Classic Hodgkin lymphoma (cHL) is a highly curable cancer.1-4 However, due in part to the young age of most patients, treatment-related toxicities and late effects, such as secondary malignant neoplasms (SMNs) and cardiovascular disease (CVD), can adversely affect survival. Cost-per-death analyses have shown that HL has the second highest cost per death or lost-productivity cost (behind only malignant melanoma),5 and productivity analyses of cancer mortality have shown HL to be the second most costly cancer in terms of lost lifetime earnings.6 In addition to these economic consequences, cHL survivors also experience significantly compromised health-related quality of life (HRQL).7
These undesirable effects are primarily due to an increased incidence of CVD, SMN, and other prominent treatment-induced morbidities that may result in excess mortality.8,9 SMN risk is known to be dependent on many clinical factors (eg, age at exposure, sex, stage) as well as several treatment-related factors (eg, type/amount of chemotherapy and radiotherapy). A Dutch analysis highlighted the impact of radiation dose/field, sex, and smoking on the risk of breast, lung, and other cancers in cHL survivors.8 Additional studies have demonstrated the impact of age and sex on incidence of major cardiac disease.10,11 However, many of the patients included in these analyses were treated before 2000. Use of ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) became widespread approximately two decades ago, and the application of radiotherapy has also decreased during this time.12 In addition, the risk of other important post-therapy morbidity and mortality with modern treatments, including infections and interstitial lung disease (ILD), have not been fully elucidated.
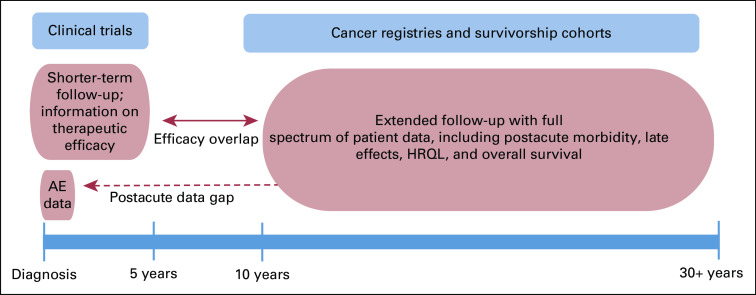
The continuum of care for patients with cHL represents a challenge to effectively study the full longitudinal spectrum that therapy has on morbidity and mortality (Fig 1). Clinical trials are important for examination of efficacy (eg, progression-free survival) and on-study acute adverse events (AEs) of therapeutic regimens. However, data are typically limited to shorter-term follow-up with little information about risk or severity of postacute morbidity or late effects.8,13 Conversely, HL registries and survivorship cohorts offer important insights on longer-term outcomes and morbidity that manifest after HL therapy, which may be leveraged to inform decision making and ultimately lead to more tailored therapy.
FIG 1.
Data sources to study the continuum of care for Hodgkin lymphoma. Ideal information to study morbidity health-related quality of life (HRQL), and mortality across the lifelong time horizon for patients with classic Hodgkin lymphoma (cHL) is not available from a single source of data. Clinical trials typically examine shorter-term efficacy (3-5 years) and acute on-study adverse events (AEs). Most clinical trials do not track AEs or morbidity that occur post-therapy, which leads to critical data gaps. Cancer registries and survivorship cohorts comprise real-world data that provide important insights with longer-term follow-up for morbidity, HRQL, and excess mortality that manifest after cHL therapy, including postacute outcomes (ie, 1-10 years after diagnosis).
In the article that accompanies this editorial, Dores et al14 analyzed cause-specific mortality among individuals with a diagnosis of cHL reported to 17 SEER registry areas (SEER-17) during 2000-2015, with follow-up to 2016. The analysis focused on a cohort of 20,007 individuals diagnosed with cHL between ages 20 and 74 years using standardized mortality ratios (SMRs) to compare cause-specific mortality risk with the general population, with excess absolute risks (EARs) per 10,000 person-years reflecting absolute increase in risk of death. With a mean follow-up of 8 years, Dores et al14 identified that the relative risk of death as a result of any cause excluding lymphoma was increased 1.8-fold in the cHL cohort compared with the general population. Furthermore, deaths due to noncancer causes accounted for many of the nonlymphoma deaths, with SMRs increased 1.6-fold for early-stage cHL and 2.4-fold for advanced-stage cHL. Table 1 highlights selected cause-specific risks based on disease stage.
TABLE 1.
Summary of Selected cHL Cause-Specific Death Rates Based on Stage From Dores et al14 (SEER-17, 2000-2016; mean follow-up, 8 years).
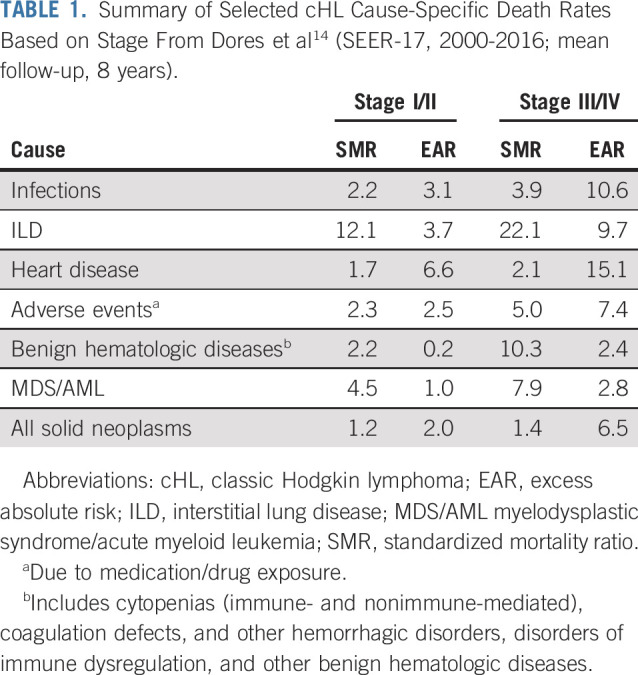
By subgroups, the authors showed that cause-specific risks of death generally did not differ by sex, regardless of stage, except for infections and medication AEs being higher in females. Interestingly, ILD SMRs decreased with advancing age; although not surprisingly, medication-related AE SMRs increased with age, being especially prevalent among the 60- to 74-year-old age group. Excess deaths from heart disease predominated among the 60- to 74-year-old age group across all stages. Moreover, death due to heart disease was significantly increased in all time intervals, with SMRs being the highest < 1 year after cHL diagnosis and lower thereafter, with EARs highest < 1 year after cHL, decreasing at 1-4 years, but increasing again at ≥ 5 years. Finally, they noted declines in more recent calendar years for stage-specific mortality from all causes; however, most rates remained elevated, including among younger patients.
Collectively, these population-based data confirm previous reports8-11,13,15-17 showing significant increases in nonlymphoma-related mortality in cHL survivors. In the current report,14 excess mortality appears to persist in the contemporary era. A limitation of the analysis was the relatively short duration of follow-up, which limits information on late effects that knowingly occur beyond 10-20 years. However, this also represents a unique strength of the data highlighting prominent postacute (ie, 1-10 years after diagnosis) morbidity and excess mortality associated with more modern, frontline therapy. The authors acknowledged an additional limitation of the data, which was their inability to directly compare morbidity or mortality rates among different treatment modalities (eg, chemotherapy alone v combined-modality therapy) because of restrictions in data use. Nonetheless, we should be cognizant that modern chemotherapy (eg, ABVD) likely contributes to postacute morbidity, late effects, and excess mortality, consistent with prior reports of patients with cHL treated with chemotherapy alone.18-20 There were other interesting findings identified in this analysis, including that many rates were increased for patients with advanced-stage disease, which may reflect the increased number of chemotherapy cycles administered compared with patients with early-stage disease. Additionally, even though younger patients had the highest ILD SMRs, EARs increased considerably with increasing age and were strikingly high for older patients (ie, EAR of 17.1 to 39.1 for patients ages 60-74 years), consistent with the recognized age-related, adverse impact of bleomycin-based therapy.21-26
In terms of next steps, an overarching question is how to optimally integrate data from the current and prior reports of treatment-related morbidity and mortality into clinical practice. A limitation of population-based analyses relates to the lack of patient-level data. Including individual patient data (IPD), which contains granular patient and disease characteristics, as well a detailed description of therapy, can delineate individual demographics (above and beyond age and sex) and parse out treatment-related factors (eg, chemotherapy cumulative dosing; radiation field size/dose) to help identify individual patients at higher risk for whom alternative treatments should be considered.
Clinicians face challenges when analyzing clinical trial and registry data in objectively assessing alternative treatment options for individual cHL patients. First, ideal information is not available across the lifelong time horizon—particularly from a single data source (Fig 1). As highlighted, clinical trials are typically limited to shorter-term follow-up (3-5 years), principally reporting on disease outcomes (eg, relapse, progression, remission) with limited information on postacute morbidity or late effects. Although longer-term follow-up data are available from cHL registries, these real-world data must be carefully culled and harmonized to ensure applicability to the contemporarily treated patient.27 Second, the benefits and risks of different therapies depend on a multitude of characteristics, such as patient age, sex, and disease stage, among other factors, which are not discernable from group-level data.
Given the impact of individual patient factors with varying treatment approaches, and the tradeoffs relative to short-term disease control versus post-therapy events, there is interest in decision modeling.28 Decision analysis, using mathematical simulation, is an ideal method on the basis of its ability to (1) incorporate best-available data collected from different study designs and populations to provide estimates for varied probabilities and parameters; (2) perform sensitivity analyses of assumptions and estimates to incorporate uncertainty; (3) validate and calibrate results against external data; and (4) identify areas of uncertainty for future research. Resultant simulation models incorporate best-available evidence regarding prevalence, efficacy, and desired outcomes to depict short- and longer-term endpoints, including HRQL, of different treatment approaches.
The development of dynamic decision models requires large numbers of IPD to account for differences across patients in terms of demographic characteristics and disease factors. Furthermore, incorporation of key biology and imaging endpoints may also be integrated into decision models to enrich prognostication and prediction of efficacy as well as treatment-related toxicities. Efforts are underway to harmonize large amounts of IPD for patients with cHL treated in recent international clinical trials and from prominent cancer registries and survivorship cohorts (ie, the HoLISTIC [Hodgkin Lymphoma International Study for Individual Care] consortium; https://www.hodgkinconsortium.com/).29 A goal of HoLISTIC is to create robust and dynamic web-based decision models with objective data on shorter- and longer-term outcomes to guide providers alongside their patients with cHL through the choices of alternative treatments that reflect individual patient characteristics, disease factors, and the preferences of the patient.30
Altogether, given the success of frontline treatments and the ability to salvage the majority of patients with cHL after recurrence, survival is high. However, this survival comes at a cost to patients in the form of postacute morbidity and late effects, which can alter both quality and length of survivorship. Analyses such as the current report underscore the continuing risks for patients, including in the modern era, and with events beginning within 1 year. Furthermore, it also endorses international clinical trial efforts during the past 10-15 years that have closely examined de-escalation of therapy for patients with cHL31-35 via positron emission tomography–based response-adapted strategies and integration of targeted therapeutic agents. Additionally, cHL clinical trials should invest in recording longer-term follow-up, including more detailed analyses of post-therapy morbidity. Finally, efforts to harmonize patient-level data on efficacy and toxicity (acute, postacute, and late) across varying treatment platforms that are applicable to diverse settings across the world to aid in decision making and delineation of individualized therapy for patients with cHL should continue.
ACKNOWLEDGMENT
A.M.E. is supported by National Cancer Institute (NCI) R01 GM127714, National Institutes of Health (NIH) R33 CA223908, NIH/NCI U01 CA187947), NIH R01 EB012521, ORIEN, Avatar Research Program, Leukemia & Lymphoma Society, and Translational Research Program. S.K.P. is supported by Leukemia & Lymphoma Society.
Footnotes
See accompanying article on page 4149
AUTHOR CONTRIBUTIONS
Conception and design: Andrew M. Evens
Administrative support: Andrew M. Evens
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Continuum of Care for Hodgkin Lymphoma: Impact of Modern Therapy on Postacute Morbidity and Mortality
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Andrew M. Evens
Honoraria: Seattle Genetics, Pharmacyclics, Verastem, Research to Practice, Miltenyi Biotec, Epizyme, Novartis, MorphoSys, Cota Healthcare, Affimed Therapeutics, Bayer, Verastem
Consulting or Advisory Role: Seattle Genetics, Bayer, Novartis, Pharmacyclics, Verastem, Miltenyi Biotec, Epizyme, MorphoSys, Cota Healthcare, Affimed Therapeutics
Research Funding: Tesaro
Travel, Accommodations, Expenses: Seattle Genetics, Research to Practice, Bayer, Affimed Therapeutics, Pharmacyclics, Novartis, Verastem
Susan K. Parsons
Consulting or Advisory Role: Seattle Genetics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hokland P, Shah M, David K, et al. doi: 10.1111/bjh.16587. How I treat advanced Hodgkin lymphoma - a global view. Br J Haematol . [epub ahead of print on June 19, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longley J, Johnson PWM. Current treatment paradigms for advanced stage Hodgkin lymphoma. Br J Haematol. 2019;184:60–71. doi: 10.1111/bjh.15622. [DOI] [PubMed] [Google Scholar]
- 3.Stephens DM, Li H, Schöder H, et al. Five-year follow-up of SWOG S0816: Limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood. 2019;134:1238–1246. doi: 10.1182/blood.2019000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Depaus J, Delcourt A, André M. Therapeutic recommendations for early stage Hodgkin lymphomas. Br J Haematol. 2019;184:9–16. doi: 10.1111/bjh.15623. [DOI] [PubMed] [Google Scholar]
- 5.Hanly P, Soerjomataram I, Sharp L. Measuring the societal burden of cancer: The cost of lost productivity due to premature cancer-related mortality in Europe. Int J Cancer. 2015;136:E136–E145. doi: 10.1002/ijc.29105. [DOI] [PubMed] [Google Scholar]
- 6.Bradley CJ, Yabroff KR, Dahman B, et al. Productivity costs of cancer mortality in the United States: 2000-2020. J Natl Cancer Inst. 2008;100:1763–1770. doi: 10.1093/jnci/djn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linendoll N, Saunders T, Burns R, et al. Health-related quality of life in Hodgkin lymphoma: A systematic review. Health Qual Life Outcomes. 2016;14:114. doi: 10.1186/s12955-016-0515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373:2499–2511. doi: 10.1056/NEJMoa1505949. [DOI] [PubMed] [Google Scholar]
- 9.Ng AK. Current survivorship recommendations for patients with Hodgkin lymphoma: Focus on late effects. Blood. 2014;124:3373–3379. doi: 10.1182/blood-2014-05-579193. [DOI] [PubMed] [Google Scholar]
- 10.Myrehaug S, Pintilie M, Tsang R, et al. Cardiac morbidity following modern treatment for Hodgkin lymphoma: Supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma. 2008;49:1486–1493. doi: 10.1080/10428190802140873. [DOI] [PubMed] [Google Scholar]
- 11.Myrehaug S, Pintilie M, Yun L, et al. A population-based study of cardiac morbidity among Hodgkin lymphoma patients with preexisting heart disease. Blood. 2010;116:2237–2240. doi: 10.1182/blood-2010-01-263764. [DOI] [PubMed] [Google Scholar]
- 12.Olszewski AJ, Shrestha R, Castillo JJ. Treatment selection and outcomes in early-stage classical Hodgkin lymphoma: Analysis of the National Cancer Data Base. J Clin Oncol. 2015;33:625–633. doi: 10.1200/JCO.2014.58.7543. [DOI] [PubMed] [Google Scholar]
- 13.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE) Lancet. 2017;390:2569–2582. doi: 10.1016/S0140-6736(17)31610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dores GM, Curtis RE, Dalal NH, et al. Cause-specific mortality following initial chemotherapy in a population-based cohort of patients with classical Hodgkin lymphoma 2000–2016.J Clin Oncol 38:4149-4162, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol. 2007;25:1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson DC, Cotton C, Crystal P, et al. Impact of early breast cancer screening on mortality among young survivors of childhood Hodgkin’s lymphoma. J Natl Cancer Inst 108djw010. 2016 doi: 10.1093/jnci/djw010. [DOI] [PubMed] [Google Scholar]
- 17.Dores GM, Metayer C, Curtis RE, et al. Second malignant neoplasms among long-term survivors of Hodgkin’s disease: A population-based evaluation over 25 years. J Clin Oncol. 2002;20:3484–3494. doi: 10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Maraldo MV, Giusti F, Vogelius IR, et al. Cardiovascular disease after treatment for Hodgkin’s lymphoma: An analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol. 2015;2:e492–e502. doi: 10.1016/S2352-3026(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 19.Swerdlow AJ, Higgins CD, Smith P, et al. Second cancer risk after chemotherapy for Hodgkin’s lymphoma: A collaborative British cohort study. J Clin Oncol. 2011;29:4096–4104. doi: 10.1200/JCO.2011.34.8268. [DOI] [PubMed] [Google Scholar]
- 20.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: A collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–214. doi: 10.1093/jnci/djk029. [DOI] [PubMed] [Google Scholar]
- 21.Evens AM, Helenowski I, Ramsdale E, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: Outcomes and prognostic factors in the modern era. Blood. 2012;119:692–695. doi: 10.1182/blood-2011-09-378414. [DOI] [PubMed] [Google Scholar]
- 22.Evens AM, Hong F, Gordon LI, et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: A comprehensive analysis from the North American intergroup trial E2496. Br J Haematol. 2013;161:76–86. doi: 10.1111/bjh.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böll B, Goergen H, Behringer K, et al. Bleomycin in older early-stage favorable Hodgkin lymphoma patients: Analysis of the German Hodgkin Study Group (GHSG) HD10 and HD13 trials. Blood. 2016;127:2189–2192. doi: 10.1182/blood-2015-11-681064. [DOI] [PubMed] [Google Scholar]
- 24.Böll B, Görgen H, Fuchs M, et al. ABVD in older patients with early-stage Hodgkin lymphoma treated within the German Hodgkin Study Group HD10 and HD11 trials. J Clin Oncol. 2013;31:1522–1529. doi: 10.1200/JCO.2012.45.4181. [DOI] [PubMed] [Google Scholar]
- 25.Stamatoullas A, Brice P, Bouabdallah R, et al. Outcome of patients older than 60 years with classical Hodgkin lymphoma treated with front line ABVD chemotherapy: Frequent pulmonary events suggest limiting the use of bleomycin in the elderly. Br J Haematol. 2015;170:179–184. doi: 10.1111/bjh.13419. [DOI] [PubMed] [Google Scholar]
- 26.Thomas TS, Luo S, Reagan PM, et al. Advancing age and the risk of bleomycin pulmonary toxicity in a largely older cohort of patients with newly diagnosed Hodgkin lymphoma. J Geriatr Oncol. 2020;11:69–74. doi: 10.1016/j.jgo.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons SK, Kelly MJ, Cohen JT, et al. Early-stage Hodgkin lymphoma in the modern era: Simulation modelling to delineate long-term patient outcomes. Br J Haematol. 2018;182:212–221. doi: 10.1111/bjh.15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evens A, Advani R, Aleman B, et al. The Hodgkin Lymphoma International Study for Individual Care (HoLISTIC): A multi-national collaborative to enhance decision making for pediatric and adult Hodgkin lymphoma (HL) Presented at the 25th European Hematology Association Congress; The Hague, Netherlands: June 11-21. [Google Scholar]
- 30.Bröckelmann PJ, McMullen S, Wilson JB, et al. Patient and physician preferences for first-line treatment of classical Hodgkin lymphoma in Germany, France and the United Kingdom. Br J Haematol. 2019;184:202–214. doi: 10.1111/bjh.15566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.André MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: Final results of the randomized EORTC/LYSA/FIL H10 Trial. J Clin Oncol. 2017;35:1786–1794. doi: 10.1200/JCO.2016.68.6394. [DOI] [PubMed] [Google Scholar]
- 32.Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
- 34.Zinzani PL, Broccoli A, Gioia DM, et al. Interim positron emission tomography response-adapted therapy in advanced-stage Hodgkin lymphoma: Final results of the phase II part of the HD0801 study. J Clin Oncol. 2016;34:1376–1385. doi: 10.1200/JCO.2015.63.0699. [DOI] [PubMed] [Google Scholar]
- 35.Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]