Abstract
Background
Almost a third of the resections in patients with colorectal liver metastases (CRLM) undergoing curative surgery, end up being tumor-margin positive (≤1 mm margin). Near-infrared fluorescent (NIRF) imaging using the fluorescent contrast agent indocyanine green (ICG) has been studied for many different applications. When administered in a relatively low dose (10 mg) 24 hours prior to surgery, ICG accumulated in hepatocytes surrounding the CRLM. This results in the formation of a characteristic fluorescent ‘rim’ surrounding CRLM when located at the periphery of the liver. By resecting the metastasis with the entire surrounding fluorescent rim, in real-time guided by NIRF imaging, the surgeon can effectively acquire margin-negative (>1 mm) resections. This pilot study aims to describe the surgical technique for using near-infrared fluorescence imaging to assess tumor-margins in vivo in patients with CRLM undergoing laparoscopic or robot-assisted resections.
Methods
Out of our institutional database we selected 16 CRLM based on margin-status (R0; n=8, R1; n=8), which were resected by a minimally-invasive approach using ICG-fluorescence. NIRF images acquired during surgery, from both the resection specimen and the wound bed, were analysed for fluorescent signal. We hypothesized that a protruding fluorescent rim at the parenchymal side of the resection specimen could indicate a too close proximity to the tumor and could be predictive for a tumor-positive surgical margin. NIRF images were correlated to final histopathological assessment of the resection margin.
Results
All lesions with a NIRF positive resection plane in vivo were reported as having a tumor-positive margin. Lesions that showcased no protruding rim in the wound bed in vivo were diagnosed as having a tumor-negative margin in 88% of cases. A 5-step surgical workflow is described to document the NIRF signal was used assess the resection margin in vivo for future clinical studies.
Conclusions
The pilot study shows that image-guided surgery using real-time ICG-fluorescence has the potential to aid surgeons in achieving a tumor-negative margin in minimally invasive liver metastasectomies. The national multi-centre MIMIC-Trial will prospectively study the effect of this technique on surgical tumor-margins (Dutch Trial Register number NL7674).
Keywords: Colorectal liver metastases (CRLM), fluorescence-guided surgery, minimal invasive liver surgery, tumor-margin, indocyanine green-fluorescence (ICG-fluorescence)
Introduction
Laparoscopic liver resection is shown to be safe and feasible with concordant oncological outcomes compared to open surgery. Unfortunately, both procedures result in a tumor-positive resection in 28% of cases (1). According to recent literature, achieving a tumor-negative resection with a margin of at least 1 mm is associated with improved long-term and disease-free survival in patients with metastatic disease isolated to the liver (2,3). Hence, in order to improve patient outcome, finding effective means to increasing tumor-negative resection rates are required.
Image-guided hepatobiliary surgery using near-infrared fluorescence (NIRF) is a technique which can improve real-time demarcation of tumors located in the liver (4). NIRF imaging, using indocyanine green (ICG), has emerged as a promising method for detecting colorectal liver metastases (CRLM) in both open and minimally invasive procedures (5). NIRF imaging studies showed an easily identifiable fluorescent rim, caused by the accumulation of ICG in the surrounding hepatocytes, which surround the CRLM (6). These studies mainly focused on the identification of additional CRLM not seen on conventional imaging modalities prior to surgery. Now, we hypothesize that the surgical removal of the entire fluorescent rim, guided by NIRF imaging, can greatly improve tumor-negative resection rates during minimal invasive procedures. Improving tumor-negative resection rates could potentially lead to prolonged disease-free and overall survival. This hypothesis is supported by the fact that NIRF signal has a penetration depth of around 8 mm. The absence of NIRF signal in the resection plane should therefore indicate a tumor-negative, or radical (R0) resection.
Recent developments in fluorescent imaging systems led to a variety of laparoscopic and robotic platforms which can display fluorescence and bright field images merged and in real-time (7). This eliminates the need to switch between imaging channels which significantly reduces any interference with the surgical procedure during fluorescent-guided resections. In addition, during NIRF guided laparoscopic liver resections, repeatedly introducing the ultrasound probe to examine the progress of the resection will become less interruptive and decisive.
While the development and adaptation of these technologies in surgery provide an immense amount of information, interpretation of these fluorescent images and mastering the technique of using fluorescence in the operating room requires training and experience. In this report, we aim to share our institute’s decade-long experience with NIRF guided liver surgery. More specifically, we describe the use of NIRF image-guided CRLM resections during minimally invasive (laparoscopic or robotic) procedures. Furthermore, we highlight the advantages of this technique in properly assessing tumor margins in vivo using ICG as a contrast agent. The technique was explored in a patient cohort composed from our institute’s database to represent a variety of possible surgical outcomes. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1999).
Methods
Patients
For the purpose of this study a cohort was composed. CRLM resected by minimally invasive approach (laparoscopic or robot-assisted) guided by ICG fluorescence imaging, were selected from the institutional database. The patient cohort represents a variety of common surgical outcomes to display the surgical technique and the interpretation of fluorescent signal in vivo, namely: eight lesions with tumor-negative margins in vivo matched by an equal number of lesions with tumor-positive margins. Lesion characteristics are summarized in Table 1.
Table 1. Lesion characteristics.
| Characteristics | Number (n=16) | % |
|---|---|---|
| Disease | ||
| Primary CRLM | 15 | 94 |
| Recurrent CRLM | 1 | 6 |
| Synchronous | 3 | 19 |
| Metachronous | 13 | 81 |
| Lesion size in mm (median; range) | 22.5 | [8–37] |
| Surgery | ||
| Laparoscopic | 8 | 50 |
| Robotically assisted | 8 | 50 |
| Liver segments | ||
| I | 0 | 0 |
| II | 3 | 19 |
| III | 1 | 6 |
| II–III (anatomical resection) | 1 | 6 |
| Iva-b | 4 | 25 |
| V | 3 | 19 |
| VI | 1 | 6 |
| VII | 1 | 6 |
| VIII | 2 | 13 |
| Blood loss in mL (median; range) | 100 | [10–500] |
CRLM, colorectal liver metastases.
CRLM were selected from patients 18 years of age and older, were diagnosed with CRLM and eligible for minimally invasive surgery with curative intend. According to the national protocol, all patients underwent either a contrast-enhanced abdominal computed tomography (CT) scan and/or gadoxetate disodium (Primovist®) enhanced magnetic resonance imaging (MRI) scan prior to surgery. All patients were administered 10 mg (5 mg/mL) ICG (Verdye, Diagnostic Green GmbH) intravenously 24 hours prior to surgery, according to the institution’s protocol. The decision to perform a laparoscopic or robot-assisted liver resection was based on the localization of the lesion(s), patient characteristics, availability of the robotic system and surgeon’s preference. Patients were selected from the institute’s database between October 2017 and September 2018. This study was conducted according to the declaration of Helsinki (as revised in 2013) and approved by the Institutional Ethics Review Board of the Leiden University Medical Center (The Netherlands, G19.012). All patient data was registered after informed consent was acquired.
Technique and signal definition
Previous data from our center consistently showed the development of a fluorescent rim which encases all CRLM (4). Hence, the basic principle of our surgical technique relies on defining this fluorescent rim as the resection margin and to acquire a tumor-negative resection by removing the entire fluorescent rim with the metastasis included (Figure 1).
Figure 1.
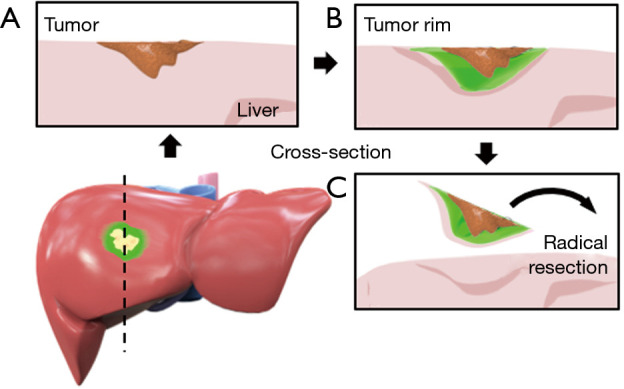
Computer-assisted model of the liver with a CRLM in segment V/VIII including a fluorescent rim surrounding the lesion. (A) Cross-section through the tumor in (B) surrounded by a fluorescent rim caused by the accumulation of ICG in immature hepatocytes surrounding the tumor. (C) Removing the entire rim should result in a tumor-negative resection. CRLM, colorectal liver metastases.
No real-time quantitative method for defining the fluorescent signal intensity during the procedure is yet available. Therefore, in vivo fluorescent signals were registered by the following classification system: (I) no fluorescent signal (Figure 2A); (II) bright fluorescent signal; where a very intense fluorescent signal from liver parenchyma is observed, that correlates to the intensity of the fluorescent rim at the liver capsula (Figure 2B,C). This signal is caused by the presence of ICG in liver tissue and indicates a protruding tumor rim; (III) intermediate fluorescent signal; where a fluorescent light is scattered by normal liver parenchyma. Surgical fluorescent images from the procedure were analyzed. A bright fluorescent signal after resection of the CRLM either produced by the resection specimen and/or the wound bed was registered as a potential tumor-positive resection.
Figure 2.
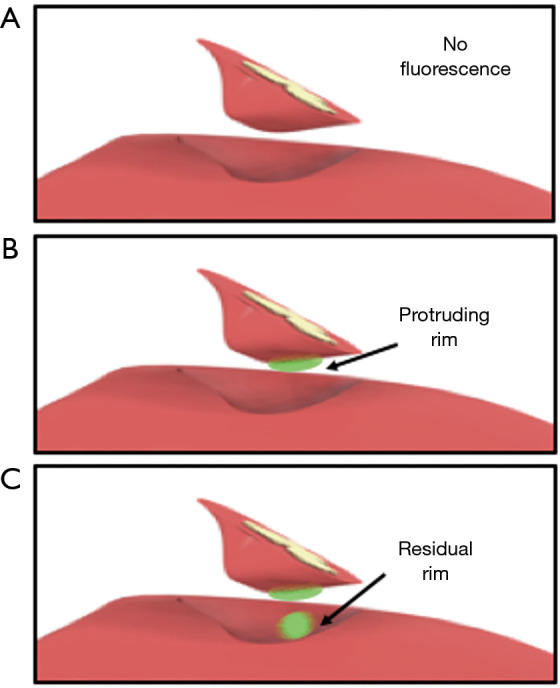
A computer-aided design of the liver showcasing three different scenarios. (A) No fluorescent signal produced by either the liver’s wound bed or the resection specimen. (B) Fluorescent rim protruding trough the liver tissue of the resection specimen indicating a potential tumor-positive resection margin and (C) bright fluorescent signal in both the wound bed and protruding trough the liver tissue of the resection specimen (potential resection with tumor-positive margin).
NIRF systems
The minimally invasive surgical procedures were performed either laparoscopically or robot-assisted. The following systems were used: (I) Viscera Elite II IR-s200 light engine (Olympus Medical Systems, Hamburg, Germany); (II) 1588 AIM platform (Stryker, Waardenburg, The Netherlands); (III) da Vinci® firefly (Intuitive Surgical, Inc., Sunnyvale, California, USA). All are clinically approved and CE (Conformité Européenne) marked. All laparoscopic systems were commercially available and equipped with both NIRF imaging and regular white light imaging (WLI) modules. This allowed for a real-time coloured overlay to be produced. During the procedures a 10 mm 30-degree angled laparoscope was used.
Surgical procedure
Access to the abdominal cavity was acquired by placing a 12 mm port in the umbilicus. Peritoneal disease was excluded with laparoscopy before any additional ports were placed; pending the location of the target lesion(s) and under the surgeon’s discretion. First, the liver was mobilized to facilitate visual inspection of the liver surface and in some cases to better access to the metastasis. The liver’s surface is thoroughly inspected visually and the entire liver parenchyma is assessed using laparoscopic IOUS. Subsequently, NIRF imaging is used to identify the target lesion(s) (Figure 3A,B) and, if present, any additional lesions. All acquired NIRF and bright-field images were stored and documented. Fresh frozen sections were collected for histopathological confirmation of any suspected lesions. All lesions were registered on a case registration form (CRF), according to their anatomical location in the liver according to Couinaud’s liver segments (8).
Figure 3.
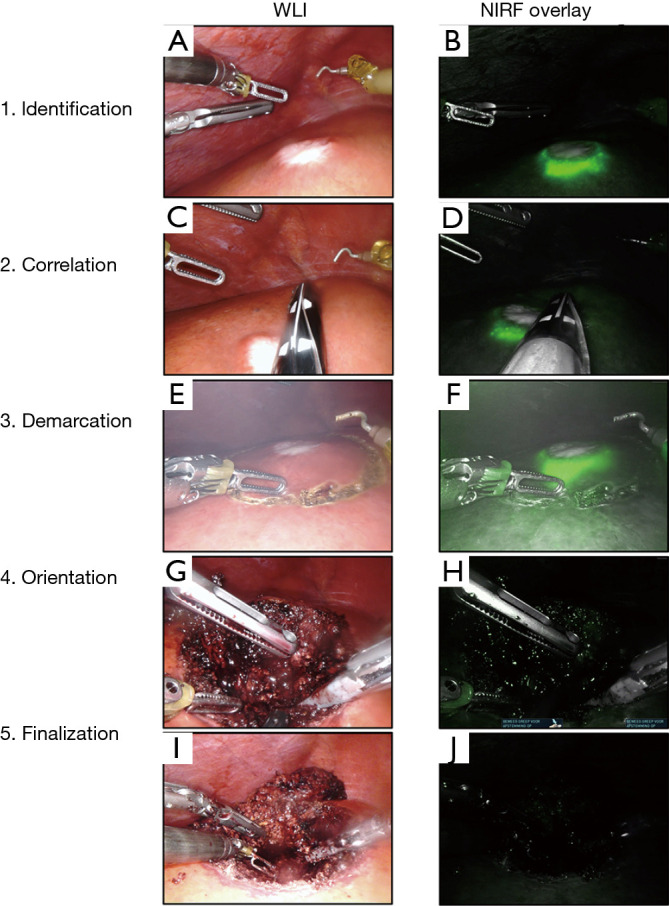
Identification and resection of a CRLM using IOUS and NIRF guidance. Surgical workflow for the identification and resection of CRLM. (A) Identification of the lesion in segment V using WLI and (B) using NIRF. (C) correlation of the lesion’s size with (D) the fluorescent rim using IOUS. (E) Demarcation of the lesion with the permanent cautery hook using WLI and (F) using NIRF. (G) Regular WLI of the resection plane and (H) frequent NIRF imaging is performed to check for potential fluorescent spots (no spots on image). (I) The wound bed and resection specimen in white light and (J) NIRF imaging to diagnose fluorescent spots (no fluorescent spots on image). CRLM, colorectal liver metastases; IOUS, intraoperative ultrasound; WLI, white light imaging; NIRF, near-infrared fluorescence.
The fluorescent rim was identified and correlated to the tumor’s location and size using IOUS (Figure 3C,D), after which the lesion is demarcated using an electrocoagulation device. After demarcation (Figure 3E,F), the resection is performed using both a vessel sealing device (Ligasure™, Medtronic, USA) and a laparoscopic compatible Cavitron Ultrasonic Surgical Aspirator (CUSA) in case of a laparoscopic procedure. In the robot-assisted procedures, the da Vinci® Xi Permanent Cautery Hook was used for demarcation and the da Vinci® Xi vessel sealer with EndoWrist in combination with bipolar cautery was used to perform the parenchymal transection. During the resection, inspection of the wound bed and resection plane using NIRF imaging was performed routinely (Figure 3G,H). Any fluorescent signal in the wound bed or resection specimen was identified and documented. All NIRF images were later analysed and correlated to histopathology. After finalizing the resection, in vivo WLI and NIRF images of the wound bed and both sides of the tissue specimen were acquired (Figure 3I,J). Figure 4A,B,C,D,E,F shows the chronological documentation of the procedure where a NIRF positive resection specimen was observed.
Figure 4.
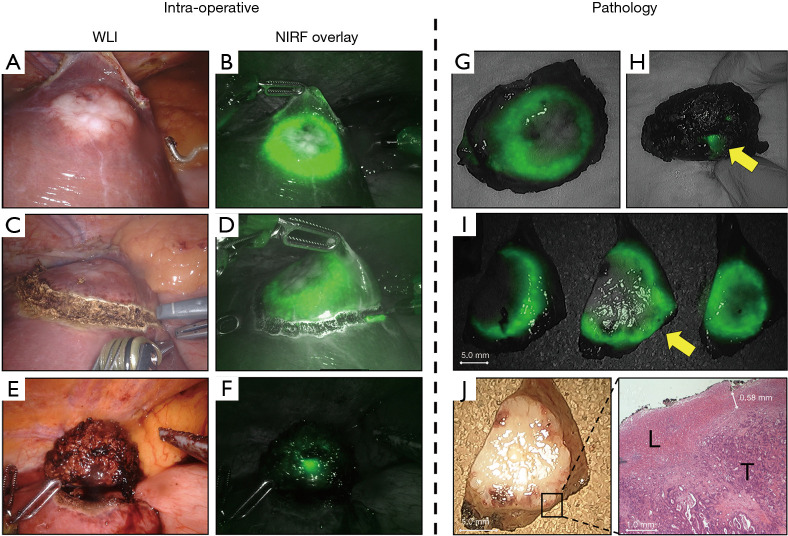
Correlation between in vivo and ex vivo NIRF imaging. (A) Resection of a CRLM in liver segment IVb in normal white light and, (B) with NIRF imaging. (C) Too close proximity of the demarcation line to the (D) fluorescent rim might result in a resection with tumor-positive margin. (E) Suspected resection with tumor-positive margin using WLI and (F) confirmed with NIRF. Ex vivo NIRF imaging of the (G) front of the lesion and (H) from the back show the same protruding rim. (I) Gross sectioning reveals a potential resection with tumor-positive margin, indicated by the yellow arrow. Which is confirmed by NIRF imaging. Later histopathological assessment of the suspected tumor-margin in (J) is confirmed by H&E staining. WLI, white light imaging; NIRF, near-infrared fluorescence; H&E, haematoxylin and eosin; L, normal liver tissue; T, tumor tissue.
Histopathology
Directly after surgery, the resected specimen is conserved on ice and transported to the pathology department. Prior to gross sectioning, white light images and NIRF images were acquired from the complete resected specimen (Figure 4G,H). Within 24 hours after surgery and after fixation with formalin, gross sectioning was performed and all bread loafs were imaged consecutively to identify the fluorescent rim. Discontinuities of the fluorescent rim or the absence of overlaying liver tissue was documented as a potentially tumor-positive resection margin (Figure 4I,J). Subsequently the macroscopic resection margin is determined by the pathologist and the tissue was paraffin embedded. Slides with a 3 µm thickness were sectioned from fresh frozen and paraffin embedded (FFPE) tissue blocks and stained with haematoxylin and eosin (H&E) to microscopically assess the lesion and the resection margin. A resection margin with a microscopical distance of more than 1 mm from the resection plane to the tumor burden is classified as a radical or margin-negative resection (R0). While resection margins with less than 1 mm were considered as margin-positive resections (R1) (2,3).
Statistical analysis
Continuous variables related to the surgical procedure were displayed in median values followed by the range. All tumor margins were providing in millimeters.
Results
NIRF guided resection
All target lesions were identified using NIRF imaging and displayed a fluorescent rim at the liver capsula in vivo (Figure 3B). Sixteen lesions were resected and sent for histopathological examination. In one patient, diagnosed with a local recurrence after ablative therapy, a deviant fluorescent pattern was observed. ICG had accumulated around the previously ablated liver tissue causing residual fluorescent signal in both the wound bed and the resection specimen after resection of the metastasis. While in all the other cases, the fluorescent rim correlated with the tumor location and size. In total, out of the 16 lesions, seven displayed a positive fluorescent signal from the specimen’s resection plane in vivo, produced by a protruding rim (Figure 4F). Out of these seven cases, three also showed NIRF signal in the corresponding plane of the wound bed of the residual liver tissue (Figure 2C). All NIRF signals were scored as a direct fluorescent signal generated by the lesion’s rim and, therefore, registered as potential tumor-positive resections. In addition, the other nine lesions showed the presence of the normal rim feature on the liver surface with no fluorescent signal at either side of the resection plane or wound bed (Table 2).
Table 2. In vivo tumor-margin assessment with NIRF.
| Lesion No. | Tumor margin determined by | T-margin (in mm) | |
|---|---|---|---|
| In vivo NIRF | Histopathology | ||
| 1 | Negative | Negative | 6 |
| 2 | Negative | Negative | 16 |
| 3.1 | Negative | Negative | 1.2 |
| 4 | Negative | Negative | 8 |
| 5 | Negative | Negative | 4 |
| 6 | Negative | Negative | 1.7 |
| 7 | Negative | Negative | 3 |
| 8.1 | Negative | Positive | <1 |
| 8.2 | Positive | Positive | <1 |
| 9 | Positive | Positive | <1 |
| 10 | Positive | Positive | <1 |
| 11 | Positive | Positive | <1 |
| 3.2 | Positive | Positive | 0 |
| 12 | Positive | Negative* | <1† |
| 13 | Positive | Negative* | 0† |
| 14 | Positive | Positive | 0 |
†, assessment of initial resection specimen; *, assessment after additional resection. NIRF, near-infrared fluorescence.
Histopathology
All lesion showed the presence of a fluorescent rim during ex vivo examination. The median lesion size based on histopathological examination was 22.5 mm (range, 8–37 mm). Median reported resection margin was 5.5 mm for R0 resections. All resection specimens showing a protruding rim in vivo and ex vivo were reported as a R1 resection (Figure 4I,J). An additional lesion which was identified by NIRF inspection was reported as a 15 mm CRLM with a tumor margin smaller than 1 mm. This lesion showed no protruding fluorescent rim in vivo and was therefore reported as false-negative. All other in vivo NIRF negative lesions (n=8) were reported as tumor-negative resections.
Discussion
In this study, we report a technique which can improve surgical margin determination in vivo during minimal invasive resections of CRLM, using real-time NIRF imaging with ICG. Injecting a low dose of ICG (10 mg) 24 hours prior to surgery, enables the surgeon to visualize a fluorescent rim surrounding the metastasis. Current results show the concordance between the appearance of fluorescent signal in vivo and the histopathological resection margin, indicating that complete resection of the fluorescence rim is sufficient for obtaining a resection with tumor-negative margins. Although this cohort is not a representation of the patient population in terms of resection margins, this report does showcase the technique’s ability to differentiate between tumor positive and negative margins in a realistic in vivo environment.
Patients with primary CRLM (synchronous and metachronous) or recurrent patients with newly developed metastases could benefit from using this technique. We were able to distinguish between tumor margins with high accuracy using our intraoperative NIRF workflow (Figure 3, Video S1). If the resection plane of the specimen shows a direct fluorescent signal produced by the protruding tumor rim, a tumor-positive margin is very likely. Residual fluorescent signal in the wound bed resulted in a resection with tumor-positive margins in all cases, but is found to be less predictive than a protruding rim. In addition, in case no fluorescent signal was observed in the resection plane during the procedure, we were able to predict a tumor-negative resection in almost all cases.
Video S1.
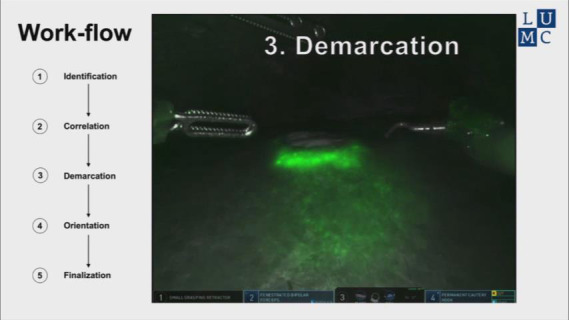
Instructional video for in vivo ICG-fluorescence guided detection of tumor margins during minimal invasive resections of colorectal liver metastases.
This study pointed out a subset of patients who might not benefit from this surgical technique: patients with local recurrence at the site of previous resection or ablative therapy might display a deviant tumor rim. Regenerative tissue or scar tissue from the liver appears to retain ICG, therefore, we hypothesize that ICG accumulation in regenerative tissue is either caused by accumulation inside immature hepatocytes or caused by the Enhanced Permeability and Retention effect (EPR) (9). For the purpose of this study (e.g., to explore the possibilities of this surgical technique) patients with low tumor burden and peripheral tumor location were selected.
NIRF guided resections during minimal invasive procedures provide many advantages (5). The real-time imaging capabilities of NIRF imaging which provides an overlay of the fluorescent image on the regular bright field images. This significantly simplifies the correlation of the signal in correlation to the anatomy. Furthermore, since NIRF is directly incorporated into the laparoscopic system, it only requires one port, eliminating the need for additional ports or constantly switching between instruments required for laparoscopic IOUS, known to be technically challenging. Secondly, in our study, all lesions were located at the liver’s surface or directly below. Presenting the clinician with the opportunity to identify the lesions using the fluorescent rim. Although identification of the lesion with NIRF is more challenging when located more centrally in the liver, the fluorescent rim is still present and can therefore still be used to assess the tumor-margin after part of the parenchymal transection is performed.
Previous reports also relied on NIRF imaging with ICG to resect tumors from patients with hepatocellular carcinoma and CRLM (10). In these studies, a higher dose of ICG (0.5 mg/kg) was administered 2–14 days prior to surgery. The high doses of ICG were shown to promote passive accumulation in the tumor itself due to EPR effect. This allowed differentiation of cancerous tissue from healthy tissue through NIRF imaging. While this approach seemed very promising, we believe that highlighting the tumor itself can still provide risk of tumor-positive resection margins and potential tumor spill. Furthermore, our technique is able to better assess the resection margins by ICG accumulation around the tumor.
Image interpretation and the lack of in vivo signal quantification is still found to be one of the major hurdles in implementing fluorescent guided surgery on a large scale and incorporating it into daily practice (11). Although an effort is made to develop real-time fluorescent signal quantification in vivo (12), this technique is not available on current clinical systems. Therefore, we feel that implementing the use of fluorescence guided surgery in new clinical centers might be achieved during a study collaboration to transfer knowledge between clinicians and to train new users.
ICG has been used in many surgical interventions as a diagnostic agent (13-15). The relatively low costs and stability in patients make it a favorable contrast agent. Nevertheless, ICG is a non-specific fluorescent dye that is either used to visualize organ perfusion (e.g., liver perfusion, biliary tract demarcation) or tumors using the EPR-effect. A variety of tumor specific fluorescent dyes are being developed to become the next generation of diagnostic fluorescent dyes. These dyes visualize the cell’s molecular biology by binding to overexpressed receptors specific to one type of tumor (16), or in some cases specific to multiple types of cancer (17-20). This might enable a more binary approach to discriminate between tumor and healthy tissue, since a positive in vivo signal resembles almost certain a resection with tumor-positive margin.
When combining a non-specific dye and a tumor-specific protein-dye conjugate that emits fluorescent light in separable wavelengths, the surgeon would be able to use the benefits of both techniques. In this ‘dual-channel’ approach, tumor-specific dyes allow tumor detection beyond the liver (e.g., peritoneum, lymph nodes) for more accurate disease staging and preventing surgery in advanced stage metastasized patients (16). The use of tumor-targeted imaging agents will allow for a more specific identification of metastases. Earlier studies with a CEA-targeted NIRF labelled antibody have shown to accurately identify peritoneal disease up to one cubic millimeter in size, and might in that perspective also benefit sensitivity (21). However, in this study, an additional subcapsular lesion of 15 mm in diameter was identified using the rim enhancement pattern, which was not visualized using IOUS nor during visual inspection. This further emphasizes the need for collaboration between clinicians, probe developers and camera technicians to further expand the translatability and quantification.
To further study the ability and potential limitation of our technical approach, we initiated a large multicenter prospective cohort study. In this study the technique’s ability to increase tumor-negative resection margins will be investigated in a realistic patient cohort. This trial also poses the ability to transfer knowledge to other clinicians and to implement this technique into other clinics. As part of the study’s endpoints, disease recurrence and patient survival will be monitored and analyzed at the 6 months and 5-year time point. In this Minimally Invasive, ICG-guided Metastasectomy in Patients with Colorectal liver metastases Trial (MIMIC Trial, Dutch Trial Register number NL7674).
Conclusions
Using a fluorescent rim surrounding CRLM has the potential to aid the surgeon in acquiring a tumor-negative resection in minimal invasive resections. The presence of a protruding fluorescent tumor-rim is predictive for a tumor-positive resection. Respectively, the absence of NIRF signal is indicative for a tumor-negative resection.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: The work in this manuscript was funded by the Dutch Cancer Society (Young Investigator Grant 11289).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted according to the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Ethics Review Board of the Leiden University Medical Center (The Netherlands, G19.012). All patient data was registered after informed consent was acquired.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1999
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1999
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1999). Dr. FBA reports grants from Dutch Cancer Society, during the conduct of the study. Dr. RPJM reports that he is a member of the study team performing the SGM-101 phase III study (NCT03659448). He does not receive funding in this perspective or has other financial conflicts related to this study. Dr. RJS reports grants from Dutch Cancer Society, during the conduct of the study. The other authors have no conflicts of interest to declare.
References
- 1.Fretland AA, Dagenborg VJ, Bjornelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. 10.1097/SLA.0000000000002353 [DOI] [PubMed] [Google Scholar]
- 2.Hamady ZZ, Lodge JP, Welsh FK, et al. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg 2014;259:543-8. 10.1097/SLA.0b013e3182902b6e [DOI] [PubMed] [Google Scholar]
- 3.Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, et al. Impact of Surgical Margin Width on Recurrence and Overall Survival Following R0 Hepatic Resection of Colorectal Metastases: A Systematic Review and Meta-analysis. Ann Surg 2018;267:1047-55. 10.1097/SLA.0000000000002552 [DOI] [PubMed] [Google Scholar]
- 4.van der Vorst JR, Schaafsma BE, Hutteman M, et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013;119:3411-8. 10.1002/cncr.28203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boogerd LS, Handgraaf HJ, Lam HD, et al. Laparoscopic detection and resection of occult liver tumors of multiple cancer types using real-time near-infrared fluorescence guidance. Surg Endosc 2017;31:952-61. 10.1007/s00464-016-5007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handgraaf HJM, Boogerd LSF, Hoppener DJ, et al. Long-term follow-up after near-infrared fluorescence-guided resection of colorectal liver metastases: A retrospective multicenter analysis. Eur J Surg Oncol 2017;43:1463-71. 10.1016/j.ejso.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vahrmeijer AL, Hutteman M, van der Vorst JR, et al. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol 2013;10:507-18. 10.1038/nrclinonc.2013.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couinaud C. Le foie, études anatomiques et chirurgicales. Paris: Masson & Cie; 1957. [Google Scholar]
- 9.Tummers QR, Hoogstins CE, Peters AA, et al. The Value of Intraoperative Near-Infrared Fluorescence Imaging Based on Enhanced Permeability and Retention of Indocyanine Green: Feasibility and False-Positives in Ovarian Cancer. PLoS One 2015;10:e0129766. 10.1371/journal.pone.0129766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki T, Murakami M, Koizumi T, et al. Determination of the surgical margin in laparoscopic liver resections using infrared indocyanine green fluorescence. Langenbecks Arch Surg 2018;403:671-80. 10.1007/s00423-018-1685-y [DOI] [PubMed] [Google Scholar]
- 11.Judy RP, Keating JJ, DeJesus EM, et al. Quantification of tumor fluorescence during intraoperative optical cancer imaging. Sci Rep 2015;5:16208. 10.1038/srep16208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koller M, Qiu SQ, Linssen MD, et al. Implementation and benchmarking of a novel analytical framework to clinically evaluate tumor-specific fluorescent tracers. Nat Commun 2018;9:3739. 10.1038/s41467-018-05727-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlek SL, van Dam DA, Rubinstein SM, et al. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc 2017;31:2731-42. 10.1007/s00464-016-5318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada T, Kawada K, Takahashi R, et al. ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 2017;31:4184-93. 10.1007/s00464-017-5475-3 [DOI] [PubMed] [Google Scholar]
- 15.Knackstedt R, Couto RA, Ko J, et al. Indocyanine Green Fluorescence Imaging with Lymphoscintigraphy for Sentinel Node Biopsy in Melanoma: Increasing the Sentinel Lymph Node-Positive Rate. Ann Surg Oncol 2019;26:3550-60. 10.1245/s10434-019-07617-z [DOI] [PubMed] [Google Scholar]
- 16.Gutowski M, Framery B, Boonstra MC, et al. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surg Oncol 2017;26:153-62. 10.1016/j.suronc.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 17.Miller SE, Tummers WS, Teraphongphom N, et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J Neurooncol 2018;139:135-43. 10.1007/s11060-018-2854-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal EL, Warram JM, de Boer E, et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res 2015;21:3658-66. 10.1158/1078-0432.CCR-14-3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korb ML, Hartman YE, Kovar J, et al. Use of monoclonal antibody-IRDye800CW bioconjugates in the resection of breast cancer. J Surg Res 2014;188:119-28. 10.1016/j.jss.2013.11.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day KE, Sweeny L, Kulbersh B, et al. Preclinical comparison of near-infrared-labeled cetuximab and panitumumab for optical imaging of head and neck squamous cell carcinoma. Mol Imaging Biol 2013;15:722-9. 10.1007/s11307-013-0652-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boogerd LSF, Hoogstins CES, Schaap DP, et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose-escalation pilot study. Lancet Gastroenterol Hepatol 2018;3:181-91. 10.1016/S2468-1253(17)30395-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as