Abstract
PURPOSE
Mortality for patients with classical Hodgkin lymphoma (cHL) treated during an era characterized in the United States by widespread use of doxorubicin, bleomycin, vinblastine, and dacarbazine and diminishing use of radiotherapy is not well understood.
PATIENTS AND METHODS
We identified 20,007 individuals diagnosed with stage I/II (early) or III/IV (advanced) cHL between age 20 and 74 years treated with initial chemotherapy in US population-based cancer registries during 2000-2015 (follow-up through 2016). We used standardized mortality ratios (SMRs) to compare cause-specific relative mortality risk following cHL to that expected in the general population and estimated excess absolute risks (EARs; per 10,000 patient-years) to quantify disease-specific death burden.
RESULTS
We identified 3,380 deaths in the cHL cohort, including 1,321 (39%) not attributed to lymphoma. Overall, noncancer SMRs were increased 2.4-fold (95% CI, 2.2 to 2.6; observed, 559; EAR, 61.6) and 1.6-fold (95% CI, 1.4 to 1.7; observed, 473; EAR, 18.2) for advanced- and early-stage cHL, respectively, compared with the general US population. SMRs and EARs differed substantially by cause of death and cHL stage. Among the highest EARs for noncancer causes of death were those for heart disease (EAR, 15.1; SMR, 2.1), infections (EAR, 10.6; SMR, 3.9), interstitial lung disease (ILD; EAR, 9.7; SMR, 22.1), and adverse events (AEs) related to medications/drugs (EAR, 7.4; SMR, 5.0) after advanced-stage cHL and heart disease (EAR, 6.6; SMR, 1.7), ILD (EAR, 3.7; SMR, 13.1), and infections (EAR, 3.1; SMR, 2.2) after early-stage cHL. Strikingly elevated SMRs for ILD, infections, and AEs were observed < 1 year after cHL. Individuals age 60-74 years with advanced-stage cHL experienced a disproportionate excess of deaths as a result of heart disease, ILD, infections, AEs, and solid tumors.
CONCLUSION
Despite evolving cHL treatment approaches, patients continue to face increased nonlymphoma mortality risks from multiple, potentially preventable causes. Surveillance, early interventions, and cHL treatment refinements may favorably affect patient longevity, particularly among high-risk subgroups.
INTRODUCTION
The long-term Hodgkin lymphoma (HL) mortality experience has been well characterized among patient cohorts established in an era predominated by mechlorethamine, vincristine, procarbazine, and prednisone (MOPP) chemotherapy and extended-field radiotherapy.1-7 Most previous mortality studies were based on data from academic centers or clinical trials and generally focused on younger (age < 50 years) patients with HL and those surviving long-term.1-7
CONTEXT
Key Objective
To characterize stage- and cause-specific mortality risks among individuals diagnosed during 2000-2015 (followed-up through 2016) with classical Hodgkin lymphoma (cHL) in the United States and treated with chemotherapy in an era predominated by use of doxorubicin, bleomycin, vinblastine, and dacarbazine and decreasing radiotherapy.
Knowledge Generated
Heart disease, interstitial lung disease (ILD), infections, and adverse events (AEs) related to medications/drugs accounted for the greatest number of excess deaths among adults diagnosed with cHL, particularly those with stage III/IV disease, with the highest estimated risks observed < 1 year after cHL diagnosis. Older individuals (age 60-74 years) experienced disproportionately increased death as a result of heart disease, ILD, infections, AEs, and solid tumors.
Relevance
We identified patient subgroups and time frames during which patients treated with chemotherapy were likely to experience a substantial burden of nonlymphoma deaths following cHL diagnosis. These high-risk groups may benefit most from surveillance and early interventions to reduce mortality.
Doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) was introduced in the 1970s, but results of comparative efficacy trials with MOPP did not emerge until the 1990s.8 Because of its more favorable toxicity profile, ABVD subsequently replaced MOPP as the preferred frontline chemotherapy regimen for all stages of HL in the United States.9,10 In addition, among US adults, frontline HL therapy with radiation alone decreased from nearly 50% in the 1970s to < 10% during 1999-2008,11 with decreases over the past two decades observed for early- and advanced-stage classical HL (cHL).12,13
The modifications in HL treatment approaches have resulted in improved overall survival over the past four decades13,14; however, the mortality experience for patients treated more recently is not well understood. With the expansion of the SEER Program in 2000 to include approximately 28% of the US population, our primary analysis sought to better understand mortality patterns of a cHL patient population treated with initial chemotherapy during an era characterized by widespread use of ABVD and diminishing use of radiotherapy.
PATIENTS AND METHODS
Patient Characteristics and Follow-Up
In our primary analyses of 31,748 individuals with a diagnosis of first primary cHL reported to 17 SEER cancer registry areas (SEER-17) during 2000-2015 and followed through 2016, we identified a cohort of 24,985 treated with initial chemotherapy after excluding 6,763 patients who had unknown or no initial chemotherapy, unknown Ann Arbor cHL stage, or known HIV infection15 (Fig 1). Our primary investigation focused on the cohort of 20,007 individuals diagnosed with cHL between 20 and 74 years of age; results for individuals < 20 and ≥ 75 years of age are provided in the Data Supplement (online only).
FIG 1.
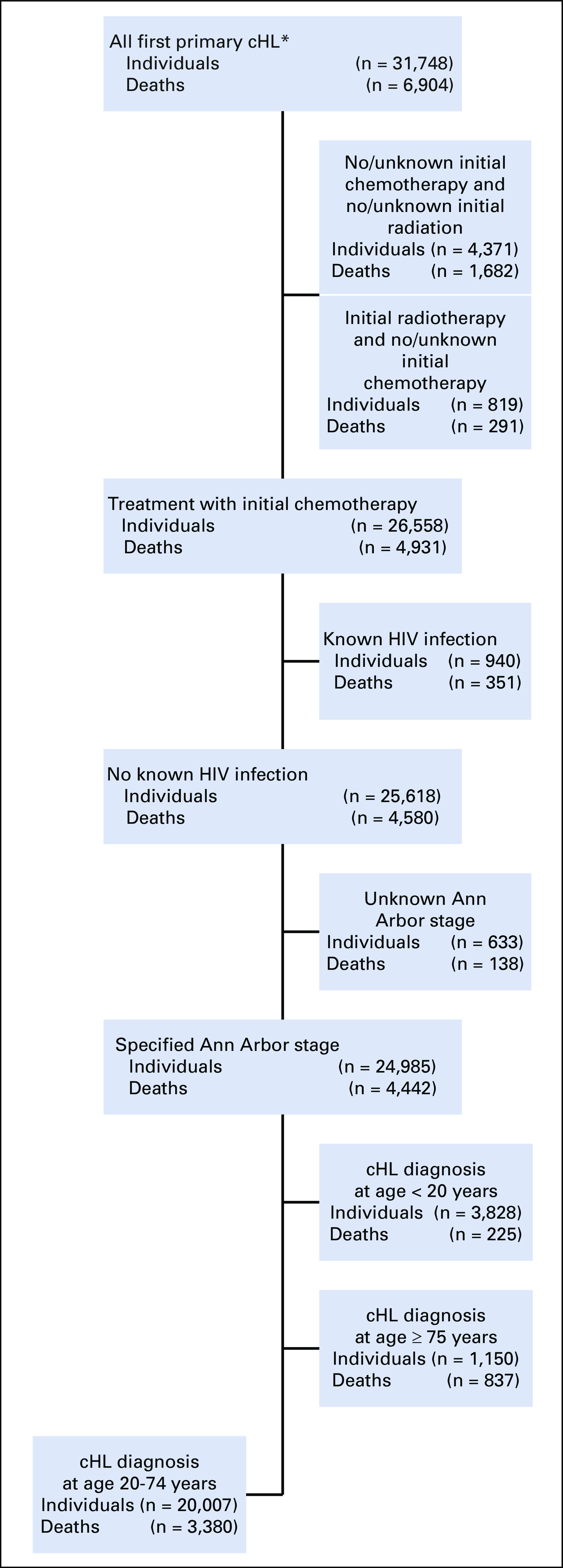
Flow diagram of individuals meeting inclusion criteria for the classical Hodgkin lymphoma (cHL) study population, 17 SEER cancer registry areas, 2000-2015 (followed through 2016). (*)Limited to patients with cHL who were not diagnosed by autopsy or death certificate only and patients with known age and sex.
The SEER Program collects general information (yes v no/unknown) on initial treatment (eg, chemotherapy, radiotherapy) only, and information on subsequent therapy is not available. However, ABVD is an established standard initial chemotherapeutic regimen for all stages of cHL in the United States.9,10 Reporting of vital status and date of last contact is required for at least 95% of all individuals reported in the SEER Program, whether living or deceased and irrespective of migration outside the cancer registry area.16 For deceased patients, the SEER Program obtains the underlying cause of death through the National Center for Health Statistics,17 which is recoded to the International Classification of Diseases, 10th Revision (ICD-10). We assessed cause-specific mortality disease groupings guided by the WHO ICD-10 (Data Supplement). Because of the potential for HL to be poorly classified as lymphoma on death certificates, we combined all HL, lymphoma (unspecified), and non-Hodgkin lymphoma deaths into one category entitled lymphoma deaths.
Statistical Analyses
Person-years of follow-up were accumulated beginning at cHL diagnosis until date of death, loss to follow-up, or study end date (December 31, 2016), whichever occurred first. Expected mortality in the cHL population was calculated for each specified cause of death by multiplying mortality rates in the general population (stratified by age, sex, race, and calendar year period) by stratum-specific person-years of follow-up.
We estimated relative risks of death by calculating standardized mortality ratios (SMRs) and corresponding exact 95% CIs using SEER*Stat software.18 The SMR compares the observed number of cHL deaths with that expected in the (age-, sex-, race-, and calendar year–matched) general population of the same SEER areas. This observed (Obs)/expected ratio reflects the strength of association for each cause of death. We estimated SMRs separately for early-stage (Ann Arbor stage I/II) versus advanced-stage (Ann Arbor stage III/IV) cHL as a surrogate of extent of therapy. For the most commonly occurring causes of death, we also estimated SMRs by latency (time from cHL diagnosis to death), sex, and age at cHL diagnosis for our primary analytic population (SEER-17, 2000-2016).
We constructed multivariable Poisson regression models to test for statistically significant (two-sided P < .05) heterogeneity of the SMRs between patient subgroups using the AMFIT module of Epicure version 2.0 software (Risk Sciences International, Ottawa, Ontario, Canada). The Poisson models were stratified by age at cHL diagnosis, sex, latency, and stage and included the log of the expected number of cases as an offset to indirectly adjust for attained age (age at time of death) and calendar year.19,20 P values for heterogeneity were calculated using likelihood ratio tests comparing model fit with and without the variable of interest.
We also calculated excess absolute risks (EARs) per 10,000 person-years (EAR = [Obs – expected] × 10,000 / person-years).18 The EAR reflects the absolute increase in risk of death (ie, the death burden) in the population. As another measure of absolute risk, we estimated cumulative mortality, considering deaths as a result of lymphoma, all other nonlymphoma neoplasms (hereafter referred to as other neoplasms), and all noncancers18,21,22 (details in Data Supplement) using SAS 9.4 statistical software (SAS Institute, Cary, NC).
Mortality Trends, 1983-2016
In a secondary analysis, we estimated the impact of treatment changes over time on cHL mortality among 10,109 patients diagnosed with first primary cHL between ages 20 and 74 years treated with initial chemotherapy during 1983-2009 and followed through 2016. We aimed to capture MOPP-, MOPP- and/or ABVD-, and ABVD-predominant treatment eras in the background of decreasing radiotherapy use. Because SEER-17 data are not available before 2000, we assessed mortality in nine SEER cancer registry areas (SEER-9) over three calendar periods that roughly correlated with prevalent chemotherapy regimens: MOPP (1983-1991, followed through 1998), MOPP/ABVD (1992-2000, followed through 2007), and ABVD (2001-2009, followed through 2016; Data Supplement).15
RESULTS
Among our primary analytic cohort of 20,007 individuals diagnosed with cHL between 20 and 74 years of age, 40% had stage III/IV cHL, and 32% were 10-year survivors (mean follow-up, 8.0 years; Data Supplement). We observed 3,380 deaths, of which 59% (n = 1,978) were attributed to lymphoma, 31% (n = 1,032) to noncancers, 9% (n = 289) to other neoplasms, and 2% (n = 81) to unknown causes. Deaths as a result of all causes occurred disproportionately among males (62%), nodular sclerosis subtype (52%), and patients treated with initial chemotherapy alone (80%).
Cumulative Mortality
Throughout 12 years of follow-up, cumulative mortality for patients with cHL exceeded the estimated general population mortality for the three age groups studied (Fig 2; Data Supplement). Among individuals ages 20-44 years at cHL diagnosis, cumulative mortality rate of lymphoma deaths (stage I/II, 5.2%; stage III/IV, 12.9%) exceeded that of noncancer deaths (stage I/II, 2.0%; stage III/IV, 4.0%) at 12 years, irrespective of stage. Among those age 45-59 and 60-74 years at early-stage cHL diagnosis, the burden of lymphoma deaths and noncancer deaths at 12 years was generally similar; however, among advanced-stage cHL, lymphoma deaths (45-59 years of age, 19.3%; 60-74 years of age, 34.6%) exceeded noncancer deaths (45-59 years of age, 13.1%; 60-74 years of age, 22.4%) over the 12-year period. All age groups had a lower burden of other neoplasm deaths than lymphoma or noncancer deaths over the study period.
FIG 2.
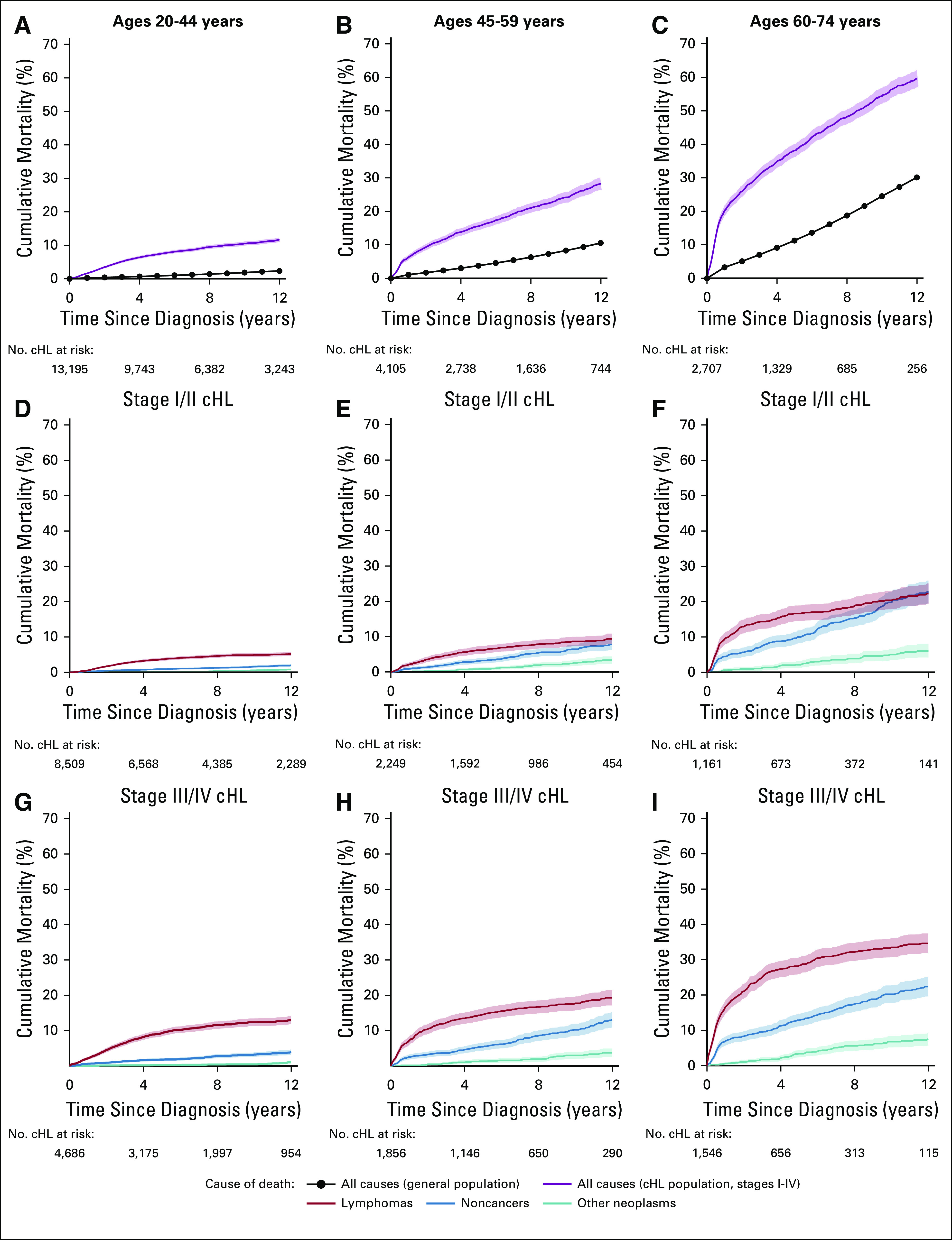
Cumulative mortality among a simulated general US population and 20,007 individuals diagnosed with cHL at ages 20-74 years and treated with initial chemotherapy: 17 SEER cancer registry areas, 2000-2015 (followed through 2016). (A-C) Cumulative mortality as a result of all causes in the general population and classical Hodgkin lymphoma (cHL) population according to age group. (D-F) Cumulative mortality as a result of lymphomas, noncancers, and other neoplasms among patients diagnosed with stage I/II cHL according to age group. (G-I) Cumulative mortality as a result of lymphomas, noncancers, and other neoplasms among patients diagnosed with stage III/IV cHL according to age group. Shaded areas (and error bars) represent the upper and lower bounds of the 95% CI for cumulative mortality.
Disease- and Stage-Specific Risks of Death
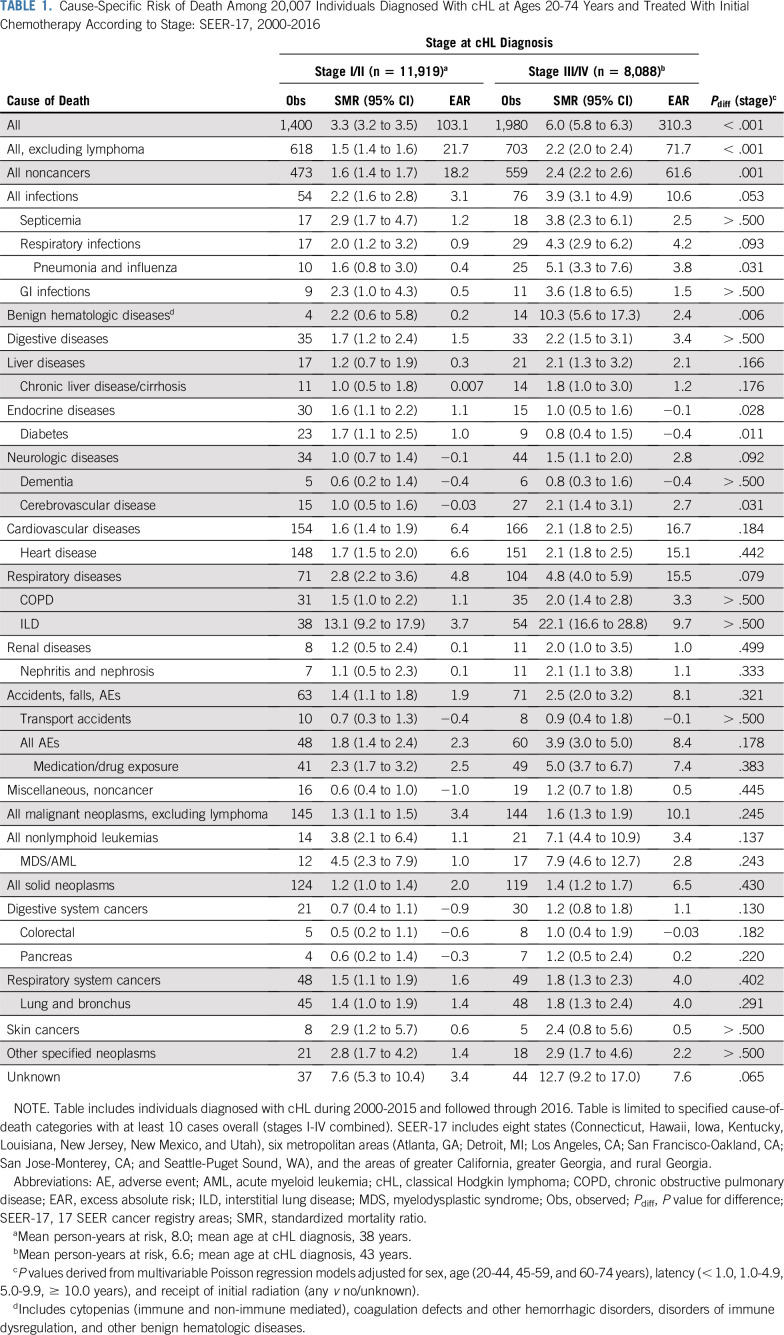
Compared with the general population, the relative risk of death as a result of any cause (excluding lymphoma) was increased 1.8-fold (95% CI, 1.7 to 1.9; Obs, 1,321) in our cHL cohort, corresponding to 39.6 excess deaths/10,000 person-years (Data Supplement). This risk was significantly higher after advanced cHL (SMR, 2.2; 95% CI, 2.0 to 2.4; Obs, 703; EAR, 71.7) than early-stage cHL (SMR, 1.5; 95% CI, 1.4 to 1.6; Obs, 618; EAR, 21.7; Pdifference [Pdiff] < .001; Table 1), with lower risks among a younger population treated with initial chemotherapy and radiation (Data Supplement).
TABLE 1.
Cause-Specific Risk of Death Among 20,007 Individuals Diagnosed With cHL at Ages 20-74 Years and Treated With Initial Chemotherapy According to Stage: SEER-17, 2000-2016
Deaths as a result of noncancer causes accounted for most nonlymphoma deaths, with the highest SMRs increased 2.4-fold (95% CI, 2.2 to 2.6; Obs, 559; EAR, 61.6) after advanced cHL and 1.6-fold (95% CI, 1.4 to 1.7; Obs, 473; EAR, 18.2) after early cHL (Pdiff = .001); however, cause-specific risks varied substantially. After advanced-stage cHL, the highest SMRs for noncancer causes of death were observed for interstitial lung disease (ILD) (SMR, 22.1; 95% CI, 16.6 to 28.8), followed by three- to 10-fold increased SMRs for benign hematologic diseases, adverse events related to medication/drug exposure (hereafter referred to as adverse events [AEs]), and infections (including septicemia, pneumonia and influenza, and GI). Lower but statistically significant SMRs (advanced-stage cHL, 1.8-2.1) also were observed for deaths as a result of chronic liver disease, cerebrovascular disease, heart disease, chronic obstructive pulmonary disease (COPD), and nephritic/nephrotic diseases. However, among patients with stage III/IV cHL, the highest EARs were observed for heart disease (15.1), ILD (9.7), infections (10.6), and AEs (7.4), with lower EARs of 2.4-3.8 for septicemia, pneumonia and influenza, benign hematologic diseases, cerebrovascular disease, and COPD. Following early-stage cHL, the highest noncancer SMRs also were observed for ILD (13.1; 95% CI, 9.2 to 17.9), with two- to threefold increased SMRs for infections, benign hematologic diseases, and AEs and lower (SMR, 1.7), but significantly increased risks for digestive diseases, diabetes, and heart disease. The greatest noncancer death burden for stage I/II cHL was a result of heart disease (EAR, 6.6), ILD (EAR, 3.7), infections (EAR, 3.1), and AEs (EAR, 2.5).
Other neoplasms accounted for nearly 25% of all nonlymphoma deaths regardless of cHL stage (Obsstage I/II, 145; Obsstage III/IV, 144). Risks were significantly elevated for death as a result of myelodysplastic syndrome/acute myeloid leukemia (MDS/AML; SMRstage I/II, 4.5 [95% CI, 2.3 to 7.9]; SMRstage III/IV, 7.9 [95% CI, 4.6 to 12.7]). Lung cancer accounted for the majority of all solid neoplasm deaths, with elevated risks among early-stage cHL (SMR, 1.4; 95% CI, 1.0 to 1.9) and advanced-stage cHL (SMR, 1.8; 95% CI, 1.3 to 2.4). Excess deaths as a result of other neoplasms accounted for approximately 15% of the total excess deaths in the cHL cohort, after excluding lymphomas (EARstage I/II, 3.4; EARstage III/IV, 10.1), with the largest excess deaths occurring for all solid neoplasms (EAR, 6.5), lung cancer (EAR, 4.0), and MDS/AML (EAR, 2.8) following advanced-stage cHL.
Risks of Death by Patient Subgroup
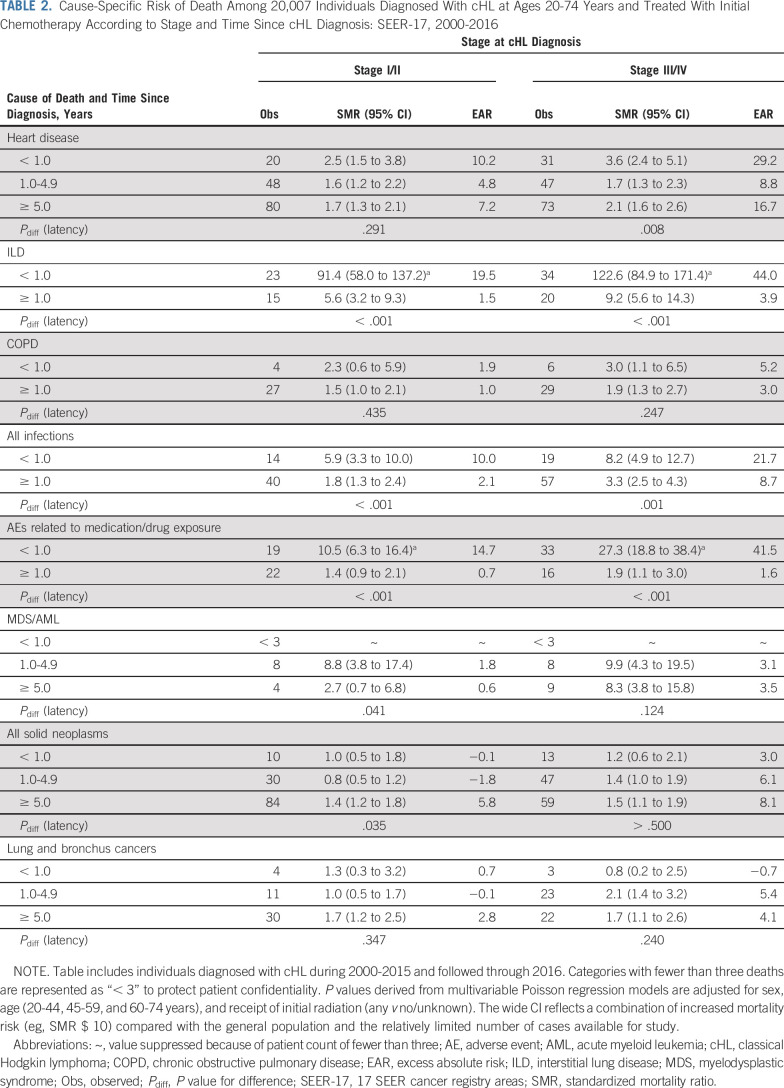
We further explored the mortality patterns by patient subgroup for the most common specific causes of death. In analyses by time since cHL diagnosis (Table 2), risk for death as a result of heart disease was significantly increased in all time intervals. Whereas SMRs for heart disease were highest < 1 year following cHL diagnosis and lower thereafter, EARs were highest < 1 year following cHL, decreased at 1-4 years, but increased again at ≥ 5 years. In contrast, strikingly elevated SMRs and EARs for death as a result of ILD, infections, and AEs were most notable within the first year of cHL diagnosis (P ≤ .001 for all comparisons). The SMR patterns for lung cancer deaths did not differ statistically over the three time periods, irrespective of cHL stage.
TABLE 2.
Cause-Specific Risk of Death Among 20,007 Individuals Diagnosed With cHL at Ages 20-74 Years and Treated With Initial Chemotherapy According to Stage and Time Since cHL Diagnosis: SEER-17, 2000-2016
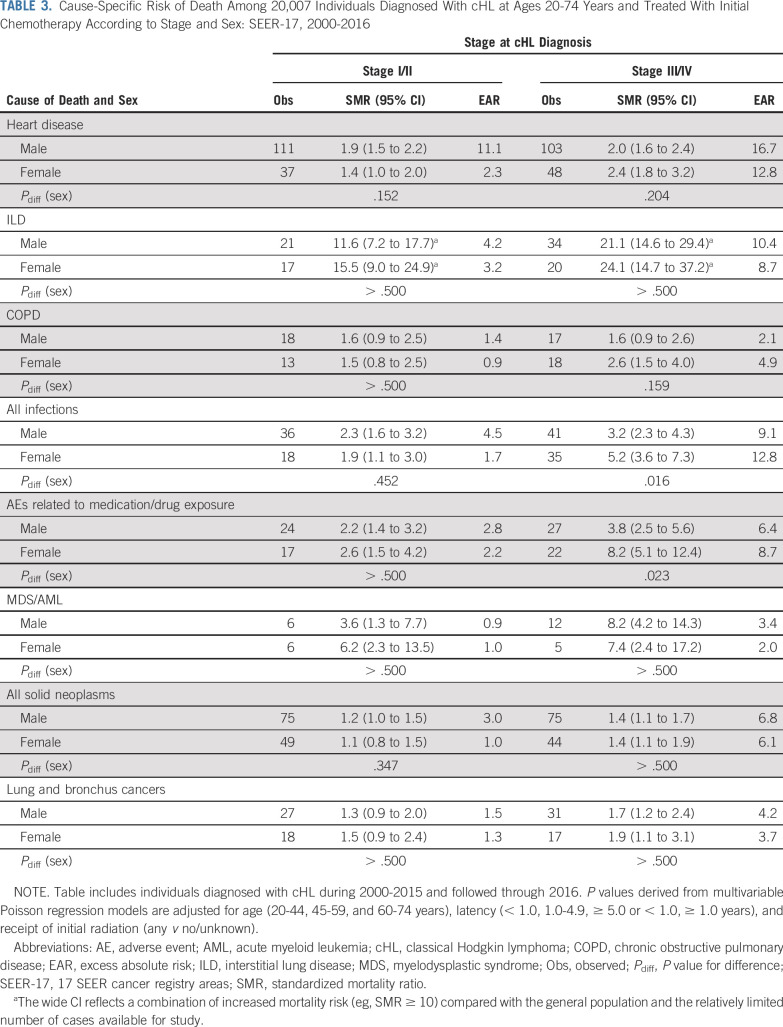
Cause-specific risks of death generally did not differ by sex, irrespective of cHL stage (Table 3). Exceptions were observed for infections and AEs after advanced cHL, where SMRs were significantly higher among females than males (infections: SMRmales, 3.2, SMRfemales, 5.2 [Pdiff = .016]; AEs: SMRmales, 3.8, SMRfemales, 8.2 [Pdiff = .023]); EARs followed a similar pattern.
TABLE 3.
Cause-Specific Risk of Death Among 20,007 Individuals Diagnosed With cHL at Ages 20-74 Years and Treated With Initial Chemotherapy According to Stage and Sex: SEER-17, 2000-2016
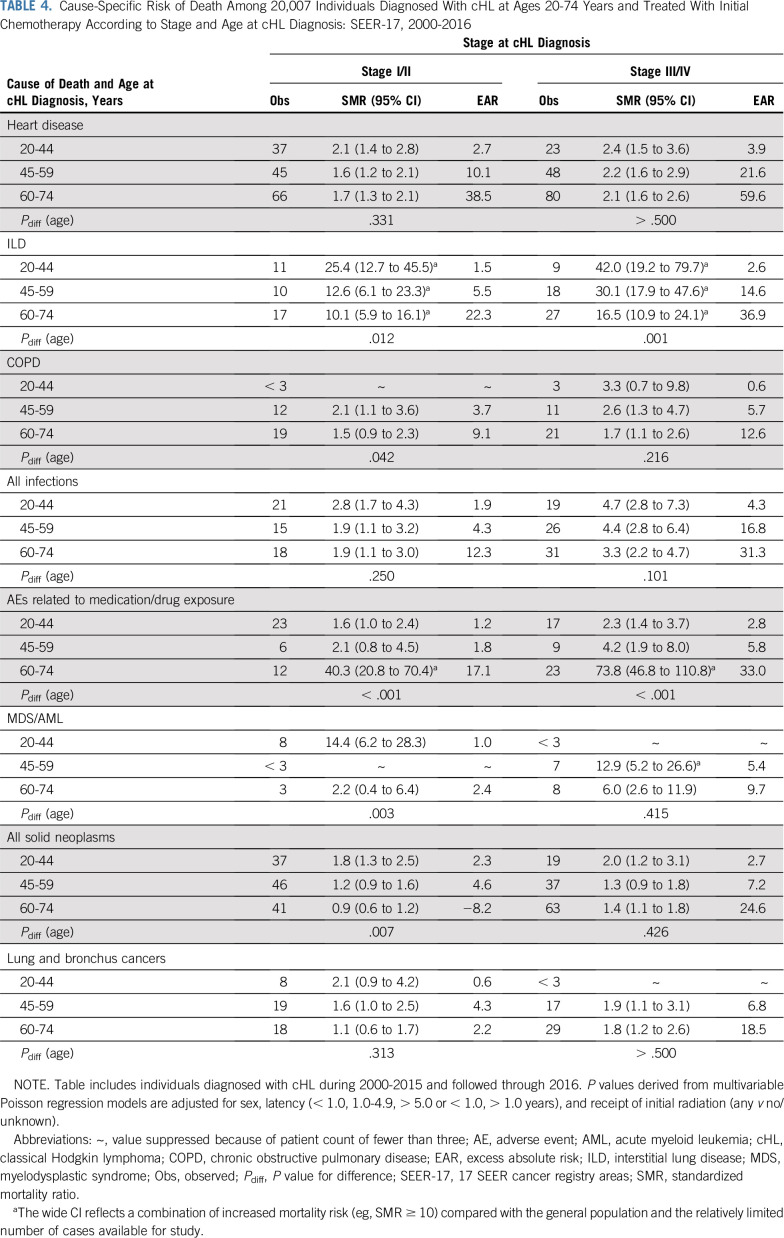
In age-specific analyses of noncancer deaths (Table 4), SMRs related to heart disease, COPD (advanced-stage cHL), and infections did not differ significantly by age, in contrast to ILD, COPD (early-stage cHL), and AEs. ILD SMRs decreased with advancing age, whereas AE SMRs increased with age, with strikingly elevated risks among the 60-74–year age group (SMRstage I/II, 40.3 [Pdiff < .001]; SMRstage III/IV, 73.8 [Pdiff < .001]). EAR patterns differed from those of SMRs, with the highest EARs uniformly seen among the 60-74–year age group, particularly for deaths as a result of heart disease (EARstage I/II, 38.5; EARstage III/IV, 59.6). Subgroup analyses demonstrated that excess deaths as a result of heart disease predominated among the 60-74–year age group across all follow-up periods and stage groups (Data Supplement). Among advanced-stage cHL, the pattern of substantially higher EARs among the 60-74–year age group was observed among males and females, with older females having a greater excess of death as a result of heart disease, AEs, solid neoplasms, and lung cancer (Data Supplement).
TABLE 4.
Cause-Specific Risk of Death Among 20,007 Individuals Diagnosed With cHL at Ages 20-74 Years and Treated With Initial Chemotherapy According to Stage and Age at cHL Diagnosis: SEER-17, 2000-2016
Mortality Trends, 1983-2016
Among our secondary analytic cohort of 10,109 adult cHL survivors in SEER-9 (mean follow-up, 7.5-10.2 years), we noted significant declines in more recent calendar years in stage-specific mortality as a result of all causes, all noncancer causes, and all other neoplasms (excluding lymphoma; Data Supplement). There also was a suggestive decline in SMR for heart disease for early-stage cHL (Ptrend = .069) but not for advanced-stage cHL.
DISCUSSION
Using large-scale, population-based data, we provide a comprehensive assessment of mortality among individuals treated with chemotherapy for cHL in the 21st century, an era characterized by decreasing radiotherapy and widespread use of ABVD in the United States. While risk of death declined progressively since the 1980s, with > 20,000 adult cHL survivors in our cohort diagnosed during 2000-2015 and more than 6,000 10-year survivors, we found that the risk of death as a result of noncancer causes and other neoplasms remains significantly elevated compared with the general population. At 12 years of follow-up, cHL all-cause mortality exceeded that in the general population, and noncancer cumulative mortality comprised a substantial fraction of total deaths, particularly among patients diagnosed with cHL between 45-74 years of age. The highest noncancer SMRs and EARs were observed for ILD, AEs, and infections, particularly among those with stage III/IV cHL and in the first year after diagnosis. Heart disease was associated with lower, although significantly increased SMRs but accounted for the greatest number of disease-specific excess deaths. Risks of death generally were attenuated for stage I/II compared with stage III/IV cHL and varied by selected patient subgroups for certain causes, which provides a roadmap for targeted efforts to further reduce the mortality burden after cHL.
Heart disease has been one of the most studied noncancer causes of morbidity and mortality following HL, with established associations for anthracyclines and radiation23-35 as well as non–treatment-related cardiac risk factors (eg, hypertension, obesity, smoking).26,27 Despite decreasing use of frontline radiotherapy, our findings among chemotherapy-treated patients diagnosed during 2000-2015 demonstrate early (< 1 year) and late increased risks of heart disease–related deaths, irrespective of cHL stage and age. Male sex has been variably identified as a risk factor for cardiac mortality.30,32 We did not identify a sex predilection for heart disease mortality overall when accounting for sex-specific baseline risk, but findings suggested an interplay of stage and age in sex-specific risks. Although our reliance on death certificate data precluded reliable evaluation of specific types of heart disease, our results emphasize the importance of adhering to ASCO guidelines to minimize the risk of cardiac dysfunction in cancer survivors.36
Bleomycin therapy has been associated with ILD and other pulmonary syndromes,37 and our finding of significant relative and excess risk of mortality as a result of ILD is consistent, particularly within the first year, with clinical reports describing substantial bleomycin-induced pulmonary toxicity during therapy or within 9 months of HL diagnosis.38-41 Although younger patients had the highest ILD SMRs, EARs increased substantially with increasing age and remained strikingly elevated for older patients, consistent with previous reports and supporting a possible role for age-related impaired renal clearance.39,42-46 Smoking, use of granulocyte colony-stimulating factor, oxygen therapy, and mediastinal radiation also may have contributed to the mortality patterns observed for ILD.37,42,43,47-54 Our results highlight the importance of continuing to pursue clinical trials that limit or substitute alternate agents for bleomycin.49,52,55-61
A study that supplemented death certificates with medical record information has raised the possibility that death certificate–based findings may underestimate infection-related mortality in HL.62 Despite this possibility, we found that infection remains an important cause of death following chemotherapy for cHL, particularly within the first year. Increased infection-related mortality risks observed ≥ 1-year after diagnosis could be related to therapy for relapsed/refractory cHL or longer-term HL-related immune defects, perhaps explaining the increased risk of death as a result of benign hematologic diseases (eg, neutropenia, thrombocytopenia).1,4,63 Even with availability of effective antibiotics, growth factors, and other supportive measures, the results from our study and previous investigations1,56,64,65 have emphasized the importance of addressing infection risks irrespective of stage, sex, age, or time since diagnosis.
While early deaths as result of AEs might be attributed to chemotherapy-related toxicities, other potential culprits include medication interactions and polypharmacy. Our observation of the highest burden of adverse events among individuals 60-74 years of age at cHL diagnosis, particularly in the first year, underscores the importance of careful monitoring of medication effects, especially among the elderly.46,66
Significantly increased mortality risks were observed for chronic liver disease, COPD, nephritis/nephrosis, and cerebrovascular disease among patients with advanced cHL. These stage-specific findings suggest a potential role for chemotherapy-related toxicity given that patients with advanced-stage cHL are likely to receive more chemotherapy (cumulatively) than those with early-stage disease. Primary biliary cirrhosis,67,68 glomerulonephritides,69 and COPD have been infrequently reported in association with cHL or its treatment. Anthracyclines are associated with a > 10% risk of hepatitis B reactivation,70 which in conjunction with hepatitis infection may contribute to chronic liver disease mortality. While chemotherapy was not found to be a risk factor for stroke and transient ischemic attacks in young 5-year survivors of cHL treated in a radiation-predominant era,71 our findings suggest a possible role for chemotherapy in cerebrovascular disease in an era of diminishing radiotherapy use.
Despite the long-time focus on second cancers among HL survivors, little is known about these risks with decreasing radiation use and during an ABVD-prevalent treatment era. We observed an increased risk of MDS/AML ≥ 1 year following cHL, which is consistent with population-based incidence studies72,73 and clinical trials.74,75 Risks were lower than reported in previous studies, consistent with the lower leukemogenicity of ABVD and regimens administered in more recent years76 compared with MOPP-based regimens.77-79 We also observed an increased risk of lung cancer deaths during the first few years after cHL diagnosis, in agreement with the short latency period previously described.80,81 Chemotherapy, radiotherapy, and immune suppression associated with cHL and/or its therapy81,82 may have contributed to the increased risk of death as a result of lung cancer and other neoplasms we observed. The long latency characterizing most second solid neoplasms72 highlights the need for longer follow-up of this cohort to more comprehensively assess second cancer mortality risks in the current era.
Strengths of our study include the focus on the recent calendar years (2000-2016) and the large population-based cHL cohort that allowed us to quantify stage- and patient subgroup–specific risks for detailed causes of death across a wide age range. The size of our study population enabled us to more precisely quantify risk of death by age, sex, and time since diagnosis, particularly deaths occurring < 1 year after cHL diagnosis for which a paucity of population-based data exists.
Even with the large cohort size, we had limited numbers of deaths in pediatric patients and in some disease categories, as reflected in imprecise SMR estimates. Longer follow-up will be required to better assess these cause-specific mortality risks and to define mortality as a result of second neoplasms and heart diseases that may be associated with longer latency periods.35,83 Although we present data separately by initial chemotherapy and radiotherapy, the SEER Program lacks information on chemotherapy drugs/doses, radiation fields/doses, subsequent therapy, and prognostic factors, and together with potential underascertainment of chemotherapy, direct treatment comparisons could be misleading. Finally, we cannot exclude potential underestimation of noncancer deaths, particularly if treatment-related (nonlymphoma) deaths were coded as lymphoma-related deaths.84
In summary, we describe stage- and cause-specific relative and absolute excess mortality risks in one of the largest population-based cHL studies during an era of widespread use of ABVD and diminishing use of radiotherapy. Despite evolving cHL treatment approaches, cumulative mortality as a result of noncancer deaths accounted for a substantial fraction of deaths, irrespective of cHL stage. Patterns of excess deaths as a result of heart disease, ILD, infections, and AEs support a need for close monitoring and intervention beginning at the time of cHL diagnosis to reduce mortality for all patients, with particular attention paid to those with advanced-stage cHL and who are diagnosed at older ages. Continuing efforts are needed to develop surveillance protocols and interventions, refined treatment approaches, and new cHL therapeutics to improve patient outcomes.
ACKNOWLEDGMENT
We thank Jeremy Miller (Information Management Services) for computing support and Ruth Pfeiffer, PhD (Division of Cancer Epidemiology, National Cancer Institute), for helpful discussions on cumulative mortality.
PRIOR PRESENTATION
Presented at the 61st American Society of Hematology Annual Meeting, Orlando, FL, December 7-10, 2019.
SUPPORT
Supported by the Intramural Research Program, National Cancer Institute, (ZIA CP010131). NHD was supported by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the National Institutes of Health (NIH) and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF grant 2014194), Genentech, Elsevier, and other private donors.
The opinions and information in this article are those of the authors, and do not represent the views and/or policies of the US Food and Drug Administration.
See accompanying editorial on page 4131
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Disclosures provided by the authors and data availability statement (if applicable) are available with this article at DOI https://doi.org/10.1200/JCO.20.00264.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Lindsay M. Morton
Collection and assembly of data: Graça M. Dores, Nicole H. Dalal
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cause-Specific Mortality Following Initial Chemotherapy in a Population-Based Cohort of Patients With Classical Hodgkin Lymphoma, 2000-2016
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol. 2003;21:3431–3439. doi: 10.1200/JCO.2003.07.131. [DOI] [PubMed] [Google Scholar]
- 2.Bhuller KS, Zhang Y, Li D, et al. Late mortality, secondary malignancy and hospitalisation in teenage and young adult survivors of Hodgkin lymphoma: Report of the Childhood/Adolescent/Young Adult Cancer Survivors Research Program and the BC Cancer Agency Centre for Lymphoid Cancer. Br J Haematol. 2016;172:757–768. doi: 10.1111/bjh.13903. [DOI] [PubMed] [Google Scholar]
- 3.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kero AE, Järvelä LS, Arola M, et al. Late mortality among 5-year survivors of early onset cancer: A population-based register study. Int J Cancer. 2015;136:1655–1664. doi: 10.1002/ijc.29135. [DOI] [PubMed] [Google Scholar]
- 5.Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol. 2002;20:2101–2108. doi: 10.1200/JCO.2002.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Favier O, Heutte N, Stamatoullas-Bastard A, et al. Survival after Hodgkin lymphoma: Causes of death and excess mortality in patients treated in 8 consecutive trials. Cancer. 2009;115:1680–1691. doi: 10.1002/cncr.24178. [DOI] [PubMed] [Google Scholar]
- 7.Matasar MJ, Ford JS, Riedel ER, et al. Late morbidity and mortality in patients with Hodgkin’s lymphoma treated during adulthood. J Natl Cancer Inst. 2015;107:djv018. doi: 10.1093/jnci/djv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeVita VT., Jr A selective history of the therapy of Hodgkin’s disease. Br J Haematol. 2003;122:718–727. doi: 10.1046/j.1365-2141.2003.04541.x. [DOI] [PubMed] [Google Scholar]
- 9.Canellos GP, Rosenberg SA, Friedberg JW, et al. Treatment of Hodgkin lymphoma: A 50-year perspective. J Clin Oncol. 2014;32:163–168. doi: 10.1200/JCO.2013.53.1194. [DOI] [PubMed] [Google Scholar]
- 10.Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin. 2018;68:116–132. doi: 10.3322/caac.21438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121:2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olszewski AJ, Shrestha R, Castillo JJ. Treatment selection and outcomes in early-stage classical Hodgkin lymphoma: Analysis of the National Cancer Data Base. J Clin Oncol. 2015;33:625–633. doi: 10.1200/JCO.2014.58.7543. [DOI] [PubMed] [Google Scholar]
- 13.Guru Murthy GS, Szabo A, Hamadani M, et al. Contemporary outcomes for advanced-stage classical Hodgkin lymphoma in the U.S.: Analysis of Surveillance, Epidemiology, and End Results database. Oncologist. 2019;24:1488–1495. doi: 10.1634/theoncologist.2019-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016. Bethesda, MD: National Cancer Institute; 2019. [Google Scholar]
- 15. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated Total U.S. (1969-2016) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2018. Underlying mortality data provided by NCHS (www.cdc.gov/nchs)
- 16.Mariotto AB, Noone AM, Howlader N, et al. Cancer survival: An overview of measures, uses, and interpretation. J Natl Cancer Inst Monogr. 2014;2014:145–186. doi: 10.1093/jncimonographs/lgu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention: National Center for Health Statistics. https://www.cdc.gov/nchs.
- 18. Surveillance Research Program, National Cancer Institute: SEER*Stat software, version 8.3.6. https://seer.cancer.gov/seerstat/
- 19.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–1113. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 20.Preston D, Lubin J, Pierce D, et al. Epicure Risk Regression and Person-Year Computation Software: Command Summary and User Guide. Ottawa, ON, Canada: Risk Sciences International; 2015. [Google Scholar]
- 21.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury JB. Non-parametric confidence interval estimation for competing risks analysis: Application to contraceptive data. Stat Med. 2002;21:1129–1144. doi: 10.1002/sim.1070. [DOI] [PubMed] [Google Scholar]
- 23.Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117:412–418. doi: 10.1182/blood-2010-06-291328. [DOI] [PubMed] [Google Scholar]
- 24.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993;270:1949–1955. [PubMed] [Google Scholar]
- 25.Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 26.van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 27.van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 28.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 29.Al-Kindi SG, Abu-Zeinah GF, Kim CH, et al. Trends and disparities in cardiovascular mortality among survivors of Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2015;15:748–752. doi: 10.1016/j.clml.2015.07.638. [DOI] [PubMed] [Google Scholar]
- 30.Eloranta S, Lambert PC, Sjöberg J, et al. Temporal trends in mortality from diseases of the circulatory system after treatment for Hodgkin lymphoma: A population-based cohort study in Sweden (1973 to 2006) J Clin Oncol. 2013;31:1435–1441. doi: 10.1200/JCO.2012.45.2714. [DOI] [PubMed] [Google Scholar]
- 31.Maraldo MV, Giusti F, Vogelius IR, et al. Cardiovascular disease after treatment for Hodgkin’s lymphoma: An analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol. 2015;2:e492–e502. doi: 10.1016/S2352-3026(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 32.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: A collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–214. doi: 10.1093/jnci/djk029. [DOI] [PubMed] [Google Scholar]
- 33.Heidenreich PA, Schnittger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol. 2007;25:43–49. doi: 10.1200/JCO.2006.07.0805. [DOI] [PubMed] [Google Scholar]
- 34.Myrehaug S, Pintilie M, Yun L, et al. A population-based study of cardiac morbidity among Hodgkin lymphoma patients with preexisting heart disease. Blood. 2010;116:2237–2240. doi: 10.1182/blood-2010-01-263764. [DOI] [PubMed] [Google Scholar]
- 35.de Vries S, Schaapveld M, van Nimwegen FA, et al. High burden of subsequent malignant neoplasms and cardiovascular disease in long-term Hodgkin lymphoma survivors. Br J Cancer. 2018;118:887–895. doi: 10.1038/bjc.2017.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 37.Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120:617–624. doi: 10.1378/chest.120.2.617. [DOI] [PubMed] [Google Scholar]
- 38.Jalali A, Ha FJ, Chong G, et al. Hodgkin lymphoma: An Australian experience of ABVD chemotherapy in the modern era. Ann Hematol. 2016;95:809–816. doi: 10.1007/s00277-016-2611-4. [DOI] [PubMed] [Google Scholar]
- 39.Martin WG, Ristow KM, Habermann TM, et al. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005;23:7614–7620. doi: 10.1200/JCO.2005.02.7243. [DOI] [PubMed] [Google Scholar]
- 40.Stamatoullas A, Brice P, Bouabdallah R, et al. Outcome of patients older than 60 years with classical Hodgkin lymphoma treated with front line ABVD chemotherapy: Frequent pulmonary events suggest limiting the use of bleomycin in the elderly. Br J Haematol. 2015;170:179–184. doi: 10.1111/bjh.13419. [DOI] [PubMed] [Google Scholar]
- 41.Van Barneveld PW, Sleijfer DT, van der Mark TW, et al. Natural course of bleomycin-induced pneumonitis. A follow-up study. Am Rev Respir Dis. 1987;135:48–51. doi: 10.1164/arrd.1987.135.1.48. [DOI] [PubMed] [Google Scholar]
- 42.Azambuja E, Fleck JF, Batista RG, et al. Bleomycin lung toxicity: Who are the patients with increased risk? Pulm Pharmacol Ther. 2005;18:363–366. doi: 10.1016/j.pupt.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Shippee BM, Bates JS, Richards KL. The role of screening and monitoring for bleomycin pulmonary toxicity. J Oncol Pharm Pract. 2016;22:308–312. doi: 10.1177/1078155215574294. [DOI] [PubMed] [Google Scholar]
- 44.Sun HL, Atenafu EG, Tsang R, et al. Bleomycin pulmonary toxicity does not adversely affect the outcome of patients with Hodgkin lymphoma. Leuk Lymphoma. 2017;58:2607–2614. doi: 10.1080/10428194.2017.1307980. [DOI] [PubMed] [Google Scholar]
- 45. doi: 10.1016/j.jgo.2019.09.009. Thomas TS, Luo S, Reagan PM, et al: Advancing age and the risk of bleomycin pulmonary toxicity in a largely older cohort of patients with newly diagnosed Hodgkin lymphoma. J Geriatr Oncol 11:69-74, 2020. [DOI] [PubMed] [Google Scholar]
- 46.Evens AM, Carter J, Loh KP, et al. Management of older Hodgkin lymphoma patients. Hematology (Am Soc Hematol Educ Program) 2019;2019:233–242. doi: 10.1182/hematology.2019000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abou Yehia Z, Mikhaeel GN, Smith G, et al. Does bleomycin lung toxicity increase the risk of radiation pneumonitis in Hodgkin lymphoma? Int J Radiat Oncol Biol Phys. 2016;96:951–958. doi: 10.1016/j.ijrobp.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Alessandrino EP, Bernasconi P, Colombo A, et al. Pulmonary toxicity following carmustine-based preparative regimens and autologous peripheral blood progenitor cell transplantation in hematological malignancies. Bone Marrow Transplant. 2000;25:309–313. doi: 10.1038/sj.bmt.1702154. [DOI] [PubMed] [Google Scholar]
- 49.Böll B, Bredenfeld H, Görgen H, et al. Phase 2 study of PVAG (prednisone, vinblastine, doxorubicin, gemcitabine) in elderly patients with early unfavorable or advanced stage Hodgkin lymphoma. Blood. 2011;118:6292–6298. doi: 10.1182/blood-2011-07-368167. [DOI] [PubMed] [Google Scholar]
- 50.Cella L, Liuzzi R, D’Avino V, et al. Pulmonary damage in Hodgkin’s lymphoma patients treated with sequential chemo-radiotherapy: Predictors of radiation-induced lung injury. Acta Oncol. 2014;53:613–619. doi: 10.3109/0284186X.2013.850739. [DOI] [PubMed] [Google Scholar]
- 51.De A, Guryev I, LaRiviere A, et al. Pulmonary function abnormalities in childhood cancer survivors treated with bleomycin. Pediatr Blood Cancer. 2014;61:1679–1684. doi: 10.1002/pbc.25098. [DOI] [PubMed] [Google Scholar]
- 52.Friedberg JW, Neuberg D, Kim H, et al. Gemcitabine added to doxorubicin, bleomycin, and vinblastine for the treatment of de novo Hodgkin disease: Unacceptable acute pulmonary toxicity. Cancer. 2003;98:978–982. doi: 10.1002/cncr.11582. [DOI] [PubMed] [Google Scholar]
- 53.Senan S, Paul J, Thomson N, et al. Cigarette smoking is a risk factor for bleomycin-induced pulmonary toxicity. Eur J Cancer. 1992;28:2084. doi: 10.1016/0959-8049(92)90262-z. [DOI] [PubMed] [Google Scholar]
- 54.Evens AM, Helenowski I, Ramsdale E, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: Outcomes and prognostic factors in the modern era. Blood. 2012;119:692–695. doi: 10.1182/blood-2011-09-378414. [DOI] [PubMed] [Google Scholar]
- 55.Böll B, Goergen H, Behringer K, et al. Bleomycin in older early-stage favorable Hodgkin lymphoma patients: Analysis of the German Hodgkin Study Group (GHSG) HD10 and HD13 trials. Blood. 2016;127:2189–2192. doi: 10.1182/blood-2015-11-681064. [DOI] [PubMed] [Google Scholar]
- 56.Böll B, Görgen H, Fuchs M, et al. ABVD in older patients with early-stage Hodgkin lymphoma treated within the German Hodgkin Study Group HD10 and HD11 trials. J Clin Oncol. 2013;31:1522–1529. doi: 10.1200/JCO.2012.45.4181. [DOI] [PubMed] [Google Scholar]
- 57.Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bredenfeld H, Franklin J, Nogova L, et al. Severe pulmonary toxicity in patients with advanced-stage Hodgkin’s disease treated with a modified bleomycin, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone, and gemcitabine (BEACOPP) regimen is probably related to the combination of gemcitabine and bleomycin: A report of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2004;22:2424–2429. doi: 10.1200/JCO.2004.09.114. [DOI] [PubMed] [Google Scholar]
- 59.Pavlakis N, Bell DR, Millward MJ, et al. Fatal pulmonary toxicity resulting from treatment with gemcitabine. Cancer. 1997;80:286–291. doi: 10.1002/(sici)1097-0142(19970715)80:2<286::aid-cncr17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 60.Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: A phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14:1348–1356. doi: 10.1016/S1470-2045(13)70501-1. [DOI] [PubMed] [Google Scholar]
- 61.Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378:331–344. doi: 10.1056/NEJMoa1708984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Vries S, Schaapveld M, Kardaun JW, et al. Comparing causes of death of Hodgkin lymphoma and breast cancer patients between medical records and cause-of-death statistics. Clin Epidemiol. 2018;10:1523–1531. doi: 10.2147/CLEP.S161359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nonas S. Pulmonary manifestations of primary immunodeficiency disorders. Immunol Allergy Clin North Am. 2015;35:753–766. doi: 10.1016/j.iac.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the Stanford V regimen and ABVD in the treatment of advanced Hodgkin’s Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390–5396. doi: 10.1200/JCO.2009.23.3239. [DOI] [PubMed] [Google Scholar]
- 65.Proctor SJ, Wilkinson J, Jones G, et al. Evaluation of treatment outcome in 175 patients with Hodgkin lymphoma aged 60 years or over: The SHIELD study. Blood. 2012;119:6005–6015. doi: 10.1182/blood-2011-12-396556. [DOI] [PubMed] [Google Scholar]
- 66.Klepin HD. Ready for prime time: Role for geriatric assessment to improve quality of care in hematology practice. Blood. 2019;134:2005–2012. doi: 10.1182/blood.2019001299. [DOI] [PubMed] [Google Scholar]
- 67.Kanellopoulou T, Alexopoulou A, Kontopidou FN, et al. Development of non-Hodgkin’s lymphoma in a patient with primary biliary cirrhosis: A case report and review of the literature. Ann Gastroenterol. 2011;24:125–128. [PMC free article] [PubMed] [Google Scholar]
- 68.Yoneda K, Sugimoto K, Shiraki K, et al. Primary biliary cirrhosis following chemotherapy for Hodgkin’s lymphoma. Intern Med. 2008;47:419–420. doi: 10.2169/internalmedicine.47.0306. [DOI] [PubMed] [Google Scholar]
- 69.Sánchez M, Enríquez R, Sirvent AE, et al. Membranous glomerulonephritis in a patient with Hodgkin’s lymphoma in remission. Nefrologia. 2013;33:745–747. doi: 10.3265/Nefrologia.pre2013.Apr.11963. [DOI] [PubMed] [Google Scholar]
- 70.Pattullo V. Prevention of hepatitis B reactivation in the setting of immunosuppression. Clin Mol Hepatol. 2016;22:219–237. doi: 10.3350/cmh.2016.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Bruin ML, Dorresteijn LD, van’t Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928–937. doi: 10.1093/jnci/djp147. [DOI] [PubMed] [Google Scholar]
- 72.Dores GM, Metayer C, Curtis RE, et al. Second malignant neoplasms among long-term survivors of Hodgkin’s disease: A population-based evaluation over 25 years. J Clin Oncol. 2002;20:3484–3494. doi: 10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]
- 73.Schonfeld SJ, Gilbert ES, Dores GM, et al. Acute myeloid leukemia following Hodgkin lymphoma: A population-based study of 35,511 patients. J Natl Cancer Inst. 2006;98:215–218. doi: 10.1093/jnci/djj017. [DOI] [PubMed] [Google Scholar]
- 74.Eichenauer DA, Thielen I, Haverkamp H, et al. Therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with Hodgkin lymphoma: A report from the German Hodgkin Study Group. Blood. 2014;123:1658–1664. doi: 10.1182/blood-2013-07-512657. [DOI] [PubMed] [Google Scholar]
- 75.Josting A, Wiedenmann S, Franklin J, et al. Secondary myeloid leukemia and myelodysplastic syndromes in patients treated for Hodgkin’s disease: A report from the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3440–3446. doi: 10.1200/JCO.2003.07.160. [DOI] [PubMed] [Google Scholar]
- 76.Koontz MZ, Horning SJ, Balise R, et al. Risk of therapy-related secondary leukemia in Hodgkin lymphoma: The Stanford University experience over three generations of clinical trials. J Clin Oncol. 2013;31:592–598. doi: 10.1200/JCO.2012.44.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boivin JF, Hutchison GB, Zauber AG, et al. Incidence of second cancers in patients treated for Hodgkin’s disease. J Natl Cancer Inst. 1995;87:732–741. doi: 10.1093/jnci/87.10.732. [DOI] [PubMed] [Google Scholar]
- 78.Tucker MA, Coleman CN, Cox RS, et al. Risk of second cancers after treatment for Hodgkin’s disease. N Engl J Med. 1988;318:76–81. doi: 10.1056/NEJM198801143180203. [DOI] [PubMed] [Google Scholar]
- 79.van Leeuwen FE, Chorus AM, van den Belt-Dusebout AW, et al. Leukemia risk following Hodgkin’s disease: Relation to cumulative dose of alkylating agents, treatment with teniposide combinations, number of episodes of chemotherapy, and bone marrow damage. J Clin Oncol. 1994;12:1063–1073. doi: 10.1200/JCO.1994.12.5.1063. [DOI] [PubMed] [Google Scholar]
- 80.Travis LB, Curtis RE, Bennett WP, et al. Lung cancer after Hodgkin’s disease. J Natl Cancer Inst. 1995;87:1324–1327. doi: 10.1093/jnci/87.17.1324. [DOI] [PubMed] [Google Scholar]
- 81.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94:182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 82.Shree T, Li Q, Glaser SL, et al. Impaired immune health in survivors of diffuse large B-cell lymphoma. J Clin Oncol. 2020;38:1664–1675. doi: 10.1200/JCO.19.01937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyer RM, Ambinder RF, Stroobants S. Hodgkin’s lymphoma: Evolving concepts with implications for practice. Hematology (Am Soc Hematol Educ Program) 2004;2004:184–202. doi: 10.1182/asheducation-2004.1.184. [DOI] [PubMed] [Google Scholar]
- 84.Welch HG, Black WC. Are deaths within 1 month of cancer-directed surgery attributed to cancer? J Natl Cancer Inst. 2002;94:1066–1070. doi: 10.1093/jnci/94.14.1066. [DOI] [PubMed] [Google Scholar]