Abstract
Background
The role of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) for hepatocellular carcinoma (HCC) remains controversial.
Methods
The records of 23 consecutive patients with hepatitis B virus (HBV)-related HCC who underwent ALPPS at our center between November 2013 and June 2018 were retrospectively reviewed. Oncological results were compared between patients who received ALPPS and those that received transarterial chemoembolization (TACE) using propensity score matching (PSM) analysis.
Results
In patients with a single tumor (n=12) the median tumor diameter was 13.0 (range: 5.1–20.0) cm, whereas in patients with multiple tumors (n=11) the median total tumor diameter was 6.3 (range: 2.3–26.0) cm. After the stage-1 ALPPS, the median future liver remnant (FLR) increased by 50.0%. The stage-2 ALPPS was completed in 20 patients (87.0%) after a median of 12 days. The 90-day mortality rate was 13% (3/23). The overall survival (OS) rates at 1-, 2-, and 5-year were 61.1%, 34.9%, and 8.7%, respectively, whereas the disease-free survival (DFS) rates at 1-, 2-, and 5-year were 27.8%, 27.8%, and 0.0%, respectively. PSM analysis showed no difference in OS between patients who underwent ALPPS and those that received TACE [P=0.178, Barcelona Clinic Liver Cancer (BCLC) stage A–C patients; P=0.241, BCLC stage B and C patients].
Conclusions
ALPPS is a safe and effective treatment option for unresectable HBV-related HCC. However, for HBV-related intermediate and advanced HCC patients, ALPPS may not be superior to TACE.
Keywords: Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), hepatocellular carcinoma (HCC), transarterial chemoembolization (TACE), propensity score matching (PSM)
Introduction
Hepatocellular carcinoma (HCC) is now the fourth most common cause of cancer-related deaths worldwide. About 78,000 patients died from HCC in 2018 (1,2). Though surgical resection is still the mainstay treatment for patients with HCC, unfortunately only 20% to 30% of patients are candidates for liver resection (3). One of the pivotal reasons for the low resection rate is an insufficient future liver remnant (FLR) volume and the risk of post-hepatectomy liver failure (PHLF) because approximately 80% of HCCs are associated with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infections, liver fibrosis, and/or cirrhosis (4,5).
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is considered one of the most important recent surgical innovations in liver surgical oncology because the technique induces a rapid increase in the FLR volume (40% to 160% within 1 to 2 weeks) (6-9). As such, this makes the tumor resection an option in patients with an FLR that has been determined to be insufficient. However, ALPPS has been associated with high operative morbidity and mortality. Although some statistics in the ALPPS registry indicated a favorable outcome rate of 98.4% and a mortality rate of 8.8% (10), a majority of patients included in this analysis were patients with liver metastasis from colorectal cancer. In patients with primary liver cancer, the international ALPPS registry demonstrated a 90-day mortality rate of 31% (35 patients included in the analysis) (11). In other studies with fewer patients, the 90-day mortality rates ranged from 0% to 12.5% (12-22). Therefore, caution is recommended when considering HCC patients for ALPPS, and some studies have even suggested that HCC is a relative contraindication for ALPPS (19).
Wang et al. (23) recently reported the overall survival (OS) of 45 patients with HCC who received ALPPS was remarkably superior to those treated with transarterial chemoembolization (TACE) (1- and 3-year OS rates after ALPPS of 64.2% and 60.2%, respectively, and 1- and 3-year OS rates after TACE of 50.0% and 7.1%, respectively, P=0.004, Kaplan-Meier log-rank test) and similar to those after one-stage liver resection (1- and 3-year OS rates after one-stage liver resection of 68.9% and 43.5%, respectively, P=0.514, Kaplan-Meier log-rank test). Thus, the authors considered ALPPS as a viable treatment option for patients with unresectable HCC. However, data of ALPPS outcomes for the treatment of HCC are still limited to a few case reports and small cohort studies.
The purpose of this present study was to report our experience of using ALPPS to treat patients with unresectable HBV-related HCC and to compare the outcomes of ALPPS with TACE using propensity score matching (PSM) analysis.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-2420).
Methods
All the procedures in this study were conducted following the Declaration of Helsinki (as revised in 2013) and arranged strictly with the approval of the First Affiliated Hospital of Sun Yat-sen University Ethics Committee [2015(124)] and informed written consent was taken from all the patients.
Patients
The records of patients with HBV-related HCC treated at the First Affiliated Hospital of Sun Yat-sen University from November 2013 to June 2018 were retrospectively reviewed. Patients included in the analysis were HBV surface antigen (HBsAg)-positive, or they had detectable HBV DNA, or were both HBV e-antibody (HBeAb) and HBV c-antibody (HBcAb) positive. All patients were negative for anti-HCV antibody. All patients received routine preoperative computed tomography (CT) or magnetic resonance imaging (MRI).
Patients were stratified according to the Barcelona Clinic Liver Cancer (BCLC) staging system (2). Surgical and anatomic designations were based on Couinaud’s classification of the liver and the Brisbane 2000 Terminology of Liver Anatomy and Resections (24). The surgical techniques used to perform ALPPS were those that have been described elsewhere (25). Postoperative complications (POCs) were defined according to Clavien-Dindo criteria (26). Patients were defined as having postoperative liver insufficiency when at least two of the following parameters were observed including serum total bilirubin (TBIL) >60 µmol/L, a prothrombin time (PT) rate <30% of the normal level, alteration of consciousness, and asterixis (27). The postoperative hospital stay was calculated from the date of surgery to the date of discharge. The overall hospital stay was defined as the sum of days that a patient stayed at the hospital.
Hematoxylin-eosin (HE) stained slides and immunohistochemical (IHC) analysis of surgical specimens were reviewed, and fibrosis was scored according to the METAVIR scoring system (28). Briefly, a score of 0 to 4 was given according to the degree of fibrosis: 0 (no fibrosis), 1 (mild fibrosis), 2 (moderate fibrosis), 3 (severe fibrosis), and 4 (cirrhosis). Tumor pathological grade was based on the Edmondson-Steiner grading system (29). The albumin-bilirubin (ALBI) score, a marker of the liver functional reserve, was calculated (30). The diagnosis of tumor recurrence was based on clinical examination, laboratory data, and radiological examinations [MRI, CT, and positron emission thermography (PET) scan].
Volumetric assessment
About 1 week after the stage-1 ALPPS, CT, or MRI was performed to measure liver volumes. CT data were analyzed and reconstructed using medical image analysis software (Myrian XP Liver; Intrasense; France). MRI data were analyzed and reconstructed using a 3.0-T system (Siemens Healthineers) with an 8-channel phased-array coil and scanning. The standard liver volume (SLV) was calculated by the Urata formula (31). When the FLR/SLV ratio reached >40%, performing the stage-2 ALPPS was considered to be safe for patients with cirrhosis, whereas a ratio >30% was sufficient for patients with no evidence of cirrhosis. The correlation between the FLR volume at the baseline (V0) and after the stage-1 ALPPS (V1) was calculated by the formula: %FLR volume increase = (V1 – V0)/V0 ×100 to evaluate the FLR volume increment (32). The kinetic growth rate (KGR), which reflects the daily increase in the volume of the FLR, was also calculated.
Follow-up
After discharge, patients were seen in the clinic monthly for the first 6 months, and then every 3 months, as described in our previous study (33). Telephone follow-up was also performed every 6 months.
PSM analysis
PSM was used to compare the outcomes of patients who underwent ALPPS with those treated by TACE during the same period. TACE was performed as described elsewhere (34). PSM was used to minimize the effect of confounding influences on measured covariates. ALPPS and TACE were matched 1:1 as closely as possible using the following variables: tumor number, tumor diameter, ascites, portal vein tumor thrombus (PVTT), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and age. The covariate balance of the matched cohorts was assessed by Student’s t-test, Mann-Whitney U test, Chi-Squared, or two-tailed Fisher exact tests. The primary endpoint was the OS. The mortality rates after treatment were also calculated.
Statistical analysis
Continuous variables with normal distribution were expressed as mean ± standard deviation, or median with range for non-normally distributed data. Continuous data were compared with the parametric Student’s t-test when normality and homogeneity of variance assumptions were satisfied; otherwise, the non-parametric Mann-Whitney U test was used. For categorical variables, unordered categorical variables were analyzed by Fisher’s exact or Chi-Square test, and ordinal categorical variables were analyzed by the non-parametric Mann-Whitney U test. Repeated measures ANOVA was used for comparisons of repeated measurements of biochemical parameters. Comparisons were first performed using Mauchly’s test of sphericity; if the value of P was >0.05, one-way ANOVA was used, otherwise, the Greenhouse-Geisser method was used to correct the one-way ANOVA. Correlation analysis was performed to identify relative factors for the KGR. Logistic regression analysis was performed to identify independent risk factors for complications and mortality. OS was defined as the time from the date of the stage-2 ALPPS until death or last follow-up and disease-free survival (DFS) was defined as the time from the date of the stage-2 ALPPS to initial tumor recurrence, metastasis or death. The survival analysis was conducted using the Kaplan-Meier method and compared with the log-rank test. Univariate and multivariate Cox regression analyses were applied to examine prognostic factors significantly associated with OS and DFS. All statistical analyses were performed using SPSS statistical software, version 23.0 (IBM, Chicago, IL, USA) and line graphs were drawn by GraphPad Prism Version 8 (San Diego, CA, USA). A two-sided value of P<0.05 was considered to be statistically significant.
Results
Patients
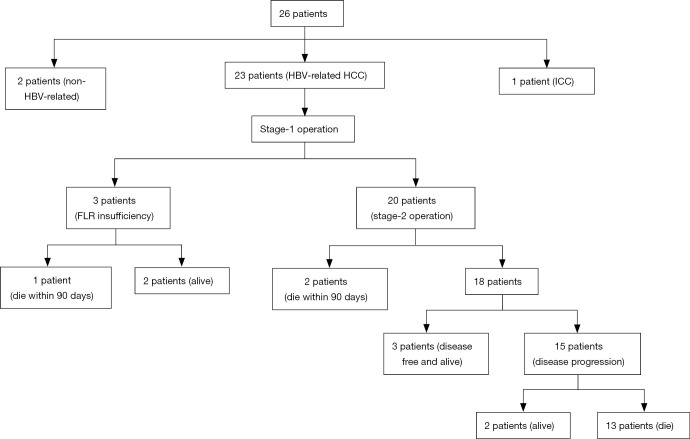
Twenty-six patients underwent ALPPS for HCC (n=25) and intrahepatic cholangiocarcinoma (ICC) (n=1) during the study period. Among the 26 patients, 23 patients with HBV-related HCC were analyzed (Figure 1), including 22 male patients (95.7%) and 1 female patient (4.3%). The median age of the 23 patients was 40 (range: 32–66) years. Twelve patients had a single tumor with a median diameter of 13.0 (range: 5.1–20.0) cm, and 11 patients had multiple tumors with a median diameter of 6.3 (range: 2.3–26.0) cm. According to the BCLC staging system, the number of stage A, B, and C patients was 7 (30.4%), 6 (26.1%), and 10 (43.5%), respectively. Seven (30.4%) patients had cirrhosis (METAVIR grade IV) (Table 1). PVTT, microvascular invasion (MVI), and lymph node metastasis (LNM) were present in 7 (30.4%), 6 (26.1%) and 5 (21.7%) patients, respectively. Among them, there were 2 patients with both PVTT and LNM, 2 patients with both PVTT and MVI and 1 patient with both LNM and MVI (Table 2).
Figure 1.
Flow chart of the patients who were treated by ALPPS at our center. ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; FLR, future liver remnant.
Table 1. Preoperative patient characteristics.
| Patient characteristics | Patient data |
|---|---|
| Age, median [range], y | 40 [32–66] |
| Sex, male/female, n (%) | 22/1 (95.7/4.3) |
| Single tumor (n=12) | |
| Diameter of tumor, median (range), cm | 13.0 (5.1–20.0) |
| Multiple tumors (n=11) | |
| Sum of diameters, median (range), cm | 6.3 (2.3–26.0) |
| BCLC staging, n (%) | |
| A (early) | 7 (30.4) |
| B (intermediate) | 6 (26.1) |
| C (advanced) | 10 (43.5) |
| METAVIR staging of liver fibrosis, n (%) | |
| 1 (mild fibrosis) | 4 (17.4) |
| 2 (moderate fibrosis) | 5 (21.7) |
| 3 (severe fibrosis) | 3 (13.0) |
| 4 (cirrhosis) | 7 (30.4) |
| Unclear† | 4 (17.4) |
| Edmondson-Steiner grading, n (%) | |
| II | 9 (39.1) |
| II–III | 5 (21.7) |
| III | 4 (17.4) |
| Unclear† | 5 (21.7) |
| ALBI grading‡, n (%) | |
| I | 13 (56.5) |
| II | 10 (43.5) |
| Type of ALPPS§, n (%) | |
| Right trisectionectomy ALPPS | 7 (30.4) |
| Right hemihepatectomy ALPPS | 11 (47.8) |
| Extended right hemihepatectomy ALPPS | 2 (8.7) |
†, The liver tissue submitted for pathological examination could not be discriminated due to large coagulation necrosis. ‡, The ALBI grading indicates liver functional reserve. §, Three patients did not complete the stage-2 ALPPS operations. BCLC, Barcelona Clinic Liver Cancer; ALBI, albumin-bilirubin; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy.
Table 2. Correlations among PVTT, MVI and LNM.
| PVTT | Cases, n (%) | MVI, n (%) | P value | LNM, n (%) | P value | MVI | Cases, n (%) | LNM, n (%) | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |||||||
| Yes | 7 (30.4) | 2 (28.6) | 5 (71.4) | 1 | 2 (28.6) | 5 (71.4) | 0.621 | Yes | 6 (26.1) | 1 (16.7) | 5 (83.3) | 1 |
| No | 16 (69.6) | 4 (25.0) | 12 (75.0) | 3 (18.8) | 13 (81.2) | No | 17 (73.9) | 4 (23.5) | 13 (76.5) | |||
PVTT, portal vein tumor thrombus; MVI, microvascular invasion; LNM, lymph node metastasis.
According to the ALBI, 13 (56.5%) patients were grade I and 10 (43.5%) were grade II. Of the 23 patients, 3 patients (2 patients with BCLC stage B; 1 patient with BCLC stage C) did not undergo the stage-2 ALPPS due to an insufficient FLR volume. Thus, 20 patients (87.0%, 20/23) received the stage-2 ALPPS a median of 12 (range: 7–17) days after the first stage (Table S1). Of the 20 patients, there were 7 (30.4%) right trisectorectomies, 11 (47.8%) right hepatectomies, and 2 (8.7%) extended right hemihepatectomies.
Liver volumes
Liver volumes pre- and post-stage-1 and FLR volume increase are shown in Table 3, Table S2. The median FLR volume increase was 50.0% (range: 15.9–94.9%). The median absolute KGR was 20.1 (range: 2.8–46.6) mL/day, and the median relative KGR (RKGR) was 5.1% (range: 1.1–13.6%) per day.
Table 3. Pre- and post-stage 1 operative increases of FLR volume.
| Variables | Median [range] |
|---|---|
| SLV (Urata formula, mL) | 1,241 [1,060–1,348] |
| Pre-stage-1 operation | |
| FLR (mL) | 330 [207–510] |
| FLR/SLV (%) | 28 [16–39] |
| Post-stage-1 operation | |
| FLR (mL) | 493 [302–706] |
| FLR/SLV (%) | 41 [23–54] |
| FLR increase post-stage-1 operation (mL) | 176.0 [45.2–3260.0] |
| FLR increase post-stage-1 operation (%) | 50.0 [15.9–94.9] |
| Absolute KGR (mL/day) | 20.1 [2.8–46.6] |
| RKGR (%/day) | 5.1 [1.1–13.6] |
FLR, future liver remnant; SLV, standard liver volume; KGR, kinetic growth rate; RKGR, relative KGR.
We further investigated the relations between RKGR and short-term overall poor outcomes (stage-1 POC, stage-1 postoperative hepatic insufficiency and 90-day mortality). The optimal cut-off value of the RKGR was calculated to be 0.0504, corresponding to maximum sensitivity and specificity of the RKGR for predicting short-term overall poor outcomes in receiver operating characteristic (ROC) curve analysis [Youden index =0.356; area under the ROC curve (AUC) =0.678; 95% confidence interval (CI): 0.420–0.936), P=0.233]. Patients were then categorized as RKGR-high (RKCG >0.0504) or RKGR-low (RKGR ≤0.0504) (Figure S1). The analysis indicated there was no significant correlation between RKGR and short-term overall poor outcomes (P=0.317). In addition, there were no statistical differences in the levels of ALT (P=0.758), AST (P=0.399), TBIL (P=0.406), albumin (ALB) (P=0.384), PT (P=0.119) and international normalized ratio (INR) (P=0.633) between the RKGR-high group and the RKGR-low group.
Intraoperative and postoperative data
For stage-1 ALPPS, the median operating time was 271 min and the median blood loss was 200 mL, while for stage-2 ALPPS the values were 205 min and 300 mL, respectively. Intraoperative RBC transfusions occurred in 13% (3/23) patients during the stage-1 operation and in 35% (7/20) patients during the stage-2 operation. The largest volume of RBC transfusion was only 4 units (during the stage-1 and the stage-2). During the stage-1 procedure, blood loss in patients with multiple tumors (median: 600 mL, range: 200–2,000 mL) was greater than that in patients with a single tumor (median: 100 mL, range: 30–800 mL) (P=0.004). The median postoperative hospital stay was 13 (range: 8–34) days, and the median overall hospital stay was 33 (range: 22–62) days (Table S1).
Overall, there were six POCs, including three cases after the stage-1 procedure and three cases after the stage-2 procedure. After the first stage, two patients had grade I complications (hepatic and renal insufficiency, and pleural effusion), and one patient had a grade II complication (peritoneal abscess). After the second stage, two patients had grade II complications (hepatic insufficiency), and one patient had a grade III complication (Thoracic and abdominal effusion) (Table S1).
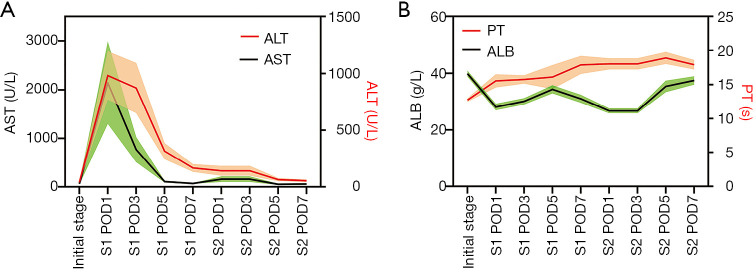
Laboratory data after stage-1 and stage-2, such as AST, ALT, ALB and PT, are shown in Figure 2. Logistic regression showed that PT was an independent predictor of POC after the stage-1 operation (P=0.044) [logit(P) = –26.561 + 1.852 × PT].
Figure 2.
Changes in laboratory findings after ALPPS procedures. (A) Dual Y-axes represented AST (black) on the left Y-axis and ALT (red) on the right. Green and orange areas indicated the error range. After the stage-1 operation, there were sharp increases and quick drops in AST and ALT. After the stage-2 operation, AST and ALT showed no inordinate increases and gradually returned to normal; (B) dual Y-axes represented ALB (black) on the left Y-axis and PT (red) on the right. Green and orange areas indicated the error range. ALB showed a marked fall in the first day after stage-1 and stage-2 operation, but steadily increased after that. PT rose from the first day after the stage-1 operation and had been at a relatively high level since then. ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALB, albumin; PT, prothrombin time; S1, stage-1 operation; S2, stage-2 operation; POD, post-operative day.
Outcomes
Three patients died within 90 days after ALPPS, including one patient with BCLC stage B after stage-1 operation; one patient with BCLC stage B and one patient with stage C after the stage-2 operation due to HCC recurrence or progression. Therefore, the 90-day mortality rate was 13% (3/23). These two patients that died within 90 days after the stage-2 operation and the three patients without undergoing the stage-2 procedure were excluded from the survival analysis. The median OS of the remaining 18 patients was 19.0 (95% CI: 2.6–35.4) months, and the 1-, 2-, and 5-year OS rates were 61.1%, 34.9%, and 8.7%, respectively. The median DFS was 5.0 (95% CI: 3.3–6.7) months, and the 1-, 2-, and 5-year DFS rates were 27.8%, 27.8%, and 0.0%, respectively. Fifteen patients developed local recurrences and/or distant metastases during follow-up. Thirteen of the 15 patients died from tumor progression, whereas the remaining two patients were alive with recurrence at the time of the censor of this study.
Univariate cox analysis showed that ALT (P=0.046) and AST (P=0.021) were significant predictors of OS, and PVTT (P=0.037), ascites (P=0.043) and AST (P=0.018) were significant predictors of DFS. Multivariate analysis showed that number of lesions (P=0.035) and AST (P=0.008) were independent risk factors for OS.
During the study period, 76 patients with HBV-related advanced HCC (BCLC stage A–C) were treated with TACE. Fourteen of these patients were selected to match as closely as possible to 14 patients undergoing ALPPS on the known risk predictors of long-term survival. The baseline characteristics of the two groups of patients are shown in Table S3.
The 1- and 2-year OS rates of patients treated with ALPPS were 50.0% and 30.0%, respectively, and the 1- and 2-year OS rates of patients treated with TACE were 84.6% and 65.8%, respectively. The median OS of patients treated with TACE was 30.0 (95% CI: 1.1–58.9) months, and the median OS of patients treated with ALPPS was 8.0 (95% CI: 0.0–20.9) months. However, the PSM analysis showed that there was no difference in the long-term survival outcome between patients who received ALPPS and those that received TACE (P=0.178) (Figure 3).
Figure 3.
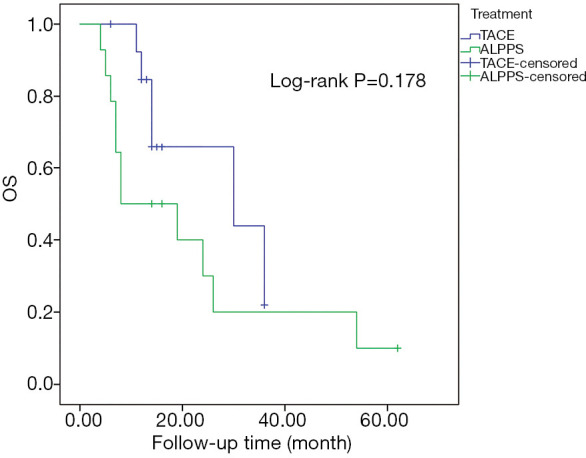
The OS rates of HBV-related HCC patients using PSM comparative analyses of ALPPS with TACE. There was no statistical difference in OS rates of HBV-related HCC patients between ALPPS and TACE (P=0.178). OS, overall survival; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; PSM, propensity score matching; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; TACE, transarterial chemoembolization.
Another PSM was conducted in which 10 BCLC stage B and C patients treated with TACE were matched to 10 BCLC stage B and C patients with ALPPS. The baseline characteristics of the two groups of patients are shown in Table S4. The 1- and 2-year OS rates of patients who received ALPPS were 50.0% and 16.7%, respectively, and the 1- and 2-year OS rates of patients treated with TACE were 88.9% and 25.0%, respectively. The median OS of patients who received TACE was 19.0 (95% CI: 11.1–26.9) months and the median OS of patients that received ALPPS was 8.0 (95% CI: 0.0–18.1) months. Likewise, the PSM analysis also showed there was no difference in the long-term survival outcome between patients with intermediate/advanced HCC receiving ALPPS and TACE (P=0.241) (Figure 4).
Figure 4.
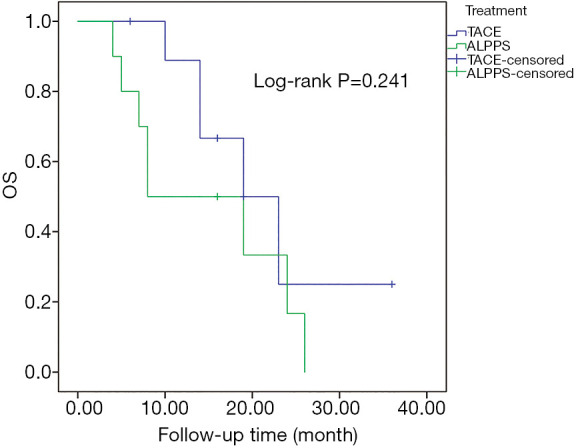
The OS rates of BCLC stage B and C HBV-related HCC patients using PSM comparative analyses of ALPPS with TACE. No statistical difference was observed in OS rates of HBV-related HCC patients between ALPPS and TACE (P=0.241). OS, overall survival; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; PSM, propensity score matching; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; TACE, transarterial chemoembolization.
Discussion
High postoperative morbidity and mortality rates have been reported for HCC patients who are treated with ALPPS. Analysis of the International ALPPS Registry indicated a 90-day mortality rate of 31% (11). López-López et al. reviewed the outcomes of 101 HCC patients who received ALPPS and reported that the morbidity rate ≥ III was 20.7%, and the postoperative mortality rate was 16.1% (35). Wang et al. (23) studied 45 HCC patients who received ALPPS and reported a stage-1 morbidity rate of 37.8%, a stage-2 morbidity rate of 56.1%, and a 90-day mortality rate of 11.1%. In our study, the median FLR volume increase, the completion rate, and the 90-day mortality rate were 50.0%, 87.0%, and 13.0%, which are similar to the results reported by Wang et al. (56.8%, 91.1%, and 11.1%, respectively).
In our study, there were no procedure-related deaths and the morbidity rates after stage-1 and stage-2 were only 13.0% and 15.0%, respectively. Of the complications, there was only one grade III complication after the stage-2 operation. The median operating time and median blood loss were not more in stage-1 and stage-2 and most patients did not receive the treatment of RBC transfusions. The postoperative hospital stay and the overall hospital stay were also not longer. Besides, we found that the volume of blood loss was positively related to the tumor number.
With respect to ALPPS outcomes, we observed a median OS of 19.0 (95% CI: 2.6–35.4) months, with 1-, 2-, and 5-year OS rates of 61.1%, 34.9%, and 8.7%, respectively. The median DFS was 5.0 (95% CI: 3.3–6.7) months, with 1-, 2-, and 5-year DFS rates of 27.8%, 27.8%, and 0.0%, respectively. Importantly, 2 BCLC stage A patients survived for more than 50 months after ALPPS (54 and 62 months, respectively) (Table S5). Taken together, our results suggest that ALPPS is a safe operation, and can provide a treatment opportunity for patients who are not suitable for liver resection because of an inadequate FLR volume.
In 2013, Shindoh et al. proposed the concept of KGR, which reflects the regenerative capacity of the liver (36). Kambakamba et al. (37) found that a KGR >6% per day significantly reduced the risk of PHLF after the second stage of ALPPS, and perioperative outcomes were improved when a KGR >6% per day was combined with a standardized FLR (sFLR) >30%. In our study, the absolute KGR was 20.1 mL/day and the RKGR was 5.1% per day, which were better than those reported in the study by Wang et al. (14.4 mL/day and 4.9% per day) (23). Nevertheless, there was no significant correlation between RKGR and short-term overall poor outcomes, and there were no differences in laboratory data between the RKGR-high (RKGR >0.0504) and RKGR-low group (RKGR ≤0.0504). Furthermore, the RKGR of 2 patients who died within 90 days after ALPPS was >0.0504.
This phenomenon can be explained by the results of animal studies, which indicate that a rapid increase in liver volume after ALPPS within a short time represents liver hypertrophy (a volume increase of hepatocytes), rather than hyperplasia (a true functional gain) (38). Matsuo et al. (39) examined liver specimens from patients undergoing ALPPS, and histological, electron microscopy and IHC staining examinations all revealed immature, fast-growing hepatocytes. Thus, it is necessary to have a useful method to predict FLR before ALPPS. Our team has also studied a combined intratumoral and peritumoral radiomics model based on gadolinium-ethoxybenzyl-diethylenetriamine (Gd-EOB-DTPA)-enhanced MRI to accurately assess liver reserve function (40).
Although ALPPS is a safe operation and it makes the tumor resection possible in patients without a sufficient FLR, the optimal selection of patients is crucial. Wang et al. (23) reported 1- and 3-year OS rates after ALPPS of 64.2% and 60.2%, respectively, and 1- and 3-year DFS rates of 47.6% and 43.9%, respectively. These outcomes after ALPPS are significantly better than those after TACE (OS, P=0.004) and similar to those after one-stage liver resection (OS, P=0.514). However, our results were poorer than those of Wang et al. In our study, the 1-, 2-, and 5-year OS rates were only 61.1%, 34.9%, and 8.7%, respectively, and the 1-, 2-, and 5-year DFS rates were only 27.8%, 27.8%, and 0.0% respectively. Furthermore, long-term outcomes of ALPPS treatment of HBV-related HCC were not better than those of TACE. We further studied the data from Wang et al. (23) and found that in their study 42.2% of the patients were BCLC stage A, 20% were stage B and 37.8% were stage C. Whereas, in our study 30.4% of patients were BCLC stage A, 26.1% were stage B and 43.5% were stage C. Thus, we postulate that the effect of ALPPS for intermediate and advanced HCC might not be better than that of TACE.
On the one hand, it is well-known that TACE is a preferred treatment for intermediate stage HCC and is recommended by most clinical practice guidelines, including the BCLC (2), the European Association for the Study of the Liver (41), the American Association for the Study of Liver Diseases (AASLD) (42,43), the Asian Pacific Association for the Study of the Liver (44), and the Japan Society of Hepatology (JSH) (45). On the other hand, García-Pérez et al. (46) reported that ALPPS seemed to induce a hypoxic environment, which enhances hepatic HIF1-α and VEGF expression, and may promote pro-inflammatory polarization of Kupffer cells (KCs) and pro-regenerative polarization of tumor-associated macrophages (TAMs). It is clear that HIF1-α and VEGF expression play a significant role in the metastatic progression and are correlated with disease stage and poor prognosis (47). Also, there is increasing evidence that vasculogenic factors are secreted in response to hypoxia-induced by portal vein occlusion techniques and play a vital role in tumorigenesis (48). Similarly, ALPPS can promote the hypoxic condition, and induce the release of these factors in response to hypoperfusion of transected lobes and the secretion of pro-regenerative and pro-inflammatory cytokines by KCs, which may contribute to tumor progression (46). The aforementioned factors may explain why in our study the results of ALPPS for patients with intermediate and advanced HCC were relatively poor.
Critical evaluation of risks and benefits of each strategy is crucial for choosing the best management for individual patients. We agree that ALPPS is a safe and effective treatment for HCC patients with an inadequate FLR volume. However, our results also reminded us that for intermediate and advanced HBV-related HCC patients, the outcome of ALPPS is not good enough and TACE may be the preferred treatment. Therefore, our data are valuable to surgeons to reexamine treatment strategies for intermediate and advanced HBV-related HCC patients with insufficient liver function. It should be noted that the efficacy of a single treatment is limited and with the comprehensive therapy, a part of unresectable HCC can be downstaged to become resectable, which thus allows for salvage surgery and better long-term survival (49,50).
The main limitations of this study are that it was conducted retrospectively at a single center, and the number of patients was small. However, the results suggest that well-designed, multicenter randomized controlled trials are warranted.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors acknowledge Cuncun Lu (Evidence-Based Medicine Center, Lanzhou University, Lanzhou, China) for his methodological assistance.
Funding: This study was supported by grants from the Science and Technology Project of Guangzhou City (No. 201707010387).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the procedures in this study were conducted following the Declaration of Helsinki (as revised in 2013) and arranged strictly with the approval of the First Affiliated Hospital of Sun Yat-sen University Ethics Committee [2015(124)] and informed written consent was taken from all the patients.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-2420
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2420
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2420). The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002;35:519-24. 10.1053/jhep.2002.32089 [DOI] [PubMed] [Google Scholar]
- 4.van Mierlo KM, Schaap FG, Dejong CH, et al. Liver resection for cancer: New developments in prediction, prevention and management of postresectional liver failure. J Hepatol 2016;65:1217-31. 10.1016/j.jhep.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-73.e1. 10.1053/j.gastro.2011.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. 10.1097/SLA.0b013e31824856f5 [DOI] [PubMed] [Google Scholar]
- 7.Alvarez FA, Ardiles V, Sanchez Claria R, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg 2013;17:814-21. 10.1007/s11605-012-2092-2 [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, et al. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery 2015;157:194-201. 10.1016/j.surg.2014.08.041 [DOI] [PubMed] [Google Scholar]
- 9.Knoefel WT, Gabor I, Rehders A, et al. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg 2013;100:388-94. 10.1002/bjs.8955 [DOI] [PubMed] [Google Scholar]
- 10.Schadde E, Raptis DA, Schnitzbauer AA, et al. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the international ALPPS registry. Ann Surg 2015;262:780-5; discussion 785-6. 10.1097/SLA.0000000000001450 [DOI] [PubMed] [Google Scholar]
- 11.D'Haese JG, Neumann J, Weniger M, et al. Should ALPPS be used for liver resection in intermediate-stage HCC? Ann Surg Oncol 2016;23:1335-43. 10.1245/s10434-015-5007-0 [DOI] [PubMed] [Google Scholar]
- 12.Björnsson B, Sparrelid E, Hasselgren K, et al. Associating liver partition and portal vein ligation for primary hepatobiliary malignancies and non-colorectal liver metastases. Scand J Surg 2016;105:158-62. 10.1177/1457496915613650 [DOI] [PubMed] [Google Scholar]
- 13.Brustia R, Scatton O, Perdigao F, et al. Vessel identifications tags for open or laparoscopic associating liver partition and portal vein ligation for staged hepatectomy. J Am Coll Surg 2013;217:e51-5. 10.1016/j.jamcollsurg.2013.08.020 [DOI] [PubMed] [Google Scholar]
- 14.Chan AC, Poon RT, Chan C, et al. Safety of ALPPS procedure by the anterior approach for hepatocellular carcinoma. Ann Surg 2016;263:e14-6. 10.1097/SLA.0000000000001272 [DOI] [PubMed] [Google Scholar]
- 15.Chan AC, Poon RT, Lo CM. Modified anterior approach for the ALPPS procedure: how we do it. World J Surg 2015;39:2831-5. 10.1007/s00268-015-3174-6 [DOI] [PubMed] [Google Scholar]
- 16.de Santibañes E, Alvarez FA, Ardiles V, et al. Inverting the ALPPS paradigm by minimizing first stage impact: the Mini-ALPPS technique. Langenbecks Arch Surg 2016;401:557-63. 10.1007/s00423-016-1424-1 [DOI] [PubMed] [Google Scholar]
- 17.Petrowsky H, Gyori G, de Oliveira M, et al. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg 2015;261:e90-2. 10.1097/SLA.0000000000001087 [DOI] [PubMed] [Google Scholar]
- 18.Røsok BI, Björnsson B, Sparrelid E, et al. Scandinavian multicenter study on the safety and feasibility of the associating liver partition and portal vein ligation for staged hepatectomy procedure. Surgery 2016;159:1279-86. 10.1016/j.surg.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 19.Schadde E, Ardiles V, Robles-Campos R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg 2014;260:829-36; discussion 836-8. 10.1097/SLA.0000000000000947 [DOI] [PubMed] [Google Scholar]
- 20.Vennarecci G, Grazi GL, Sperduti I, et al. ALPPS for primary and secondary liver tumors. Int J Surg 2016;30:38-44. 10.1016/j.ijsu.2016.04.031 [DOI] [PubMed] [Google Scholar]
- 21.Vivarelli M, Vincenzi P, Montalti R, et al. ALPPS procedure for extended liver resections: a single centre experience and a systematic review. PLoS One 2015;10:e0144019. 10.1371/journal.pone.0144019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao L, Li JW, Zheng SG. Totally laparoscopic ALPPS in the treatment of cirrhotic hepatocellular carcinoma. Surg Endosc 2015;29:2800-1. 10.1007/s00464-014-4000-1 [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Peng Y, Hu J, et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis B virus-related hepatocellular carcinoma: a single center study of 45 patients. Ann Surg 2020;271:534-41. 10.1097/SLA.0000000000002942 [DOI] [PubMed] [Google Scholar]
- 24.Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford) 2002;4:99; author reply 99-100. 10.1080/136518202760378489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vennarecci G, Laurenzi A, Santoro R, et al. The ALPPS procedure: a surgical option for hepatocellular carcinoma with major vascular invasion. World J Surg 2014;38:1498-503. 10.1007/s00268-013-2296-y [DOI] [PubMed] [Google Scholar]
- 26.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Annals of surgery 2000;232:665-72. 10.1097/00000658-200011000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289-93. 10.1002/hep.510240201 [DOI] [PubMed] [Google Scholar]
- 29.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462-503. [DOI] [PubMed] [Google Scholar]
- 30.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. 10.1200/JCO.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urata K, Hashikura Y, Ikegami T, et al. Standard liver volume in adults. Transplant Proc 2000;32:2093-4. 10.1016/S0041-1345(00)01583-9 [DOI] [PubMed] [Google Scholar]
- 32.Tschuor C, Croome KP, Sergeant G, et al. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion -- an extension of the ALPPS approach. Eur J Surg Oncol 2013;39:1230-5. 10.1016/j.ejso.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 33.Ji F, Fu SJ, Shen SL, et al. The prognostic value of combined TGF-beta1 and ELF in hepatocellular carcinoma. BMC Cancer 2015;15:116. 10.1186/s12885-015-1127-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan WZ, Yang JY, Lu MD, et al. Transcatheter arterial chemoembolization plus percutaneous thermal ablation in large hepatocellular carcinoma: clinical observation of efficacy and predictors of prognostic factors. Zhonghua Yi Xue Za Zhi 2011;91:2190-4. [PubMed] [Google Scholar]
- 35.López-López V, Robles-Campos R, Brusadin R, et al. Tourniquet-ALPPS is a promising treatment for very large hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Oncotarget 2018;9:28267-80. 10.18632/oncotarget.25538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shindoh J, Truty MJ, Aloia TA, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg 2013;216:201-9. 10.1016/j.jamcollsurg.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kambakamba P, Stocker D, Reiner CS, et al. Liver kinetic growth rate predicts postoperative liver failure after ALPPS. HPB (Oxford) 2016;18:800-5. 10.1016/j.hpb.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Pérez R, Revilla-Nuin B, Martínez CM, et al. Associated liver partition and portal vein ligation (ALPPS) vs selective portal vein ligation (PVL) for staged hepatectomy in a rat model. Similar regenerative response? PLoS One 2015;10:e0144096. 10.1371/journal.pone.0144096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuo K, Murakami T, Kawaguchi D, et al. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery 2016;159:1289-98. 10.1016/j.surg.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 40.Feng ST, Jia Y, Liao B, et al. Preoperative prediction of microvascular invasion in hepatocellular cancer: a radiomics model using Gd-EOB-DTPA-enhanced MRI. Eur Radiol 2019;29:4648-59. 10.1007/s00330-018-5935-8 [DOI] [PubMed] [Google Scholar]
- 41.European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 42.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 43.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. 10.1007/s12072-017-9799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kudo M, Trevisani F, Abou-Alfa GK, et al. Hepatocellular carcinoma: therapeutic guidelines and medical treatment. Liver Cancer 2016;6:16-26. 10.1159/000449343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García-Pérez R, Ferrer Fábrega J, Varona-Bosque A, et al. Role of Kupffer cells in the progression of CRC liver metastases after the first stage of ALPPS. Sci Rep 2018;8:8089. 10.1038/s41598-018-26082-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ioannou M, Paraskeva E, Baxevanidou K, et al. HIF-1alpha in colorectal carcinoma: review of the literature. J buon 2015;20:680-9. [PubMed] [Google Scholar]
- 48.Schadde E, Tsatsaris C, Swiderska-Syn M, et al. Hypoxia of the growing liver accelerates regeneration. Surgery 2017;161:666-79. 10.1016/j.surg.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 49.Lau WY, Ho SK, Yu SC, et al. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg 2004;240:299-305. 10.1097/01.sla.0000133123.11932.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma--a strategy to increase resectability. Ann Surg Oncol 2007;14:3301-9. 10.1245/s10434-007-9549-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as