Abstract
Background & aims
Iron is an essential trace element to almost all organism, and the delicate balance between host defend system and viral proliferation plays an important role in infective conditions. While the association of the iron metabolism with the prognosis of COVID-19 remains poorly understood. We aimed to estimate the associations of systemic iron metabolism parameters with the severity and risks of adverse outcomes in COVID-19.
Methods
In this retrospective cohort study, we included 158 confirmed COVID-19 patients in Tongji Hospital, Wuhan, China (27 January to 5 April, 2020). Demographic data, comorbidities, laboratory examinations, treatments, and clinical outcomes were all collected. Multivariable Poisson regression was used to estimate the association of iron parameter levels with the severity and risks of adverse outcomes in COVID-19 patients.
Results
We identified 60 (38%) severe cases in 158 COVID-19 patients. The median age was 63 years (interquartile range [IQR]: 54–73) and the median length of hospital stay was 28 days (IQR: 17–40). After adjusting for age, sex, IL-6, and pre-existing comorbidities, all iron parameters were associated with the severity of COVID-19 with adjusted risk ratio of 0.42 [95% CI: 0.22–0.83], 4.38 [95% CI: 1.86–10.33], 0.19 [95% CI: 0.08–0.48], and 0.25 [95% CI: 0.10–0.58] for serum iron, ferritin, transferrin, and total iron-binding capacity, respectively. These iron indices were also related to the risk of ARDS, coagulopathy, acute cardiac injury, acute liver injury, and acute kidney injury in COVID-19 patients and high cytokine concentrations.
Conclusions
Patients with low serum iron status likely suffered from severe condition and multiple–organ injury in COVID-19. The iron metabolism parameters might be risk factors and clinical biomarkers for COVID-19 prognosis.
Keywords: COVID-19, Iron, Cytokine storm, Interleukin-6, ARDS, Multiple–organ injury
Abbreviations
- ACI
acute cardiac injury
- AKI
acute kidney injury
- ALI
acute liver injury
- ALT
alanine transaminase
- ARDS
acute respiratory distress syndrome
- aRR
adjusted relative risk
- AST
aspartate transaminase
- CI
confidence interval
- COVID-19
Coronavirus disease 19
- DIC
disseminated intravascular coagulation
- ICU
intensive care units
- IL-2R
interleukin-2 receptor
- IL-6
interleukin-6
- IL-8
interleukin-8
- IMV
invasive mechanical ventilation
- IQR
interquartile range
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- sTfR
soluble transferrin receptor
- TIBC
total iron-binding capacity
- TNF-α
tumor necrosis factor-α
- TSAT
transferrin saturation
- UIBC
unsaturated iron-binding capacity
- ULN
upper limit of normal
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a pandemic [1]. As of September 14, 2020, more than 29 million cases and 920,000 deaths are confirmed over 200 countries, which causes a burden to the global medical system. Acute respiratory distress syndrome (ARDS) and multiorgan failure are major causes of mortality in COVID-19 patients [2], and the host excessive inflammation response or “cytokine storm” is part of the reason for ARDS and multiorgan failure [3,4].
Iron is required for various fundamental biological processes ranging from DNA synthesis to ATP generation in human and pathogens [5]. After viral invasion, the rapid proliferation puts iron in the center of a competition between host cells and virus, indicating that iron status may affect viral replication and damage to host cells [6]. As a β-coronavirus, SARS-CoV-2 attacks host cells through angiotensin-converting enzyme 2 and causes damages to almost all tissue and organs, and the immune system plays an important role in the viral pathogenic mechanism [7]. Iron homeostasis is crucial for the host immune defense system, because the property of gaining and losing electrons results in the generation of superoxide anions, and its content in macrophage regulates the production of pro-inflammatory cytokines [8]. The association of iron status with COVID-19 prognosis has been preliminarily explored. A previous study in SARS found that serum iron level decreased in confirmed patients [9], and studies on COVID-19 found that low serum iron level was an independent risk factor for the severity of hypoxemic respiratory failure and death in COVID-19 patients [10,11]. High concentration of serum ferritin was also found to be associated with poor outcomes in COVID-19 patients [12]. However, recent studies were descriptive case series or had a relatively small sample size. In the present study, we aimed to depict the systemic iron status in COVID-19 patients and assess the association between the iron metabolism parameters and poor prognosis of COVID-19.
2. Methods
2.1. Patients
This retrospective cohort study was conducted in Tongji Hospital, which was one of the designated centers for COVID-19 treatment in Wuhan, China. In this study, we included 169 patients with iron metabolism indices. After excluding patients who were pregnant, younger than 20 years, and possessed acute lethal organ injury on admission or hematological diseases, 158 patients were included in the final analysis. All patients were confirmed of SARS-CoV-2 infection according to the guidance of the National Health Commission of China and the World Health Organization [13,14]. All enrolled patients were followed up until death or discharge. The informed consent was waived because of the urgent need to collect data on the newly emerged pathogen. This study was approved by the Ethics Committees of the Tongji Medical College, Huazhong University of Science and Technology.
2.2. Data collection
Demographic data, medical records, laboratory findings, arterial blood gas analysis, in-hospital treatments, and clinical outcomes were collected from the hospital electronic medical record system. Detailed demographic information, symptoms, and comorbidities of all patients were recorded or diagnosed on admission. Laboratory findings primarily included complete blood count, lymphocyte subsets, inflammatory factors, cytokines, and biomarkers of cardiac, liver, and kidney injury. According to patients’ condition, these laboratory indices were tested at least once during the follow-up.
2.3. Laboratory procedure
The serum levels of iron, ferritin, transferrin, soluble transferrin receptor (sTfR), and unsaturated iron-binding capacity (UIBC) were tested by a fully automated analyzer (Roche/Hitachi Cobas c 701/702, Roche Diagnostics GmbH) using validated laboratory methods at admission. Meanwhile, transferrin saturation (TSAT) and total iron-binding capacity (TIBC) were calculated simultaneously by the analyzer. All kits needed in the tests were purchased from Roche Diagnostics.
2.4. Outcomes
The severity of the disease was defined at admission according to the Chinese management guideline for COVID-19 (version 7.0) [14]. In our study, adverse outcomes included ARDS, acute cardiac injury (ACI), acute liver injury (ALI), acute kidney injury (AKI), and coagulopathy. We also described disseminated intravascular coagulation (DIC), septic shock, and death. ARDS was diagnosed according to the Berlin Definition [15]. Coagulopathy was confirmed if prothrombin time was extended by 3 s or activated partial thromboplastin time was extended by 5 s [16]. ACI was identified if the serum level of high-sensitivity cardiac troponin I (hs-cTnI) was above the upper limit of normal (ULN) [17]. ALI was confirmed if serum level of alanine transaminase (ALT) or aspartate aminotransferase (AST) was threefold higher than the ULN, or alkaline phosphatase (ALP), γ-glutamyl transpeptidase, or total bilirubin were two times higher than the ULN [18]. AKI was defined when the increment of the serum creatinine level was over 0.3 mg/dL (26.5 mmol/L) within 48 h [19]. DIC was assessed by a scoring algorithm using platelet count, D-dimer, international normalized ratio of prothrombin time, and fibrinogen level. A score of ≥5 points was compatible with DIC [20]. Septic shock was identified according to the International Consensus Definitions for Sepsis-3 [21] and doctoral reports.
2.5. Statistical analysis
Given that most of the variables were skewed distribution, continuous variables were expressed as median (interquartile range [IQR]) and compared using non-parametric Mann–Whitney U-test. Categorical variables were expressed as numbers (percentage) and compared using chi-squared test or Fisher's exact test as appropriate. We calculated the adjusted mean of the iron metabolism parameters in patients with or without undesirable outcomes using the generalized linear model after adjusting for age, sex, IL-6 concentration, and pre-existing comorbidities (including hypertension, diabetes, cardiac disease, cerebrovascular disease, chronic pulmonary disease, chronic kidney disease, chronic liver disease, and tumor) to control the confounding factors of iron status. We categorized the iron indices into tertiles and calculated multivariable–adjusted relative risk (aRR) and 95% confidence interval (CI) for each outcome using the Poisson regression model. When IL-6 was included in the model, the missing values of it (n = 4) were imputed with median value. In addition, a multivariable-adjusted linear regression model was used to estimate the association of log-transformed iron indices with lymphocyte counts, cytokines, and organ injury biomarkers. All analyses were performed using R software (The R Foundation, http://www.r-project.org, version 4.0.0). A two-sided P < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics and laboratory indices in severe and non-severe patients
Baseline characteristics and laboratory indices by the illness severity of 158 confirmed COVID-19 patients were shown in Supplemental Table 1. Among the patients (38% severe cases [n = 60], 49% male [n = 77]), the median age was 63 years (IQR: 54–73) and the median length of hospital stay was 28 days (IQR: 17–40). Among all comorbidities, hypertension was the commonest (n = 61, 39%). Compared with non-severe patients, severe patients had higher levels of pro-inflammatory cytokines at baseline, including interleukin-6, interleukin-8, interleukin-2 receptor, and tumor necrosis factor-α. The levels of infection biomarkers such as high-sensitivity C-reactive protein and procalcitonin were higher in severe patients. As for lymphocytes, we found that cell counts (including lymphocytes, total T cells, CD4+ T cells, CD8+ T cells, total B cells, and natural killer cells) at admission were lower in severe patients, but subset proportions had no significant difference in the two groups of patients. At the end of follow-up, five (3%) deaths occurred in severe patients. During hospitalization, the most common complication in all patients was ARDS (n = 64, 41%), followed by ACI (n = 44, 28%) and ALI (n = 43, 27%), then coagulopathy (n = 33, 21%), AKI (n = 22, 14%), DIC (n = 8, 5%), and septic shock (n = 5, 3%). The incidences of these undesirable outcomes were augmented in severe patients compared with non-severe patients (all P < 0.001).
3.2. Adjusted mean of the baseline serum iron metabolism parameters by outcomes
The correlation between iron metabolism parameters levels in our study was shown in Fig. 1 . We compared the iron metabolism indices of COVID-19 patients who suffered from different adverse outcomes with patients who did not progress to the relevant outcome to assess their iron status. After adjusting for sex, age, and comorbidities (including hypertension, diabetes, cardiac disease, cerebrovascular disease, chronic pulmonary disease, chronic kidney disease, chronic liver disease, and tumor), we found that the adjusted mean of the serum iron metabolism parameters at baseline differed significantly in patients with or without adverse outcomes (Table 1 ). In particular, severe patients had a higher serum ferritin level and lower serum levels of iron, transferrin, total iron-binding capacity, and unsaturated iron-binding capacity than those in non-severe condition. The iron metabolism parameters also differed in patients with or without multiple-organ injury. The serum level of iron was lower in patients who suffered from ARDS, coagulopathy, ACI, AKI, DIC, and septic shock during hospitalization, and ICU/IMV/death (P = 0.003 for AKI, P = 0.005 for septic shock, and P < 0.001 for others). Similarly, we found that serum concentration of transferrin and total iron-binding capacity value were lower in patients with adverse outcomes (all P < 0.05), and unsaturated iron-binding capacity value was lower in patients who suffered from ARDS, coagulopathy, ALI, DIC, and septic shock during follow-up (all P < 0.05). However, serum ferritin was higher in those who had ARDS, coagulopathy, ACI, ALI, DIC, and septic shock, and ICU/IMV/death (P = 0.016 for coagulation disorders and P < 0.001 for the rest). In addition, the serum level of TSAT was lower in patients with ACI, AKI, and DIC, whereas sTfR was not significantly different in all groups.
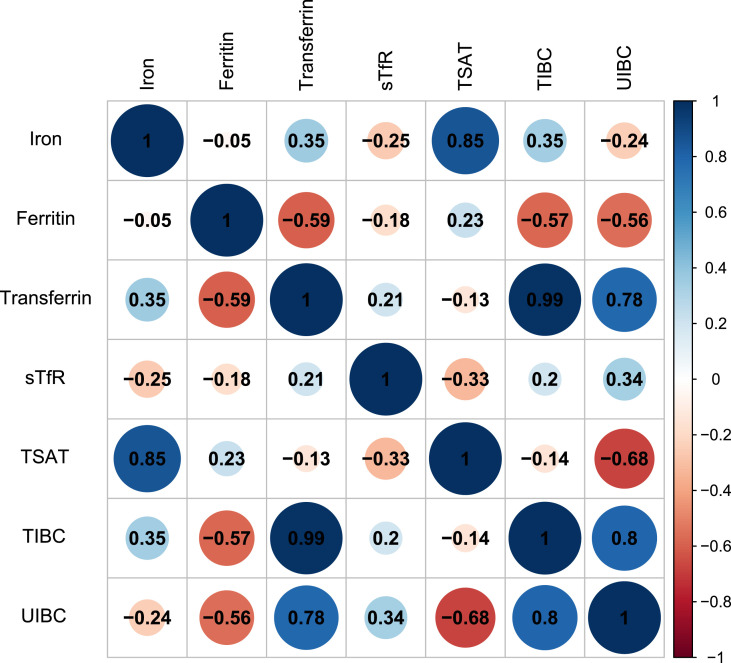
Fig. 1.
The correlation between iron metabolism indices among COVID-19 patients. Abbreviations: sTfR = soluble transferrin receptor; TIBC = total iron-binding capacity; TSAT = transferrin saturation; UIBC = unsaturated iron-binding capacity.
Table 1.
Multivariable-adjusted mean of baseline iron metabolism indices value in COVID-19 patients.
| Serum iron, μmol/L | Serum ferritin, ng/mL | Serum transferrin, g/L | Soluble transferrin receptor, ng/mL | Transferrin saturation, % | Total iron binding capacity, μmol/L | Unsaturated iron binding capacity, μmol/L | ||
|---|---|---|---|---|---|---|---|---|
| Severe | No (n = 98) | 15.4 (14.3, 16.6) | 328.1 (184.4, 471.7) | 2.0 (1.9, 2.1) | 3.2 (2.9, 3.5) | 36.6 (33.6, 39.6) | 43.1 (41.5, 44.6) | 27.6 (25.9, 29.3) |
| Yes (n = 60) | 11.3 (9.8, 12.9) | 1088.7 (900.7, 1276.7) | 1.6 (1.5, 1.7) | 2.9 (2.5, 3.4) | 33.4 (29.5, 37.3) | 34.6 (32.5, 36.6) | 23.2 (21.0, 25.4) | |
| P-value | <0.001 | <0.001 | <0.001 | 0.396 | 0.226 | <0.001 | 0.003 | |
| ARDS | No (n = 94) | 15.5 (14.3, 16.7) | 325.4 (177.3, 473.5) | 2.0 (1.9, 2.1) | 3.2 (2.9, 3.5) | 36.7 (33.7, 39.7) | 43.1 (41.5, 44.7) | 27.6 (25.9, 29.3) |
| Yes (n = 64) | 11.5 (10.0, 13.0) | 1045.1 (862.4, 1227.8) | 1.6 (1.5, 1.7) | 2.9 (2.5, 3.3) | 33.5 (29.7, 37.2) | 35.1 (33.1, 37.1) | 23.5 (21.4, 25.7) | |
| P-value | <0.001 | <0.001 | <0.001 | 0.3 | 0.21 | <0.001 | 0.006 | |
| Coagulopathy | No (n = 92) | 14.9 (13.8, 15.9) | 534.1 (397.7, 670.5) | 1.9 (1.8, 2.0) | 3.2 (2.9, 3.4) | 36.6 (34.0, 39.1) | 41.6 (40.2, 43.0) | 26.7 (25.2, 28.2) |
| Yes (n = 33) | 10.1 (8.0, 12.2) | 930.5 (651.7, 1209.3) | 1.5 (1.4, 1.6) | 2.8 (2.3, 3.4) | 30.8 (25.6, 36.1) | 33.2 (30.4, 36.1) | 23.1 (20.1, 26.1) | |
| P-value | <0.001 | 0.016 | <0.001 | 0.319 | 0.061 | <0.001 | 0.04 | |
| ACI | No (n = 114) | 15.5 (14.4, 16.6) | 427.6 (287.8, 567.5) | 1.9 (1.9, 2.0) | 3.0 (2.7, 3.3) | 37.5 (34.7, 40.2) | 42.0 (40.5, 43.5) | 26.5 (24.9, 28.1) |
| Yes (n = 44) | 9.7 (7.9, 11.5) | 1107.3 (867.5, 1347.1) | 1.6 (1.4, 1.7) | 3.3 (2.8, 3.8) | 30.0 (25.3, 34.7) | 34.2 (31.6, 36.8) | 24.5 (21.8, 27.2) | |
| P-value | <0.001 | <0.001 | <0.001 | 0.386 | 0.011 | <0.001 | 0.241 | |
| ALI | No (n = 115) | 14.2 (13.1, 15.3) | 463.4 (327.3, 599.4) | 1.9 (1.8, 2.0) | 3.2 (2.9, 3.5) | 35.4 (32.7, 38.1) | 41.1 (39.6, 42.6) | 26.9 (25.4, 28.4) |
| Yes (n = 43) | 13.0 (11.2, 14.8) | 1027.5 (801.4, 1253.7) | 1.6 (1.5, 1.8) | 2.8 (2.3, 3.3) | 35.4 (30.9, 39.8) | 36.4 (33.9, 38.9) | 23.4 (20.9, 25.9) | |
| P-value | 0.281 | <0.001 | 0.001 | 0.173 | 0.994 | 0.002 | 0.023 | |
| AKI | No (n = 136) | 14.6 (13.6, 15.7) | 612.0 (474.1, 750.0) | 1.9 (1.8, 1.9) | 3.0 (2.8, 3.3) | 36.7 (34.1, 39.2) | 40.8 (39.3, 42.3) | 26.1 (24.7, 27.6) |
| Yes (n = 22) | 9.2 (6.0, 12.3) | 647.2 (224.3, 1070.0) | 1.6 (1.3, 1.8) | 3.4 (2.6, 4.3) | 27.4 (19.6, 35.2) | 33.9 (29.4, 38.4) | 24.8 (20.2, 29.3) | |
| P-value | 0.003 | 0.884 | 0.012 | 0.442 | 0.037 | 0.008 | 0.594 | |
| DIC | No (n = 150) | 14.4 (13.5, 15.3) | 566.9 (446.9, 686.9) | 1.9 (1.8, 1.9) | 3.1 (2.8, 3.3) | 36.1 (33.8, 38.3) | 40.7 (39.4, 41.9) | 26.3 (25.0, 27.6) |
| Yes (n = 8) | 4.6 (0.6, 8.7) | 1554.8 (1014.6, 2095.0) | 1.1 (0.8, 1.3) | 3.2 (2.1, 4.3) | 22.5 (12.2, 32.7) | 24.4 (18.9, 30.0) | 19.8 (13.8, 25.7) | |
| P-value | <0.001 | <0.001 | <0.001 | 0.879 | 0.012 | <0.001 | 0.039 | |
| Septic shock | No (n = 153) | 14.1 (13.2, 15.1) | 576.6 (458.4, 694.8) | 1.9 (1.8, 1.9) | 3.1 (2.8, 3.3) | 35.6 (33.3, 37.9) | 40.3 (39.1, 41.6) | 26.2 (24.9, 27.5) |
| Yes (n = 5) | 6.3 (1.1, 11.6) | 1849.2 (1179.6, 2518.7) | 1.1 (0.7, 1.4) | 3.2 (1.8, 4.6) | 28.6 (15.6, 41.6) | 24.7 (17.5, 31.9) | 18.4 (11.0, 25.8) | |
| P-value | 0.005 | <0.001 | <0.001 | 0.846 | 0.301 | <0.001 | 0.044 | |
| ICU/IMV/Death | No (n = 142) | 14.5 (13.5, 15.4) | 480.9 (371.6, 590.1) | 1.9 (1.8, 1.9) | 3.1 (2.9, 3.4) | 36.2 (33.8, 38.5) | 40.7 (39.4, 42.1) | 26.3 (24.9, 27.6) |
| Yes (n = 16) | 8.6 (5.7, 11.5) | 1824.2 (1491.9, 2156.5) | 1.4 (1.2, 1.6) | 2.9 (2.1, 3.7) | 28.5 (21.4, 35.7) | 31.8 (27.8, 35.8) | 23.2 (19.0, 27.3) | |
| P-value | <0.001 | <0.001 | <0.001 | 0.586 | 0.051 | <0.001 | 0.17 |
Data were presented as adjusted mean (95% CI) and adjusted for age, sex, and pre-existing comorbidities (including hypertension, diabetes, cardiac disease, cerebrovascular disease, chronic pulmonary disease, chronic kidney disease, chronic liver disease, and tumor).
Abbreviations: ACI = acute liver injury; ALI = acute liver injury; AKI = acute kidney injury; ARDS = acute respiratory distress syndrome; COVID-19 = coronary disease 2019; DIC = disseminated intravascular coagulation; ICU = intensive care units; IMV = invasive mechanical ventilation.
3.3. Baseline serum levels of the iron metabolism parameters were associated with various adverse outcomes
In the multivariable Poisson regression model, the baseline serum levels of the iron metabolism parameters were significantly associated with the risk of various adverse outcomes (Table 2 ). Compared with the lowest tertile, patients with the highest tertile of serum iron level had lower risks of severe illness (aRR 0.42, 95% CI: 0.22–0.83), ARDS (aRR 0.44, 95% CI: 0.22–0.87), coagulopathy (aRR 0.27, 95% CI: 0.10–0.76), ACI (aRR 0.24, 95% CI: 0.09–0.63), and AKI (aRR 0.16, 95% CI: 0.04–0.63). No significant association was found between serum iron and ALI. Similarly, the serum levels of transferrin, TIBC, and UIBC were inversely associated with the risks of undesirable outcomes. By comparing the highest tertile with the lowest tertile of the iron parameters, the high level of transferrin was associated with low risks of severe illness (aRR 0.21, 95% CI: 0.08–0.52), ARDS (aRR 0.24, 95% CI: 0.10–0.56), and coagulation disorders (aRR 0.19, 95% CI: 0.04–0.85). The serum transferrin level was not associated with ACI, ALI and AKI. High TIBC value was related to low risk of severe illness (aRR 0.27, 95% CI: 0.11–0.64), ARDS (aRR 0.30, 95% CI: 0.13–0.68), coagulopathy (aRR 0.19, 95% CI: 0.04–0.89), and ACI (aRR 0.27, 95% CI: 0.09–0.81). No significant association was found for ALI and AKI. Compared with the lowest tertile, patients with the highest tertile of ferritin level had a higher risk of severe illness (aRR 4.02, 95% CI: 1.67–9.69), ARDS (aRR 3.13, 95% CI: 1.40–6.98), coagulation disorders (aRR 5.86, 95% CI: 1.27–26.97), ACI (aRR 2.91, 95% CI: 1.03–8.27), and ALI (aRR 3.61, 95% CI: 1.43–9.09), whereas no significant difference was found for AKI. Serum TSAT, sTfR and UIBC values were not associated with these adverse outcomes.
Table 2.
Multivariable-adjusted RR and 95% CI for adverse outcomes in COVID-19 patients.
| Levels of serum iron metabolism indices |
P-trend | Per SD increment | |||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||
| Serum iron, μmol/L | <10.7 | 10.7–15.8 | ≥15.8 | ||
| Severe | 1 | 0.42 (0.22, 0.84) | 0.44 (0.22, 0.87) | 0.01 | 0.71 (0.53, 0.94) |
| ARDS | 1 | 0.52 (0.28, 0.98) | 0.48 (0.25, 0.92) | 0.02 | 0.72 (0.55, 0.95) |
| Coagulopathy | 1 | 0.30 (0.11, 0.81) | 0.27 (0.10, 0.76) | 0.01 | 0.53 (0.34, 0.80) |
| ACI | 1 | 0.45 (0.21, 0.96) | 0.24 (0.09, 0.63) | 0.002 | 0.55 (0.39, 0.79) |
| ALI | 1 | 0.84 (0.38, 1.83) | 0.75 (0.35, 1.59) | 0.45 | 0.91 (0.67, 1.24) |
| AKI | 1 | 0.53 (0.18, 1.54) | 0.16 (0.04, 0.63) | 0.008 | 0.55 (0.34, 0.89) |
| Serum ferritin, ng/mL | <269.7 | 269.7–561.6 | ≥561.6 | ||
| Severe | 1 | 2.28 (0.93, 5.57) | 4.02 (1.67, 9.69) | 0.001 | 1.37 (1.16, 1.63) |
| ARDS | 1 | 1.86 (0.83, 4.21) | 3.13 (1.40, 6.98) | 0.004 | 1.34 (1.14, 1.59) |
| Coagulopathy | 1 | 2.84 (0.58, 13.77) | 5.86 (1.27, 26.97) | 0.01 | 1.21 (0.92, 1.58) |
| ACI | 1 | 2.34 (0.84, 6.51) | 2.91 (1.03, 8.27) | 0.07 | 1.36 (1.11, 1.66) |
| ALI | 1 | 1.33 (0.46, 3.83) | 3.61 (1.43, 9.09) | 0.002 | 1.33 (1.09, 1.63) |
| AKI | 1 | 1.20 (0.28, 5.10) | 1.73 (0.39, 7.66) | 0.41 | 1.02 (0.61, 1.69) |
| Serum transferrin, g/L | <1.6 | 1.6–2.0 | ≥2.0 | ||
| Severe | 1 | 0.50 (0.28, 0.91) | 0.21 (0.08, 0.52) | 0.0002 | 0.52 (0.38, 0.70) |
| ARDS | 1 | 0.56 (0.32, 1.00) | 0.24 (0.10, 0.56) | 0.0004 | 0.55 (0.41, 0.74) |
| Coagulopathy | 1 | 0.41 (0.17, 0.96) | 0.19 (0.04, 0.85) | 0.005 | 0.39 (0.25, 0.63) |
| ACI | 1 | 0.35 (0.16, 0.75) | 0.40 (0.16, 1.02) | 0.007 | 0.49 (0.34, 0.72) |
| ALI | 1 | 0.70 (0.33, 1.45) | 0.49 (0.20, 1.17) | 0.09 | 0.69 (0.49, 0.96) |
| AKI | 1 | 0.21 (0.06, 0.72) | 0.33 (0.08, 1.32) | 0.02 | 0.54 (0.32, 0.92) |
| Soluble transferrin receptor, ng/mL | <2.5 | 2.5–3.2 | ≥3.2 | ||
| Severe | 1 | 0.65 (0.34, 1.25) | 0.72 (0.38, 1.35) | 0.32 | 0.87 (0.63, 1.21) |
| ARDS | 1 | 0.69 (0.37, 1.29) | 0.70 (0.37, 1.30) | 0.26 | 0.86 (0.62, 1.18) |
| Coagulopathy | 1 | 0.41 (0.16, 1.10) | 0.51 (0.21, 1.23) | 0.12 | 0.69 (0.38, 1.23) |
| ACI | 1 | 0.61 (0.27, 1.40) | 0.96 (0.46, 1.98) | 0.98 | 1.10 (0.81, 1.49) |
| ALI | 1 | 0.79 (0.37, 1.71) | 0.78 (0.36, 1.66) | 0.52 | 0.77 (0.52, 1.16) |
| AKI | 1 | 1.17 (0.35, 3.88) | 1.56 (0.50, 4.85) | 0.42 | 1.17 (0.80, 1.72) |
| Transferrin saturation, % | <28.0 | 28.0–39.8 | ≥39.8 | ||
| Severe | 1 | 0.71 (0.37, 1.36) | 0.61 (0.30, 1.22) | 0.16 | 0.93 (0.72, 1.21) |
| ARDS | 1 | 0.86 (0.47, 1.59) | 0.59 (0.30, 1.17) | 0.13 | 0.92 (0.72, 1.19) |
| Coagulopathy | 1 | 0.57 (0.23, 1.42) | 0.35 (0.13, 0.96) | 0.04 | 0.76 (0.52, 1.11) |
| ACI | 1 | 0.81 (0.39, 1.70) | 0.60 (0.26, 1.41) | 0.24 | 0.80 (0.59, 1.09) |
| ALI | 1 | 0.78 (0.34, 1.78) | 1.01 (0.48, 2.17) | 0.91 | 1.03 (0.75, 1.41) |
| AKI | 1 | 0.65 (0.20, 2.13) | 0.33 (0.10, 1.12) | 0.07 | 0.70 (0.45, 1.10) |
| Total iron-binding capacity, μmol/L | <35.6 | 35.6–44.4 | ≥44.4 | ||
| Severe | 1 | 0.50 (0.27, 0.90) | 0.27 (0.11, 0.64) | 0.0007 | 0.55 (0.40, 0.74) |
| ARDS | 1 | 0.56 (0.31, 0.99) | 0.30 (0.13, 0.68) | 0.002 | 0.58 (0.43, 0.77) |
| Coagulopathy | 1 | 0.39 (0.17, 0.93) | 0.19 (0.04, 0.89) | 0.005 | 0.40 (0.25, 0.64) |
| ACI | 1 | 0.40 (0.19, 0.83) | 0.27 (0.09, 0.81) | 0.003 | 0.51 (0.35, 0.74) |
| ALI | 1 | 0.86 (0.42, 1.77) | 0.45 (0.19, 1.12) | 0.10 | 0.71 (0.51, 0.99) |
| AKI | 1 | 0.38 (0.13, 1.16) | 0.22 (0.04, 1.08) | 0.02 | 0.53 (0.32, 0.90) |
| Unsaturated iron-binding capacity, μmol/L | <21.3 | 21.3–29.2 | ≥29.2 | ||
| Severe | 1 | 0.56 (0.29, 1.07) | 0.50 (0.24, 1.02) | 0.04 | 0.72 (0.53, 0.97) |
| ARDS | 1 | 0.62 (0.33, 1.16) | 0.54 (0.27, 1.09) | 0.06 | 0.75 (0.56, 1.00) |
| Coagulopathy | 1 | 0.47 (0.19, 1.17) | 0.46 (0.16, 1.35) | 0.08 | 0.71 (0.46, 1.09) |
| ACI | 1 | 0.46 (0.21, 1.04) | 0.68 (0.31, 1.50) | 0.20 | 0.82 (0.59, 1.15) |
| ALI | 1 | 0.64 (0.31, 1.35) | 0.50 (0.20, 1.21) | 0.10 | 0.73 (0.50, 1.06) |
| AKI | 1 | 0.74 (0.28, 1.98) | 0.76 (0.21, 2.74) | 0.57 | 0.87 (0.52, 1.45) |
Multivariable Poisson regression model was adjusted for age, sex, IL-6, and pre-existing comorbidities (including hypertension, diabetes, cardiac disease, cerebrovascular disease, chronic pulmonary disease, chronic kidney disease, chronic liver disease, and tumor).
Linear trend test was conducted using the median value of each category of every iron metabolism parameter.
Abbreviations: ACI = acute liver injury; AKI = acute kidney injury; ALI = acute liver injury; ARDS = acute respiratory distress syndrome; COVID-19 = coronary disease 2019; RR = relative risk.
The associations of the iron metabolism parameters and organ injury markers were shown in Supplemental Table 2. After adjusting for age, sex, and pre-existing diseases in multivariable linear regression model, the high serum iron level was associated with low D-dimer and TNI concentrations (P < 0.05 for both). Serum transferrin, TIBC, and UIBC were all negatively related to D-dimer and creatine, and high transferrin and TIBC values were related to low ALT activity (all P < 0.05). Furthermore, serum ferritin was positively related to D-dimer concentration and ALT and AST activities (all P < 0.05). Similarly, serum TSAT and sTfR values were not associated with these acute organ injury markers. The relationship between iron metabolism parameters and D-dimer was presented in Supplemental Fig. 1.
3.4. Baseline serum levels of the iron metabolism parameters were associated with subset lymphocyte counts and cytokine concentrations
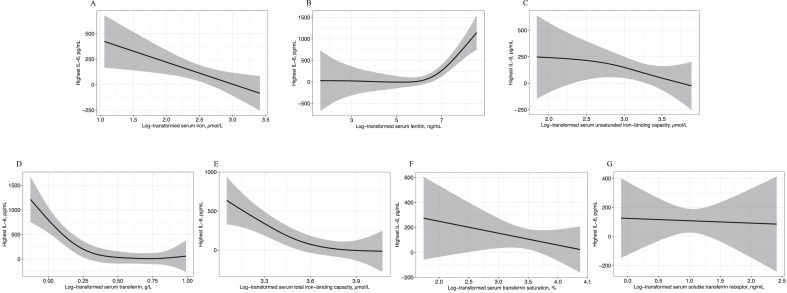
Considering the important role of immune cells and cytokines in the progression of COVID-19, we used the multivariable linear regression model to analyze the dose–response relationship between serum iron metabolism parameters and lymphocyte subset counts, cytokines, infection biomarkers, and indices for acute organ injuries. β-Coefficients and 95% CI of all iron metabolism indicators with these indices were presented in Supplemental Table 2. After log-transforming and adjusting for age, sex, and pre-existing comorbidities, we found that the serum iron status was significantly associated with the expression of pro-inflammatory cytokines. Serum iron was negatively related to the levels of IL-6, IL-8, and IL-2R. In addition, transferrin, TIBC, and UIBC were negatively associated with IL-6, IL-8, IL-2R, and TNF-α (all P < 0.05). As an inflammatory indicator, serum ferritin was positively related to high levels of IL-6, IL-8, IL-2R, and TNF-α (all P < 0.01). The dose–response relationship between systemic iron status and IL-6 concentration was shown in Fig. 2 , and the association of iron indices with IL-8 and IL-2R was presented in Supplemental Figs. 2 and 3. In our study, serum TSAT and sTfR values were not associated with cytokine content.
Fig. 2.
Association between log-transformed serum levels of iron metabolism parameters and IL-6 concentration in COVID-19 patients. All iron metabolism parameters were log-transformed in the linear regression. The model was adjusted for age, sex, and pre-existing comorbidities (including hypertension, diabetes, cardiac disease, cerebrovascular disease, chronic pulmonary disease, chronic kidney disease, chronic liver disease, and tumor). The shaded area shows the 95% CIs. A: Log-transformed serum iron. B: Log-transformed serum ferritin. C: Log-transformed serum transferrin. D: Log-transformed serum total iron-binding capacity. E: Log-transformed serum unsaturated iron-binding capacity. F: Log-transformed serum transferrin saturation. G: Log-transformed serum soluble transferrin receptor.
4. Discussion
In this study, we systemically described iron status in severe and non-severe patients and reported a significant association of iron metabolism disorders with COVID-19 severity and the risk of various adverse outcomes. In particular, increased serum ferritin, decreased serum iron, transferrin, TIBC, and UIBC were associated with the increased risk of severe COVID-19, ARDS, and acute organ injuries. We did not estimate the associations of the iron metabolism parameters with DIC, septic shock and death, because the low incidence of these outcomes would limit the statistical power. No significant relations were found for TSAT and sTfR. Based on previous reports, this longitudinal study was the first to systemically investigate the association of iron metabolism parameters with the risk of various adverse COVID-19 outcomes.
Former studies have indicated a U-shape of iron status and the risk of infection [22]. However, whether the iron metabolism parameters are risk factors for COVID-19 prognosis remains poorly understood. A previous study in Austria found that ferritin and transferrin were both associated with the risk for ICU admission in COVID-19 patients, and no significant relations were found in iron and other indices [23]. This study indicated the relation between iron metabolism and the undesirable outcome of COVID-19, whereas the association with specific comorbidities warrants further investigation. In our study, we found high serum iron level was associated with disease severity in COVID-19 patients. A case series of 30 ICU patients in UK found that serum iron was low in severe patients and related to the severity of hypoxemic respiratory failure [11], and another study of 50 patients in China found that low posttreatment serum iron was an independent risk factor of death in COVID-19 patients (β = −0.011, 95% CI: −0.019, −0.003) [10]. The evidence from these studies supported our results, whereas the relatively small sample size and non-controlling confounding factors limited the statistical power. As an acute–phase protein, ferritin is the most commonly investigated iron index in COVID-19, and in line with previous studies, we found that high ferritin level was associated with the severity of COVID-19 [24]. In our study, except for iron and ferritin, we also found that the serum transferrin, and TIBC levels were all inversely associated with the severity of COVID-19 after adjusting for age, sex, IL-6, and pre-existing comorbidities, whereas UIBC, sTfR and TSAT were not associated with poor prognosis. In acute infectious diseases such as COVID-19, these indices have limited clinical value due to their relative stability [25]. Previous studies on SARS have observed a decrease in the transferrin level in patients at the progressive phase compared with patients with mild condition [26], which was in line with our findings. However, transferrin is also an acute–phase protein and decreases under infection. Meanwhile, TIBC declines under infection because it primarily reflects the transferrin level in the circulation, and we observed a strong correlation between them in this analysis (r2 = 0.99). Given the retrospective nature, we cannot confirm the causality between the iron metabolism parameters and the severity of COVID-19, but our findings indicated that these indices could be used as biomarkers of disease severity in hospitalized patients and stratifying patients for appropriate treatment. One of the strengths of our study is that we explore the association of systemic iron status using relatively complete iron metabolism parameters with disease severity in COVID-19 patients, which adds more weight to the evidence body than using iron or ferritin only.
Current evidence shows that respiratory failure from ARDS was the leading cause of death in COVID-19, and coagulopathy was also a common implication related to adverse clinical outcomes of this disease. In our analysis, we observed the significant associations between the iron metabolism parameters and the risk of ARDS, coagulopathy, ACI, ALI, and AKI in COVID-19 in-hospital patients. Consistent with our results, previous studies reported a low serum level of iron and transferrin in patients with ARDS and the predictive effect of increased ferritin concentration for the risk of ARDS [27,28]. Previous study have found a low serum iron level was associated with increased plasma level of coagulation factor VIII and venous thromboembolic risk in other diseases [29]. Similarly, we found that decreased serum iron, transferrin, TIBC, and UIBC and increased ferritin were all related to the increased D-dimer concentration and the high risk of coagulopathy in COVID-19 patients. This association might result from the roles of iron in the interaction with proteins of the coagulation cascade and transferrin in maintaining coagulation balance by interacting with coagulation and anti-coagulation factors [30], and the increased iron concentration as well as the decreased transferrin in COVID-19 patients resulted in the increased coagulation and the impaired anti-coagulation, which led to coagulopathy finally.
Experimental studies have illustrated the possible mechanisms of iron metabolism in infective diseases. After viral infection, cytokines, particularly IL-6, upregulate the secretion of hepcidin, an essential regulatory hormone for iron status homeostasis, through STAT3 signaling [6,31]. By promoting the degradation of ferroportin, hepcidin inhibits cellular iron efflux from duodenal enterocytes to the circulation [6]. Meanwhile, interferon-γ and TNF-α upregulate the expression of divalent metal transporter-1, resulting in an increased uptake of iron into active macrophages [32]. These regulations finally lead to the decreased serum level of iron. Thus, the low serum iron status observed in our study indicates the increased iron accumulation in macrophage and other cells that store iron in normal condition. Given the important role of iron in nearly all microorganisms, the increased intracellular iron content facilitates the replication of SARS-CoV-2 in host cells which exacerbates the damage to host [33]. Except for the direct effect on virus, iron status also has an impact on the host immune system. Through the generation of reactive oxygen species and activation of IκB kinase, increased intracellular iron resulting from infection upregulates the expression of inflammatory factors such as IL-6, IL-8, and TNF-α, which in turn aggravates the iron accumulation in host cells [34]. This feedback loop contributes to the “cytokine storm” in COVID-19, which further increases the secretion of vascular endothelial growth factors, monocyte chemoattractant protein-1, as well as reduced E-cadherin on endothelial cells, leading to the vascular hyperpermeability [35], and results in ARDS, multiorgan failure, and eventually death when the high cytokine concentrations are unabated over time [36,37]. Moreover, increased serum ferritin level reflects the activation of the reticuloendothelial system and generation of reactive oxygen species, thereby resulting in multiple organ dysfunction [38]. Meanwhile, the accumulation of iron in host cells might be involved in the injury of multiple organs, including heart, lung, liver, and kidney through ferroptosis, which is a type of cell death triggered by excessive intracellular iron induced peroxidation [39]. In our study, low serum iron status was associated with high level of pro-inflammatory cytokines, including IL-6, IL-8, IL-2R, and TNF-α in the circulation, and we observed a dose–response relationship between iron status and IL-6 concentration, which could explain the association of low serum iron status with the risk of adverse outcomes of COVID-19. These findings gave a hint that manage the hypoferremia might be a possible way for COVID-19 treatment. In addition, there are several clinical trials aimed to assess the effect of iron chelator in COVID-19 patients (NCT04333550, NCT04333550) have been in progress. However, this is only a preliminary hypothesis and more evidence is warranted.
In the present study, the serum iron concentration of most COVID-19 patients was in normal range. A longitudinal observation study of ICU patients showed that serum iron increased to a normal range during their hospitalization [40]. Given the emergency of medical situation, the iron metabolism parameters were evaluated during their treatment rather than at the onset of their hospitalization, but the symptomatic treatments might increase iron content in the circulation, as the iron level in our study was comparable to posttreatment iron concentration in a recent case series [10]. However, in a relatively normal range, iron level was still a risk factor of disease severity and multiple–organ injury, which indicated that including the iron metabolism parameters in routine admission laboratory tests and strengthening surveillance of iron metabolism in COVID-19 patients might have a positive effect on the management of in-hospital patients during the COVID-19 pandemic.
Our study has several limitations. First, as an observational study, though we performed multivariable-adjusted model to control confounding effects, residual confounders and potential bias could not be completely ruled out. Second, we did not estimate the association of iron status with mortality because of low incidence. Third, although our study has a larger sample size than other studies in assessing the relationship between systemic iron status and incidence of adverse outcomes, more prospective and larger scale studies are warranted. Forth, hepcidin was not measured in our study, which affected the interpretation of iron metabolism regulation under the attack of this new pathogen. Finally, we did not monitor the dynamic changes of the iron metabolism parameters in the whole course of disease progression, and more evidence available in this area might allow a wider understanding of SARS-CoV-2 pathophysiology.
5. Conclusions
We demonstrated that low serum iron status was associated with high risk of severity and adverse outcomes in COVID-19 patients, and serum iron status was inversely related to the level of pro-inflammatory cytokines. These findings indicated that low serum iron status might be a risk factor for clinical outcomes, and strengthening surveillance of iron metabolism might add clinical value to predict disease progression among COVID-19 patients.
Authors’ contributions
LC, Q.Wei and LL had full access to all the data in the study, and took responsibility for the integrity of the data and the accuracy of the data analysis. LC, Q.Wei and LL made substantial contributions to the study concept and design. LC, YL, X.Liang, MG, X.Liu, and Q.Wang took responsibility for obtaining ethical approval, collecting samples, and confirming data accuracy. LC and YL were in the charge of the statistical analysis. YL and LC were in the charge of the manuscript draft. All authors contributed to critical revision of the report. All authors reviewed and approved the final version.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgement
We mourn all the lives lost during this pandemic. We thank all study participants and staff in our study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2020.11.033.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 Aug 25;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P., Porter J.C., Manson J.J., Isaacs J.D., Openshaw P.J.M., McInnes I.B., et al. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. The Lancet Respiratory medicine. 2020;8(8):822–830. doi: 10.1016/S2213-2600(20)30267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drakesmith H., Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6(7):541–552. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- 6.Drakesmith H., Prentice A.M. Hepcidin and the iron-infection axis. Science. 2012;338(6108):768–772. doi: 10.1126/science.1224577. [DOI] [PubMed] [Google Scholar]
- 7.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganz T., Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15(8):500–510. doi: 10.1038/nri3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang T.J., Zhou X.Z., Zhao M., Zhou Z.P., Jiang S.C., Ye W.H., et al. [Analysis of severe acute respiratory syndrome in Beijing] Zhonghua nei ke za zhi. 2003;42(6):369–372. [PubMed] [Google Scholar]
- 10.Zhao K., Huang J., Dai D., Feng Y., Liu L., Nie S. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open forum infectious diseases. 2020;7(7):ofaa250. doi: 10.1093/ofid/ofaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah A., Frost J.N., Aaron L., Donovan K., Drakesmith H. Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Crit Care. 2020;24(1):320. doi: 10.1186/s13054-020-03051-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. 1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health O . World Health Organization; Geneva: 2020. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020.WHO/nCoV/Clinical/2020.3 Contract No.: [Google Scholar]
- 14.National Health Commission of the People’s Republic of China . 2020. Chinese management guideline for COVID-19.http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf (version 7.0, in Chinese). Availble from: Published March 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Force A.D., et al. Acute respiratory distress syndrome: the Berlin Definition. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai Q., Huang D., Yu H., Zhu Z., Xia Z., Su Y., et al. COVID-19: abnormal liver function tests. J Hepatol. 2020 Sep;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 20.Taylor F.B., Toh C.H., Hoots W.K., Wada H., Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemostasis. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 21.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international Consensus Definitions for Sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohus R.M., Paulsen J., Gustad L., Askim Å., Mehl A., DeWan A.T., et al. Association of iron status with the risk of bloodstream infections: results from the prospective population-based HUNT Study in Norway. Intensive Care Med. 2018;44(8):1276–1283. doi: 10.1007/s00134-018-5320-8. [DOI] [PubMed] [Google Scholar]
- 23.Bellmann-Weiler R., Lanser L., Barket R., Rangger L., Schapfl A., Schaber M., et al. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med. 2020;9(8) doi: 10.3390/jcm9082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng F., Huang Y., Guo Y., Yin M., Chen X., Xiao L., et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis : IJID: Off Pub Int Sci. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodnough L.T., Nemeth E., Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116(23):4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 26.Wan J., Sun W., Li X., Ying W., Dai J., Kuai X., et al. Inflammation inhibitors were remarkably up-regulated in plasma of severe acute respiratory syndrome patients at progressive phase. Proteomics. 2006;6(9):2886–2894. doi: 10.1002/pmic.200500638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mumby S., Upton R.L., Chen Y., Stanford S.J., Quinlan G.J., Nicholson A.G., et al. Lung heme oxygenase-1 is elevated in acute respiratory distress syndrome. Crit Care Med. 2004;32(5):1130–1135. doi: 10.1097/01.ccm.0000124869.86399.f2. [DOI] [PubMed] [Google Scholar]
- 28.Stites S.W., Nelson M.E., Wesselius L.J. Transferrin concentrations in serum and lower respiratory tract fluid of mechanically ventilated patients with COPD or ARDS. Chest. 1995;107(6):1681–1685. doi: 10.1378/chest.107.6.1681. [DOI] [PubMed] [Google Scholar]
- 29.Livesey J.A., Manning R.A., Meek J.H., Jackson J.E., Kulinskaya E., Laffan M.A., et al. Low serum iron levels are associated with elevated plasma levels of coagulation factor VIII and pulmonary emboli/deep venous thromboses in replicate cohorts of patients with hereditary haemorrhagic telangiectasia. Thorax. 2012;67(4):328–333. doi: 10.1136/thoraxjnl-2011-201076. [DOI] [PubMed] [Google Scholar]
- 30.Tang X., Fang M., Cheng R., Zhang Z., Wang Y., Shen C., et al. Iron-deficiency and estrogen are associated with ischemic stroke by up-regulating transferrin to induce hypercoagulability. Circ Res. 2020 Aug 14;127(5):651–663. doi: 10.1161/CIRCRESAHA.119.316453. [DOI] [PubMed] [Google Scholar]
- 31.Silva I., Peccerella T., Mueller S., Rausch V. IL-1 beta-mediated macrophage-hepatocyte crosstalk upregulates hepcidin under physiological low oxygen levels. Redox Biol. 2019;24:101209. doi: 10.1016/j.redox.2019.101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss G., Goodnough L.T. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 33.Kalyanaraman B. Do free radical NETwork and oxidative stress disparities in African Americans enhance their vulnerability to SARS-CoV-2 infection and COVID-19 severity? Redox Biology. 2020:101721. doi: 10.1016/j.redox.2020.101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong S., She H., Takeuchi H., Han B., Engelhardt J.F., Barton C.H., et al. Signaling role of intracellular iron in NF-kappaB activation. J Biol Chem. 2003;278(20):17646–17654. doi: 10.1074/jbc.M210905200. [DOI] [PubMed] [Google Scholar]
- 35.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 36.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. The Lancet Respiratory medicine. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meduri G.U., Kohler G., Headley S., Tolley E., Stentz F., Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108(5):1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 38.Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y., Chen P., Zhai B., Zhang M., Xiang Y., Fang J., et al. The emerging role of ferroptosis in inflammation. Biomed Pharmacother. 2020;127:110108. doi: 10.1016/j.biopha.2020.110108. [DOI] [PubMed] [Google Scholar]
- 40.Bolondi G., Russo E., Gamberini E., Circelli A., Meca M.C.C., Brogi E., et al. Iron metabolism and lymphocyte characterisation during Covid-19 infection in ICU patients: an observational cohort study. World J Emerg Surg. 2020;15(1):41. doi: 10.1186/s13017-020-00323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.