced a greater reduction in systolic BP than exercise intervention (~90 min supervised gym-walk per time, 1–2 times/week) (Akerman et al. 2019).
Despite these promising clinical outcomes, the results for human studies could be attributed to both psychological and physiological factors (Pickering et al. 2005). In contrast, when evaluating the peripheral sympathetic and BP responses in animal studies, we can exclude the effects of the CNS by decerebration. Meanwhile, from an ethical perspective, Tm evaluation is invasive, and indirect means of estimating Tm are therefore more preferable for human studies (Brajkovic & Ducharme, 2005; Flouris et al. 2015). Moreover, it is challenging to examine the mechanism of molecular signals in the nervous system of human participants. Nonetheless, in animal studies, more direct and accurate means of monitoring Tm are allowed by inserting a detecting probe into the muscle. In addition, by evaluating receptor alterations in the primary sensory neurons of the DRG, the underlying molecular mechanisms can be studied precisely. Thus, in the present study, we used a rat model of PAD induced by femoral artery occlusion. By completely ligating the femoral artery, a dramatic shortage of blood supply occurs in the lower limb (Waters et al. 2004). Using this model, we first evaluated Tm in both control limbs and ligated limbs. Compared with that in controls, Tm fell by ~1.5°C following femoral artery ligation. Based on the degree of Tm decrease, a heat pad was applied to elevate Tm until it returned to the range that was similar to that of the non-heated control limb. As a consequence, we selected a temperature increment of 1.5°C in the HT groups.
ATP is one of the chief energy sources for muscle contraction. It is mostly located in the intracellular space under normal conditions. However, under ischaemic conditions, ATP efflux through the swelling membrane is increased (Boudreault & Grygorczyk, 2002). In addition, a decrease in Tm induces a decelerated rate of ATP turnover (Grey et al. 2006), which probably leads to an elevation of ATP concentration in the extracellular space.
P2X receptors, which sense ATP, are a group of ionotropic receptors and are mainly present on the peripheral thin fibre nerves and central sensory nerves involved in processing numerous sensory signals (Burnstock, 2017). In general, seven subtypes of P2X receptors (P2X1–P2X7) are included in this family. Specifically, the P2X3 receptor has been discovered to be mainly expressed in DRG neurons and to be less present in other neurons, such as sympathetic, enteric and CNS neurons (Chen et al. 1995). The P2X3 receptor is also involved in the coding process of non-noxious warm stimuli (Souslova et al. 2000). Moreover, expression of the P2X3 receptor is upregulated in the muscle afferents, and its functions are amplified as a result of femoral artery occlusion (Liu et al. 2011; Xing et al. 2013).
It has been found that the protein expression of the P2X3 receptor is increased beginning 24 h after ligation and continues to be elevated 72 h after ligation (Liu et al. 2011). Immunostaining evidence further suggests that the location of P2X3 increase is mainly in the neuronal cells (Xing et al. 2013), rather than other types of cells in the DRG. In the present study, the increase in P2X3 protein expression was similar to that observed by Liu et al. (2011). Importantly, the elevation of the P2X3 expression and its functions were attenuated when HT was applied in PAD rats. With the elevation in Tm (induced by HT in the present study), the rate of ATP turnover was probably enhanced. Moreover, as Tm recovers to the level observed under pre-ischaemic conditions by warming the muscle, the aberrant alterations are also likely to be abolished. Therefore, the augmentation of P2X3 expression and current response was attenuated. Notwithstanding the temperature-dependent nature of the P2X3 receptor, HT may also enhance blood flow directed to the lower limbs (Thomas et al. 2017). This is likely to enhance the elimination of H+ from ischaemic muscle tissue. The acid-sensing ion channel (ASIC) is co-expressed with the P2X3 receptor, and their functions interact (Stephan et al. 2018). The reduction in H+ concentration may reduce the expression and/or functions of the ASIC–P2X3 complex. Without detecting the levels of pH and/or H+ in active muscles, however, this remains a speculation.
After elucidating the alterations in the P2X3 receptor following HT in PAD rats, we examined the subsequent BP response in PAD rats. First, we evaluated the exercise pressor reflex initiated by static muscle contraction. With a similar amount of tension, the BP response to contraction in PAD rats was exaggerated compared with that in control rats. Following HT, the augmentation of the BP response was significantly attenuated. This is consistent with findings in human studies, suggesting that HT has a positive impact on the abnormal exercise pressor reflex. Second, injection of αβ-me ATP is used to determine whether stimulation of P2X3 was involved in the BP response during the HT process. Similar to previous findings (Hanna et al. 2002; Li & Sinoway, 2002), αβ-me ATP injected into the femoral artery stimulated the P2X3 receptor in the muscle afferents and this injection amplified arterial BP in PAD rats. With HT, the enhancement of the BP response was also remarkably suppressed in PAD rats. This supported our assumption that HT has a beneficial effect on the amplified exercise pressor reflex in PAD rats probably via a reduction in the activity of the P2X receptor pathway.
A previous study has shown that arterial injection of an antagonist of the P2X receptor attenuates the BP responses to muscle contraction in rats with 72 h of femoral artery occlusion (Stone et al. 2014). Thus, in the current study, we did not examine whether blockade of the P2X receptor would attenuate the exaggerated pressor response in PAD rats or PAD rats exposed to HT. In the current study, we observed that HT partly reduces the expression and function of the P2X receptor in PAD rats. We speculated that a P2X antagonist would attenuate the BP response to muscle contraction in both PAD rats and PAD rats exposed to HT, and the effects of blocking the P2X receptor would be smaller in PAD rats exposed to HT. This result would indicate that there is a reduced P2X receptor response in PAD rats exposed to HT than in PAD rats not exposed to HT, probably due to a reduction in the activity of the P2X receptor pathway. Nevertheless, our current study has provided evidence showing decreases in the expression of the P2X receptor and amplitude of the P2X currents in PAD rats exposed to HT.
Study limitation
We used a rat model of femoral artery occlusion to evoke the exercise pressor reflex via static muscle contraction. Static exercise is not a surrogate for the more dynamic forms of activity performed by PAD patients (i.e. walking); thus, care should be taken when extrapolating the findings of the current study to dynamic exercise. This also suggests the need to incorporate animal and human experiments to explore thematically linked questions. Additionally, limb blood flow was not measured in the current study. Therefore, we cannot discern the effects of heat on the development of intermittent claudication during exercise in PAD. Moreover, Tm was increased by the same magnitude (i.e. 1.5°C) in both control and PAD rats during the heating protocol. One may therefore question whether increasing Tm to the same absolute level in PAD animals as that in control animals (i.e. 37°C) would have elicited even greater improvements in BP and further reductions in P2X3 receptor expression. It is likely that the lower P2X3 expression induced by increasing Tm to a greater degree would lead to the lower BP response. However, a greater increase in Tm is also likely to stimulate muscle mechanosensitive afferents. A previous study showed that local heating (increasing the forearm Tm from ~34°C to 39°C) of an isometric exercising forearm muscle group augmented the increase in muscle SNA during fatiguing exercise (Ray & Gracey, 1997). Thus, in the current study, we decided to raise Tm by ~1.5°C, which is likely to lead to different effects on the muscle afferents at the receptor level, allowing us to study the P2X receptor in the exercise pressor reflex in PAD after HT. An additional limitation of this study is that only male animals were included because sex discrepancies were beyond the scope of our current study. To address the issue of sex discrepancies requires further studies.
Conclusion
The results of the present study demonstrate that HT attenuates the amplified pressor response evoked by static muscle contraction and by stimulation of the P2X receptor in muscle afferents in PAD rats. HT has beneficial effects on the exaggerated exercise pressor reflex in PAD, and a reduction in the activity of the P2X3 receptor pathway is probably a part of the mechanism mediating this improvement.
Supplementary Material
Translational perspective.
We tested the hypothesis that prior heat exposure (heat treatment) plays a beneficial role in modifying the exaggeration of the exercise pressor reflex in peripheral artery disease partly via a reduction in the activity of the P2X receptor pathway. We found that prior heat exposure attenuates the upregulation of the P2X receptor and the amplified amplitude of P2X currents in dorsal root ganglion neurons of rats with femoral artery occlusion. We further observed that heat treatment alleviates the amplified blood pressure response to static muscle contraction and to stimulation of P2X in muscle afferent nerves in occluded rats. Data from the current animal study provide receptor mechanisms (e.g. P2X in muscle afferents), which have implications for future research examining the long-term effects of heat treatment on the exaggerated sympathetic responsiveness observed in patients with peripheral artery disease and thereby leading to improvement of walking ability in this disease.
Key points.
During exercise, the blood pressure (BP) response is exaggerated in peripheral artery disease (PAD).
We examined whether heat treatment (HT) has beneficial effects on the exaggerated exercise pressor reflex in PAD rats.
With HT (increase in basal muscle temperature of ~1.5°C for 30 min, twice daily for three continuous days), the amplified BP response to muscle contraction is alleviated in PAD.
We demonstrated that HT attenuates the enhancement of the BP response induced by stimulation of P2X in muscle afferent nerves of PAD rats. HT also attenuates the upregulation of the P2X3 and the increase in P2X currents in the muscle afferent neurons of PAD rats.
Previous heat exposure plays a beneficial role in modifying the exaggeration of the exercise pressor reflex in PAD and a reduction in the activity of the P2X receptor pathway is probably a part of the mechanism mediating this improvement.
Acknowledgements
The authors thank Chunying Yang for outstanding technical assistance.
Funding
This study was supported by NIH P01 HL134609 and R01 HL141198.
Biography
 Lu Qin received her PhD from The Chinese University of Hong Kong in 2017. Dr Qin is currently a postdoctoral scholar at the Heart & Vascular Institute in the Pennsylvania State University College of Medicine at Hershey. Her long-term research goals are to examine neural control pathways of the circulation during exercise, and interventions for improvements of the abnormal neural responses to exercise in cardiovascular diseases, especially peripheral artery disease.
Lu Qin received her PhD from The Chinese University of Hong Kong in 2017. Dr Qin is currently a postdoctoral scholar at the Heart & Vascular Institute in the Pennsylvania State University College of Medicine at Hershey. Her long-term research goals are to examine neural control pathways of the circulation during exercise, and interventions for improvements of the abnormal neural responses to exercise in cardiovascular diseases, especially peripheral artery disease.
Footnotes
Competing interests
None.
Additional information
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Statistical Summary Document
References
- Akerman AP, Thomas KN, van Rij AM, Body ED, Alfadhel M & Cotter JD (2019). Heat therapy vs. supervised exercise therapy for peripheral arterial disease: a 12-wk randomized, controlled trial. Am J Physiol Heart Circ Physiol 316, H1495–H1506. [DOI] [PubMed] [Google Scholar]
- Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S & Catalano M (1999). The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50, 361–374. [DOI] [PubMed] [Google Scholar]
- Bailey MA, Griffin KJ & Scott DJ (2014). Clinical assessment of patients with peripheral arterial disease. Semin Intervent Radiol 31, 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakke EF, Hisdal J, Jorgensen JJ, Kroese A & Stranden E (2007). Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33, 20–25. [DOI] [PubMed] [Google Scholar]
- Boudreault F & Grygorczyk R (2002). Cell swelling-induced ATP release and gadolinium-sensitive channels. Am J Physiol Cell Physiol 282, C219–226. [DOI] [PubMed] [Google Scholar]
- Brajkovic D & Ducharme MB (2005). Confounding factors in the use of the zero-heat-flow method for non-invasive muscle temperature measurement. Eur J Appl Physiol 94, 386–391. [DOI] [PubMed] [Google Scholar]
- Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, Lohse MJ & Hein L (2002). Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation 106, 2491–2496. [DOI] [PubMed] [Google Scholar]
- Burnstock G (2017). Purinergic signalling: therapeutic developments. Front Pharmacol 8, 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G &Wood JN (1995). A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377, 428–431. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB & Rector T (1984). Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311, 819–823. [DOI] [PubMed] [Google Scholar]
- Collins MN & Billman GE (1989). Autonomic response to coronary occlusion in animals susceptible to ventricular fibrillation. Am J Physiol Heart Circ Physiol 257, H1886–H1894. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM & Perez-Gonzalez JF (1971). The reflex nature of the pressor response to muscular exercise. J Physiol 215, 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouris AD, Dinas PC, Tsitoglou K, Patramani I, Koutedakis Y & Kenny GP (2015). Non-invasive measurement of tibialis anterior muscle temperature during rest, cycling exercise and post-exercise recovery. Physiol Meas 36, N103–113. [DOI] [PubMed] [Google Scholar]
- Gao Z, Kehoe V, Xing J, Sinoway L & Li J (2006). Temperature modulates P2X receptor-mediated cardiovascular responses to muscle afferent activation. Am J Physiol Heart Circ Physiol 291, H1255–1261. [DOI] [PubMed] [Google Scholar]
- Garcia-Villalon AL, Padilla J, Monge L, Fernandez N, Gomez B & Dieguez G (1997). Role of the purinergic and noradrenergic components in the potentiation by endothelin-1 of the sympathetic contraction of the rabbit central ear artery during cooling. Br J Pharmacol 122, 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI & Mitchell JH (1972). Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol (London) 226, 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SR, De Vito G, Nimmo MA, Farina D & Ferguson RA (2006). Skeletal muscle ATP turnover and muscle fiber conduction velocity are elevated at higher muscle temperatures during maximal power output development in humans. Am J Physiol Regul Integr Comp Physiol 290, R376–382. [DOI] [PubMed] [Google Scholar]
- Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC & O’Leary DS (2001). Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol 90, 55–61. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Hayes SG & Kaufman MP (2002). αβ-Methylene ATP elicits a reflex pressor response arising from muscle in decerebrate cats. J Appl Physiol (1985) 93, 834–841. [DOI] [PubMed] [Google Scholar]
- Kaufman MP & Forster HV (1996). Reflexes controlling circulatory, ventilatory and airway responses to exercise In Handbook of Physiology - Section 12, Exercise: Regulation and Integration of Multiple Systems, ed. Rowell LB & Shepherd JT, pp. 381–447. Oxford University Press, New York. [Google Scholar]
- Kenny GP, Reardon FD, Zaleski W, Reardon ML, Haman F & Ducharme MB (2003). Muscle temperature transients before, during, and after exercise measured using an intramuscular multisensor probe. J Appl Physiol (1985) 94, 2350–2357. [DOI] [PubMed] [Google Scholar]
- Kluess HA, Buckwalter JB, Hamann JJ & Clifford PS (2005). Elevated temperature decreases sensitivity of P2X purinergic receptors in skeletal muscle arteries. J Appl Physiol (1985) 99, 995–998. [DOI] [PubMed] [Google Scholar]
- Li J & Sinoway LI (2002). ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol 283, H2636–2643. [DOI] [PubMed] [Google Scholar]
- Li J & Xing J (2012). Muscle afferent receptors engaged in augmented sympathetic responsiveness in peripheral artery disease. Front Physiol 3, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li JD, Lu J, Xing J & Li J (2011). Contribution of nerve growth factor to upregulation of P2X3 expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 301, H1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Xing J & Li J (2013). Bradykinin B2 receptor contributes to the exaggerated muscle mechanoreflex in rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 304, H1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ & Clark E (2004). Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA 292, 453–461. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP & Iwamoto GA (1983). The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol 45, 229–242. [DOI] [PubMed] [Google Scholar]
- Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB & Sinoway LI (2012). Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590, 6237–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff D, Kuhlenhoelter AM, Lin C, Wong BJ, Motaganahalli RL & Roseguini BT (2016). Thermotherapy reduces blood pressure and circulating endothelin-1 concentration and enhances leg blood flow in patients with symptomatic peripheral artery disease. Am J Physiol Regul Integr Comp Physiol 311, R392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG & Roccella EJ (2005). Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111, 697–716. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A & Coats AJ (1999). A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J 137, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ & Piepoli MF (2001). Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation 104, 2324–2230. [DOI] [PubMed] [Google Scholar]
- Ray CA & Gracey KH (1997). Augmentation of exercise-induced muscle sympathetic nerve activity during muscle heating. J Appl Physiol 82, 1719–1725. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Naylor HL, Hogeman CS & Sinoway LI (1999). Blood flow dynamics in heart failure. Circulation 99, 3002–3008. [DOI] [PubMed] [Google Scholar]
- Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R & Zelis R (1989). Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol (1985) 66, 429–436. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH & Garry MG (2001). Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537, 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR & Rudan I (2019). Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health 7, e1020–e1030. [DOI] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenuis-Oosthuizen D, Smith AJ, Kidd EJ &Wood JN (2000). Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 407, 1015–1017. [DOI] [PubMed] [Google Scholar]
- Stephan G, Huang L, Tang Y, Vilotti S, Fabbretti E, Yu Y, Norenberg W, Franke H, Goloncser F, Sperlagh B, Dopychai A, Hausmann R, Schmalzing G, Rubini P & Illes P (2018). The ASIC3/P2X3 cognate receptor is a pain-relevant and ligand-gated cationic channel. Nat Commun 9, 1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AJ, Yamauchi K & Kaufman MP (2014). Purinergic 2X receptors play a role in evoking the exercise pressor reflex in rats with peripheral artery insufficiency. Am J Physiol Heart Circ Physiol 306, H396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KN, van Rij AM, Lucas SJ & Cotter JD (2017). Lower-limb hot-water immersion acutely induces beneficial hemodynamic and cardiovascular responses in peripheral arterial disease and healthy, elderly controls. Am J Physiol Regul Integr Comp Physiol 312, R281–R291. [DOI] [PubMed] [Google Scholar]
- Vanoli E & Schwartz PJ (1990). Sympathetic–parasympathetic interaction and sudden death. Basic Res Cardiol 85 (Suppl 1), 305–321. [DOI] [PubMed] [Google Scholar]
- Victor RG, Bertocci LA, Pryor SL & Nunnally RL (1988). Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82, 1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop TG, Eldridge FL, Iwamoto GA & Mitchell JH (1996). Central neural control of respiration and circulation during exercise In Handbook of Physiology - Section 12, Exercise: Regulation and Integration of Multiple Systems, ed. Rowell LB & Shepherd JT, pp. 333–380. Oxford University Press, New York. [Google Scholar]
- Waters RE, Terjung RL, Peters KG & Annex BH (2004). Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol (1985) 97, 773–780. [DOI] [PubMed] [Google Scholar]
- Wilson JR & Mancini DM (1993). Factors contributing to the exercise limitation of heart failure. J Am Coll Cardiol 22, 93A–98A. [DOI] [PubMed] [Google Scholar]
- Xing J, Lu J & Li J (2013). Augmented P2X response and immunolabeling in dorsal root ganglion neurons innervating skeletal muscle following femoral artery occlusion. J Neurophysiol 109, 2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J & Li J (2018). Role of TNF-α in regulating the exercise pressor reflex in rats with femoral artery occlusion. Front Physiol 9, 1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziganshin AU, Rychkov AV, Ziganshina LE & Burnstock G (2002). Temperature dependency of P2 receptor-mediated responses. Eur J Pharmacol 456, 107–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.