Abstract
Background
Hepatocellular carcinoma (HCC) is a common and biologically aggressive malignancy linked to cirrhotic and pre-cirrhotic changes in the liver. We analyzed degrees of fibrosis in affected patients as indices of survival, to establish an effective prognostic nomogram.
Methods
Eligible patients with HCC and hepatic fibrosis, of varying degrees, were selected from the Surveillance, Epidemiology, and End Results (SEER) database for propensity score matching (PSM). The prognostic value of data was determined using Kaplan-Meier and Cox proportional hazards model. A nomogram based on variables derived from multivariate analyses was established and subjected to internal validation. Its predictive accuracy was tested by concordance index (C-index) and calibration plots.
Results
In this propensity score-matched cohort, advanced fibrosis/cirrhosis (vs. none-to-moderate fibrosis) correlated with poorer survival [hazard ratio (HR): 1.131, 95% confidence interval (CI): 1.032–1.240; P=0.009]. Multivariate analysis identified the following as independent risk factors for HCC: age >63 years, higher fibrosis score, American Joint Cancer Committee (AJCC) stages T3–4, distant metastasis (M1), tumor size >1 cm, major vascular invasion, and elevated alpha-fetoprotein (AFP) level. A nomogram that integrated these factors offered a superior prognostic prediction for HCC patients (C-index: 0.749, 95% CI: 0.7485–0.7495) relative to conventional tumor staging the AJCC tumor-node-metastasis (TNM) staging system (0.730). In calibration plots, optimal agreement between nomogram-predicted and observed survival was evident.
Conclusions
Increased fibrosis was an independent risk factor for survival of HCC patients. A prognostic nomogram integrating fibrosis score and other independent risk factors offered more accurate depictions in this regard.
Keywords: Hepatocellular carcinoma (HCC), fibrosis, nomogram, prognosis
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer, accounting for up to 10% of cancer-related mortality worldwide and carrying a 70–100% cumulative risk of recurrence within 5 years after surgery (1-3). In the vast majority of cases (70–90%), HCC arises within advanced fibrosis and cirrhosis as a consequence of various etiologic insults (4). Fibrosis is a protective wound healing response to chronic liver damage that progresses to fibrous scarring or cirrhosis if injurious conditions persist (5). Often associated with hepatic dysfunction, fibrosis and cirrhosis are considered premalignant states that heighten the risk of developing HCC (6).
The American Joint Cancer Committee (AJCC) tumor-node-metastasis (TNM) staging system (7th ed.) is commonly used for tumor classification and remains the prognostic standard in this setting (7). Nonetheless, its practical application is not entirely satisfactory, especially in patients with advanced disease (8). One possible explanation for inconsistencies lies in the heterogeneity of patient backgrounds and its failure to include other clinicopathological characteristics (such as demographics and tumor profiles) that are important to predict survival. As effective and convenient statistical tools, nomograms incorporate all prognostic variables and have been generated for a variety of cancer types (9-11).
Using Surveillance, Epidemiology, and End Results (SEER) analysis, we established a prognostic nomogram that integrated fibrosis scores and other independent variables to predict patient survival as a function of hepatic fibrosis. This model offers greater accuracy that may aid clinicians in routine practice.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3267).
Methods
Patients selection and data extraction
We performed a retrospective cohort analysis by querying the SEER-18 regs research data (November 2017 submission) (https://seer.cancer.gov/) for patients diagnosed with HCC [International Classification of Diseases for Oncology (3rd ed., ICD-O-3): HCC, histologic codes 8170-8175; liver, site code C22.0] between 2004 and 2015. Ethics approval and informed consent were waived because SEER data are freely available and our investigation was retrospective in nature.
The following exclusion criteria were applied to HCC patients in this study: (I) age <18 years, (II) voids in survival time or fibrosis score, (III) ID number duplication, (IV) diagnosis prior to 2004, and (V) no evidence of primary cancer. Fibrosis (or Ishak) scores were categorized as F0–4 (code 0, none-to-moderate) or F5–6 (code 1, severe fibrosis or cirrhosis), to compare these subsets in terms of patient survival. Alpha-fetoprotein (AFP) levels were likewise grouped as positive/elevated (code 10), negative/normal or within normal limits (code 20), or unclear results. Most tumors (90%) were <1 cm; therefore, the related groupings were ≤1 cm (code 0–991), >1 cm (code 992–996), or unclear. Vascular invasion was recorded as absent, minor (codes 200/350/370/380/400/520/550), major (codes 630/635/660), or unclear. Data on age, sex, AJCC TNM stage, fibrosis score, AFP level, tumor size, vascular invasion, and survival (months) were ultimately extracted from the SEER database.
Statistical analysis
As previously described (12), 1:1 propensity score matching (PSM) was implemented to minimize selection bias and balance baseline covariates in F0–4 and F5–6 subsets of hepatic fibrosis. No overt covariate imbalances emerged in pertinent testing, supporting the adequacy of execution. Statistical analysis of SEER data relied on standard software (SPSS v19.0; IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD) values and analyzed via unpaired t-tests. Chi-square or the Fisher’s exact test was used to compare categorical variables. Survival curves were generated by Kaplan-Meier method (log-rank test). Factors significantly impacting survival in the univariable analysis were further tested by multivariate Cox proportional hazards model. Due to unclear data recorded for some variables, four sensitivity analyses were undertaken to gauge the robustness of their influence (after PSM) on overall survival (OS), repeating all statistical maneuvers to accommodate the following adjustments: (I) all unclear T stages classified as T1–2; (II) all unclear T stages classified as T3–4; (III) all unclear M stages classified as M0; and (IV) all unclear M stages classified as M1. The prognostic nomogram utilized variables of multivariate analyses and was constructed in R software (rms package; R Foundation for Statistical Computing, Vienna, Austria). The predictive accuracy and discriminative ability were determined by concordance index (C-index) and calibration curves. Statistical significance was set at P<0.05.
Results
Clinicopathologic characteristics
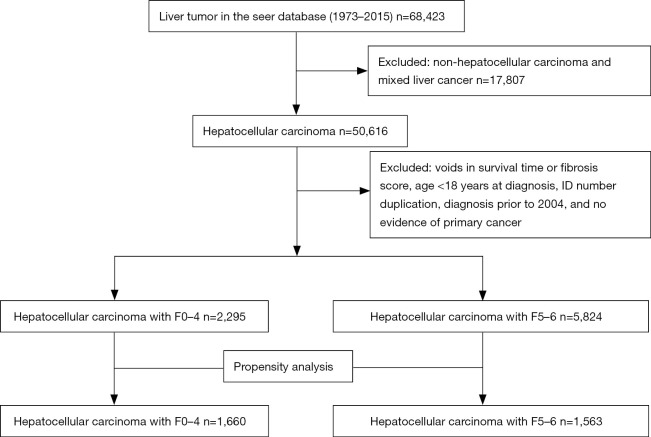
A total of 8,119 patients listed in the SEER database met our eligibility criteria, each diagnosed with HCC between 2004 and 2015. Follow-up intervals ranged from 0–143 (median, 15) months. The F0–4 and F5–6 fibrosis subsets accounted for 2,295 and 5,824 patients, respectively (Figure 1). Those with advanced fibrosis and cirrhosis were more apt to be younger (61.76±9.54, P<0.001) and male (P=0.027), with T1–2 tumors (P<0.001), no nodal metastasis (P=0.0065), no distant metastasis (P=0.0002), and elevated AFP levels (P<0.001). Those with none-to-moderate fibrosis were more often older and female, with T3–4 tumors and no AFP elevations. Because differing degrees of hepatic fibrosis may be confounded by differences in baseline patient characteristics, we used PSM to distinguish 1:1 matched pairs (F0–4, 1,660; F5–6, 1,563). Covariates of the two groups were thus well balanced and demonstrated no significant differences at baseline (P>0.05 for all; Table 1 and Figure S1).
Figure 1.
Flowchart of selecting patients with HCC using SEER database. HCC, hepatocellular carcinoma; SEER, Surveillance, Epidemiology, and End Results.
Table 1. The baseline characteristics of patients with HCC of different fibrosis scores.
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| F0–4 (n=2,295) | F5–6 (n=5,824) | P value | F0–4 (n=1,660) | F5–6 (n=1,563) | P value | ||
| Age, mean ± SD | 63.84±12.13 | 61.76±9.54 | <0.001 | 63.54±9.62 | 63.43±9.24 | 0.248 | |
| Sex, n | 0.027 | 0.614 | |||||
| Male | 1,742 | 4,555 | 1,320 | 1,254 | |||
| Female | 553 | 1,269 | 340 | 309 | |||
| AJCC T, 7th ed., n | <0.001 | 0.755 | |||||
| T1–T2 | 922 | 2,707 | 729 | 705 | |||
| T3–T4 | 361 | 762 | 201 | 180 | |||
| Unclear | 1,012 | 2,355 | 730 | 678 | |||
| AJCC N, 7th ed., n | 0.0065 | 0.721 | |||||
| N0 | 1,211 | 3,283 | 909 | 869 | |||
| N1 | 72 | 197 | 19 | 14 | |||
| Unclear | 1,012 | 2,344 | 732 | 680 | |||
| AJCC M, 7th ed., n | 0.0002 | 0.950 | |||||
| M0 | 1,175 | 3,250 | 890 | 844 | |||
| M1 | 143 | 387 | 47 | 46 | |||
| Unclear | 977 | 2,187 | 723 | 673 | |||
| AFP, n | <0.001 | 0.749 | |||||
| Positive/elevated | 1,217 | 3,531 | 958 | 911 | |||
| Negative | 648 | 1,411 | 432 | 413 | |||
| Unclear | 430 | 882 | 270 | 239 | |||
| Tumor size, n | 0.347 | 0.896 | |||||
| ≤1 cm | 2,091 | 5,262 | 1,583 | 1,492 | |||
| >1 cm | 7 | 12 | 0 | 0 | |||
| Unclear | 197 | 550 | 77 | 71 | |||
| Vascular invasion, n | <0.001 | 0.909 | |||||
| Absent | 920 | 2,219 | 761 | 713 | |||
| Minor | 284 | 399 | 138 | 120 | |||
| Major | 185 | 475 | 91 | 87 | |||
| Unclear | 906 | 2,731 | 670 | 643 | |||
HCC, hepatocellular carcinoma; PSM, propensity score matching; AJCC, American Joint Cancer Committee; AFP, alpha-fetoprotein.
Progression of fibrosis correlates with worse prognosis in patients with HCC
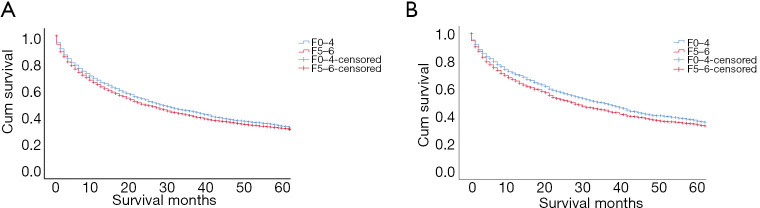
Prior to matching, patients of the F0–4 (vs. F5–6) subset displayed significantly higher cumulative 1-, 3-, and 5-year OS rates (65.8%, 44.5%, and 33.9% vs. 62.3%, 41.2%, and 32.0%, respectively). The prognosis in those with advanced fibrosis/cirrhosis was significantly worse (P=0.029) (Figure 2A), and the median OS was significantly lower (23 vs. 27 months, P=0.029). After 1:1 PSM, the 1-, 3-, and 5-year cumulative OS rates still diverged (F0–4, 70.6%, 49.6%, and 37.6% vs. F5–6, 65.8%, 47.7%, and 34.7%, respectively). In these patients, the median OS was again significantly lower (28 vs. 36 months, P=0.028), and patient prognosis was significantly worse in those with advanced fibrosis/cirrhosis (P=0.028) (Figure 2B). In univariate analysis of all matched patients, age (P<0.001), sex (P=0.008), primary tumor (P<0.001), nodal metastasis (P<0.001), distant metastasis (P<0.001), vascular invasion (P<0.001), AFP level (P<0.001), fibrosis score (P=0.028), and tumor size (P<0.001) were closely associated with OS (Table 2). Multivariate Cox proportional hazards analyses, using factors proven significant by univariate analysis, identified advanced fibrosis/cirrhosis [hazard ratio (HR): 1.131, 95% confidence interval (CI): 1.032–1.240; P=0.009], age >63 years (HR: 1.365; P<0.001), T3–4 staging (HR: 1.810; P<0.001), distant metastasis (M1) (HR: 3.460; P<0.001), tumor size >1 cm (HR: 2.536; P<0.001), major vascular invasion (HR: 2.321; P<0.001), and elevated AFP level (HR: 1.511; P<0.001) as independent risk factors for increased mortality (Table 3).
Figure 2.
Analyses of patient survival in F0–4 and F5–6 subsets of hepatic fibrosis (A) before and (B) after PSM, underscoring related prognostic ramifications. PSM, propensity score matching.
Table 2. Univariate analysis of prognostic factors for OS.
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| All patients | Survival time (months), median (95% CI) | P value | All patients | Survival time (months), median (95% CI) | P value | ||
| Group | 0.029 | 0.028 | |||||
| F0–4 | 2,295 | 27 (23.999–0.001) | 1,660 | 36 (32.191–39.809) | |||
| F5–6 | 5,824 | 23 (21.465–24.535) | 1,563 | 28 (24.265–31.735) | |||
| Age | <0.001 | <0.001 | |||||
| ≤63 | 4,695 | 30 (27.516–32.484) | 1,692 | 40 (35.293–44.707) | |||
| >63 | 3,424 | 20 (18.51–21.49) | 1,531 | 25 (22.395–27.605) | |||
| Sex | 0.025 | 0.008 | |||||
| Male | 6,297 | 24 (22.491–25.509) | 2,574 | 30 (26.955–33.045) | |||
| Female | 1,822 | 27 (23.862–30.138) | 649 | 37 (29.635–44.365) | |||
| AJCC T, 7th ed. | <0.001 | <0.001 | |||||
| T1–T2 | 3,629 | 44 (39.689–48.311) | 1,434 | 56 | |||
| T3–T4 | 1,123 | 7 (6.046–7.954) | 381 | 9 (7.077–10.923) | |||
| Unclear | 3,367 | 19 (17.365–20.635) | 1,408 | 22 (19.305–24.695) | |||
| AJCC N, 7th ed. | <0.001 | <0.001 | |||||
| N0 | 4,494 | 32 (29.665–34.335) | 1,778 | 42 (37.736–46.264) | |||
| N1 | 269 | 4 (3.095–4.905) | 33 | 4 (1.908–6.092) | |||
| Unclear | 3,356 | 20 (18.367–21.633) | 1,412 | 22 (19.307–24.693) | |||
| AJCC M, 7th ed. | <0.001 | <0.001 | |||||
| M0 | 4,425 | 33 (30.567–35.433) | 1,734 | 42 (37.38–46.62) | |||
| M1 | 530 | 3 (2.494–3.506) | 93 | 4 (3.191–4.809) | |||
| Unclear | 3,164 | 21 (19.190–22.81) | 1,396 | 23 (20.243–25.757) | |||
| Vascular invasion | <0.001 | <0.001 | |||||
| Absent | 3,139 | 52 (47.312–56.688) | 1,474 | 59 (49.969–68.031) | |||
| Minor | 683 | 30 (25.482–34.518) | 258 | 36 (24.221–47.779) | |||
| Major | 660 | 4 (3.281–4.719) | 178 | 7 (4.961–9.039) | |||
| Unclear | 3,637 | 17 (15.722–18.278) | 1,313 | 17 (14.85–19.15) | |||
| AFP | <0.001 | <0.001 | |||||
| Positive/elevated | 4,748 | 19 (17.785–20.215) | 1,869 | 23 (20.536–25.464) | |||
| Negative | 2,059 | 47 (41.161–52.839) | 845 | 57 (48.156–65.844) | |||
| Unclear | 1,312 | 21 (17.714–24.286) | 509 | 25 (19.72–30.28) | |||
| Tumor size | <0.001 | <0.001 | |||||
| ≤1 cm | 7,353 | 30 (28.238–31.762) | 3,075 | 35 (32.288–37.712) | |||
| >1 cm | 19 | 13 (0–27.348) | 0 | – | |||
| Unclear | 747 | 2 (1.568–2.432) | 148 | 2 (1.069–2.931) | |||
OS, overall survival; PSM, propensity score matching; CI, confidence interval; AJCC, American Joint Cancer Committee; AFP, alpha-fetoprotein.
Table 3. Multivariate analysis of factors predictive of patients’ OS.
| Variables | Before PSM | After PSM | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Group, F5–6 (vs. F0–4) | 1.111 (1.042–1.185) | 0.001 | 1.131 (1.032–1.240) | 0.009 | |
| Age, >63 (vs. ≤63) | 1.447 (1.366–1.533) | <0.001 | 1.365 (1.243–1.498) | <0.001 | |
| Sex, female (vs. male) | 0.917 (0.856–0.983) | 0.014 | |||
| AJCC T, 7th ed., T3–4 (vs. T1–2) | 1.683 (1.517–1.867) | <0.001 | 1.810 (1.517–2.159) | <0.001 | |
| AJCC N, 7th ed., N1 (vs. N0) | 1.364 (1.168–1.594) | <0.001 | |||
| AJCC M, 7th ed., M1 (vs. M0) | 2.328 (2.068–2.621) | <0.001 | 3.460 (2.705–4.424) | <0.001 | |
| Tumor size, >1 cm (vs. ≤1 cm) | 1.417 (0.837–2.399) | 0.194 | 2.536 (2.096–3.068) | <0.001 | |
| Vascular invasion, minor (vs. absent) | 1.267 (1.132–1.418) | <0.001 | 1.202 (1.000–1.445) | 0.050 | |
| Vascular invasion, major (vs. absent) | 2.401 (2.134–2.701) | <0.001 | 2.321 (1.885–2.857) | <0.001 | |
| AFP, negative (vs. positive/elevated) | 0.670 (0.622–0.721) | <0.001 | 0.662 (0.587–0.746) | <0.001 | |
OS, overall survival; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Cancer Committee; AFP, alpha-fetoprotein.
Predictors of survival in patients with advanced fibrosis/cirrhosis patients
Using multivariate analysis, predictors of survival among patients with advanced fibrosis/cirrhosis patients were assessed. As shown in Table 4, survival worsened significantly at an older age (>63 years) in patients with advanced fibrosis/cirrhosis (HR: 1.480, 95% CI: 1.297–1.689; P<0.001), and in the presence of advanced fibrosis/cirrhosis patients, T3–4 staging (HR: 1.900, 95% CI: 1.477–2.444; P<0.001), distant metastasis (M1) (HR: 3.270, 95% CI: 2.297–4.655; P<0.001), tumor size >1 cm (HR: 2.809, 95% CI: 2.152–3.668; P<0.001), and major vascular invasion (HR: 2.457, 95% CI: 1.819–3.319; P<0.001). Patient survival was also significantly better in the absence (vs. presence) of AFP elevation (HR: 0.702, 95% CI: 0.594–0.831; P<0.001).
Table 4. Multivariate analysis of predictors of survival among patients with advanced fibrosis/cirrhosis patients.
| Variables | HR (95% CI) | P value |
|---|---|---|
| Age, >63 (vs. ≤63) | 1.480 (1.297–1.689) | <0.001 |
| Derived AJCC T, 7th ed., T3–4 (vs. T1–2) | 1.900 (1.477–2.444) | <0.001 |
| Derived AJCC M, 7th ed., M1(vs. M0) | 3.270 (2.297–4.655) | <0.001 |
| Tumor size, >1 cm (vs. ≤1 cm) | 2.809 (2.152–3.668) | <0.001 |
| Vascular invasion, minor (vs. absent) | 1.200 (0.918–1.569) | 0.181 |
| Vascular invasion, major (vs. absent) | 2.457 (1.819–3.319) | <0.001 |
| AFP, negative (vs. positive/elevated) | 0.702 (0.594–0.831) | <0.001 |
HR, hazard ratio; CI, confidence interval; AJCC, American Joint Cancer Committee; AFP, alpha-fetoprotein.
Construction and internal validation of prognostic nomogram
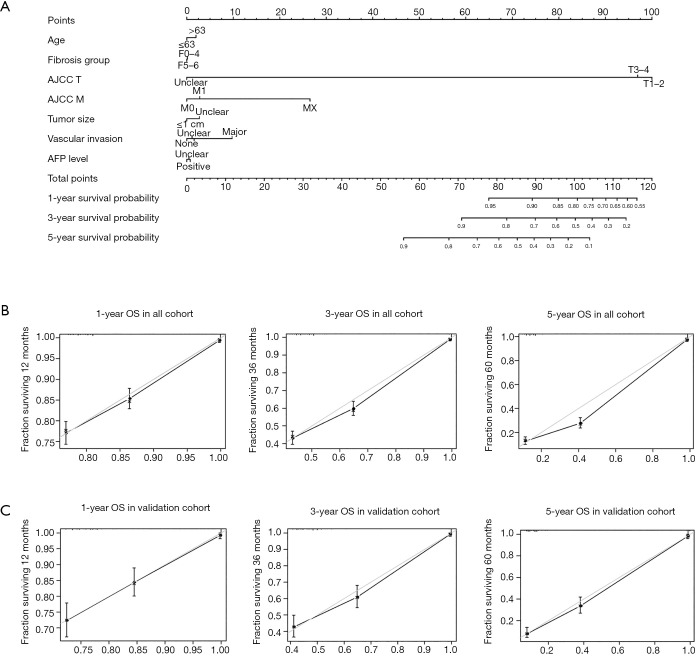
Our prognostic nomogram integrated all significant independent factors determined by multivariate analyses, achieving a C-index of 0.749 (95% CI: 0.7485–0.7495) for predicting survival (Figure 3A). Calibration plots for probabilities of survival at 1, 3, and 5 years also indicated optimal agreement between predictions by nomogram and actual observations (Figure 3B). A portion of the cohort (30%) was then selected at random for internal validation using R open-source software. The C-index of internal validation for this nomogram was 0.761 (95% CI: 0.759–0.763), and the calibration curves indicated good agreement between the nomogram-predicted and observed OS at 1, 3, and 5 years (Figure 3C). Hence, the integration of fibrosis score and other factors (i.e., age, AJCC T stage, distant metastasis, tumor size, vascular invasion, and AFP level) in our nomogram provided a reliable means to predict survival in patients with HCC.
Figure 3.
HCC survival nomogram and calibration curves. (A) Prognostic nomogram integrating fibrosis score and other independent risk factors for predicting OS and (B,C) calibration curves for predicting patient survival at 1, 3, and 5 years in all patients and in a validation cohort. HCC, hepatocellular carcinoma; OS, overall survival; AJCC, American Joint Cancer Committee; AFP, alpha-fetoprotein.
Performance of nomogram vs. independent prognostic factors or conventional staging
The high C-indices recorded for our model underscore its predictive accuracy. In the cohort overall, the C-index for predicting survival by nomogram was 0.749 (95% CI: 0.7485–0.7495), which exceeded that of conventional staging AJCC TNM (0.730), tumor size (0.506), vascular invasion (0.509), AFP level (0.55), or patient age (0.536). Internal validation of the nomogram produced a C-index of 0.761 (95% CI: 0.759–0.763) again outperforming conventional staging AJCC TNM (0.742), tumor size (0.502), vascular invasion (0.520), AFP level (0.539), or patient age (0.534). It appears that the nomogram we generated is a useful and reliable model for predicting survival in patients with HCC, surpassing independent prognostic factors and AJCC TNM staging in this regard.
Sensitivity analyses in the propensity score-matched cohort
To check the robustness of our results, a series of sensitivity analyses were performed. As already elaborated, age, sex, primary tumor, nodal metastasis, distant metastasis (M1), vascular invasion, AFP level, fibrosis score, and tumor size proved to be closely associated with OS. Advanced fibrosis/cirrhosis, age >63 years, T3–4 staging, distant metastasis (M1), tumor size >1 cm, major vascular invasion, and AFP level further emerged as independent risk factors for increased mortality in multivariate Cox proportional hazards analysis. However, once adjustments were made (unclear T stages classified as T1–2; unclear M stages classified as M1), minor vascular invasion was identified as an independent risk factor of OS (Tables S1-S6). Consequently, the association between minor vascular invasion and OS is not particularly robust and requires caution in its interpretation.
Discussion
Findings of the present study have demonstrated that advanced fibrosis/cirrhosis is independently associated with survival of patients with HCC. We used this information to generate an internally validated fibrosis score-based prognostic nomogram. This predictive model is reliable and compelling, with practical ramifications in a clinical setting.
HCC is an aggressive malignancy closely linked to hepatic fibrosis and end stage cirrhosis. Nearly all affected patients (~80–90%) harbor some underlying fibrotic changes, roughly one in three patients with cirrhosis are likely to develop HCC in their lifetime. In patients with advanced hepatic fibrosis/cirrhosis, the 5-year incidence of HCC ranges from 5–30% (6,13). However, the prognostic impact of fibrosis scores in conjunction with HCC, has yet to be fully explored (14-17). A better understanding of the role that fibrosis plays in this setting and the use of a nomogram that includes fibrosis score, may yield a more accurate prediction for patient survival.
According to the AJCC, fibrosis (or Ishak) scores are categorized as either none-to-moderate or severe fibrosis/cirrhosis. While adopting these same subsets, we conducted PSM to minimize selection bias. Patients designated as such did not differ significantly by age, sex, AJCC TNM stage, AFP level, tumor size, or vascular invasion; therefore, patient distribution was well balanced. Our results indicate a worse prognosis in patients with HCC who displayed advanced fibrosis/cirrhosis, rather than those with none-to-moderate fibrotic change. Notably, Noda et al. found no significant relationship between hepatic fibrosis and OS in patients with HCC (P=0.1185) (18). Similarly, Suh et al. found no significant difference between OS and fibrosis, regardless of the fibrosis score (mild vs. severe; P=0.267) (14). However, severe fibrosis/cirrhosis constituted as an independent risk factor for OS in our analysis of the data. The outcomes herein are consistent with those of a recent meta-analysis by Zhang et al. and another study by Toyoda et al. (19,20). Prior discrepancies may be partly attributable to differences in patient enrollment criteria. Noda et al. focused on patients with HCC of non-viral origins (18), whereas Suh et al. investigated Child‐Pugh A status and single HCC lesions <5 cm (14). Approximately 70% of patients with HCC have viral hepatitis infections (6). Child-Pugh B status and hepatitis C viral positivity also carry poor prognoses in conjunction with HCC (21).
Aside from fibrosis score, other factors (i.e., age >63 years, T3–4 staging, M1, tumor size >1 cm, major vascular invasion, and elevated AFP level) emerged as independent predictors of poor prognosis in multivariate analyses of our patients with HCC, which is consistent with past reports (8,22-25). The fact that we did not find sex or nodal metastasis to be predictive of patient survival, is also consistent with past reports (25,26). Furthermore, patients with advanced fibrosis/cirrhosis who were older (>63 years) demonstrated worse survival. Additionally, T3–4 staging, M1, tumor size >1 cm, major vascular invasion, or elevated AFP level worsened patient survival in the presence of advanced fibrosis/cirrhosis. The prognostic nomogram that we developed (C-index: 0.749) incorporated these factors comprehensively and was internally validated (C-index: 0.761). Our prognostic nomogram also outperformed AJCC TNM staging, in both all (C-index: 0.730) and validation (C-index: 0.742) cohorts. Calibration plots for 1-, 3-, and 5-year probabilities of survival, indicated optimal agreement between predictions by nomogram and actual observations in both all and validation cohorts.
There were certain limitations of this study that might have influenced our results to some extent. The SEER database lacked any records pertaining to etiologic origins of HCC (e.g., viral hepatitis), liver function indices, Child-Pugh classifications, degrees of portal hypertension, or scoring of performance status. Thus, our nomogram and other commonly used systems, including Barcelona Clinic Liver Cancer (BCLC) staging, Hong Kong Liver Cancer (HKLC) staging, the model to estimate survival for hepatocellular carcinoma (MESH), or the Cancer of the Liver Italian Program (CLIP) score, could not be compared in terms of predictive accuracy (27-30). Some variables were also unclear or insufficiently detailed. For example, although Hsu et al. invoked AFP thresholds of 20 and 400 ng/mL to predict long-term outcomes of patients with HCC, the SEER database merely records AFP levels as elevated or negative (31).
It is well known that therapeutics have tremendous bearing on the clinical outcomes of patients with HCC. Use of nucleos(t)ide analogs for hepatitis B viral suppression and eradication of hepatitis C virus through direct-acting antivirals or pegylated interferon, may significantly diminish the prospect of hepatic decompensation and ultimately improve patient survival. In the absence of fibrosis, patient eligibility for curative treatment is often heightened. However, SEER-18 Regs Research data (November 2017 submission) offered no information on related treatment modalities.
Finally, the retrospective nature of this study made it difficult to avert bias from other confounding factors, despite our implementation of PSM. Further validation through multicenter prospective recruitment is clearly warranted to augment this initial assessment of our novel prognostic nomogram.
Conclusions
Based on our findings, it is evident that patients with HCC have poorer outcomes if there is advanced fibrosis/cirrhosis rather than lesser degrees of fibrosis. We have successfully generated a reliable and superior nomogram that incorporates fibrosis score and other independent risk factors to accurately predict patient prognoses in this setting.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the Innovation Fund of Science and Technology Commission of Shanghai Municipality (No. 15411950501, 15411950507, and 17140902700).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3267
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3267). The authors have no conflicts of interest to declare.
References
- 1.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 2.Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg 2011;253:453-69. 10.1097/SLA.0b013e31820d944f [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002;235:373-82. 10.1097/00000658-200203000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. 10.1053/j.gastro.2007.04.061 [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 2011;6:425-56. 10.1146/annurev-pathol-011110-130246 [DOI] [PubMed] [Google Scholar]
- 6.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50. 10.1053/j.gastro.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Carducci MA, Compton CC, et al. AJCC cancer staging manual. 7th ed. Chicago: Springer, 2009:237. [Google Scholar]
- 8.Chun YH, Kim SU, Park JY, et al. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer 2011;47:2568-75. [DOI] [PubMed] [Google Scholar]
- 9.Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer- specific survival nomogram. J Clin Oncol 2007;25:1316-22. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. 10.1200/JCO.2012.41.5984 [DOI] [PubMed] [Google Scholar]
- 12.Pattanayak CW, Rubin DB, Zell ER. Propensity score methods for creating covariate balance in observational studies. Rev Esp Cardiol 2011;64:897‐903. 10.1016/j.recesp.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 13.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. 10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 14.Suh SW, Choi YS. Influence of liver fibrosis on prognosis after surgical resection for resectable single hepatocellular carcinoma. ANZ J Surg 2019;89:211-5. 10.1111/ans.14732 [DOI] [PubMed] [Google Scholar]
- 15.Huang JL, Fu YP, Jing CY, et al. A novel and validated prognostic nomogram based on liver fibrosis and tumor burden for patients with hepatocellular carcinoma after curative resection. J Surg Oncol 2018;117:625-33. 10.1002/jso.24895 [DOI] [PubMed] [Google Scholar]
- 16.Taura K, Ikai I, Hatano E, et al. Influence of coexisting cirrhosis on outcomes after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery 2007;142:685-94. 10.1016/j.surg.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 17.Sasaki K, Shindoh J, Margonis GA, et al. Effect of Background Liver Cirrhosis on Outcomes of Hepatectomy for Hepatocellular Carcinoma. JAMA Surg 2017;152:e165059. 10.1001/jamasurg.2016.5059 [DOI] [PubMed] [Google Scholar]
- 18.Noda Y, Kawaguchi T, Korenaga M, et al. High serum interleukin-34 level is a predictor of poor prognosis in patients with non-viral hepatocellular carcinoma. Hepatol Res 2019;49:1046-53. 10.1111/hepr.13350 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wang R, Yang X. FIB-4 index serves as a noninvasive prognostic biomarker in patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2018;97:e13696. 10.1097/MD.0000000000013696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyoda H, Kumada T, Tada T, et al. Differences in the impact of prognostic factors for hepatocellular carcinoma over time. Cancer Sci 2017;108:2438-44. 10.1111/cas.13406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen RX, Gan YH, Ge NL, et al. Comparison of transarterial chemoembolization with radiofrequency ablation for unresectable Barcelona Clinic Liver Cancer stage 0/A hepatocellular carcinoma: a propensity score matching. J Gastroenterol Hepatol 2016;31:442-9. 10.1111/jgh.13077 [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Liu C, Tan Y, et al. Role of liver resection in treating intermediate and advanced stage adolescent and young adult hepatocellular carcinoma patients: a propensity-matching cohort study. Int J Surg 2018;54:259-64. 10.1016/j.ijsu.2018.03.051 [DOI] [PubMed] [Google Scholar]
- 23.Borzio M, Dionigi E, Rossini A, et al. External validation of the ITA.LI.CA prognostic system for patients with hepatocellular carcinoma: a multicenter cohort study. Hepatology 2018;67:2215-25. 10.1002/hep.29662 [DOI] [PubMed] [Google Scholar]
- 24.Fung J, Cheung KS, Wong DK, et al. Long-term outcomes and predictive scores for hepatocellular carcinoma and hepatitis B surface antigen seroclearance after hepatitis B e-antigen seroclearance. Hepatology 2018;68:462-72. 10.1002/hep.29874 [DOI] [PubMed] [Google Scholar]
- 25.Lin Q, Huang X, Zhong C, et al. Improved survival with radiotherapy in hepatocellular carcinoma with major vascular invasion: a propensity-matched analysis of Surveillance, Epidemiology, and End Results database. Cancer Med 2019;8:515-26. 10.1002/cam4.1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harimoto N, Yoshizumi T, Inokuchi S, et al. Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol 2018;25:3316-23. 10.1245/s10434-018-6672-6 [DOI] [PubMed] [Google Scholar]
- 27.Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61-74. 10.1055/s-0030-1247133 [DOI] [PubMed] [Google Scholar]
- 28.The Cancer of the Liver Italian Program (CLIP) investigators . A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology 1998;28:751-5. 10.1002/hep.510280322 [DOI] [PubMed] [Google Scholar]
- 29.Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-700.e3. 10.1053/j.gastro.2014.02.032 [DOI] [PubMed] [Google Scholar]
- 30.Liu PH, Hsu CY, Hsia CY, et al. Proposal and validation of a new model to estimate survival for hepatocellular carcinoma patients. Eur J Cancer 2016;63:25-33. 10.1016/j.ejca.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY, Liu PH, Lee YH, et al. Using serum α-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: what is the most optimal cutoff? PLoS One 2015;10:e0118825. 10.1371/journal.pone.0118825 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as