Abstract
BACKGROUND
Bile acids (BAs) have attracted attention in the research of irritable bowel syndrome with predominant diarrhea (IBS-D) due to their ability to modulate bowel function and their tight connection with the gut microbiota. The composition of the fecal BA pool in IBS-D patients is reportedly different from that in healthy populations. We hypothesized that BAs may participate in the pathogenesis of IBS-D and the altered BA profile may be correlated with the gut microbiome.
AIM
To investigate the role of BAs in the pathogenesis of IBS-D and the correlation between fecal BAs and gut microbiota.
METHODS
Fifty-five IBS-D patients diagnosed according to the Rome IV criteria and twenty-eight age-, sex-, and body mass index-matched healthy controls (HCs) were enrolled in this study at the gastroenterology department of China-Japan Friendship Hospital. First, clinical manifestations were assessed with standardized questionnaires, and visceral sensitivity was evaluated via the rectal distension test using a high-resolution manometry system. Fecal primary BAs including cholic acid (CA) and chenodeoxycholic acid (CDCA), secondary BAs including deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA) as well as the corresponding tauro- and glyco-BAs were examined by ultraperformance liquid chromatography coupled to tandem mass spectrometry. The gut microbiota was analyzed using 16S rRNA gene sequencing. Correlations between fecal BAs with clinical features and gut microbiota were explored.
RESULTS
Fecal CA (IBS-D: 3037.66 [282.82, 6917.47] nmol/g, HC: 20.19 [5.03, 1304.28] nmol/g; P < 0.001) and CDCA (IBS-D: 1721.86 [352.80, 2613.83] nmol/g, HC: 57.16 [13.76, 1639.92] nmol/g; P < 0.001) were significantly increased, while LCA (IBS-D: 1621.65 [58.99, 2396.49] nmol/g, HC: 2339.24 [1737.09, 2782.40]; P = 0.002] and UDCA (IBS-D: 8.92 [2.33, 23.93] nmol/g, HC: 17.21 [8.76, 33.48] nmol/g; P = 0.025) were significantly decreased in IBS-D patients compared to HCs. Defecation frequency was positively associated with CA (r = 0.294, P = 0.030) and CDCA (r = 0.290, P = 0.032) and negatively associated with DCA (r = −0.332, P = 0.013) and LCA (r = −0.326, P = 0.015) in IBS-D patients. In total, 23 of 55 IBS-D patients and 15 of 28 HCs participated in the visceral sensitivity test. The first sensation threshold was negatively correlated with CDCA (r = −0.459, P = 0.028) in IBS-D patients. Furthermore, the relative abundance of the family Ruminococcaceae was significantly decreased in IBS-D patients (P < 0.001), and 12 genera were significantly lower in IBS-D patients than in HCs (P < 0.05), with 6 belonging to Ruminococcaceae. Eleven of these genera were negatively correlated with primary BAs and positively correlated with secondary BAs in all subjects.
CONCLUSION
The altered metabolism of BAs in the gut of IBS-D patients was associated with diarrhea and visceral hypersensitivity and might be ascribed to dysbiosis, especially the reduction of genera in Ruminococcaceae.
Keywords: Bile acids, Irritable bowel syndrome, Diarrhea, Visceral hypersensitivity, Microbiota, Dysbiosis
Core Tip: This study comprehensively investigated the fecal bile acid profile of irritable bowel syndrome with predominant diarrhea (IBS-D) patients and healthy controls, and the correlations between bile acids (BAs) and clinical characteristics as well as the gut microbiota of IBS-D patients. We found that the composition of fecal BAs in IBS-D patients is featured by increased primary BAs and decreased secondary BAs, which was associated with diarrhea and visceral hypersensitivity. The abnormality of BAs might be induced by dysbiosis in IBS-D patients, especially the reduction of genera in the Ruminococcaceae family, which contains the majority of bacteria that are capable of converting primary BAs into secondary BAs.
INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic and sometimes intractable functional bowel disorder, characterized by recurrent abdominal pain related to defecation or accompanied by changes in stool frequency or form. It affects 1.1%–35.5% of the population across different countries[1], with a prevalence of 6.5% in the Chinese population[2]. IBS is classified into four subtypes based on the predominant stool form of the patients: IBS with predominant diarrhea (IBS-D), IBS with predominant constipation (IBS-C), IBS with mixed bowel habits, and IBS unclassified[3]. As the most common subtype in China, IBS-D accounts for 47.1%–66.3% of all IBS cases[4,5]. The exact pathogenesis of IBS is incompletely understood. Motility disturbances and visceral hypersensitivity are generally thought to be involved, and gut dysbiosis, immune dysregulation, intestinal barrier dysfunction, and brain-gut interaction disorder may also be implicated in the pathophysiology of IBS[6].
Recently, bile acids (BAs) metabolism has attracted attention in IBS. Apart from facilitating the absorption of lipids, BAs can also affect gastrointestinal motility, mucosal permeability, and the secretion of water, electrolytes, and mucus in the gut[7]. Changes in BAs metabolism between IBS patients and healthy populations have been reported[8-10], but the data delineating BAs metabolism in Chinese IBS-D patients are still sparse. Moreover, BA sequestrants, which can bind intraluminal BAs, can attenuate diarrhea and decrease the score of the IBS Symptom Severity Scale (IBS-SSS) in IBS-D patients[11-13]. Therefore, the involvement of BAs in the pathogenesis of IBS-D is worthy of particular note.
Primary BAs, including cholic acid (CA) and chenodeoxycholic acid (CDCA), are formed from cholesterol in hepatocytes, excreted through the biliary tree into the gallbladder after conjugation with glycine or taurine, and further released into the duodenum in response to meal ingestion. Conjugated CA and CDCA are then chemically modified by the intestinal microbiota through deconjugation and 7α-dehydroxylation, converting them into secondary BAs, i.e. deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. Apart from LCA, a small part of CDCA is transformed into another kind of secondary BA, ursodeoxycholic acid (UDCA), by microbiota through 7α/β-epimerization[14,15]. DCA, LCA, and UDCA can also be conjugated with glycine or taurine. Over 90% of conjugated BAs are reabsorbed in the terminal ileum and return to the liver through the portal circulation, while unconjugated BAs can be reabsorbed by passive diffusion across the epithelium of the small intestine and colon[7,16]. Because of the critical position of the gut microbiome in BAs synthetic regulation, gut dysbiosis may lead to dysmetabolism of BAs[14]. Ample evidence has suggested that the gut microbiota of IBS-D patients differs from that of healthy controls (HCs)[17-21], and gut dysbiosis has been considered to be involved in the putative pathophysiology of IBS[22-24]. However, information about correlations between fecal BAs and the gut flora in IBS-D patients is limited.
Due to the evidence of altered fecal BA profile and gut microbiota in IBS-D patients, we hypothesized that BAs may participate in the pathogenesis of IBS-D, and the gut dysbiosis may contribute to the abnormity of BAs metabolism. To investigate this hypothesis, BAs-related metabolomic analyses and 16S rRNA gene sequencing of feces were performed in an IBS-D cohort and matched HCs to explore the association between the composition of fecal BA pool and the gut microbiota.
MATERIALS AND METHODS
Subject recruitment and sample collection
A total of 55 IBS-D patients aged between 18 and 60 years along with 28 age-, sex-, and body mass index (BMI)-matched HCs were recruited in this study. The sample size was calculated with PASS 08.0.3 (NCSS LLC., Kaysville, UT, United States), based on the proportions of primary BAs, secondary BAs, and conjugated BAs in IBS-D patients and HCs in a previous study[25]. A sample size of 22 patients and 11 HCs will have 90% power to detect the difference in fecal BAs composition at a significance level of 0.05.
All patients visited the gastroenterology department of China-Japan Friendship Hospital from May 2019 to October 2019 and were diagnosed with IBS-D according to the Rome IV criteria. The score of IBS-SSS of the patients was required to be above 75 to ensure that they were symptomatic at the study entry. HCs were recruited through public advertisements. Subjects with a history of organic diseases (including gastrointestinal diseases and other major organ injuries), major abdominal surgery, endoscopic retrograde cholangiopancreatography, psychiatric disorders, and abuse of alcohol were excluded. Pregnant or lactating female subjects and those with dysmenorrhea were also excluded. Subjects who took probiotics, antibiotics, prokinetics, antispasmodics, analgesics, non-steroidal anti-inflammatory drugs, and antidepressants within 4 wk, or corticosteroids, immunosuppressants, BA sequestrants, and lipid-lowering agents within 6 mo before the study were excluded. Informed consent was obtained from each subject before his/her entry to the study. This study was approved by the Ethics Committee of the China-Japan Friendship Hospital (No. 2019-64-K44).
Fresh stool samples were collected with sterile plastic tubes from all subjects in China-Japan Friendship Hospital and were immediately transferred to the laboratory and stored at −80°C until analysis. Each sample was homogenized and divided into at least 2 parts for BAs and microbiota analyses. Considering the circadian rhythm in BAs synthesis and metabolism[26], all of the samples were collected between 07:00 am and 10:00 am. All subjects were asked to maintain their usual dietary habits at least 1 wk before the collection of the stool samples and until all of the assessments were finished. The pharmacological agents aforementioned were not allowed throughout the study period.
Clinical and psychological assessments
Disease severity was evaluated with the IBS-SSS, a validated questionnaire including five items (severity and frequency of abdominal pain, abdominal bloating, bowel habit dissatisfaction, and overall interference with quality of life)[27]. The highest score was 100 for each item and the total score ranges from 0 to 500. All subjects were asked to report their stool consistency according to the Bristol stool form scale (BSFS) and defecation frequency per day during the preceding 2 wk.
Psychological characteristics were assessed with the hospital anxiety and depression scale (HADS) consisting of 7 items for anxiety and 7 items for depression. The maximum score is 21 for both of the dimensions, with a score of less than 8 for non-cases, a score of 8-10 for doubtful cases, and a score of more than 10 for definite cases of anxiety or depression[28]. In addition, the visceral sensitivity index (VSI) scale was used to measure gastrointestinal symptoms-specific anxiety (GSA)[29], with 15 items ranging from score 0 (no GSA) to 5 (severe GSA) and a maximum score of 75.
Visceral sensitivity was assessed with a high-resolution manometry system that has been used previously in our laboratory[30]. Briefly, after completely emptying the rectum with a glycerin enema, an anorectal catheter with a latex balloon at the tip was inserted into the rectum of subjects. After subjects adapted to the catheter in the rectum, the balloon was manually inflated with air through a 100-mL syringe at a speed of 10 mL/5 s, and subjects were asked to report their feelings of initial perception, defecation sensation, and discomfort/pain during the process, with the corresponding balloon volumes recorded as the first sensation threshold, defecating sensation threshold, and maximum tolerable threshold, respectively.
BAs analysis
Chemicals: All 15 standards were obtained from Steraloids Inc. (Newport, RI, United States) and TRC Chemicals (Toronto, ON, Canada) including CA, CDCA, DCA, LCA, UDCA, glyco-cholic acid, glyco-chenodeoxycholic acid, glyco-deoxycholic acid, glyco-lithocholic acid, glyco-ursodeoxycholic acid (GUDCA), tauro-cholic acid (TCA), tauro-chenodeoxycholic acid, tauro-deoxycholic acid, tauro-lithocholic acid, and tauro-ursodeoxycholic acid (TUDCA). Six deuterated internal standards were obtained from Steraloids Inc. (Newport, RI, United States) and C/D/N Isotopes Inc. (Quebec, Canada).
Sample preparation: To diminish sample degradation, feces samples were thawed in an ice-bath at first. Next, approximately 10 mg of each sample was weighed and transferred to a tube together with 25 mg zirconium oxide beads and 200 μL acetonitrile/methanol (8/2) containing 10 μL internal standards. The sample was centrifuged at 13500 g for 20 min at 4 °C after homogenization. Then, 10 μL supernatant was diluted with 90 μL of the mixture containing the equal amount of acetonitrile/methanol (8/2) and ultrapure water. After centrifugation, the supernatant was used to quantitate the BAs with the ultraperformance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) system (ACQUITY UPLC-Xevo TQ-S, Waters Corp., MA, United States).
UPLC-MS/MS analysis: Each sample was injected splitless into an ACQUITY UPLC Cortecs C18 1.6 μM VanGuard precolumn (2.1 mm × 5 mm) and an ACQUITY UPLC Cortecs C18 1.6 μM analytical column (2.1 mm × 100 mm). The mobile phases consisted of 10 mmol/L ammonium acetate with 0.25% acetate acid (mobile phase A) and acetonitrile/methanol/isopropanol (8/1/1) (mobile phase B). The flow rate was 0.40 mL/min with the following mobile phase gradient: 0-0.3 min (5% B), 0.3-0.5 min (5%-10% B), 0.5-2 min (10%-15% B), 2-3 min (15%-30% B), 3-6 min (30% B), 6-8 min (30%-35% B), 8-9 min (35%-40% B), 9-10 min (40% B), 10-15 min (40%-75% B), 15-15.5 min (75%-100% B), 15.5-16.2 min (100% B), 16.2-16.3 min (100%-5% B), 16.3-17 min (5% B). The column temperature was 30°C and the injection volume of each sample was 5 μL. The capillary voltage was 2.0 kV in negative ion mode. The source temperature was maintained at 150°C and the desolvation gas temperature was maintained at 550°C.
Data processing: The raw data files generated by UPLC-MS/MS were processed using MassLynx software (v4.1; Waters, MA, United States) to perform peak integration, calibration, and quantitation for BAs in the samples. The BAs analysis was conducted by the Metabo-profile Biotechnology (Shanghai, China).
16S rRNA gene sequencing analysis
Microbial DNA was extracted from the fecal samples of the subjects using a QIAamp Fast DNA Stool Mini Kit (Qiagen, Valencia, United States). DNA quality was determined by 1% agarose gel electrophoresis and Thermo NanoDrop 2000 (Thermo Fisher Scientific, MA, United States). The amplification of the hypervariable V3-V4 region was conducted using primers 341F (5’-CCTACGGGRSGCAGCAG-3’) and 806R (5’-GGACTACVVGGGTATCTAATC-3’). The KAPA HiFi Hotstart ReadyMix PCR Kit (Kapa Biosystems, Massachusetts, United States) was used for PCR, containing 15 μL of 2 × Kapa Library Amplification ReadyMix, 1 μL forward primer, 1 μL reverse primer, and 50 ng template DNA. The amplicons were then extracted with 2% agarose gels and further purified using an AxyPrep DNA Gel Extraction Kit (Axygen, CA, United States), and quantified using a Qubit dsDNA HS Assay Kit (Invitrogen, MA, United States). After the library was constructed, sequencing was performed using the Illumina NovaSeq PE250 platform (Illumina, CA, United States). Fecal 16S rRNA analysis was conducted by the Realbio Genomics Institute (Shanghai, China).
Sequences with similarity ≥ 97% were defined as operational taxonomic units. Alpha-diversity was analyzed with QIIME (V1.9.1), including the Shannon and Simpson indexes to depict microbial diversity as well as the Chao1 index to depict microbial richness. Beta diversity was assessed by principal coordinate analysis (PCoA) based on weighted and unweighted UniFrac distance metrics analysis. The Mann-Whitney U-test was employed to compare the relative abundance of bacterial taxa between the IBS-D group and the control group after logarithmic transformation. The distinguishing features of the fecal microbiota were analyzed using linear discriminant analysis (LDA) effect size (LEfSe), which can identify floras both significantly different and biologically meaningful[31]. The LEfSe P value of 0.05 and the LDA score threshold of 2.0 were used in this study.
Statistical analysis
Data are presented as the mean ± SD or the median (Q1, Q3). Comparisons between groups were performed using the independent samples t-test or the nonparametric Mann-Whitney U-test, and qualitative data were analyzed using the Chi-squared test, with a two-sided P < 0.05 considered statistically significant. False discovery rate (FDR) correction following the Benjamin-Hochberg method was applied when comparing the concentrations of BAs and the relative abundances of gut microbiota in the two groups. The relationships of BAs with the other clinical and microbial parameters were analyzed by Spearman’s correlation. Statistical analyses were performed using SPSS version 23.0 (SPSS Inc., Chicago, IL, United States). Statistical charts were generated using Graph Prism version 6.0 (GraphPad Software Inc., La Jolla, CA, United States).
Apart from the absolute concentrations of the different BAs, several parameters were also analyzed. The total BAs is the sum of the 15 BAs. The unconjugated BAs is the sum of CA, CDCA, DCA, LCA, and UDCA, and the conjugated BAs is the sum of their corresponding glyco- and tauro-BAs. The primary BAs is the sum of CA and CDCA and their glyco- and tauro- derivatives, and similarly, the secondary BAs is the sum of DCA, LCA, UDCA, and their glyco- and tauro-derivatives.
RESULTS
Demographics and clinical characteristics
Fifty-five IBS-D patients (41 men, 14 women) and 28 age-, sex-, and BMI-matched HCs (20 men, 8 women) participated in the study. Their demographic and clinical characteristics are presented in Table 1. The median duration of disease was 3.0 years (range, 0.5–30 years) and the median IBS-SSS score was 180.0 (range, 100–410) in the IBS-D group. The scores of abdominal pain severity and frequency on the IBS-SSS were 40.0 (20.0, 50.0) and 30.0 (20.0, 40.0) in the IBS-D group, respectively. The defecation frequency and BSFS scores were both significantly higher in IBS-D patients than in HCs (P < 0.001). Meanwhile, scores of HADS-anxiety, HADS-depression, and VSI were significantly increased in patients compared to controls (P < 0.01), indicating that IBS-D patients were prone to suffering from comorbid anxiety and depression.
Table 1.
Demographics and clinical characteristics of patients with diarrhea-predominant irritable bowel syndrome and healthy controls
|
Features
|
IBS-D patients
|
Controls
|
P
value
|
| n | 55 | 28 | NA |
| Age in yr | 35.1 ± 9.8 | 34.4 ± 10.6 | 0.754 |
| Gender, male: Female | 41: 14 | 5: 2 | 0.761 |
| Body mass index in kg/m2 | 23.3 ± 3.4 | 22.6 ± 3.1 | 0.335 |
| Duration of disease in yr | 3.0 (1.5, 7.0) | NA | NA |
| Defecation frequency | 3.5 (2.5, 4.0) | 1.0 (0.7, 1.0) | < 0.001 |
| BSFS score | 5.5 (5.0, 6.0) | 4.0 (4.0, 4.0) | < 0.001 |
| IBS-SSS | 180.0 (150.0, 240.0) | NA | NA |
| Abdominal pain severity | 40.0 (20.0, 50.0) | NA | NA |
| Abdominal pain frequency | 30.0 (20.0, 40.0) | NA | NA |
| Abdominal bloating | 10.0 (0.0, 20.0) | NA | NA |
| Bowel habit dissatisfaction | 60.0 (60.0, 70.0) | NA | NA |
| Overall interference with QOL | 30.0 (30.0, 50.0) | NA | NA |
| HADS anxiety score | 5.0 (3.0, 10.0) | 2.0 (0.0, 3.8) | < 0.001 |
| HADS depression score | 4.0 (1.0, 7.0) | 1.5 (0.0, 3.8) | 0.009 |
| VSI score | 30.0 (19.0, 42.0) | 4.0 (0.0, 13.3) | < 0.001 |
The data are presented as the mean ± SD or the median (Q1, Q3). BSFS: Bristol stool form scale; HADS: Hospital anxiety and depression scale; IBS-D: Irritable bowel syndrome with predominant diarrhea; IBS-SSS: Irritable bowel syndrome symptom severity scale; NA: Not applicable; QOL: Quality of life; VSI: Visceral sensitivity index.
In total, 23 of the 55 IBS-D patients and 15 of the 28 HCs participated in the visceral sensitivity test, while the other subjects refused this examination due to the concern of the discomfort caused by the catheter in the rectum. The maximum tolerable threshold was significantly lower in the patients compared to that in HCs (P < 0.001). The defecating sensation threshold tended to decrease in the patients, but the difference was not statistically significant (P = 0.073). The difference between the first sensation thresholds of the two groups was not significant (Table 2).
Table 2.
Visceral sensation thresholds in patients with diarrhea-predominant irritable bowel syndrome and healthy controls
|
Visceral sensation threshold in mL
|
IBS-D patients, n = 23
|
Controls, n = 15
|
P
value
|
| First sensation threshold | 40 (20, 50) | 40 (35, 60) | 0.235 |
| Defecating sensation threshold | 60 (50, 85) | 70 (65, 100) | 0.073 |
| Maximum sensation threshold | 105 (90, 120) | 160 (145, 190) | < 0.001 |
The data are expressed as median (Q1, Q3). IBS-D: Irritable bowel syndrome with predominant diarrhea.
Fecal BA pool composition
The absolute concentrations of the 15 fecal BAs measured were available for all of the subjects and are summarized in Table 3. Compared to HCs, primary BAs, including CA, CDCA, and corresponding conjugated BAs, were significantly elevated in IBS-D patients (P < 0.01). Moreover, IBS-D patients displayed a significant decrease of LCA (P < 0.01) and a decreased trend of DCA although not significantly (P = 0.084). In addition, TUDCA and GUDCA were also significantly increased (P < 0.01), while UDCA was significantly decreased in IBS-D patients compared to HCs (P < 0.05). The level of total fecal BAs was significantly elevated in the IBS-D group (P < 0.05).
Table 3.
Levels of fecal bile acids in patients with diarrhea-predominant irritable bowel syndrome and controls
|
Bile acids in nmol/g
|
IBS-D patients, n = 55
|
Controls, n = 28
|
P
value
|
| CA | 3037.66 (282.82, 6917.47) | 20.19 (5.03, 1304.28) | < 0.001 |
| CDCA | 1721.86 (352.80, 2613.83) | 57.16 (13.76, 1639.92) | < 0.001 |
| DCA | 2012.66 (232.57, 2659.34) | 2159.78 (1676.03, 3094.08) | 0.084 |
| LCA | 1621.65 (58.99, 2396.49) | 2339.24 (1737.09, 2782.40) | 0.002 |
| UDCA | 8.92 (2.33, 23.93) | 17.21 (8.76, 33.48) | 0.025 |
| TCA | 5.36 (0.62, 26.39) | 0.72 (0.46, 2.11) | 0.004 |
| TCDCA | 6.85 (1.54, 22.89) | 1.41 (0.37, 3.58) | 0.001 |
| TDCA | 1.53 (0.93, 8.08) | 1.75 (0.86, 6.63) | 1.000 |
| TLCA | 0.88 (0.57, 1.85) | 1.03 (0.36, 2.80) | 0.908 |
| TUDCA | 1.43 (0.68, 2.61) | 0.37 (0.07, 1.23) | 0.002 |
| GCA | 4.36 (2.31, 17.52) | 2.23 (1.39, 3.55) | < 0.001 |
| GCDCA | 17.47 (5.61, 51.56) | 5.17 (2.56, 10.51) | < 0.001 |
| GDCA | 3.32 (0.63, 10.63) | 2.67 (1.44, 6.83) | 0.867 |
| GLCA | 0.64 (0.39, 1.61) | 0.91 (0.41, 1.28) | 0.282 |
| GUDCA | 1.27 (0.56, 4.76) | 0.65 (0.38, 0.87) | 0.002 |
| Total BAs | 8227.35 (5218.49, 12464.03) | 5220.28 (3971.35, 8272.29) | 0.011 |
| Unconjugated BAs | 8197.73 (5135.49, 11622.72) | 5183.71 (3945.65, 8253.23) | 0.017 |
| Conjugated BAs | 55.28 (27.09, 198.08) | 28.51 (9.68, 36.32) | 0.002 |
| Tauro-BAs | 23.00 (5.03, 58.65) | 6.34 (2.52, 18.05) | 0.006 |
| Glyco-BAs | 28.03 (13.20, 69.07) | 12.77 (6.18, 23.15) | < 0.001 |
| CBA/UBA ratio | 0.0080 (0.0036, 0.0172) | 0.0035 (0.0019, 0.0058) | 0.004 |
| Primary BAs | 5534.22 (672.24, 10546.54) | 85.88 (27.08, 3197.10) | < 0.001 |
| Secondary BAs | 3759.10 (336.36, 4694.96) | 4675.59 (3543.60, 5623.73) | 0.009 |
| PBA/SBA ratio | 1.66 (0.22, 11.14) | 0.02 (0.01, 0.57) | < 0.001 |
The bile acids data are presented as the median (Q1, Q3). The P value after FDR correction was presented in the table. BAs: Bile acids; CA: Cholic acid; CBA/UBA ratio: The ratio of conjugated BAs to unconjugated BAs; CDCA: Chenodeoxycholic acid; DCA: Deoxycholic acid; GCA: Glyco-cholic acid; GCDCA: Glyco-chenodeoxycholic acid; GDCA: Glyco-deoxycholic acid; GLCA: Glyco-lithocholic acid; GUDCA: Glyco-ursodeoxycholic acid; IBS-D: Irritable bowel syndrome with predominant diarrhea; LCA: Lithocholic acid; PBA/SBA ratio: The ratio of primary BAs to secondary Bas; TCA: Tauro-cholic acid; TCDCA: Tauro-chenodeoxycholic acid; TDCA: Tauro-deoxycholic acid; TLCA: Tauro-lithocholic acid; TUDCA: Tauro-ursodeoxycholic acid; UDCA: Ursodeoxycholic acid.
In terms of unconjugated BAs and conjugated BAs, the fecal BA pool of the IBS-D group and the HC group were both predominantly composed of unconjugated BAs. The ratio of conjugated BAs to unconjugated BAs (CBA/UBA) was significantly increased in the IBS-D group (P < 0.01), with the levels of unconjugated BAs and conjugated BAs both significantly higher in IBS-D patients compared to HCs (P < 0.05). Meanwhile, the level of fecal secondary BAs was much higher than primary BAs in the control group; however, this trend was inversed in some IBS-D patients, leading to a significantly increased ratio of primary BAs to secondary BAs (PBA/SBA) in the IBS-D group (P < 0.001).
To further explore the proportion of IBS-D patients with a remarkably imbalanced fecal BAs composition, the 90th percentiles of the CBA/UBA ratio (0.0299) and the PBA/SBA ratio (1.40) in the control group were determined as cutoff values, and we found that 10 (18.2%) and 30 (54.5%) patients had a high CBA/UBA ratio (≥ 0.0299) and a high PBA/SBA ratio (≥ 1.40) among the 55 IBS-D patients, respectively. There were no significant differences in demographic indices, BMI, or duration of disease between the high CBA/UBA and low CBA/UBA groups in patients, as well as between the high PBA/SBA and low PBA/SBA groups in patients.
Correlations between fecal BAs and clinical parameters
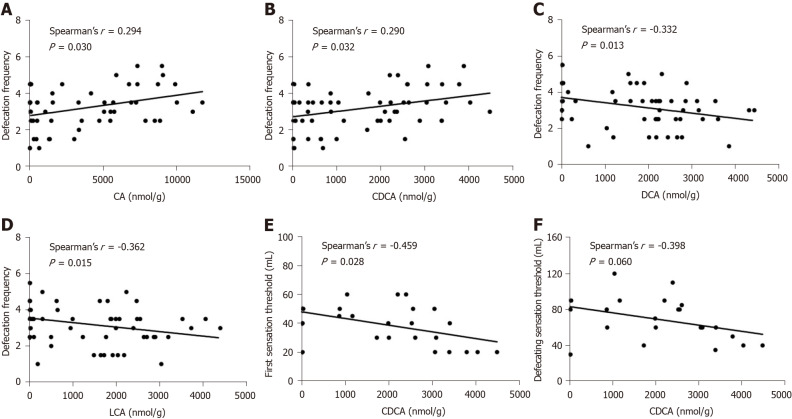
We analyzed the correlations between the fecal BAs and clinical parameters in the IBS-D group. Defecation frequency was positively associated with the levels of CA and CDCA and inversely associated with the levels of DCA and LCA (P < 0.05) (Figure 1A-D). Moreover, we observed an inverse correlation between the first sensation threshold and the concentration of CDCA (P < 0.05) (Figure 1E). The defecating sensation threshold also tended to be negatively correlated with CDCA (P = 0.060) (Figure 1F). However, the duration of disease, IBS-SSS, abdominal pain severity, abdominal pain frequency, the BSFS score, and the maximum tolerable threshold showed no significant correlations with fecal BAs. Additionally, there were no significant correlations between the HADS-anxiety score, HADS-depression score, or VSI and fecal BAs, in line with the earlier studies[9,32].
Figure 1.
Correlations between fecal bile acids and clinical parameters in patients with diarrhea-predominant irritable bowel syndrome. A-D: Defecation frequency was positively correlated with the level of cholic acid and chenodeoxycholic acid (CDCA), and negatively correlated with the level of deoxycholic acid and lithocholic acid; E: The first sensation threshold was negatively correlated with CDCA; F: The defecating sensation threshold tended to be negatively correlated with CDCA. CA: Cholic acid; CDCA: Chenodeoxycholic acid; DCA: Deoxycholic acid; LCA: Lithocholic acid.
In the IBS-D group, patients with a high PBA/SBA ratio had a significantly higher defecation frequency than those with a low PBA/SBA ratio (3.5 [2.5, 4.5] vs 3.0 [1.5, 3.5]; P = 0.038), along with an increasing trend of bowel habit dissatisfaction (70.0 [60.0, 72.5] vs 60.0 [45.0, 70.0]; P = 0.059] and abdominal pain severity (40.0 [30.0, 60.0] vs 30.0 [20.0, 50.0]; P = 0.077). However, all of the clinical parameters aforementioned showed no significant difference between patients with high and low CBA/UBA ratios.
Characteristics of the gut microbiome of IBS-D patients
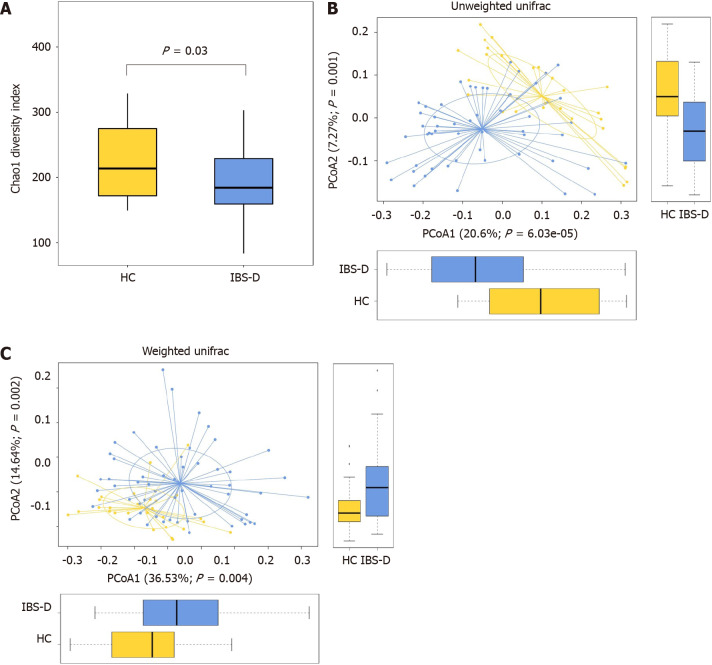
We performed 16S rRNA gene sequencing of the fecal samples from all subjects. Chao1 analysis exhibited a decrease of microbiota richness in IBS-D patients compared with HCs (P < 0.05) (Figure 2A), whereas the results of the Shannon and Simpson indices showed no evident differences in the diversity and evenness of the colonic microbiota. Unweighted and weighted UniFrac PCoA showed that the global microbiota structure of the samples of IBS-D patients differed significantly from that of HCs (P < 0.05) (Figure 2B and C). The configuration of the gut microbiota in the two groups is shown in Figure 3.
Figure 2.
Fecal bacterial structures of patients with diarrhea-predominant irritable bowel syndrome and healthy controls. A: Chao1 index in the irritable bowel syndrome with predominant diarrhea (IBS-D) group and the healthy control (HC) group, Chao1 index was decreased significantly in the IBS-D group; B and C: Weighted and unweighted principal coordinate analysis of fecal bacterial in the IBS-D and HC groups, both differed significantly between the two groups. Boxes indicate the interquartile range; lines inside the boxes indicate the medians; the two whiskers indicate the maximum and minimum of the data; the points outside the box indicate outliers. PCoA: Principal coordinate analysis.
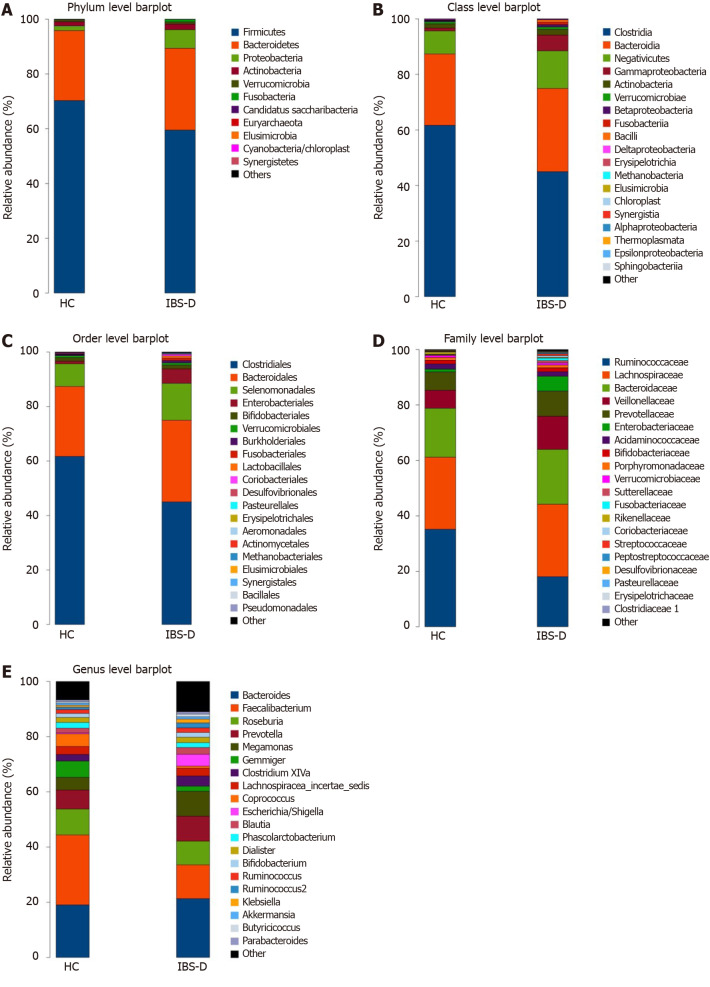
Figure 3.
Configuration of fecal bacterial of patients with diarrhea-predominant irritable bowel syndrome and healthy controls. A-E: Relative bacterial abundance of fecal bacterial at phylum (A), class (B), order (C), family (D), and genus (E) levels in the irritable bowel syndrome with predominant diarrhea group and the healthy control group. IBS-D: Irritable bowel syndrome with predominant diarrhea; HC: Healthy control.
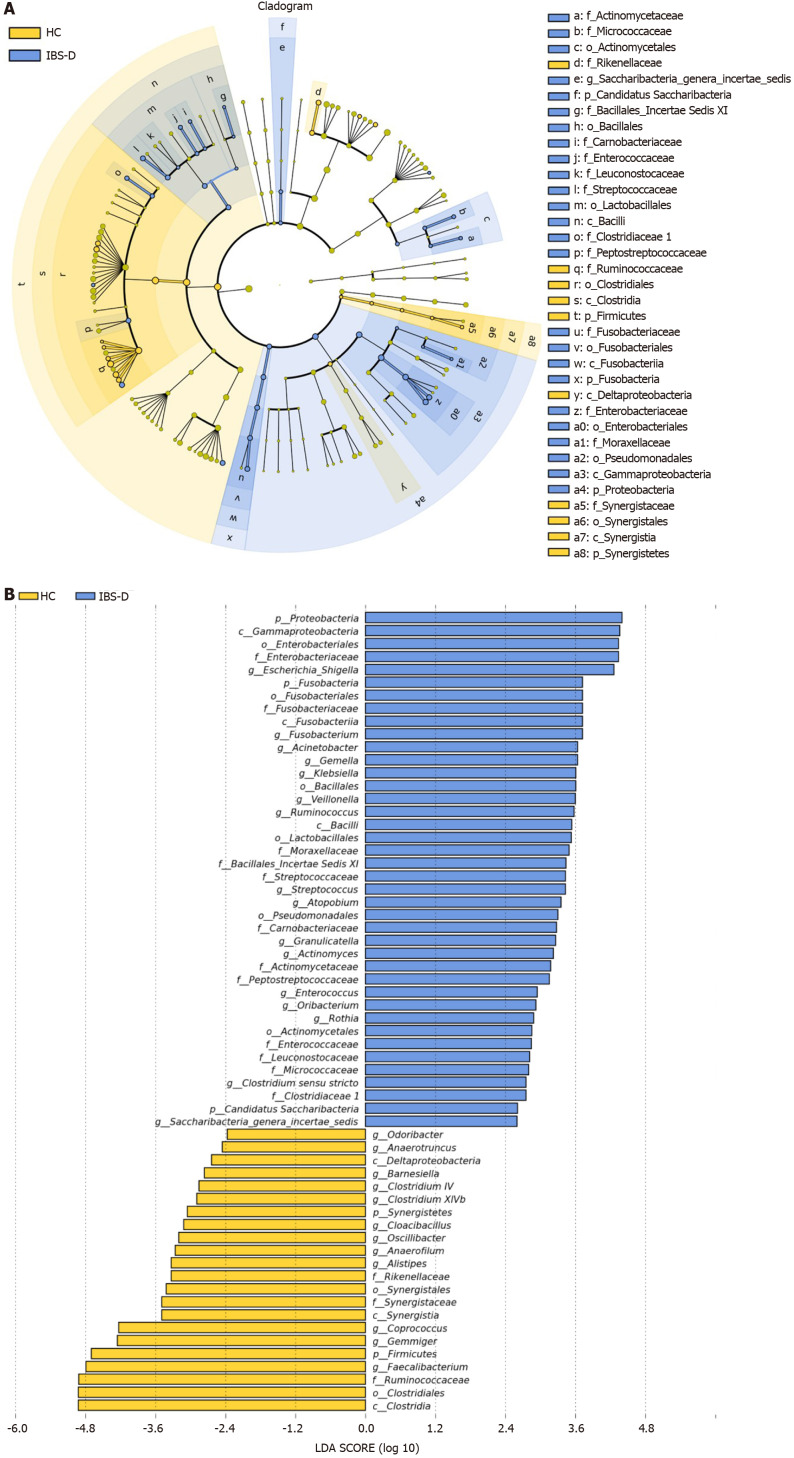
Furthermore, distinguishing phylotypes at the phylum, class, order, family, and genus levels were analyzed via LEfSe (Figure 4). As dominant phyla in human feces, the relative abundance of Proteobacteria was significantly higher in IBS-D group (P < 0.001), and Bacteroidetes and Actinobacteria tended to increase while Firmicutes tended to decrease in the IBS-D group. The gut microbiota of IBS-D patients were characterized by decreased relative abundance of the class clostridia (LDA score [log10] > 4.8, P = 0.011), the order Clostridiales (LDA score [log10] > 4.8, P = 0.011), and the family Ruminococcaceae (LDA score [log10] > 4.8, P < 0.001), and increased relative abundances of the class Gammaproteobacteria (LDA score [log10] > 3.6, P < 0.001), the order Enterobacteriales (LDA score [log10] > 3.6, P < 0.001), and the family Enterobacteriaceae (LDA score [log10] > 3.6, P < 0.001). At the genus level, 12 genera were significantly less abundant (P < 0.05) in the fecal microbiota of IBS-D patients, including 6 genera in Ruminococcaceae (Anaerofilum, Anaerotruncus, Clostridium IV, Faecalibacterium, Gemmiger, and Oscillibacter), 2 genera in the family Lachnospiraceae (Clostridium XlVb and Coprococcus), 2 genera in the family Porphyromonadaceae (Barnesiella and Odoribacter), 1 genus in the family Rikenellaceae (Alistipes) and 1 genus in the family Synergistaceae (Cloacibacillus), yet the differences of Clostridium IV, Clostridium XlVb and Barnesiella lost significance after FDR correction. In parallel, 8 genera were significantly more abundant in the IBS-D group (P < 0.05), including Escherichia/shigella, Enterococcus, Streptococcus, Rothia, Klebsiella, Saccharibacteria genera incertae sedis, Fusobacterium, and Veillonella. Ruminococcus belonging to Ruminococcaceae was also increased in the IBS-D group (P = 0.049), but it lost significance after FDR correction.
Figure 4.
Linear discriminant analysis effect size analysis of fecal bacterial of patients with diarrhea-predominant irritable bowel syndrome and healthy controls. A: The cladogram showed enriched taxa in the irritable bowel syndrome with predominant diarrhea (IBS-D) group (blue) and the healthy control (HC) group (yellow); the taxa represented by the English letters in the cladogram are shown in the legend on the right; B: Taxa enriched in the IBS-D group are indicated with a positive linear discriminant analysis (LDA) score (blue) and taxa enriched in the HC group are indicated with a negative score (yellow); only taxa with LDA effect size (LEfSe) P values < 0.05 and LDA scores ≥ 2.0 are presented.
Correlations between fecal BAs and microbiota composition
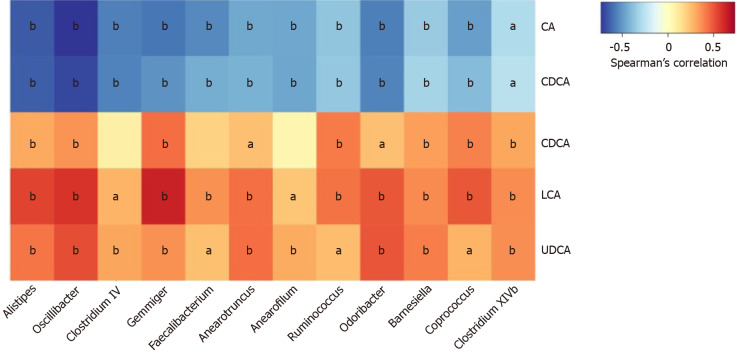
To explore whether the changes in fecal BA profile correlated with the configuration of gut flora, and given that conjugated BAs only account for a minor part of fecal BA pool, we analyzed the associations between the five unconjugated BAs (CA, CDCA, DCA, LCA, and UDCA) and the distinguishing genera in all subjects. Markedly, the genera reduced in the IBS-D group except for Cloacibacillus exhibited a negative correlation with primary BAs and a positive correlation with secondary BAs, and Ruminococcus presented a similar trend (Figure 5).
Figure 5.
Correlations between fecal bile acids and microbiota composition in all subjects. The heatmap presents the Spearman correlation coefficients between unconjugated fecal bile acids and the distinguishing genera in all subject. Seven genera belong to the family Ruminococcaceae (Oscillibacter, Clostridium IV, Gemmiger, Anaerofilum, Anaerotruncus, Faecalibacterium, and Ruminococcus); one genus belongs to the family Rikenellaceae (Alistipes); two genera belong to the family Porphyromonadaceae (Odoribacter and Barnesiella); two genera belong to the family Lachnospiraceae (Coprococcus and Clostridium XlVb). All of the genera were decreased significantly in the IBS-D group except for Ruminococcus. aP < 0.05, bP < 0.01. CA: Cholic acid; CDCA: Chenodeoxycholic acid; DCA: Deoxycholic acid; LCA: Lithocholic acid; UDCA: Ursodeoxycholic acid.
DISCUSSION
In this study, we comprehensively assessed the composition of the fecal BA pool of IBS-D patients, the correlations between clinical features and the imbalance of fecal BAs, and the correlations between fecal BAs and the gut microbiome. The fecal BA pool of IBS-D patients was characterized by increased primary BAs and decreased secondary BAs, along with increased total BAs. Correlations between defecation frequency and thresholds of rectal distention testing with fecal BAs were observed. Furthermore, we found that several genera with discrepant relative abundances between IBS-D patients and HCs were significantly related to fecal BAs, especially the genera within the family Ruminococcaceae.
Evidence is accumulating that fecal primary BAs are increased in IBS-D[9,10,25,33]. A study with 14 IBS-D patients and 18 HCs by Duboc et al[33] reported that fecal DCA was reduced in IBS-D patients, and the levels of LCA and UDCA in IBS-D patients were similar to those in HCs, whereas our results showed that LCA and UDCA were decreased significantly in IBS-D patients and DCA tended to decrease. Over half of the subjects in the IBS-D group had a higher PBA/SBA ratio than the 90th percentiles of the PBA/SBA ratio in HCs in the current study, suggesting that the imbalance between primary BAs and secondary BAs in IBS-D deserves more attention. The level of total fecal BAs in IBS-D is inconsistent in different studies, with several reporting an increase[34,35] while others showing no significant difference compared with HCs[8,10,25,33]. A meta-analysis reported that BA malabsorption (BAM) appeared in 28.1% of IBS-D patients[36]. However, Peleman et al[37] demonstrated that fecal CA and CDCA concentrations in IBS-D patients without BAM were still significantly higher than those in HCs, suggesting that abnormal BA metabolism might exist in IBS-D patients whether they have co-occurring BAM or not.
Both fecal conjugated and unconjugated BAs were increased significantly in the IBS-D group in the present study. One likely explanation is that the BA biosynthesis increases in IBS-D patients, as evidenced by elevated serum 7α-hydroxy-4-cholesten-3-one (C4, the precursor of primary BAs in the liver and thus a marker of hepatic BA synthesis[38]) in several studies[8,11,35], thereby the conjugated BAs entering the intestine may also increase, leading to more unconjugated BAs. However, only a minority of the IBS-D patients had a CBA/UBA ratio above the 90th percentiles of the CBA/UBA ratio in HC, and we found no significant difference in clinical manifestations between patients with high and low CBA/UBA ratios, suggesting that an abnormality of conversion from conjugated BAs to unconjugated BAs may not widely exist in IBS-D patients.
BAs have been previously shown as an intraluminal factor influencing the secretion function of the colon. Rectal perfusion of CDCA, DCA, and their conjugated BAs in healthy volunteers can induce water and chloride secretion[39]. Experiments in vitro revealed that CDCA, DCA, and their conjugated patterns can inhibit Cl-/OH- exchange and activate the cystic fibrosis transmembrane conductance regulator of colonic epithelial cells, attenuating the absorption and stimulating the secretion of chloride, respectively[40-42]. Besides, several studies found that instillation of TCA into the sigmoid colon and oral administration of CA and CDCA could accelerate colon motility[43-45], and the colonic transit rate of IBS-D patients showed positive correlation with the proportion of primary BAs in feces[37], indicating that primary BAs can modulate colonic motor function. The positive association between defecation frequency and primary BAs found in the current study, consistent with previous reports[9,33], might result from the promotion of secretion and motility of the colon by CA and CDCA. It is noteworthy that the relationship between colonic motility and luminal BAs is not unidirectional. Aside from BAs, abnormal motility may be induced by other factors such as neuromuscular dysfunction and chronic stress in IBS-D patients[46,47]. The variance in colonic transit may influence the passive absorption of BAs across the colon epithelium and result in changes of BAs excreted in feces[37], which is corroborated by the decrease or increase of total BAs in the feces of healthy volunteers when intervened with loperamide or senna, respectively[48]. Therefore, correlations cannot be equated with causal associations in this observational study, and a longitudinal study is required to verify these results.
Previous studies mainly focused on the relationship between gastrointestinal motility rather than visceral sensitivity and BAs in IBS-D[9,10,35]. Experiments in animals and healthy volunteers demonstrated that instillation of CDCA and DCA could increase the sensitivity to rectal distension[49-51]. Consistently, we observed that the first sensation threshold and the defecating sensation threshold inversely associated with fecal CDCA in IBS-D patients, suggesting that increased CDCA in the colon might induce a decline in the colonic sensory thresholds, which could be one of the potential mechanisms of visceral hypersensitivity of IBS-D patients. Still, these observational results should be viewed with caution, and further studies on the link between hypersensitivity and BAs in IBS-D are warranted.
We next compared the composition of the gut microbiome in IBS-D patients and HCs. As expected, gut dysbiosis was observed in IBS-D patients. In agreement with a previous study[52], we found that the richness of the gut flora was reduced in IBS-D while the diversity and evenness showed no significant difference between IBS-D patients and HCs, and we also found that Firmicutes tended to decrease while Bacteroidetes tended to increase in IBS-D. The proportions of the Clostridiales order and the Ruminococcaceae family within it have been reported to decrease in IBS[52,53], which is supported by our findings. In particular, we observed six genera in Ruminococcaceae were significantly decreased in IBS-D, including Anaerofilum, Anaerotruncus, Clostridium IV, Faecalibacterium, Gemmiger, and Oscillibacter.
The conversion of BAs in the intestine depends on the microbial community. Tauro- and glyco- BAs are first deconjugated into taurine/glycine and unconjugated BAs under the action of bile salt hydrolase (BSH) enzymes in various bacteria, as a prerequisite for further BAs metabolism by the microbiome[14]. Subsequently, the formation of DCA and LCA from CA and CDCA through 7α-dehydroxylation was carried out by bacteria with BA-inducible (bai) genes[14]. BSH distributes widely at the phylum level including Firmicutes, Bacteroidetes, and Actinobacteria in humans[54], while the capability to convert primary BAs to secondary BAs is limited to only a small number of species, mainly in Clostridiales, with an overwhelming majority of the strains belonging to Ruminococcaceae, and a marginal part belonging to the Lachnospiraceae and Peptostreptococcaceae families[55-57].
Given the imbalance of primary and secondary BAs in IBS-D and the essential position of microbiota in the metabolism of BAs, we further investigated the association between fecal BAs and the gut microbiota. The multiple genera of Ruminococcaceae and Lachnospiraceae decreased in IBS-D patients were positively associated with secondary BAs while negatively associated with primary BAs, indicating that the altered composition of the fecal BA pool might be ascribed to the reduction of bacteria with bai, which could lower the efficiency of conversion from primary BAs to secondary BAs. Ruminococcaceae has been reported to be positively associated with DCA in the feces of cirrhotic patients[58]. Kwan et al[59] also provided evidence that DCA and LCA in serum were positively correlated with Ruminococcaceae and Lachnospiraceae in feces although fecal BAs were not reported, supporting the importance of these two families in the metabolism of BAs. However, aside from microbial factors, faster colonic transit can result in shorter time for biotransformation of BAs and may be another cause for the imbalance between primary and secondary BAs[37], and the inverse association between DCA and defecation frequency in IBS-D patients in our study may be explained by the shortened CA exposure time caused by accelerated colonic transit, despite that DCA is considered as a secretory BA.
It must be noted that there are reciprocal regulations between the microorganism and BAs[60,61]. The antibacterial activity of BAs[62,63] may affect the gut community structure, thus the abnormal BAs concentration in the gut may, in turn, influence the gut microbiota in IBS-D patients. Increased CA in the gut could induce increased Gammaproteobacteria[64], which could be a potential reason for increased Gammapro-teobacteria in IBS-D patients. Interestingly, animal studies found that CA intake induced expansion of Firmicutes, especially the groups capable of 7α-dehydroxylation, and reduction of Bacteroidetes[64,65], contrary to the features of the microflora we observed in IBS-D patients, which support to some extent that the reduction of the bacteria with bai genes might happen prior to the increase of CA in the gut of IBS-D patients. However, further animal experiments and longitudinal research in humans are required, and BA sequestrants may contribute to the exploration of causal associations between BAs dysmetabolism and gut dysbiosis in IBS-D patients.
There were several limitations in this preliminary study. First, in consideration that women only accounted for approximately 25% of our patients, the findings in this study need to be verified in the future with more female patients recruited. The sex disparity in the distribution of IBS subtypes may be the major reason for the sex imbalance in this study, with IBS-D more prevalent in men and IBS-C more prevalent in women[66,67]. Besides, women with dysmenorrhea were excluded in this study, resulting in a further reduction of available female patients. Second, although BAs can theoretically affect the secretory function of the colon, we failed to observe a correlation between stool form and BAs, which might be a result of the similarity in the BSFS score in most IBS-D patients. Hence, feces moisture, a more precise parameter than the BSFS score, can be a better choice in a future study. Third, we did not supply a standardized diet for subjects during the study period, though subjects were required not to change their daily dietary habits throughout the study period, considering that the short-term modification of a diet can rapidly disturb the gut microbiota[68]. A standardized diet can largely control the influence of disparate eating habits on the microbiota and BA profile. However, the difference between the standardized diet and the usual dietary habits of patients may cause perturbation to the gut microbiota, masking the gut microbiota under usual dietary habits. Therefore, the measures of gut microbiota and BAs before and after a standardized diet combined with a detailed assessment of the usual dietary habits of patients are necessary for a future study.
CONCLUSION
In conclusion, our study depicted the composition of fecal BA pool in IBS-D patients, which was characterized by increased primary BAs and decreased secondary BAs and associated with diarrhea as well as visceral hypersensitivity. The imbalance of primary and secondary BAs might be induced by dysbiosis in IBS-D, especially the reduction of Ruminococcaceae. These preliminary findings may offer insight into the complicated pathophysiology of IBS-D, and provide evidence for BAs modulation in the treatment of IBS-D.
ARTICLE HIGHLIGHTS
Research background
Bile acids (BAs) have attracted attention in irritable bowel syndrome with predominant diarrhea (IBS-D) because of the effects on gastrointestinal motility and secretion function of the intestine. Experiments in animals and healthy volunteers also indicated that hypersensitivity can be caused by BAs, which is a major patho-physiological abnormality in IBS-D. The metabolism of BAs in the intestine depends on the gut microbiota. Therefore, it could be hypothesized that BAs may be involved in the pathogenesis of IBS-D and the altered BA profile in the intestine may be associated with microbiota.
Research motivation
Although a few studies have portrayed the composition of fecal BAs in IBS-D patients, the data in Chinese IBS-D patients are still sparse. Besides, few studies have explored the correlations between the gut flora and BAs in IBS-D patients. The main topics of this study included identifying the correlations of BAs with clinical features containing rectoanal sensory parameters of IBS-D patients, and exploring the correlations between the composition of fecal BAs and the gut microbiome. The findings may add insight to the pathogenesis of IBS-D, and provide evidence for regulating intestinal BAs to treat IBS-D.
Research objectives
The present study aimed to evaluate the correlations of BAs with clinical features of IBS-D patients and to explore whether the composition of fecal BAs was associated with the gut microbiome in IBS-D patients.
Research methods
Subjects underwent clinical and psychological assessments, including IBS symptom severity system, the grade of Bristol stool form scale and defecation frequency per day in the preceding 2 wk, hospital anxiety and depression scale, and visceral sensitivity index, along with visceral sensitivity testing with a high-resolution manometry system. Fecal BAs were measured by ultraperformance liquid chromatography coupled to tandem mass spectrometry. The gut microbiota was analyzed using 16S rRNA gene sequencing. Relationships between fecal BAs and clinical characteristics as well as gut microbiota were explored.
Research results
Cholic acid, chenodeoxycholic acid (CDCA), and their conjugated BAs were significantly increased, while lithocholic acid (LCA) and ursodeoxycholic acid were significantly decreased and deoxycholic acid (DCA) tended to decrease in IBS-D patients. Defecation frequency was positively associated with primary BAs and inversely associated with DCA and LCA in IBS-D patients. The first sensation threshold was negatively correlated, and the defecating sensation threshold tended to be negatively correlated with CDCA in IBS-D patients. Furthermore, several genera were significantly reduced in IBS-D patients compared with HCs and exhibited a negative correlation with primary BAs and a positive correlation with secondary BAs, especially the genera in the Ruminococcaceae family.
Research conclusions
This study presented evidence that the composition of the fecal BA pool was characterized by increased primary BAs and decreased secondary BAs in IBS-D, which was associated with diarrhea and visceral hypersensitivity in IBS-D. The dysmetabolism of BAs in IBS-D might be ascribed to gut dysbiosis especially the reduction of Ruminococcaceae.
Research perspectives
In the future, careful evaluation of the usual dietary habit of subjects is required, and diet needs to be standardized during the study period. Large multicenter studies are also necessary to verify the conclusions drawn in this study. Notably, BA sequestrant may contribute to the studies on the involvement of BAs in IBS-D pathophysiology as well as the association between BAs and microbiota.
ACKNOWLEDGEMENTS
We thank Dr. Du SY, Dr. Zhang MG, Dr. Li YM, Dr. Fang L, Dr. Bai RX, Dr. Qin G and Dr. Chen S for enrollment of participants.
Footnotes
Institutional review board statement: This study was approved by the Ethics Committee of China-Japan Friendship Hospital (No. 2019-64-K44).
Informed consent statement: All study participants provided written informed consent prior to study enrollment.
Conflict-of-interest statement: All authors report no conflicts of interest.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: August 17, 2020
First decision: September 12, 2020
Article in press: October 13, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: O'Malley D S-Editor: Zhang H L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Wei Wei, Graduate School, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China; Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China.
Hui-Fen Wang, Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China.
Yu Zhang, Graduate School, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China; Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China.
Yan-Li Zhang, Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China.
Bing-Yu Niu, Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China; Graduate School, Beijing University of Chinese Medicine, Beijing 100029, China.
Shu-Kun Yao, Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China. shukunyao@126.com.
Data sharing statement
No additional data are available.
References
- 1.Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, Hungin APS, Kang JY, Minhu C, Schmulson M, Bolotin A, Friger M, Freud T, Whitehead W. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66:1075–1082. doi: 10.1136/gutjnl-2015-311240. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Duan L, Liu Y, Leng Y, Zhang H, Liu Z, Wang K. [A meta-analysis of the prevalence and risk factors of irritable bowel syndrome in Chinese community] Zhonghua Nei Ke Za Zhi. 2014;53:969–975. [PubMed] [Google Scholar]
- 3.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016:Epub ahead of print. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Yao X, Yang YS, Cui LH, Zhao KB, Zhang ZH, Peng LH, Guo X, Sun G, Shang J, Wang WF, Feng J, Huang Q. Subtypes of irritable bowel syndrome on Rome III criteria: a multicenter study. J Gastroenterol Hepatol. 2012;27:760–765. doi: 10.1111/j.1440-1746.2011.06930.x. [DOI] [PubMed] [Google Scholar]
- 5.Long Y, Huang Z, Deng Y, Chu H, Zheng X, Yang J, Zhu Y, Fried M, Fox M, Dai N. Prevalence and risk factors for functional bowel disorders in South China: a population based study using the Rome III criteria. Neurogastroenterol Motil. 2017;29 doi: 10.1111/nmo.12897. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016:Epub ahead of print. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Appleby RN, Walters JR. The role of bile acids in functional GI disorders. Neurogastroenterol Motil. 2014;26:1057–1069. doi: 10.1111/nmo.12370. [DOI] [PubMed] [Google Scholar]
- 8.Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, Dyer RB, Lamsam J, Zinsmeister AR. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol 2012; 10: 1009-15. :e3. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin A, Camilleri M, Vijayvargiya P, Busciglio I, Burton D, Ryks M, Rhoten D, Lueke A, Saenger A, Girtman A, Zinsmeister AR. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013; 11: 1270-1275. :e1. doi: 10.1016/j.cgh.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijayvargiya P, Camilleri M, Chedid V, Carlson P, Busciglio I, Burton D, Donato LJ. Analysis of Fecal Primary Bile Acids Detects Increased Stool Weight and Colonic Transit in Patients With Chronic Functional Diarrhea. Clin Gastroenterol Hepatol 2019; 17: 922-929. :e2. doi: 10.1016/j.cgh.2018.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajor A, Törnblom H, Rudling M, Ung KA, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. 2015;64:84–92. doi: 10.1136/gutjnl-2013-305965. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M, Acosta A, Busciglio I, Boldingh A, Dyer RB, Zinsmeister AR, Lueke A, Gray A, Donato LJ. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:438–448. doi: 10.1111/apt.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, Lamsam J, Singh R, Zinsmeister AR. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long SL, Gahan CGM, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med. 2017;56:54–65. doi: 10.1016/j.mam.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Mekhjian HS, Phillips SF, Hofmann AF. Colonic absorption of unconjugated bile acids: perfusion studies in man. Dig Dis Sci. 1979;24:545–550. doi: 10.1007/BF01489324. [DOI] [PubMed] [Google Scholar]
- 16.Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuang X, Xiong L, Li L, Li M, Chen M. Alterations of gut microbiota in patients with irritable bowel syndrome: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:28–38. doi: 10.1111/jgh.13471. [DOI] [PubMed] [Google Scholar]
- 19.Labus JS, Hollister EB, Jacobs J, Kirbach K, Oezguen N, Gupta A, Acosta J, Luna RA, Aagaard K, Versalovic J, Savidge T, Hsiao E, Tillisch K, Mayer EA. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. 2017;5:49. doi: 10.1186/s40168-017-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krogsgaard LR, Andersen LO', Johannesen TB, Engsbro AL, Stensvold CR, Nielsen HV, Bytzer P. Characteristics of the bacterial microbiome in association with common intestinal parasites in irritable bowel syndrome. Clin Transl Gastroenterol. 2018;9:161. doi: 10.1038/s41424-018-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T, Gu X, Li LX, Li M, Li B, Cui X, Zuo XL. Microbial and metabolomic profiles in correlation with depression and anxiety co-morbidities in diarrhoea-predominant IBS patients. BMC Microbiol. 2020;20:168. doi: 10.1186/s12866-020-01841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. 2014;11:497–505. doi: 10.1038/nrgastro.2014.40. [DOI] [PubMed] [Google Scholar]
- 24.Rea K, Dinan TG, Cryan JF. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dior M, Delagrèverie H, Duboc H, Jouet P, Coffin B, Brot L, Humbert L, Trugnan G, Seksik P, Sokol H, Rainteau D, Sabate JM. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol Motil. 2016;28:1330–1340. doi: 10.1111/nmo.12829. [DOI] [PubMed] [Google Scholar]
- 26.Chiang JYL, Ferrell JM. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018;18:71–87. doi: 10.3727/105221618X15156018385515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 28.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 29.Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Qin G, Liu DR, Wang Y, Yao SK. Increased expression of brain-derived neurotrophic factor is correlated with visceral hypersensitivity in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2019;25:269–281. doi: 10.3748/wjg.v25.i2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aziz I, Mumtaz S, Bholah H, Chowdhury FU, Sanders DS, Ford AC. High Prevalence of Idiopathic Bile Acid Diarrhea Among Patients With Diarrhea-Predominant Irritable Bowel Syndrome Based on Rome III Criteria. Clin Gastroenterol Hepatol 2015; 13: 1650-5. :e2. doi: 10.1016/j.cgh.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Duboc H, Rainteau D, Rajca S, Humbert L, Farabos D, Maubert M, Grondin V, Jouet P, Bouhassira D, Seksik P, Sokol H, Coffin B, Sabaté JM. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:513–520, e246. doi: 10.1111/j.1365-2982.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhao L, Yang W, Chen Y, Huang F, Lu L, Lin C, Huang T, Ning Z, Zhai L, Zhong LL, Lam W, Yang Z, Zhang X, Cheng C, Han L, Qiu Q, Shang X, Huang R, Xiao H, Ren Z, Chen D, Sun S, El-Nezami H, Cai Z, Lu A, Fang X, Jia W, Bian Z. A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J Clin Invest. 2020;130:438–450. doi: 10.1172/JCI130976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camilleri M, Shin A, Busciglio I, Carlson P, Acosta A, Bharucha AE, Burton D, Lamsam J, Lueke A, Donato LJ, Zinsmeister AR. Validating biomarkers of treatable mechanisms in irritable bowel syndrome. Neurogastroenterol Motil. 2014;26:1677–1685. doi: 10.1111/nmo.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015;42:3–11. doi: 10.1111/apt.13227. [DOI] [PubMed] [Google Scholar]
- 37.Peleman C, Camilleri M, Busciglio I, Burton D, Donato L, Zinsmeister AR. Colonic Transit and Bile Acid Synthesis or Excretion in Patients With Irritable Bowel Syndrome-Diarrhea Without Bile Acid Malabsorption. Clin Gastroenterol Hepatol 2017; 15: 720-727. :e1. doi: 10.1016/j.cgh.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mekjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971;50:1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keely SJ, Scharl MM, Bertelsen LS, Hagey LR, Barrett KE, Hofmann AF. Bile acid-induced secretion in polarized monolayers of T84 colonic epithelial cells: Structure-activity relationships. Am J Physiol Gastrointest Liver Physiol. 2007;292:G290–G297. doi: 10.1152/ajpgi.00076.2006. [DOI] [PubMed] [Google Scholar]
- 41.Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC. Chenodeoxycholic acid stimulates Cl(-) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. Am J Physiol Cell Physiol. 2013;305:C447–C456. doi: 10.1152/ajpcell.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alrefai WA, Saksena S, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholate modulates apical Cl-/OH- exchange activity in Caco2 cells. Dig Dis Sci. 2007;52:1270–1278. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 43.Kirwan WO, Smith AN, Mitchell WD, Falconer JD, Eastwood MA. Bile acids and colonic motility in the rabbit and the human. Gut. 1975;16:894–902. doi: 10.1136/gut.16.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao AS, Wong BS, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, Burton D, Carlson P, Lamsam J, Singh R, Zinsmeister AR. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology 2010; 139: 1549-1558, 1558. :e1. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mascolo N, Gaginella TS, Izzo AA, Di Carlo G, Capasso F. Nitric oxide involvement in sodium choleate-induced fluid secretion and diarrhoea in rats. Eur J Pharmacol. 1994;264:21–26. doi: 10.1016/0014-2999(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 46.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2013;368:578–579. doi: 10.1056/NEJMc1214185. [DOI] [PubMed] [Google Scholar]
- 47.Chang L, Di Lorenzo C, Farrugia G, Hamilton FA, Mawe GM, Pasricha PJ, Wiley JW. Functional Bowel Disorders: A Roadmap to Guide the Next Generation of Research. Gastroenterology. 2018;154:723–735. doi: 10.1053/j.gastro.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Lewis S, Cochrane S. Alteration of sulfate and hydrogen metabolism in the human colon by changing intestinal transit rate. Am J Gastroenterol. 2007;102:624–633. doi: 10.1111/j.1572-0241.2006.01020.x. [DOI] [PubMed] [Google Scholar]
- 49.Li WT, Luo QQ, Wang B, Chen X, Yan XJ, Qiu HY, Chen SL. Bile acids induce visceral hypersensitivity via mucosal mast cell-to-nociceptor signaling that involves the farnesoid X receptor/nerve growth factor/transient receptor potential vanilloid 1 axis. FASEB J. 2019;33:2435–2450. doi: 10.1096/fj.201800935RR. [DOI] [PubMed] [Google Scholar]
- 50.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 51.Edwards CA, Brown S, Baxter AJ, Bannister JJ, Read NW. Effect of bile acid on anorectal function in man. Gut. 1989;30:383–386. doi: 10.1136/gut.30.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhuang X, Tian Z, Li L, Zeng Z, Chen M, Xiong L. Fecal Microbiota Alterations Associated With Diarrhea-Predominant Irritable Bowel Syndrome. Front Microbiol. 2018;9:1600. doi: 10.3389/fmicb.2018.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sundin J, Aziz I, Nordlander S, Polster A, Hu YOO, Hugerth LW, Pennhag AAL, Engstrand L, Törnblom H, Simrén M, Öhman L. Evidence of altered mucosa-associated and fecal microbiota composition in patients with Irritable Bowel Syndrome. Sci Rep. 2020;10:593. doi: 10.1038/s41598-020-57468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA. 2008;105:13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vital M, Rud T, Rath S, Pieper DH, Schlüter D. Diversity of Bacteria Exhibiting Bile Acid-inducible 7α-dehydroxylation Genes in the Human Gut. Comput Struct Biotechnol J. 2019;17:1016–1019. doi: 10.1016/j.csbj.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, Lawley TD, Finn RD. A new genomic blueprint of the human gut microbiota. Nature. 2019;568:499–504. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pasolli E, Asnicar F, Manara S, Zolfo M, Karcher N, Armanini F, Beghini F, Manghi P, Tett A, Ghensi P, Collado MC, Rice BL, DuLong C, Morgan XC, Golden CD, Quince C, Huttenhower C, Segata N. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 2019; 176: 649-662. :e20. doi: 10.1016/j.cell.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949–955. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwan SY, Jiao J, Qi J, Wang Y, Wei P, McCormick JB, Fisher-Hoch SP, Beretta L. Bile Acid Changes Associated With Liver Fibrosis and Steatosis in the Mexican-American Population of South Texas. Hepatol Commun. 2020;4:555–568. doi: 10.1002/hep4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C, Zhu C, Shao L, Ye J, Shen Y, Ren Y. Role of Bile Acids in Dysbiosis and Treatment of Nonalcoholic Fatty Liver Disease. Mediators Inflamm. 2019;2019:7659509. doi: 10.1155/2019/7659509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sung JY, Shaffer EA, Costerton JW. Antibacterial activity of bile salts against common biliary pathogens. Effects of hydrophobicity of the molecule and in the presence of phospholipids. Dig Dis Sci. 1993;38:2104–2112. doi: 10.1007/BF01297092. [DOI] [PubMed] [Google Scholar]
- 63.Sannasiddappa TH, Lund PA, Clarke SR. In Vitro Antibacterial Activity of Unconjugated and Conjugated Bile Salts on. Staphylococcus aureus Front Microbiol. 2017;8:1581. doi: 10.3389/fmicb.2017.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 65.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4:382–387. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 67.Kim YS, Kim N. Sex-Gender Differences in Irritable Bowel Syndrome. J Neurogastroenterol Motil. 2018;24:544–558. doi: 10.5056/jnm18082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.