Abstract
BACKGROUND
Pleomorphic adenoma (PA) is the most common benign tumor that occurs in the salivary glands; however, tracheobronchial PA is rarely observed. To the best of our knowledge, fewer than 50 cases have been reported in the literature. We report a 49-year-old woman who had been treated for asthma for 2 years before being diagnosed with PA of the trachea.
CASE SUMMARY
A 49-year-old woman was referred to our hospital due to dyspnea upon exertion and chronic cough with wheezing for 2 years. Laboratory tests showed an elevated white blood cell count, absolute neutrophil count, and percentage of neutrophils. A chest computerized tomography scan showed a well-defined, soft-tissue density lesion measuring 2.4 cm × 2.1 cm in the lower trachea. Flexible bronchoscopy revealed that nearly 90% of the tracheal lumen was obstructed. The histopathological and immunohistochemistry features suggested PA of the trachea. Furthermore, we review the characteristics of 29 patients with tracheobronchial PA over the last 30 years.
CONCLUSION
Tracheobronchial PA occurs without gender predominance, mostly in the lower or upper trachea, and has a low recurrence rate. The median age at diagnosis is 48 years. The most common symptoms are cough, stridor, dyspnea, and wheezing.
Keywords: Pleomorphic adenoma, Trachea, Bronchoscopy, Review, Diagnosis, Case report
Core Tip: Pleomorphic adenoma of the trachea is a rare benign tumor with slow growth. However, no standards for management have been established, and the clinical course has not yet been defined. In this study, 29 cases of tracheobronchial pleomorphic adenoma are reviewed with regard to the most common symptoms, clinical course, and treatment. For early and accurate diagnosis, chest computerized tomography and bronchoscopy should be performed initially in suspected cases.
INTRODUCTION
Pleomorphic adenoma (PA) is an unusual type of salivary-gland neoplasm that occurs in the trachea[1]. The tumor is composed of recognizable epithelial tissue mixed with mucoid, myxoid, and chondroid tissues, which can also be observed in the soft palate, hard palate, upper lip, nasal septum, nasopharynx, orbital area, lower eyelid, buccal mucosa, cheek, and external auditory canal[2]. To the best of our knowledge, fewer than 50 cases have been reported[3-6]. Due to the lack of early specific symptoms, PA of the trachea is usually misdiagnosed as asthma[6-9]. In addition, cases of PA can progress to malignant tumors[10]. We present a case of PA of the trachea that was successfully treated by bronchoscopic interventions.
CASE PRESENTATION
Chief complaints
Dyspnea upon exertion and chronic cough with wheezing for 2 years.
History of present illness
A 49-year-old woman was referred to our hospital for dyspnea upon exertion and chronic cough with wheezing for 2 years. The above symptoms worsened with white mucus sputum for the past one week with no complaints of fever, chest tightness, chest pain, or hemoptysis.
History of past illness
The patient was previously diagnosed with asthma and treated with inhaled glucocorticoids for 2 mo.
Personal and family history
There was no history of tobacco use, and the patient denied having a personal or family history of other diseases.
Physical examination
In the physical examination, lip cyanosis, three depression signs (suprasternal fossa, supraclavicular fossa, and intercostal space), and expiratory and inspiratory wheezing were observed, and the sound of her lungs was decreased with crackles, but she did not have lymphadenopathy or weight loss. Furthermore, we could hear stridor in the trachea and neck.
Laboratory examinations
Routine blood tests showed an elevated white blood cell count (14.70 × 109 cells/L; range, 3.5-9.5 × 109 cells/L), absolute neutrophil count (11.36 × 109 cells/L; range, 1.8-6.3 × 109 cells/L), and neutrophil percentage (77.3%; range, 40%-75%); the serum potassium level was found to be decreased in the blood biochemistry results (2.78 mmol/L; range, 3.5-5.5 mmol/L). The tumor markers were normal. The arterial blood gas test suggested respiratory acidosis combined with metabolic alkalosis.
Imaging examinations
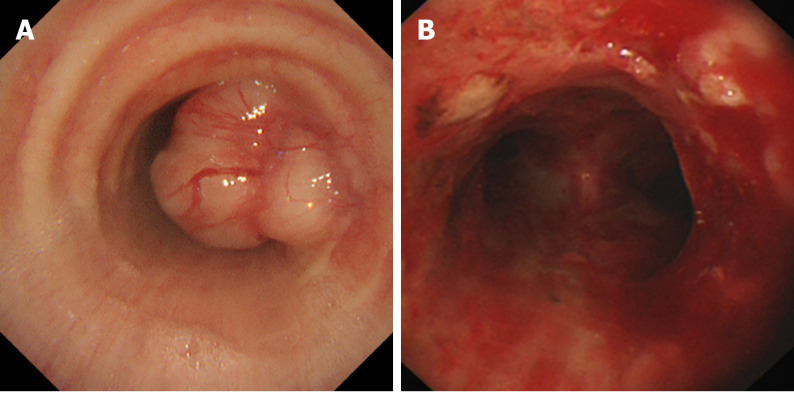
Pneumonia was detected from the chest X-ray, with no other abnormalities. A computed tomographic (CT) scan of the chest showed a sign of pulmonary infection, and computed tomographic virtual bronchoscopy (CTVB) showed a well-defined, soft-tissue density lesion measuring 2.4 cm × 2.1 cm in the lower trachea, located 2 cm above the carina (Figure 1). Fiberoptic bronchoscopy revealed that the surface of the mass was smooth and vasodilatory, and nearly 90% of the tracheal lumen was obstructed, so the bronchoscope failed to pass through (Figure 2).
Figure 1.
Computed tomographic presentation of the patient. A: Mediastinal computed tomographic scan of the chest showed a 2.4 cm × 2.1 cm homogenous well-defined, dense soft tissue lesion in the left lateral inner wall of the trachea (orange arrows); B: Computed tomographic scan with multiplanar reconstruction showed a round lesion in the lower trachea (black arrow); C: A tumor in the inner trachea observed by computed tomographic virtual bronchoscopy (blue arrow).
Figure 2.
Bronchoscopic findings. A: A polypoid and vasodilatory mass originated from the right side of the lower trachea; B: After endoscopic resection, the tumor was removed almost completely, and the airway patency was restored.
Pathological examination
Histopathological analysis revealed that the tumor was composed of epithelial and myxoid mesenchymal elements and was characterized by the presence of ductal structures that appeared to contain double-layered cells in a mucoid or hyaline stroma. Notably, there was no sign of necrosis or mitosis (Figure 3). Immunohistochemically, the tumor cells did not express thyroid transcription factor-1 and cytokeratin 7 (CK 7), but were positive for CK, CK 5/6, p63, and the S-100 protein, with low expression of Ki-67 (10%). Moreover, the basement membrane was immunoreactive for AB/ para-aminosailcylic acid. After immunohistochemical staining, the definite diagnosis was determined to be PA of the trachea.
Figure 3.
Pathological presentation of the patient. The tumor was composed of epithelial and myxoid mesenchymal elements and characterized by the presence of ductal structures that appeared to contain double-layered cells in a mucoid or hyaline stroma. No signs of necrosis or mitosis were observed (hematoxylin-eosin staining, × 100).
FINAL DIAGNOSIS
The patient was finally diagnosed with PA of the trachea.
TREATMENT
Considering that the patient's vital signs were stable, intratracheal tumor resection was performed by electron bronchoscopy under conscious sedation induced using intravenous midazolam. Finally, tumor tissues were excised with an electro-surgical snare and cryotherapy. Then, the edges and base of the mucosal defect were treated with argon plasma coagulation (APC) to enhance tumor clearance. There was no significant bleeding or perforation from the wound (Figure 2). After resection, the tracheal lumen was completely unobstructed, and there were no new organisms.
OUTCOME AND FOLLOW-UP
The patient's wheezing symptoms were remarkably relieved after the operation, but cough and expectoration remained. Regarding the sign of pulmonary infection from the chest CT, the patient was discharged 9 d after anti-infection treatment and remained asymptomatic at the 3-mo follow-up.
DISCUSSION
PA originating from the trachea is rare. According to Fitchett et al[11], it accounts for 1% of lung carcinomas and between 2% to 9% of all cases of PA. This type of PA consists of myoepithelial cells mixed with neoplastic ducts and stroma. The demographics and presenting characteristics of the 29 cases are shown in Table 1. Likewise, the major clinical features of the patients are listed in Table 2. According to the review, no gender predominance was found. The age of the patients ranged from 8 to 83 years, with a median age of 48 years, and there were four minors. More than half of these tumors were located in the lower or upper trachea; however, two cases originated from the airway and grew outward into the thyroid or mediastinum. Although a few patients presented with hemoptysis, the most common symptoms were cough, stridor, dyspnea, and wheezing, depending on the site and degree of airway obstruction. The patient in this case had a 2-year history of dyspnea upon exertion and chronic cough with wheezing before being properly diagnosed with PA of the trachea. The median clinical course was 5.5 mo, and the longest course was 10 years, which may reflect the benign nature of the tumor. In addition, it results in low recurrence rates at follow-ups.
Table 1.
Summary of presenting characteristics of tracheobronchial pleomorphic adenoma reported in the English medical literature
|
Ref.
|
Age
|
Sex
|
Clinical presentation
|
Course (mo)
|
Tumor site
|
Tumor size (cm)
|
Immunohistochemical staining
|
Treatment
|
Comorbidities
|
Complications
|
Clinical follow-up period (mo)
|
| Heifetz et al[18], 1992 | 15 | M | Asthma, wheezing, and dyspnea | 12 | Upper trachea (level of the fourth ring) | 2.5 × 2.5 × 2.5 | +: CK AE1/3, S-100, actin, vimentin, EMA, GFAP | CO2 laser bronchoscopy | No | No | Alive with no evidence of recurrence (6) |
| Basaklar et al[19], 1994 | 11 | F | Nonproductive harsh cough, high fever, nausea, vomiting, and night sweats | 1.5 | Right upper lobe bronchus | 2 | Not available | Surgical resection | Atelectasis, multiple mediastinal and peribronchial lymphadenopathies | No | Not available |
| Sweeney et al[20], 1996 | 27 | M | Incidental (asymptomatic) | Not available | Right lower lobe bronchus | 3 × 5 | +: CK, EMA, S 100, SMA | A lower lobectomy | No | No | Not available |
| Paik et al[21], 1996 | 50 | M | Mild dyspnea upon exertion | 3 | Mid trachea (4 cm above the carina) | 2 × 2 | Not available | Right thoracotomy with segmental resection and end-to-end anastomosis | No | No | Alive with no evidence of recurrence (18 d) |
| Bizal et al[22], 1997 | 27 | M | Dyspnea upon exertion and intermittent wheezing | 12 | Lower trachea (2 cm above the carina) | 2.5 | Not available | Surgical resection and primary anastonosis performed through right thoracotomy | No | No | Alive with no evidence of recurrence (6) |
| Paik et al[23], 1997 | 48 | F | Dyspnea upon exertion and productive cough with wheezing | 3 | Lower trachea | 1.5 × 1.2 | +: Vimentin, CK, S-100, GFAP, SMA | Tracheal wedge resection | No | No | Not available |
| Pomp et al[24], 1998 | 79 | F | Increasing stridor, dyspnea and a dry cough | 2 | Upper trachea (level of fifth ring) | 2 | Not available | Radiotherapy, excision through rigid bronchoscopy | No | Recurrent PA of the trachea | Not available |
| Pomp et al[24], 1998 | 58 | F | Increasing dyspnea and stridor | 6 | Upper trachea (below the larynx) | 90% occlusion | Not available | Excision via tracheotomy | No | No | Alive with no evidence of recurrence (12) |
| Kim et al[25], 2000 | 15 | M | Asthma, dyspnea and stridor | 5 | Upper trachea | 1.5 | Not available | Segmental tracheal resection and end-to-end anastomosis | No | No | Alive with no evidence of recurrence (12) |
| Baghai-Wadji et al[7], 2006 | 8 | M | Asthma, fever, productive cough, severe wheezing, and respiratory distress | 10 d | Lower trachea | 90% occlusion | +: Chromogranin, NSE, CK | Surgical resection and tracheal reconstruction (pericardial patch graft) | Pneumonia | No | Alive with no evidence of recurrence (6) |
| Aribas et al[8], 2007 | 42 | F | Asthma, severe dyspnea | 2 yr | Lower trachea | 2 × 2 | +: Vimentin, GFAP, S-100 | Segmental tracheal resection and end-to-end anastomosis | No | Tracheal stenosis | Alive with no evidence of recurrence (5 yr) |
| Ashwaq et al[26], 2007 | 37 | M | Spontaneous hemoptysis | 8 | Mid trachea | 2 × 2 | Not available | Excision with cold instrument via suspension laryngoscopy | No | No | Alive with no evidence of recurrence (3) |
| Matsubara et al[27], 2008 | 71 | M | Incidental (asymptomatic) | Not available | Left main bronchus | Not available | +: polyclonal anti-S-100, anti-GFAP | Endoscopic resection with electrosurgical snaring and APC | No | No | Alive with no evidence of recurrence (6) |
| Fitchett et al[11], 2008 | 65 | M | Hoarse barking cough | 5 | Right main bronchus | 1.3 | Not available | Endoscopic resection with diathermy snare | No | No | Not available |
| Kamiyoshihara et al[28], 2009 | 34 | F | Dyspnea upon exertion | 3 | Left main bronchus | 1.2 × 1.1 | Not available | Surgical resection with wedge bronchiectomy | No | No | Alive with no evidence of recurrence (11) |
| Tanaka et al[13], 2010 | 57 | F | A neck mass | 10 yr | Right lobe of the thyroid (originating from the trachea) | 3.25 × 2.09 | +: SMA, 34bE12; -: P53 and ki67 | Surgical resection and direct anastomosis | No | No | Not available |
| Kajikawa et al[9], 2010 | 55 | M | Asthma, dyspnea with wheezing | 2 yr | Lower trachea | Not available | Not available | Endoscopic resection with APC, electrocautery and rigid bronchoscopic coring | No | No | Alive with no evidence of recurrence (7) |
| Lin et al[29], 2011 | 36 | F | Bronchial asthma, worsening shortness of breath | 6 | Lower trachea(3 cm above the carina) | 2 × 2 × 2 | Not available | Segmental tracheal resection and anastomosis | Allergic rhinitis | No | Not available |
| Goto et al[30], 2011 | 71 | M | Progressive dyspnea | Not available | Left main bronchus | 2.5 × 2 | +: CK AE1/3, SMA | Endoscopic resection with electrosurgical snaring | Chronic obstructive pulmonary disease, squamous cell; carcinoma (pT2N0M0, stage IB) | No | Alive with no evidence of recurrence (2) |
| Solak et al[15], 2012 | 46 | F | Severe dyspnea | 12 | Upper trachea | 3 × 2 | Not available | Collar incision with partial sternotomy and end-to-end anastomosis | No | No | Alive with no evidence of recurrence (1) |
| Park et al[16], 2013 | 59 | M | Dyspnea upon exertion | 3 | Mid trachea | 2 × 2 | +: CK, CK 19, EMA, S100, p63 | Right thoracotomy with segmental resection and end-to-end anastomosis | Active pulmonary tuberculosis | No | Alive with no evidence of recurrence (5 yr) |
| Lee et al[31], 2014 | 54 | F | Blunt chest pain upon bending forward | 2 wk | Posterior mediastinum (originating from the left main bronchus) | 6.0 × 4.5 × 2.5 | +: P63 and SMA | Video-assisted thoracic surgery | No | No | Alive with no evidence of recurrence (2 yr) |
| Casillas-Enríquez et al[32], 2014 | 33 | F | Productive cough, wheezing, and occasional hemoptysis | 4 yr | Upper trachea | 80% occlusion | Not available | Endoscopic resection with APC | No | No | Alive with no evidence of recurrence (8) |
| Sim et al[33], 2014 | 32 | F | Dyspnea upon exertion and chronic cough with wheezing | 8 | Lower trachea | 1.8 × 1.6 | Not available | Endoscopic resection with rigid forceps and APC | Situs inversus | No | Alive with no evidence of recurrence (1) |
| Zhu et al[3], 2018 | 38 | F | Progressive shortness of breath | 5 yr | Right main bronchus | 1.42 × 0.96 | Not available | Endoscopic resection with electrosurgical snare and APC | No | No | Alive with no evidence of recurrence (3) |
| Kim et al[4], 2018 | 49 | M | Exacerbation of dyspnea upon exertion, cough and sputum | 3 | Lower trachea | 1.5 × 1.3 × 1.3 | +: CK 5/6, CK, p53 | Right thoracotomy with segmental resection and anastomosis with tracheobronchoplasty | Active pulmonary tuberculoma | No | Alive with no evidence of recurrence (3) |
| David et al[5], 2020 | 83 | F | Worsening shortness of breath and waking up with blood in her oropharynx | 1 | Upper trachea (3.0 cm below the vocal fold edge) | 1.6 × 1.3 | +: P63, SMA; -: Chromogranin, synaptophysin | Endoscopic excision with fiber-based CO2 laser and rigid bronchoscope | Hypertension, rheumatoid arthritis | No | Not available |
| Takahashi et al[6], 2019 | 51 | F | Asthma, cough and wheezing at night | 2 | Upper trachea (periphery 30 mm from the glottis) | 1.5 | Not available | Endoscopic resection with electrosurgical snaring and forceps | No | No | Alive with no evidence of recurrence (30) |
| Our case | 49 | F | Dyspnea upon exertion and chronic cough with wheezing | 2 yr | Lower trachea | 2.4 × 2.1 | + :CK, CK 5/6, p63, S-100, Ki-67 (10%); - :TTF-1, CK 7 | Endoscopic resection electrosurgical snare, cryotherapy and APC | No | No | Alive with no evidence of recurrence (3) |
CK: Cytokeratin; EMA: Epithelial membrane antigen; GFAP: Glial fibrillary acidic protein; SMA: Smooth muscle actin; NSE: Neuron-specific enolase; APC: Argon plasma coagulation; TTF-1: Thyroid transcription factor-1; M: Male; F: Female; CK 7: Cytokeratin 7.
Table 2.
Outline of major features characterizing presentation of 29 cases of tracheobronchial pleomorphic adenoma
|
Variable
|
n
(%) or median (IQR)
|
| Sex | |
| Female | 16 (55.17) |
| Male | 13 (44.83) |
| Age, yr | |
| Median (range) | 48 (8-83) |
| Symptoms | |
| Asymptomatic | 2 (6.90) |
| Respiratory symptoms (wheezing, dyspnea, cough, stridor, hemoptysis) | 24 (82.76) |
| Fever | 2 (6.90) |
| Gastrointestinal symptoms (vomiting, diarrhea) | 1 (3.45) |
| Night sweats | 1 (3.45) |
| Chest pain | 1 (3.45) |
| Neck mass | 1 (3.45) |
| Clinical course | |
| Median (range) | 5.5 m (10 d-10 y) |
| Location | |
| Upper trachea | 8 (27.59) |
| Mid trachea | 3 (10.34) |
| Lower trachea | 9 (31.03) |
| Bronchus | 7 (24.14) |
| Thyroid | 1 (3.45) |
| Posterior mediastinum | 1 (3.45) |
| Size (largest diameter), cm | |
| Median (range) | 2 (1.2-6) |
| Recurrence | 1 (3.45) |
IQR: Interquartile range.
Tracheal tumors are difficult to identify in chest radiographs. Moreover, patients initially present with non-alarming symptoms mimicking asthma[11]. The patient in this case was previously misdiagnosed with asthma and treated with inhaled glucocorticoids for 2 mo. Therefore, chest CT and bronchoscopy play a critical role in making early and proper diagnoses. CTVB involves the three-dimensional reconstruction of high-resolution helical CT images of the tracheobronchial tree, which can facilitate the analysis of bronchial lesions beyond the limits of bronchoscopy and the assessment of airway patency distal to high-grade obstructions[12]. However, CTVB cannot be used to identify the nature of a lesion, while bronchoscopy can be used to complete this by biopsy.
Histologically, PA is also known as a “mixed tumor”, which describes its pleomorphic appearance rather than its dual origin from epithelial and mesenchymal components. The stroma may be mucoid, myxoid, cartilaginous, or hyaline. Approximately 6% of tumors have the potential to transform into carcinoma ex pleomorphic adenoma[10]. When it presents with atypical cells, an abnormal chromatin pattern, and necrosis, the diagnosis of carcinoma ex pleomorphic adenoma is made. Regarding immunohistochemistry findings, the tumor shows positive staining for creatine kinase, p63, S-100 protein, epithelial membrane antigen, and glial fibrillary acidic protein. S-100 protein and glial fibrillary acidic protein may be helpful markers in differentiating PA and adenoid cystic carcinoma[13]. In addition, the patient in our study had a Ki-67 index of 10%. This marker is widely known as a proliferative marker, and numerous studies have shown a positive correlation between Ki-67 expression and the proliferative cell fraction in tumors[14].
Given the rarity of tracheal PA, no standards for management have been established, but it is clear that the main goal is to remove the lesion and restore airway patency. Surgical resection and airway anastomosis have traditionally been applied in many studies[4,15,16]. Compared with surgery, endoscopic resection is less traumatic and allows a faster recovery after the operation. Endobronchial intervention using a rigid and flexible bronchoscope is widely performed in cases of airway stenosis. In our case, we successfully applied bronchoscopic interventional therapy to remove the tumor, such as electrosurgical snare, cryotherapy and argon plasma coagulation. Due to its rarity, its biological behavior and clinical course have not been well described. One case of tracheal PA was reported to be recurrent in 2020 after surgical resection and end-to-end anastomosis were performed 10 years previously[17]. Therefore, long-term follow-ups are essential for patients. According to the medical literature, there is no clearly recommended follow-up period or interval, of which the longest follow-up period is 5 years without recurrence[8]. We will follow this patient by periodic chest CT and flexible bronchoscopy at least 10 years after the tumor resection.
CONCLUSION
Overall, we summarize the clinical presentation, clinical course, treatment, and prognosis of tracheobronchial PA according to the literature over the last 30 years[18-33].PA of the trachea is extremely rare, and patients initially present with non-specific symptoms mimicking asthma. Chest CT and bronchoscopy play a critical role in making an early diagnosis, whereas a definite diagnosis is made on the basis of histopathological and immunohistochemistry features. Although surgical resection is traditionally performed, this article supports the notion that bronchoscopic interventions for PA of the trachea are viable treatment options.
Footnotes
Informed consent statement: The patient and her legal guardian provided informed written consent during the treatment.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest to disclose.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: April 22, 2020
First decision: September 29, 2020
Article in press: November 2, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Handra-Luca A S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Xing YX
Contributor Information
Qian-Nuan Liao, Department of Pulmonary and Critical Care Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China.
Ze-Kui Fang, Department of Pulmonary and Critical Care Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China.
Shu-Bing Chen, Department of Pulmonary and Critical Care Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China.
Hui-Zhen Fan, Department of Pulmonary and Critical Care Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China.
Li-Chang Chen, Department of Pulmonary and Critical Care Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China.
Xi-Ping Wu, Department of Pulmonary and Critical Care Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China.
Xi He, Department of Pulmonary and Critical Care Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China.
Hua-Peng Yu, Department of Pulmonary and Critical Care Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, Guangdong Province, China. huapengyu@aliyun.com.
References
- 1.Gaissert HA, Mark EJ. Tracheobronchial gland tumors. Cancer Control. 2006;13:286–294. doi: 10.1177/107327480601300406. [DOI] [PubMed] [Google Scholar]
- 2.Kuo YL, Tu TY, Chang CF, Li WY, Chang SY, Shiao AS, Chu PY, Chan KT, Tai SK, Wang YF, Kao SC, Kao SY, Lo WL, Wu CH, Shu WH, Ma S, Wang TH. Extra-major salivary gland pleomorphic adenoma of the head and neck: a 10-year experience and review of the literature. Eur Arch Otorhinolaryngol. 2011;268:1035–1040. doi: 10.1007/s00405-010-1437-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Z, Lian X, Yang D. Right main bronchial pleomorphic adenoma: A case report and literature review. Medicine (Baltimore) 2018;97:e12648. doi: 10.1097/MD.0000000000012648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Oak CH, Jang TW, Jung MH. Tracheal pleomorphic adenoma with coexisting pulmonary tuberculoma. Yeungnam Univ J Med. 2018;35:114–120. doi: 10.12701/yujm.2018.35.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David AP, Bakos SR, Daniero JJ. Pleomorphic Adenoma of the Trachea. Ear Nose Throat J. 2020;99:235–236. doi: 10.1177/0145561319840189. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi M, Yorozuya T, Miyasaka Y, Kodama K, Yoshikawa T, Taya T, Mori Y, Ikeda K, Miyajima S, Chiba H, Takahashi H. A case of tracheal pleomorphic adenoma misdiagnosed as asthma. Oxf Med Case Reports. 2019;2019:omz111. doi: 10.1093/omcr/omz111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baghai-Wadji M, Sianati M, Nikpour H, Koochekpour S. Pleomorphic adenoma of the trachea in an 8-year-old boy: a case report. J Pediatr Surg. 2006;41:e23–e26. doi: 10.1016/j.jpedsurg.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Aribas OK, Kanat F, Avunduk MC. Pleomorphic adenoma of the trachea mimicking bronchial asthma: report of a case. Surg Today. 2007;37:493–495. doi: 10.1007/s00595-006-3441-0. [DOI] [PubMed] [Google Scholar]
- 9.Kajikawa S, Oki M, Saka H, Moritani S. Pleomorphic adenoma of the trachea. Respiration. 2010;80:433–434. doi: 10.1159/000308462. [DOI] [PubMed] [Google Scholar]
- 10.Gao HX, Li Q, Chang WL, Zhang YL, Wang XZ, Zou XX. Carcinoma ex pleomorphic adenoma of the trachea: A case report. World J Clin Cases. 2019;7:2623–2629. doi: 10.12998/wjcc.v7.i17.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitchett J, Luckraz H, Gibbs A, O'Keefe P. A rare case of primary pleomorphic adenoma in main bronchus. Ann Thorac Surg. 2008;86:1025–1026. doi: 10.1016/j.athoracsur.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein SE, Schrump DS, Nguyen DM, Hewitt SM, Kunst TF, Summers RM. Comparative evaluation of super high-resolution CT scan and virtual bronchoscopy for the detection of tracheobronchial malignancies. Chest. 2003;124:1834–1840. doi: 10.1378/chest.124.5.1834. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Shibata T, Suzuki K. Pleomorphic adenoma originating from the trachea showing the appearance of a follicular tumor of the thyroid on ultrasonography. J Med Ultrason (2001) 2010;37:27–30. doi: 10.1007/s10396-009-0245-z. [DOI] [PubMed] [Google Scholar]
- 14.Díaz KP, Gondak R, Martins LL, de Almeida OP, León JE, Mariano FV, Altemani A, Vargas PA. Fatty acid synthase and Ki-67 immunoexpression can be useful for the identification of malignant component in carcinoma ex-pleomorphic adenoma. J Oral Pathol Med. 2019;48:232–238. doi: 10.1111/jop.12820. [DOI] [PubMed] [Google Scholar]
- 15.Solak O, Ocalan K, Unlu M, Aycicek A, Aktepe F, Sivaci R. Pleomorphic adenoma of the trachea. Gen Thorac Cardiovasc Surg. 2012;60:843–846. doi: 10.1007/s11748-012-0081-8. [DOI] [PubMed] [Google Scholar]
- 16.Park KS, Sung WJ. Pleomorphic adenoma of the trachea: a case report. Korean J Pathol. 2013;47:399–401. doi: 10.4132/KoreanJPathol.2013.47.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karamustafaoglu YA, Yanık F, Yoruk Y. Palliative treatment of recurrent tracheal pleomorphic adenoma 10 years after segmental resection using the endobronchial shaver. Clin Respir J. 2020;14:495–497. doi: 10.1111/crj.13149. [DOI] [PubMed] [Google Scholar]
- 18.Heifetz SA, Collins B, Matt BH. Pleomorphic adenoma (benign mixed tumor) of the trachea. Pediatr Pathol. 1992;12:563–574. doi: 10.3109/15513819209024207. [DOI] [PubMed] [Google Scholar]
- 19.Başaklar AC, Sönmez K, Memiş L, Kale N. Pleomorphic adenoma of the main bronchus. J Pediatr Surg. 1994;29:1550–1552. doi: 10.1016/0022-3468(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney EC, McDermott M. Pleomorphic adenoma of the bronchus. J Clin Pathol. 1996;49:87–89. doi: 10.1136/jcp.49.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paik HC, Lim SH, Lee DY, Paik SY. Pleomorphic adenoma of the trachea--a case report. Yonsei Med J. 1996;37:81–85. doi: 10.3349/ymj.1996.37.1.81. [DOI] [PubMed] [Google Scholar]
- 22.Bizal JC, Righi PD, Kesler KA. Pleomorphic adenoma of the trachea. Otolaryngol Head Neck Surg. 1997;116:139–140. doi: 10.1016/S0194-59989770369-3. [DOI] [PubMed] [Google Scholar]
- 23.Paik SS, Jin YH, Park CK, Shin DH, Chung WS, Lee JD. Pleomorphic adenoma of the trachea. J Korean Med Sci. 1997;12:564–566. doi: 10.3346/jkms.1997.12.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomp J, Pannekoek BJ, Overdiep SH. Pleomorphic adenoma and severe tracheal obstruction. Respir Med. 1998;92:889–891. doi: 10.1016/s0954-6111(98)90398-5. [DOI] [PubMed] [Google Scholar]
- 25.Kim KH, Sung MW, Kim JW, Koo JW. Pleomorphic adenoma of the trachea. Otolaryngol Head Neck Surg. 2000;123:147–148. doi: 10.1067/mhn.2000.102809. [DOI] [PubMed] [Google Scholar]
- 26.Ashwaq AM, Sani A. Pleomorphic adenoma of the trachea. Med J Malaysia. 2007;62:162–163. [PubMed] [Google Scholar]
- 27.Matsubara M, Yasuo M, Tanabe T, Tsushima K, Urushihata K, Yamamoto H, Hanaoka M, Koizumi T, Fujimoto K, Kubo K, Yamazaki Y, Uehara T. Pleomorphic adenoma with an endobronchial resection. Intern Med. 2008;47:1117–1120. doi: 10.2169/internalmedicine.47.0853. [DOI] [PubMed] [Google Scholar]
- 28.Kamiyoshihara M, Ibe T, Takeyoshi I. Pleomorphic adenoma of the main bronchus in an adult treated using a wedge bronchiectomy. Gen Thorac Cardiovasc Surg. 2009;57:43–45. doi: 10.1007/s11748-008-0319-7. [DOI] [PubMed] [Google Scholar]
- 29.Lin CH, Lin MW, Chen JS, Yu CJ. Shortness of breath while lying down: a woman with orthopneic asthma. CMAJ. 2011;183:77–79. doi: 10.1503/cmaj.081801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goto T, Maeshima A, Akanabe K, Hamaguchi R, Wakaki M, Oyamada Y, Kato R. Bronchial pleomorphic adenoma coexisting with lung cancer. Ann Thorac Cardiovasc Surg. 2011;17:174–177. doi: 10.5761/atcs.cr.09.01516. [DOI] [PubMed] [Google Scholar]
- 31.Lee YK, Kim YH, Kim GY, Youn HC. Pleomorphic adenoma presenting with a mediastinal mass. Thorac Cancer. 2014;5:89–92. doi: 10.1111/1759-7714.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casillas-Enríquez JD, Álvarez-Maldonado P, Salguero-Cruz L, Navarro-Reynoso F, Cicero-Sabido R, Núñez-Pérez Redondo C. Pleomorphic adenoma of the trachea: A case report. J Bronchology Interv Pulmonol. 2014;21:51–53. doi: 10.1097/LBR.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 33.Sim DW, Oh IJ, Kim KS, Choi YD, Kwon YS. Pleomorphic adenoma of the trachea. J Bronchology Interv Pulmonol. 2014;21:230–233. doi: 10.1097/LBR.0000000000000076. [DOI] [PubMed] [Google Scholar]