Abstract
Colorectal cancer (CRC), ranking as the third commonest cancer, leads to extremely high rates of mortality. Metastasis is the major cause of poor outcome in CRC. When metastasis occurs, 5-year survival rates of patients decrease sharply, and strategies to enhance a patient’s lifetime seem limited. MicroRNAs (miRNAs) are evolutionarily conserved small non-coding RNAs that are significantly involved in manipulation of CRC malignant phenotypes, including proliferation, invasion, and metastasis. To date, accumulating studies have revealed the mechanisms and functions of certain miRNAs in CRC metastasis. However, there is no systematic discussion about the biological implications and clinical potential (diagnostic role, prognostic role, and targeted therapy potential) of metastasis-related miRNAs in CRC. This review mainly summarizes the recent advances of miRNA-mediated metastasis in CRC. We also discuss the clinical values of metastasis-related miRNAs as potential biomarkers or therapeutic targets in CRC. Moreover, we envisage the future orientation and challenges in translating these findings into clinical applications.
Keywords: miRNA, colorectal cancer, metastasis, biomarkers, therapeutic targets
Graphical Abstract
miRNAs are significantly involved in CRC metastasis. However, the concrete mechanisms have not been systematically elucidated. This review summarizes recent advances on the roles of miRNAs in CRC metastasis, including cellular processes, molecular mechanisms, and clinical application. This may provide novel views for the comprehension and further investigation of miRNAs.
Main Text
Colorectal cancer (CRC) ranks as the third most malignant form of cancer and the fourth leading cause of cancer mortality in the world.1,2 About 60% patients who diagnosed with CRC are simultaneously observed with localized or distant metastases.3 Metastasis is significantly associated with poor prognosis in CRC patients.4 The 5-year survival rates decline with the progression of tumor stage, in which metastasis is one of the principle standards to evaluate the tumor-node-metastasis (TNM) stage, and the 5-year survival rates of patients with stage II are 20% higher than those with stage III.4,5 Thus, elucidation on the mechanisms of CRC is urgently required for improving clinical outcomes.
Metastasis is a pivotal process, which can not only reveal the malignant transformation of neoplasms, but also partly reflect the clinical stage of malignant tumors.6 Numerous studies have been conducted on cancer metastasis. The most significant factors affecting cancer metastasis include epithelial-mesenchymal transition (EMT),7 angiogenesis,8 hypoxia,9 and the tumor microenvironment (TME). EMT, a process in which epithelial cells lose their adhesion and then transform to the mesenchymal phenotype, is the early stage of cancer metastasis.10 Angiogenesis refers to generation of new blood vessels at primary sites or secondary organs.11 It is a rate-determining step in metastasis because cancer cells require additional nutrition and oxygen.11 Hypoxia can lead cancer cells to acquire more aggressive phenotypes by regulating genetic programs that can facilitate cells to adapt to hypoxic conditions.12 The TME is a biological system that contains cancer-associated endothelial cells (CAECs), immune cells, cancer-associated fibroblasts (CAFs), and extracellular matrix (ECM). Transformation of the TME is essential for CRC carcinogenesis.13,14 Molecules interacting with the microenvironment (MET) may also contribute to metastasis.13
MicroRNAs (miRNAs) are short (19–25 nt), endogenous, non-coding, and regulatory RNAs, and they regulate target genes at the post-transcriptional level.15 miRNAs play crucial roles in biological and pathological processes such as metabolism, apoptosis, differentiation, cell proliferation, cell cycle, as well as invasion and metastasis.16 Accumulating studies have found that miRNA dysregulation was significantly associated with tumor metastasis. For example, miR-103 promotes metastasis of hepatoma cells by increasing vascular permeability.17 In breast cancer, miR-182 enhances metastasis by targeting transforming growth factor β (TGF-β)-induced EMT via SMAD family member 7 (SMAD7).18 Recent studies have also shed light on the role of miRNAs in CRC metastasis. In CRC, miRNAs participate in several cellular processes related to metastasis, including EMT,19 angiogenesis,20 and interaction with the MET.13 miRNAs can act as oncogenes or suppressors via different molecular mechanisms, including the classical signaling pathways,21 the long non-coding RNA (lncRNA)/miRNA/mRNA axis,22 and methylation of the DNA promoter.23 So far, the concrete mechanisms of CRC metastasis are still unknown. Researchers have been devoted to clarifying the specific mechanisms of miRNAs in CRC metastasis.21 Additionallyt, numerous biomarkers and therapeutic targets associated with CRC metastasis have been reported.24
In this review, we searched articles from the PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Science (http://apps.webofknowledge.com), and EMBASE (https://www.embase.com) databases. We used various combinations and variations of search terms, including “colorectal cancer,” “CRC,” “colon cancer,” “rectal cancer,” “metastasis,” “miRNA,” “microRNA,” “diagnosis,” “prognosis,” “therapy,” and their variants. Then, we summarized the recent advances of miRNAs in the metastatic process of CRC and commented on their applications as biomarkers or therapeutic targets in CRC management. Our review may provide new insights into the comprehension and future research on CRC metastasis.
miRNAs and Cellular Process
miRNAs and EMT Process
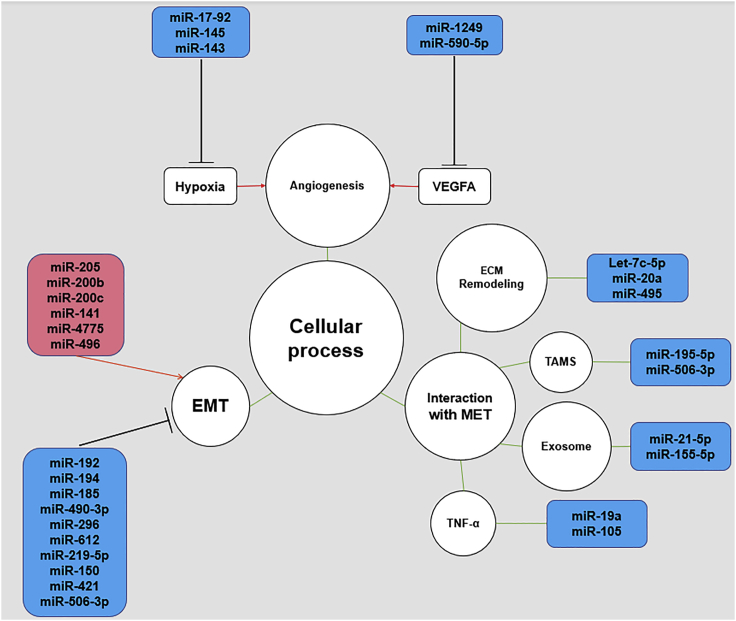
Emerging evidence has revealed that the dysregulation of miRNAs is associated with cancer metastasis.13,19 According to the published literature on the role of miRNAs in CRC metastasis, the metastasis-related cellular processes can be divided into four categories: EMT, angiogenesis, hypoxia, and interaction with the MET (Figure 1).20,23 The dysregulated miRNAs, their confirmed targets, and the corresponding cellular processes are listed in Table 1.
Figure 1.
Role of miRNAs in CRC Metastasis
The role of miRNAs in CRC metastasis is shown. The black lines indicate suppression while red lines indicate promotion of downstream targets or processes. The green lines indicate interaction with corresponding processes or molecules. EMT, epithelial-mesenchymal transition; MET, microenvironment.
Table 1.
miRNA-Related Cellular Processes in CRC Metastasis
| Cellular Processes | miRNAs | Alteration | Downstream Targets | References |
|---|---|---|---|---|
| EMT | miR-205 | ↑ | Snail, SNORD | 7,25 |
| let-7i | ↑ | |||
| miR-200b | ↑ | ZEB1, ZEB2 | 26 | |
| miR-200c | ↑ | |||
| miR-141 | ↑ | |||
| miR-4775 | ↑ | Smad7 | 27 | |
| miR-496 | ↑ | RASSF6 | 28 | |
| miR-192 | ↓ | Snail | 7,25 | |
| miR-194 | ↓ | |||
| miR-150 | ↓ | |||
| miR-490-3p | ↓ | FRAT | 29 | |
| miR-612 | ↓ | AKT2 | 30 | |
| miR-219-5p | ↓ | LEF1 | 31 | |
| miR-185 | ↓ | STIM1 | 32 | |
| miR-296 | ↓ | S100A4 | 33 | |
| miR-421 | ↓ | MTA1 | 34 | |
| Angiogenesis | miR-1249 | ↓ | VEGFA, HMGA | 35 |
| miR-590-5p | ↓ | NF90, VEGFA | 36 | |
| miR-25-3p | ↑ | KLF2, KLF4, VEGFR2, ZO-1 | 37 | |
| Hypoxia | miR-17~92 | ↓ | HIF-1α, TGFBR2, VEGFA | 8 |
| miR-145 | ↓ | IRS1, HIF-1α | 38 | |
| miR-143 | ↓ | IGF-1R | 39 | |
| ECM remodeling | let-7c-5p | ↓ | COL1A2 | 40 |
| miR-20a | ↓ | MMP2 | 41 | |
| miR-495 | ↓ | |||
| miR-155-5p | ↓ | MDEBRG1 | 42 | |
| miR-21-5p | ↓ | |||
| TAM polarization | miR-195-5p | ↑ | NOTCH2, GATA3, IL-4 | 43 |
| miR-506-3p | ↓ | IL-6, CCL2 | 44 | |
| Interaction with TME via TNF-α | miR-19a | ↓ | TNF-α | 45 |
| miR-105 | ↑ | NF-κB, TNF-α, RAP2C | 46 |
SNORD, small nucleolar RNAs with C/D motifs; ZEB1, zinc finger E-box binding homeobox 1; RASSF6, Ras association domain family member 6; STIM1, stromal interaction molecule 1; FRAT, frequently rearranged in advanced T cell lymphoma; S100A4, S100 calcium-binding protein A4; AKT2, AKT serine/threonine kinase 2; LEF1, lymphoid enhancer-binding factor 1; Gli1, GLI family zinc finger 1; MTA1, metastasis-associated protein 1; FoxQ1, forkhead box Q1; CCL2, C-C motif chemokine ligand 2; IL-6, interleukin 6; JAK2, Janus kinase 2; VEGFA, vascular endothelial growth factor A; HMGA, high mobility group A; NF90, IL enhancer binding factor 3; ZO-1, tight junction protein 1; KLF2, Krüpple-like factor 2; HIF-1α, hypoxia inducible factor-1α; IGF-1R, insulin-like growth factor 1 receptor; TGFBR2, transforming growth factor-β receptor type-2; IRS1, insulin receptor substrate 1; TNF-α, tumor necrosis factor alpha; NAT10, N-acetyltransferase 10; NF-κB, nuclear factor κB subunit 1; TAM, tumor-associated macrophage; Notch2, notch receptor 2; GATA3, GATA binding protein 3; IL-4, interleukin 4.
Previously, studies have confirmed that miRNAs directly or indirectly interact with EMT-related molecules such as the E-cadherin/β-catenin complex and N-cadherin and are essential for EMT progression.47 Some miRNAs can induce the progression of EMT, which is why they function as tumor-promoting genes.47 For instance, Snail belongs to the family of zinc finger transcription factors and is one of the transcription factors of EMT.19 miR-205 and let-7i are confirmed to be the downstream molecules of Snail, and they can induce EMT in CRC, indicating their oncogenic role in CRC metastasis.7,25 Likewise, zinc finger E-box binding homeobox 1 (ZEB1) and E-box binding homeobox 2 (ZEB2), which represent the zinc finger E-box binding homeobox family, are two additional EMT-related transcription factors.19 A recent study reported that miR-200b, miR-200c, and miR-141 can directly regulate both ZEB1 and ZEB2 in CRC, followed by the activation of EMT and metastasis.26 Moreover, several miRNA-related signaling pathways can also induce EMT. For example, miR-4775 was reported to promote EMT and metastasis by positively targeting the SMAD7/TGF-β axis.27 Additionally, hypermethylation of Ras association domain family member 6 (RASSF6) was confirmed as a direct target of miR-496. The miR-496/RASSF6 axis can promote EMT and migration in CRC through Wnt signaling.28
Conversely, some miRNAs can inhibit metastasis partly by suppressing the EMT process. Przygodzka et al.25 reported that miR-192 and miR-194 could suppress the Snail-induced EMT and metastasis. miR-150 can also inhibit metastasis of CRC by directly targeting Snail and Gli1.7 Moreover, miR-490-3p inhibits Wnt/β-catenin expression via binding frequently rearranged in advanced T cell lymphoma (FRAT) protein and subsequently suppresses EMT.29 In addition, EGFR signaling is also suppressed by certain miRNAs and finally leads to inhibition of EMT.19 miR-612 reversely regulates EMT-related process via inhibiting AKT serine/threonine kinase 2 (AKT2), and CRC metastasis is alleviated afterward.30 Overexpression of miR-219-5p can inhibit the expression of lymphoid enhancer-binding factor 1 (LEF1), resulting in the downregulation of the AKT/extracellular signal-regulated kinase (ERK) pathway, repression of EMT, and lower metastasis rate.31 Likewise, other oncogenic factors can be targeted by miRNAs, leading to the downregulation of EMT. miR-185 can inhibit EMT and metastasis of CRC cells by inhibiting stromal interaction molecule 1 (STIM1).32 miR-296 was observed to suppress CRC metastasis via manipulating the S100A4-mediated EMT process.33 Moreover, miR-421 suppresses the EMT process by repressing metastasis-associated protein 1 (MTA1).34
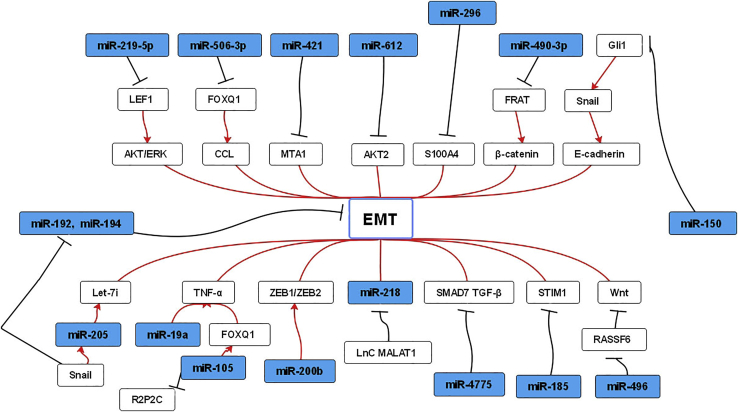
As discussed above, numerous miRNAs are reported to be associated with the EMT process (Figure 2). Therefore, preventing EMT may be a promising method for anti-metastasis.
Figure 2.
Network of miRNAs and EMT in CRC Metastasis
The interactions between miRNAs and protein-coding genes, lncRNAs, and several signaling pathways are shown. Promotion (red line) or suppression (black line) of EMT is presented.
miRNAs and Angiogenesis
The balance between angiogenic and anti-angiogenic factors is pivotal in the manipulation of angiogenesis.48 Vascular endothelial growth factor A (VEGFA) and its receptors are two important factors in angiogenesis.9 Recent researches on tumor metastasis highlighted the importance of miRNAs and angiogenesis.20 For example, pp53-induced miR-1249 inhibits angiogenesis by negatively targeting VEGFA, which then regulates AKT/mechanistic target of rapamycin kinase (mTOR) signaling pathway.35 Similarly, VEGFA and interleukin enhancer binding factor 3 (NF90) are downstream targets of miR-590-5p.36 Meanwhile, miR-25-3p can upregulate VEGF receptor 2 (VEGFR2) by targeting Krüpple-like factor 2 (KLF2) and Krüpple-like factor 4 (KLF4).37 Additionally, miR-25-3p plays an oncogenic role in stimulating the process of angiogenesis.37 Therefore, keeping the balance between angiogenesis and anti-angiogenesis may be helpful to restrain cancer cell migration.
miRNAs and Hypoxia
Hypoxia is another hallmark for solid tumors, including CRC.16 It may result in more aggressive phenotypes by modulation of the genetic programs that facilitate cellular adaption to the hypoxic environment.12 Hypoxia-inducible factor 1α (HIF-1α) and insulin-like growth factor 1 receptor (IGF-1R) are the major factors that induce a hypoxic environment.8 To date, increasing studies have found that miRNAs play a significant role in cancer metastasis under a hypoxic environment.16 For example, the miR-17∼92 cluster can negatively regulate HIF-1α and inhibit CRC metastasis.9 Yin et al.38 found that miR-145 blocked CRC metastasis by repressing the expression of HIF-1α. miR-143 inhibits angiogenesis and metastasis through suppressing IGF-1R, another major factor in hypoxia.39 Given that hypoxia is closely related to angiogenesis and metastasis, targeting hypoxia-related miRNAs may lead to significant consequences for controlling cancer metastasis.
Interaction between miRNAs and TME
TME is vital for the progression of aggressive metastasis.13 Previous studies have shown that miRNAs are closely related to TME, and that miRNAs could regulate some TME-related genes in cancer metastasis.49 Moreover, miRNAs can act as modulators among immune cells, CAECs, CAFs, and tumor cells.41,49, 50, 51, 52 For instance, let-7c-5p can interact with TME by negatively regulating the expression of collagen type I alpha 2 chain (COL1A2) in CRC.40 The balance between matrix metallopeptidases (MMPs) and tissue inhibitor of metalloproteases-2 (TIMP-2) plays a significant role in the degradation of ECM in cancer cells.53 Makondi et al.53 demonstrated that miR-20a and miR-495 suppress CRC metastasis by repressing the expression of matrix metalloproteinase, especially MMP2. Likewise, miRNAs derived from the exosomes can also interact with the MET.14 For example, the elevated expression of miR-21-5p and miR-155-5p is induced by M2 macrophage-derived exosomes (MDEs), and then they bind the S-ribonuclease binding protein (SBP) family protein (BRG1), resulting in downregulation of BRG1 and higher rates of metastasis. Importantly, the contents of MDE range with the conversion of the MET.42
Tumor-associated macrophages (TAMs) are also pivotal components of TME. During cancer development and progression, TAMs undergo M1-like or M2-like polarization and subsequently manipulate tumor metastasis.54 Some metastasis-related miRNAs are reported to widely participated in these processes.13 For example, miR-195-5p directly binds to the 3′ UTR of the notch receptor 2 (Notch2), resulting in the suppression of M2-like TAM polarization and metastasis.43 Meanwhile, CD163+ TAMs participate in the secretion of interleukin 6 (IL-6) and the downregulation of miR-506-3p. miR-506-3p then suppresses metastasis by repressing the production of C-C motif chemokine ligand 2 (CCL2), a molecular marker recruiting macrophages.44
Tumor necrosis factor (TNF)-α, a central proinflammatory cytokine, is also involved in TME transformation as the downstream target of specific miRNAs.55 Current research confirmed that miR-19a promoted TME transformation by enhancing TNF-α, which is required for TNF-α-induced metastasis.45 Similarly, miR-105 is necessary for the TNF-α-induced TME transformation process, in which the nuclear factor κB (NF-κB) subunit 1 signaling pathway is elevated to activate TNF-α,46 revealing the role of inflammatory factors in the carcinogenesis of CRC.
Thus, the interaction between miRNAs and TME may be another important point for developing the anti-metastasis strategy.
miRNAs and Molecular Mechanisms
miRNAs in Key CRC Signaling Pathways
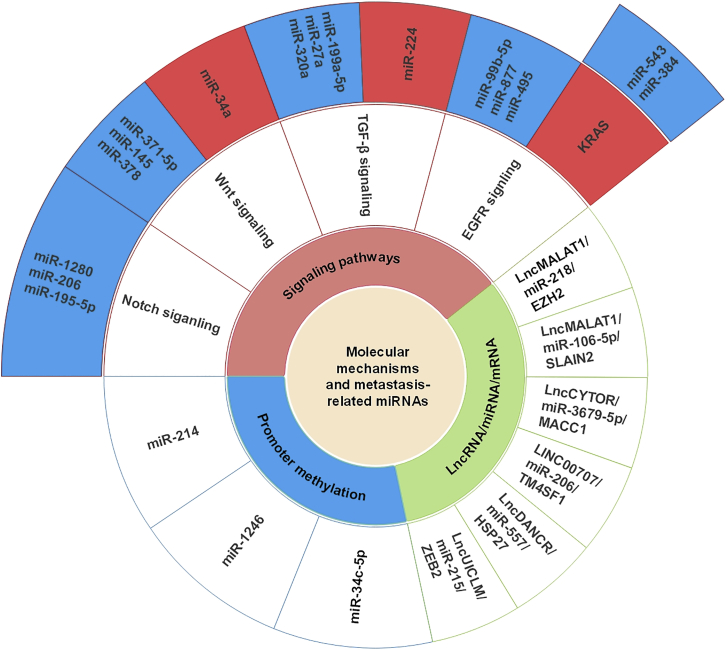
Emerging research has revealed that miRNA-mediated CRC metastasis depends on different molecular mechanisms.21 Table 2 presents these miRNAs, their targets, and the corresponding molecular mechanisms in CRC. Moreover, Figure 3 also illustrates the concrete mechanisms of miRNAs in CRC metastasis, including classical signaling pathways (such as the Notch, Wnt, EGFR, and TGF-β signaling pathways), the lncRNA/miRNA/mRNA axis, and methylation-related pathways.82
Table 2.
miRNA-Related Molecular Mechanisms in CRC Metastasis
| Mechanisms | miRNAs | Alteration | Downstream Targets | References |
|---|---|---|---|---|
| Mutation of KRAS | miR-543 | ↓ | KRAS, MTA1, HMGA2 | 56 |
| miR-384 | ↓ | KRAS, CDC42 | 57 | |
| PTEN/AKT/mTOR axis | miR-877 | ↓ | MTDH | 58 |
| miR-495 | ↓ | FAM83D | 59 | |
| miR-99b-5p | ↓ | p-mTOR | 60 | |
| TGF-β signaling | miR-224 | ↑ | USP3, Smad4 | 61,62 |
| miR-27a | ↓ | SGPP1, Smad2, p-STAT, cleaved caspase-3 | 63 | |
| miR-320a | ↓ | ΔNp63α, PKCγ, Rac1 | 64 | |
| miR-199a-5p | ↓ | ROCK1 | 65 | |
| Wnt/β-catenin signaling | miR-135 | ↓ | APC | 66,67 |
| miR-494 | ↓ | |||
| miR-371-5p | ↓ | SOX17, SOX2 | 68 | |
| miR-145 | ↓ | PRC2 | 69 | |
| miR-132 | ↓ | |||
| miR-212 | ↓ | |||
| miR-34a | ↑ | SIRT1 | 70 | |
| miR-378 | ↓ | SDAD1 | 71 | |
| Notch signaling | miR-1280 | ↓ | JAG2, Zeb1, Suz12, Gata1/3 | 72 |
| miR-200b | ↓ | |||
| lncRNA/miRNA/mRNA axis | miR-218 | ↑ | EZH2, E-cadherin | 73 |
| miR-106-5p | ↓ | SLAIN2, MT | 74 | |
| miR-3679-5p | ↑ | MACC1 | 75 | |
| miR-206 | ↓ | NOTCH3, TM4SF1 | 76 | |
| miR-577 | ↓ | HSP27 | 77 | |
| miR-215 | ↑ | ZEB2 | 78 | |
| DNA promoter methylation | miR-214 | ↑ | FOXD3, MED19 | 79 |
| miR-34c-5p | ↑ | SATB2 | 80 | |
| miR-1246 | ↑ | METTL3, SPRED2, MAPK | 81 |
JAG2, jagged canonical Notch ligand 2; Suz12, polycomb repressive complex; SOX17, SRY-box transcription factor 17; NEAT1, nuclear-enriched abundant transcript 1; SRIT1, sirtuin 1; SDAD1, SDA1 domain containing 1P; MTDH, metadherin; FAM83D, family with sequence similarity 83, member D; PTEN, phosphatase and tensin homolog; mTOR, mechanistic target of rapamycin kinase; KRAS, Kirsten Ras; HMGA2, high-mobility group AT-hook 2; USP3, ubiquitin-specific peptidase 3; SGPP1, sphingosine-1-phosphate phosphatase 1; p-STAT3, phosphorylated signal transducer and activator of transcription 3; Rac1, Rac family small GTPase 1; ROCK1, Rho-associated coiled coil-containing protein kinase 1; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; EZH2, enhancer of zeste 2 polycomb repressive complex 2 subunit; SLAIN2, SLAIN motif family member 2; MT, microtubule; CYTOR, cytoskeleton regulator RNA; MACC1, MET transcriptional regulator; NOTCH3, neurogenic locus notch homolog protein 3; TM4SF1, transmembrane 4 L6 family member 1; HSP27, heat shock protein 27; CCAT2, colon cancer-associated transcript 2; FOXD3, forkhead box 3; MED19, mediator complex subunit 19; SATB2, SATB homeobox 2; METTL3, methyltransferase-like 3; SPRED2, sprout-related EVH1 domain-containing protein 2; MAPK, mitogen-activated protein kinase; ANXA1, annexin A1; DFFA, DNA fragmentation factor subunit alpha; PDCD4, programmed cell death 4; MTSS1, metastasis suppressor 1; FBXW7, F-box and WD repeat domain containing 7; SLC6A8, solute carrier family 6 member 8; CKB, creatine kinase, brain-type; HOXB7, homeobox B7; GALNT5, polypeptide N-acetylgalactosaminyltransferase 5; CEMIP, hyaluronidase 1; ADAMTS5, ADAM metallopeptidase with thrombospondin type 1 motif 5; IGFBP5, insulin-like growth factor binding protein 5; SND1, staphylococcal nuclease domain containing-1; EIF5A2, eukaryotic translation initiation factor 5A2; MBD2, methyl-CpG binding domain protein 2; CHD5, chromodomain helicase DNA binding protein 5.
Figure 3.
Metastasis-Related miRNAs and Molecular Mechanisms
The interactions between metastasis-related miRNAs and molecular mechanisms in CRC are shown. miRNAs in blue frameworks indicate suppression while miRNAs and KRAS in red frameworks indicate promotion of relevant signaling pathways. Blank frameworks mean interaction with relevant molecular mechanisms.
miRNAs in the EGFR Signaling Pathway
Kirsten Ras (KRAS) is a key molecule involved in EGFR signaling. The mutation of KRAS is the most commonly seen mutated gene (up to 30%–60%) in CRC patients, and it is a main resistant factor to anti-EGFR-targeted therapy.23,83 As the downstream targets of miR-543, the expression levels of KRAS, MTA1, and high-mobility group AT-hook 2 (HMGA2) are inversely correlated with its expression in clinical samples, revealing the pivotal tumor suppressive role of miR-543 in CRC.56 In addition, a recent study showed that overexpression of miR-384 could inhibit KRAS and cell division cycle 42 (CDC42) and act as an inhibitor in CRC metastasis.57
The phosphatase and tensin homolog (PTEN)/AKT/mTOR axis is also generally observed to be activated by miRNAs in the EGFR pathway.58 It is approximately amplified in 15%–20% cases of CRC patients.84 For example, miR-877 suppresses the malignant phenotype of CRC by directly targeting metadherin (MTDH). As a result, the PTEN/AKT pathway is indirectly suppressed, leading to the repression of metastasis.58 miR-495 can also repress the proliferation and migration of CRC cells via the PTEN/P13K/AKT/mTOR pathway by repressing the expression of family with sequence similarity 83, member D (FAM83D).59 Similarly, miR-99b-5p is reported to bind mTOR to suppress the mTOR-activated metastasis.60
miRNAs in the TGF-β Signaling Pathway
The TGF-β signaling pathway exerts a dual function on CRC.85 TGF-β and its receptors are two major factors involved in multiple malignant progression.85 The TGF-β signaling pathway suppresses the progression of CRC in its early stage while promoting CRC in its advanced stage.85 As estimated, approximately 30% of CRC cases are associated with TGF-β type II receptor (TGF-βR) mutations.86,87 SMADs, one of key factors in the TGF-β signaling pathway, are commonly used as the downstream targets of specific miRNAs.88 Recent studies found that miR-224 could directly target SMAD family member 4 (SMAD4) and suppress the TGF-β signaling-induced metastasis.61,62 Similarly, miR-27a directly binds sphingosine-1-phosphate phosphatase 1 (SGPP1) and SMAD family member 2 (SMAD2), resulting in the downregulation of TGF-β signaling.63
Other molecules in the TGF-β signaling pathway can also interact with miRNAs. For example, ΔNp63α can indirectly impair the expression of Rac family small GTPase 1 (Rac1) (a molecule involved in TGF-β signaling) by regulating miR-320a.64 miR-199a-5p is identified as a CRC suppressor by inhibiting Rho-associated coiled coil-containing protein kinase 1 (ROCK1), another significant regulator in TGF-β signaling.65
miRNAs in Wnt Signaling Pathways
Adenomatous polyposis coli (APC), whose mutation happens in 90% of CRC, plays a suppressive role in Wnt signaling.21 Several miRNAs, including miR-135 and miR-494, are validated to be associated with APC and then result in the dysregulation of Wnt signaling.66,67 In addition, miRNAs can regulate Wnt signaling by regulating other molecules in CRC metastasis.68 For instance, miR-371-5p represses the expression of the Wnt/β-catenin signaling pathway and inhibits CRC metastasis via the SOX17/miR-371-5p/SOX2 axis.68 miR-132, miR-145, and miR-212 can all regulate the Wnt signaling pathway by targeting the TCF4-β-catenin complex and histone trimethylation complex Prc2p (PRC2).69 Additionally, it is reported that miR-34a can suppress the activation of Wnt signaling via sirtuin 1 (SIRT1) and nuclear enriched abundant transcript 1 (NEAT1).70 Zeng et al.71 revealed that miR-378 inhibited metastasis via the suppression of SDA1 domain containing 1P (SDAD1) as well as downregulation of the Wnt/β-catenin pathway.
miRNAs in the Notch Signaling Pathway
Previous studies demonstrated that the Notch signaling pathway was also involved in CRC metastasis and that miRNAs can manipulate it post-transcriptionally.89 miR-1280 can inhibit metastasis by directly inhibiting jagged canonical notch ligand 2 (JAG2), Zeb1, polycomb repressive complex (Suz12), and Gata1/3, which are activators of the Notch signaling pathway.72 miR-200b is also validated as an activator of the Notch signaling pathway.89
lncRNA/miRNA/mRNA Axis
The lncRNA/miRNA/mRNA axis plays important role in tumor metastasis.90 lncRNA widely participates in gene regulatory networks at different levels of gene expression, including chromatin modification, transcription, and post-transcription.91 lncRNA can interact with mRNAs and miRNAs directly or indirectily.92 According to studies, the regulatory network between lncRNAs, miRNAs, and mRNAs is significant and common in carcinogenesis.93 lncRNA acts as a positive transcriptional regulator of mRNAs by competitively binding miRNAs, with this process being termed as the sponge-like effect.94,95 Research on the novel lncRNA/miRNA/mRNA network is valuable to understand the mechanisms of cancer metastasis.
lncMALAT1 can activate the expression of E-cadherin and induce metastasis by interacting with miR-218.73 lncMALAT1 is also reported as a metastasis-inducing factor by suppressing miR-106-5p and stimulating the expression of SLAIN2.74 Moreover, it is reported that lncRNA CYTOR could competitively bind to miR-3679-5p and regulate the expression of MET transcriptional regulator (MACC1).75 Similarly, LINC00707 can promote CRC metastasis by sponging miR-206 and indirectly modulating the expression of neurogenic locus notch homolog protein 3 (NOTCH3) and transmembrane 4 L6 family member 1 (TM4SF1).76 While lncRNA DANCR can stimulate the expression of HSP27 and CRC metastasis via miR-577 sponging.77 Additionally, Chen et al.78 identified that miR-215, as the downstream target of lncRNA UICLM, could negatively regulate ZEB2 and enhance metastasis.
miRNAs and Methylation
Methylation, occurring on the DNA promoter or on miRNA itself, is another significant molecular mechanism related to CRC metastasis.96 Methylation of the DNA promoter located in or near the CpG island, followed by manipulation of the chromatin structure and gene transcription, is usually observed in tumor development. The specific mechanism is probably transcriptional silencing.97 Recent studies have revealed that miRNA-related methylation plays a crucial role in cancer metastasis.79 For example, forkhead box 3 (FOXD3) positively regulates miR-214 via promoter hypermethylation.79 miR-214 binds mediator complex subunit 19 (MED19) and suppresses metastasis afterward.79 Similarly, promoter methylation of miR-34c-5p can also impair the process of CRC metastasis.80 To date, researchers have made great progress in the understanding of the epigenome, and it is generally thought that a small subgroup of the thousands of epigenetic alterations is irreplaceable in driving CRC, among which DNA methylation is the most widely studied.98 The concrete mechanism is still not clearly verified on how methylation influences the activity of miRNAs.98 The prospect is broad although further investigation is necessary.
Methylation occurring on the miRNA itself may also serve as a contributor to CRC metastasis.81 Methyltransferase-like 3 (METTL3), a main methyltransferase involved in the methylation process, assists the maturation of miR-1246 by methylating pri-miR-1246. miR-1246 inhibits the expression of downstream sprout-related EVH1 domain-containing protein 2 (SPRED2) and then upregulates mitogen-activated protein kinases (MAPKs), leading to the malignant phenotype of CRC.81 We assume that modification of the methylation status of miRNAs may be a novel strategy for controlling cancer metastasis.
Clinical Value of miRNAs Involved in Metastasis in CRC
miRNAs as Biomarkers for CRC Diagnosis and Prognosis
Existing CRC screening methods have their limitations. The fecal occult blood test (FOBT) and fecal immunochemical test, convenient and inexpensive, are widely adopted. However, their sensitivity is relatively low for detecting pre-neoplastic lesions.99 The current gold standard for CRC diagnosis is colonoscopy. This method can observe the tumor from both macro and micro perspectives. It is not widely applied for its invasive feature.99 The stool DNA test is characterized by high labor intensity and cost, despite its high sensitivity and specificity. If the concentration of certain miRNAs applied for detection is too low, the result of the stool-based mRNA test varies.100 Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are the most commonly used plasma biomarkers, in which CEA is also widely applied to monitor CRC recurrence, despite their low sensitivity and specificity.100,101 In conclusion, the diagnostic and prognostic methods need to be upgraded so that early stage CRC can be detected with high accuracy and straightforward approaches.
miRNAs have attracted researchers’ attention as biomarkers for several reasons. Primarily, as is revealed from context, accumulating miRNAs have been validated to be significantly associated with CRC progression.102 Since there are abundant CRC-related miRNAs, it is promising to compare the existing miRNAs to select the most suitable and applicable ones as markers.103 Additionally, miRNAs are stable and sensitive such that their expression can be detected quantitatively and reliably from tissues, serum, plasm, stool, circulating exosomes, urine, and even saliva and sputum (Table 3).104,105 Additionally, research on multiple miRNAs and detective technologies is already at the pre-clinical stage. The results indicate that the prospect is extremely broad.2
Table 3.
miRNAs as Diagnostic or Prognostic Biomarkers
| miRNA | Expression | Clinical Application | References |
|---|---|---|---|
| Circulating Serum miRNAs as Biomarkers for CRC | |||
| miR-203 | ↑ | diagnosis | 106, 107 |
| miR-122 | ↑ | diagnosis | 105 |
| miR-200 family | ↑ | ||
| miR-126 | ↓ | diagnosis | 109 |
| miR-21 | ↑ | ||
| miR-210 | ↑ | ||
| miR-592 | ↓ | prognosis | 118 |
| miR-106-5p | ↑ | ||
| miR-615-3p | ↑ | ||
| miR-21 | ↑ | prognosis | 118 |
| miR-103 | ↑ | ||
| miR-93 | ↑ | ||
| miR-566 | ↓ | ||
| Exosomal miRNAs as Biomarkers for CRC | |||
| miR-96-5p | ↑ | diagnosis | 112 |
| miR-149 | ↑ | ||
| miR-17-5p | ↑ | diagnosis | 113 |
| miR-92a-3p | ↑ | ||
| miR-21 | ↑ | prognosis | 119 |
| miR-548c-5p | ↓ | prognosis | 120 |
| Stool-Based miRNAs as Biomarkers for CRC | |||
| miR-4478 | ↑ | diagnosis | 116 |
| miR-1295b-3p | ↑ | ||
| miR-135b | ↑ | prognosis | 122 |
| miR-92a | ↑ | prognosis | 126 |
miRNAs as Diagnostic Biomarkers
Ideal biomarkers should be beneficial for early detection and intervention of CRC. Usually, miRNAs are significantly altered in CRC development and progression, and thus they can be used as practical diagnostic biomarkers.104 Multiple studies have reported that miRNAs in the serum, exosome, and even in stool show potentials for early diagnosis.56,106 Circulating serum miRNAs are the most commonly explored ones, and even single miRNA can be used to diagnose CRC.103,106 For example, serum miR-203 has been revealed as a promising diagnostic factor in CRC patients in several studies due to its positive association with early period CRC and its stability for detection.107,108 miR-122 and the miR-200 family are also associated with early stage CRC. They are quantitatively detectable and could probably be used as one of the multiple markers in the blood test.106 Similarly, downregulation of miR-126, together with upregulation of miR-21 and miR-210, shows great value for early diagnosis of CRC.109 Indeed, a series of co-expressed miRNAs or miRNA profiles are more effective in CRC detection.110
Experts also revealed that exosomal miRNAs and stool-based miRNA detection are promising for early diagnosis.111 For example, downregulation of miR-96-5p and miR-149 in GPC1+ exosomes are observed in CRC patients, and they may serve as potential diagnosis biomarkers in CRC.112 Similarly, early stage CRC patients show increased levels of miR-17-5p and miR-92a-3p in exosomes.113 In addition, decreased expression levels of miR-4478 and miR-1295-p in stool samples represent non-invasive and effective diagnostic biomarkers for CRC patients.114,115 Downregulation of fecal miR-4478 and miR-1295b-3p can also be detected in early stage CRC, indicating their potential roles as promising diagnostic biomarkers for CRC.116
miRNAs as Prognostic Biomarkers
The expression levels of miRNAs are closely associated with progression of CRC, and they can predict the overall survival (OS), possibility of recurrence, and drug response in CRC.117 Therefore, detection of miRNAs in CRC patients can help us to make right decisions in cancer management. Several metastasis-related miRNAs have been considered as potential markers for predicting outcome in CRC patients. For instance, miR-106-5p, miR-615-3p, and miR-592 are closely correlated with the prognosis of CRC patients.118 Similarly, increased miR-21, miR-103, and miR-93, together with downregulated miR-566, are defined as a specific miRNA profile for predicting metastasis and outcome in CRC patients. This profile can be applied to distinguish between primary CRC and CRC with liver metastasis.118 Recently, a two miRNA-based signature in CRC, including let-7i and miR-10b, has also been proposed for use in distinguishing patients with higher risk of metastasis and hepatic recurrence.119
Recent studies also shed light on the roles of exosomal miRNAs in predicting the prognosis of CRC patients.111 For example, exosomal miR-21 is significantly elevated in higher TNM stage CRC patients, and it can serve as an independent risk factor for CRC.120 In addition, reduced miR-548c-5p in serum exosomes is independently correlated with shorter OS and higher TNM stage in CRC patients.121 Researchers have also shown great interest in applying stool-based miRNAs to predict prognosis in CRC patients.122 Advanced CRC patients with higher miR-135b in stool samples showed a significantly shorter OS than did those with lower miR-135b.123 miR-92a was reported as a prognostic biomarker in both serum and stool, and a high level of miR-92a is associated with poor progression-free survival in CRC.124
Theoretically, miRNAs used as promising diagnostic or prognostic biomarkers for CRC have a broad prospect. However, to date, no miRNA has been applied as a reliable biomarker in clinical practice. The causes are multifarious. First, miRNAs are always involved in various carcinomas, and few specific miRNAs in CRC have been validated.125 Second, current detection technologies are not sensitive enough. They should be upgraded so that a tiny concentration and alteration of miRNAs can be sensitively observed. Novel tools such as isothermal amplification techniques, near-infrared technology,126 and energy transfer-based photoelectrochemical127 and molecular beacons128 are all promising methods.102 Additionally, if miRNA detection is to be extensively used in the clinic, convenience and low cost must be taken into consideration.129
miRNA-based Targeted Therapy for CRC Metastasis
A single miRNA could regulate the expression of many mRNA genes, and thus targeting miRNAs may be an effective approach for the development of personalized anti-tumor regimens.130 Moreover, some miRNAs can even target the same mRNA or exert a similar function in CRC. Additionally, manipulating those miRNAs simultaneously may be an available strategy for targeted therapy.131
To date, the methods for developing miRNA-based therapy include repression of the oncogenic miRNAs or activation of the suppressors.122 For the former, we can achieve it by applying anti-miRNA oligonucleotides or blocking related oncogenes via virus-based constructs, miRNA sponges, miRNA-masking, and small molecule inhibitors.132 To realize the later goal, double-stranded miRNA mimics133 and viral/liposomal delivery systems2 can be applicable. However, these methods are inevitably disturbed by toxicity and side effects, including off-target and immune-related effects.110 Additionally, current studies on the clinical application of miRNAs are limited in the pre-clinical stage.134 For example, application of MRX34 (one miR-34 mimic encapsulated in lipid nanoparticles) was halted during the phase I clinical trial for various solid tumors (including CRC) due to severe immune-related adverse effects,135 even though previous evidence proved that it was involved in the manipulation of multi-oncogenic pathways and it displayed obvious anti-tumor effects in advanced CRC.134 Fortunately, several recent studies may change the situation. Miravirsen, an inhibitor of miR-122, was also introduced in a phase II clinical trial for HCV infection, which showed lower toxicity and side effects.136 Moreover, the-poly(amino acid)s/miR-139-5p nanoparticle complex can significantly repress the CRC migration in mice with a much better effect than mere miR-139-5p, and this delivery system is safer and more efficient.137
To develop miRNA-based targeted therapy, the most challenging problem is to explore the novel and effective delivery systems.127 Thus, the use of superior miRNA delivery systems in cancer treatment is expected in the next decade.137 Moreover, the off-target effects from non-specific miRNAs, the toxic and immune responses from the miRNA mimics and inhibitors, and the reduced efficacy due to the degradation of miRNAs are all beyond our expectation, which are anticipated to be minimized or even eliminated.127 One solution is to develop lower toxic miRNA mimics and inhibitors, and this relies on studies involving pharmacological, pharmacokinetic, and pharmacodynamic factors.126 Another approach is to reshape the miRNA expression profiles by self-adjustment of the human body.126 For example, a study has shown that some miRNAs related to urea synthesis (miR-221-3p, miR-221-5p, and miR-222-3p) could be induced in mice by giving them a low-protein diet, which leads to reduced urea synthesis.138 Additionally, the combination of miRNA-based therapy with classical chemotherapy may be the effective approach for drug-resistant CRC patients.137
Discussion
Metastasis is a malignant phenotype of CRC and it is closely related to poor prognosis. Dysregulation of abundant miRNAs contributes to this malignant process and influences the development of CRC. Therefore, reshaping the expression profiles of these miRNAs can partially control cancer metastasis, and finally improve the OS of CRC patients. In this review, we summarized the recent advances of miRNAs in CRC metastasis and discussed their roles in CRC metastasis. The clinical values (diagnostic role, prognostic role, and therapeutic potential) of certain miRNAs in CRC were also summarized. Our review attempts to provide new clues for future research about miRNAs in metastasis of CRC.
miRNAs are dysregulated and play an important role in metastasis of CRC. miRNA expression patterns in metastatic CRC can be inconsistent among different studies.139 This phenomenon can be caused by many factors, including the differences in patient selection criteria, collection methods, biological sample processing, and detection approaches.86 Thus, the normalized criteria and protocol are essential for miRNA research. Moreover, genome-wide searching and bioinformatics analysis can help us to easily identify more miRNAs involved in metastasis, which may improve the accuracy for its clinical application.117
Recently, miRNAs in plasm, blood, and stool show potential use as the diagnostic or prognostic biomarkers. However, the efficiency of these markers in the clinical setting still needs further investigation. First, it is necessary to establish miRNA detection methods with high sensitivity, high specificity, and low labor and economic cost. For instance, liquid biopsy, which refers to analysis of DNA, RNA, and proteins in body fluid (blood, urine, and cerebrospinal fluid), presents higher specificity and accuracy than do other approaches.140 Second, the exosomes containing miRNAs may also be a promising tool for early screening patients. Studies have also indicated that these exosomes can be used as prognostic indicators for cancer metastasis.85
The theories of precision medicine and personalized treatment have attracted researchers’ attention recently.21 miRNAs are promising targets for cancer treatments. miRNAs can simultaneously modulate several mRNAs, suggesting that targeting miRNAs can be more effective than targeting one single mRNA.141 So far, miRNA-based therapy includes miRNA mimics, miRNA inhibitors, and anti-miRNAs.13 These composites can be used to reshape the expression levels of dysregulated miRNAs in different diseases, including cancers. Moreover, the combination of a miRNA-based strategy with chemotherapy may have a wide application in some drug-resistant cancer patients.98 Importantly, novel and superior miRNA delivery systems are the bases for developing miRNA-based targeted therapy.
Collectively, studies on metastasis-related miRNAs have made great progress in elucidating their roles in CRC. These miRNAs also show potential in clinical application for CRC management. However, no miRNAs have already been used in clinical practice for multiple reasons. The search for applicable miRNAs and miRNA profiles, the development of detective methods and techniques, the combination of miRNA-based therapy with other chemotherapy, and the construction of delivery systems are all necessary for further research. Additionally, these improvements will finally be beneficial to improve the OS of CRC patients.
Acknowledgments
This work was supported in part by grants from the Scientific Foundation of Shaanxi Province (nos. 2019ZDLSF01-02-01 and 2018SF-240), the National Clinical Research Center for Digestive Diseases (no. 2015BAI13B07), and the State Key Laboratory of Cancer Biology (no. CBSKL2014Z13).
Author Contributions
L.N., W.Y., and L.D. conceived this manuscript. X.W., Y.L., Y.Z., and J.L. collected and prepared the related references. L.N., W.Y., and L.D. drafted the manuscript. C.X., C.L., and W.Z. drew the figures. W.Y., Q.Z., L.H., and D.F. supervised and revised the manuscript. All authors read and approved the final manuscript.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Xie X., Pan J., Han X., Chen W. Downregulation of microRNA-532-5p promotes the proliferation and invasion of bladder cancer cells through promotion of HMGB3/Wnt/β-catenin signaling. Chem. Biol. Interact. 2019;300:73–81. doi: 10.1016/j.cbi.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G.S., Barzi A., Jemal A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 5.Naxerova K., Reiter J.G., Brachtel E., Lennerz J.K., van de Wetering M., Rowan A., Cai T., Clevers H., Swanton C., Nowak M.A. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357:55–60. doi: 10.1126/science.aai8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mani S.A., Yang J., Brooks M., Schwaninger G., Zhou A., Miura N., Kutok J.L., Hartwell K., Richardson A.L., Weinberg R.A. Mesenchyme forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc. Natl. Acad. Sci. USA. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan H., Liu X., Zheng W.W., Zhuang Z.H., Wang C.D. miR-150 alleviates EMT and cell invasion of colorectal cancer through targeting Gli1. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4853–4859. [PubMed] [Google Scholar]
- 8.Xu Z., Zhu C., Chen C., Zong Y., Feng H., Liu D., Feng W., Zhao J., Lu A. CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1α/VEGF-A pathway in colorectal cancer. Cell Death Dis. 2018;9:974. doi: 10.1038/s41419-018-1010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma H., Pan J.S., Jin L.X., Wu J., Ren Y.D., Chen P., Xiao C., Han J. MicroRNA-17∼92 inhibits colorectal cancer progression by targeting angiogenesis. Cancer Lett. 2016;376:293–302. doi: 10.1016/j.canlet.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Akinc A., Zumbuehl A., Goldberg M., Leshchiner E.S., Busini V., Hossain N., Bacallado S.A., Nguyen D.N., Fuller J., Alvarez R. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J. Mol. Cell. Cardiol. 2016;97:47–55. doi: 10.1016/j.yjmcc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Kim C.W., Oh E.T., Kim J.M., Park J.S., Lee D.H., Lee J.S., Kim K.K., Park H.J. Hypoxia-induced microRNA-590-5p promotes colorectal cancer progression by modulating matrix metalloproteinase activity. Cancer Lett. 2018;416:31–41. doi: 10.1016/j.canlet.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Yang N., Zhu S., Lv X., Qiao Y., Liu Y.J., Chen J. MicroRNAs: pleiotropic regulators in the tumor microenvironment. Front. Immunol. 2018;9:2491. doi: 10.3389/fimmu.2018.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cătană C.S., Pichler M., Giannelli G., Mader R.M., Berindan-Neagoe I. Non-coding RNAs, the Trojan horse in two-way communication between tumor and stroma in colorectal and hepatocellular carcinoma. Oncotarget. 2017;8:29519–29534. doi: 10.18632/oncotarget.15706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Hara S.P., Mott J.L., Splinter P.L., Gores G.J., LaRusso N.F. MicroRNAs: key modulators of posttranscriptional gene expression. Gastroenterology. 2009;136:17–25. doi: 10.1053/j.gastro.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muhammad S., Kaur K., Huang R., Zhang Q., Kaur P., Yazdani H.O., Bilal M.U., Zheng J., Zheng L., Wang X.S. MicroRNAs in colorectal cancer: role in metastasis and clinical perspectives. World J. Gastroenterol. 2014;20:17011–17019. doi: 10.3748/wjg.v20.i45.17011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang J.H., Zhang Z.J., Shang L.R., Luo Y.W., Lin Y.F., Yuan Y., Zhuang S.M. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;68:1459–1475. doi: 10.1002/hep.29920. [DOI] [PubMed] [Google Scholar]
- 18.Yu J., Lei R., Zhuang X., Li X., Li G., Lev S., Segura M.F., Zhang X., Hu G. MicroRNA-182 targets SMAD7 to potentiate TGFβ-induced epithelial-mesenchymal transition and metastasis of cancer cells. Nat. Commun. 2016;7:13884. doi: 10.1038/ncomms13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vu T., Datta P.K. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers (Basel) 2017;9:171. doi: 10.3390/cancers9120171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J.J., Zheng S., Sun L.F., Zheng L. MicroRNA regulation network in colorectal cancer metastasis. World J. Biol. Chem. 2014;5:301–307. doi: 10.4331/wjbc.v5.i3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balacescu O., Sur D., Cainap C., Visan S., Cruceriu D., Manzat-Saplacan R., Muresan M.S., Balacescu L., Lisencu C., Irimie A. The impact of miRNA in colorectal cancer progression and its liver metastases. Int. J. Mol. Sci. 2018;19:3711. doi: 10.3390/ijms19123711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L., Cho K.B., Li Y., Tao G., Xie Z., Guo B. Long noncoding RNA (lncRNA)-mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int. J. Mol. Sci. 2019;20:5758. doi: 10.3390/ijms20225758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cekaite L., Eide P.W., Lind G.E., Skotheim R.I., Lothe R.A. MicroRNAs as growth regulators, their function and biomarker status in colorectal cancer. Oncotarget. 2016;7:6476–6505. doi: 10.18632/oncotarget.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S., Hou X., Wu C., Han L., Li Q., Wang J., Luo S. miR-645 promotes invasiveness, metastasis and tumor growth in colorectal cancer by targeting EFNA5. Biomed. Pharmacother. 2020;125:109889. doi: 10.1016/j.biopha.2020.109889. [DOI] [PubMed] [Google Scholar]
- 25.Przygodzka P., Papiewska-Pająk I., Bogusz-Koziarska H., Sochacka E., Boncela J., Kowalska M.A. Regulation of miRNAs by Snail during epithelial-to-mesenchymal transition in HT29 colon cancer cells. Sci. Rep. 2019;9:2165. doi: 10.1038/s41598-019-39200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu S.H., He X.C., Wang L. Correlation analysis of miR-200b, miR-200c, and miR-141 with liver metastases in colorectal cancer patients. Eur. Rev. Med. Pharmacol. Sci. 2017;21:2357–2363. [PubMed] [Google Scholar]
- 27.Zhao S., Sun H., Jiang W., Mi Y., Zhang D., Wen Y., Cheng D., Tang H., Wu S., Yu Y. miR-4775 promotes colorectal cancer invasion and metastasis via the Smad7/TGFβ-mediated epithelial to mesenchymal transition. Mol. Cancer. 2017;16:12. doi: 10.1186/s12943-017-0585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H., Yan B., Zhang P., Liu S., Li Q., Yang J., Yang F., Chen E. miR-496 promotes migration and epithelial-mesenchymal transition by targeting RASSF6 in colorectal cancer. J. Cell. Physiol. 2020;235:1469–1479. doi: 10.1002/jcp.29066. [DOI] [PubMed] [Google Scholar]
- 29.Zheng K., Zhou X., Yu J., Li Q., Wang H., Li M., Shao Z., Zhang F., Luo Y., Shen Z. Epigenetic silencing of miR-490-3p promotes development of an aggressive colorectal cancer phenotype through activation of the Wnt/β-catenin signaling pathway. Cancer Lett. 2016;376:178–187. doi: 10.1016/j.canlet.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Sheng L., He P., Yang X., Zhou M., Feng Q. miR-612 negatively regulates colorectal cancer growth and metastasis by targeting AKT2. Cell Death Dis. 2015;6:e1808. doi: 10.1038/cddis.2015.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L.X., Hu C.Y., Jing L., Wang M.C., Xu M., Wang J., Wang Y., Nan K.J., Wang S.H. MicroRNA-219-5p inhibits epithelial-mesenchymal transition and metastasis of colorectal cancer by targeting lymphoid enhancer-binding factor 1. Cancer Sci. 2017;108:1985–1995. doi: 10.1111/cas.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z., Liu X., Feng B., Liu N., Wu Q., Han Y., Nie Y., Wu K., Shi Y., Fan D. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer. Oncogene. 2015;34:4808–4820. doi: 10.1038/onc.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Z., Yu L., Luo S., Li M., Li J., Li Q., Sun Y., Wang C. miR-296 inhibits the metastasis and epithelial-mesenchymal transition of colorectal cancer by targeting S100A4. BMC Cancer. 2017;17:140. doi: 10.1186/s12885-017-3121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue L., Yang D. miR-421 inhibited proliferation and metastasis of colorectal cancer by targeting MTA1. J. BUON. 2018;23:1633–1639. [PubMed] [Google Scholar]
- 35.Chen X., Zeng K., Xu M., Liu X., Hu X., Xu T., He B., Pan Y., Sun H., Wang S. p53-induced miR-1249 inhibits tumor growth, metastasis, and angiogenesis by targeting VEGFA and HMGA2. Cell Death Dis. 2019;10:131. doi: 10.1038/s41419-018-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Q., Zhu Y., Wei X., Zhou J., Chang L., Sui H., Han Y., Piao D., Sha R., Bai Y. miR-590-5p inhibits colorectal cancer angiogenesis and metastasis by regulating nuclear factor 90/vascular endothelial growth factor A axis. Cell Death Dis. 2016;7:e2413. doi: 10.1038/cddis.2016.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng Z., Li Y., Pan Y., Lan X., Song F., Sun J., Zhou K., Liu X., Ren X., Wang F. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin Y., Yan Z.P., Lu N.N., Xu Q., He J., Qian X., Yu J., Guan X., Jiang B.H., Liu L.Z. Downregulation of miR-145 associated with cancer progression and VEGF transcriptional activation by targeting N-RAS and IRS1. Biochim. Biophys. Acta. 2013;1829:239–247. doi: 10.1016/j.bbagrm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Qian X., Yu J., Yin Y., He J., Wang L., Li Q., Zhang L.Q., Li C.Y., Shi Z.M., Xu Q. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle. 2013;12:1385–1394. doi: 10.4161/cc.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X.G., Huang X.L., Liang S.Y., Tang S.M., Wu S.K., Huang T.T., Mo Z.N., Wang Q.Y. Identifying miRNA and gene modules of colon cancer associated with pathological stage by weighted gene co-expression network analysis. OncoTargets Ther. 2018;11:2815–2830. doi: 10.2147/OTT.S163891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu L.F., Thai T.H., Calado D.P., Chaudhry A., Kubo M., Tanaka K., Loeb G.B., Lee H., Yoshimura A., Rajewsky K., Rudensky A.Y. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lan J., Sun L., Xu F., Liu L., Hu F., Song D., Hou Z., Wu W., Luo X., Wang J. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 43.Lin X., Wang S., Sun M., Zhang C., Wei C., Yang C., Dou R., Liu Q., Xiong B. miR-195-5p/NOTCH2-mediated EMT modulates IL-4 secretion in colorectal cancer to affect M2-like TAM polarization. J. Hematol. Oncol. 2019;12:20. doi: 10.1186/s13045-019-0708-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Wei C., Yang C., Wang S., Shi D., Zhang C., Lin X., Liu Q., Dou R., Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer. 2019;18:64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang L., Wang X., Wen C., Yang X., Song M., Chen J., Wang C., Zhang B., Wang L., Iwamoto A. hsa-miR-19a is associated with lymph metastasis and mediates the TNF-α induced epithelial-to-mesenchymal transition in colorectal cancer. Sci. Rep. 2015;5:13350. doi: 10.1038/srep13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Z., Zhou R., Liu C., Wang Y., Zhan W., Shao Z., Liu J., Zhang F., Xu L., Zhou X. MicroRNA-105 is involved in TNF-α-related tumor microenvironment enhanced colorectal cancer progression. Cell Death Dis. 2017;8:3213. doi: 10.1038/s41419-017-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin D., Fang Y., Li Z., Chen Z., Xiang J. Epithelial-mesenchymal transition-associated microRNAs in colorectal cancer and drug-targeted therapies (Review) Oncol. Rep. 2015;33:515–525. doi: 10.3892/or.2014.3638. [DOI] [PubMed] [Google Scholar]
- 48.Soheilifar M.H., Grusch M., Neghab H.K., Amini R., Maadi H., Saidijam M., Wang Z. Angioregulatory microRNAs in colorectal cancer. Cancers (Basel) 2019;12:71. doi: 10.3390/cancers12010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baumjohann D., Ansel K.M. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat. Rev. Immunol. 2013;13:666–678. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu K., Pan Q., Zhang X., Kong L.Q., Fan J., Dai Z., Wang L., Yang X.R., Hu J., Wan J.L. miR-146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting PDGFRA expression. Carcinogenesis. 2013;34:2071–2079. doi: 10.1093/carcin/bgt160. [DOI] [PubMed] [Google Scholar]
- 51.Verghese E.T., Drury R., Green C.A., Holliday D.L., Lu X., Nash C., Speirs V., Thorne J.L., Thygesen H.H., Zougman A. miR-26b is down-regulated in carcinoma-associated fibroblasts from ER-positive breast cancers leading to enhanced cell migration and invasion. J. Pathol. 2013;231:388–399. doi: 10.1002/path.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitra A.K., Zillhardt M., Hua Y., Tiwari P., Murmann A.E., Peter M.E., Lengyel E. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012;2:1100–1108. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makondi P.T., Wei P.L., Huang C.Y., Chang Y.J. Development of novel predictive miRNA/target gene pathways for colorectal cancer distance metastasis to the liver using a bioinformatic approach. PLoS ONE. 2019;14:e0211968. doi: 10.1371/journal.pone.0211968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai X., Yin Y., Li N., Zhu D., Zhang J., Zhang C.Y., Zen K. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J. Mol. Cell Biol. 2012;4:341–343. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 55.Zhou M., Chen J., Zhou L., Chen W., Ding G., Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell. Immunol. 2014;292:65–69. doi: 10.1016/j.cellimm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Fan C., Lin Y., Mao Y., Huang Z., Liu A.Y., Ma H., Yu D., Maitikabili A., Xiao H., Zhang C. MicroRNA-543 suppresses colorectal cancer growth and metastasis by targeting KRAS, MTA1 and HMGA2. Oncotarget. 2016;7:21825–21839. doi: 10.18632/oncotarget.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y.X., Chen Y.R., Liu S.S., Ye Y.P., Jiao H.L., Wang S.Y., Xiao Z.Y., Wei W.T., Qiu J.F., Liang L. miR-384 inhibits human colorectal cancer metastasis by targeting KRAS and CDC42. Oncotarget. 2016;7:84826–84838. doi: 10.18632/oncotarget.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L., Li C., Cao L., Li H., Zou H., Li H., Pei H. MicroRNA-877 inhibits malignant progression of colorectal cancer by directly targeting MTDH and regulating the PTEN/Akt pathway. Cancer Manag. Res. 2019;11:2769–2781. doi: 10.2147/CMAR.S194073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Yan L., Yao J., Qiu J. miRNA-495 suppresses proliferation and migration of colorectal cancer cells by targeting FAM83D. Biomed. Pharmacother. 2017;96:974–981. doi: 10.1016/j.biopha.2017.11.138. [DOI] [PubMed] [Google Scholar]
- 60.Li W., Chang J., Wang S., Liu X., Peng J., Huang D., Sun M., Chen Z., Zhang W., Guo W., Li J. miRNA-99b-5p suppresses liver metastasis of colorectal cancer by down-regulating mTOR. Oncotarget. 2015;6:24448–24462. doi: 10.18632/oncotarget.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z., Yang J., Di J., Cui M., Xing J., Wu F., Wu W., Yang H., Zhang C., Yao Z. Downregulated USP3 mRNA functions as a competitive endogenous RNA of SMAD4 by sponging miR-224 and promotes metastasis in colorectal cancer. Sci. Rep. 2017;7:4281. doi: 10.1038/s41598-017-04368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ling H., Pickard K., Ivan C., Isella C., Ikuo M., Mitter R., Spizzo R., Bullock M., Braicu C., Pileczki V. The clinical and biological significance of MIR-224 expression in colorectal cancer metastasis. Gut. 2016;65:977–989. doi: 10.1136/gutjnl-2015-309372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bao Y., Chen Z., Guo Y., Feng Y., Li Z., Han W., Wang J., Zhao W., Jiao Y., Li K. Tumor suppressor microRNA-27a in colorectal carcinogenesis and progression by targeting SGPP1 and Smad2. PLoS ONE. 2014;9:e105991. doi: 10.1371/journal.pone.0105991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aljagthmi A.A., Hill N.T., Cooke M., Kazanietz M.G., Abba M.C., Long W., Kadakia M.P. ΔNp63α suppresses cells invasion by downregulating PKCγ/Rac1 signaling through miR-320a. Cell Death Dis. 2019;10:680. doi: 10.1038/s41419-019-1921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Q.D., Zhou Q.Q., Dong L., Huang Z., Wu F., Deng X. miR-199a-5p inhibits the growth and metastasis of colorectal cancer cells by targeting ROCK1. Technol. Cancer Res. Treat. 2018;17 doi: 10.1177/1533034618775509. 1533034618775509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagel R., le Sage C., Diosdado B., van der Waal M., Oude Vrielink J.A., Bolijn A., Meijer G.A., Agami R. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 2008;68:5795–5802. doi: 10.1158/0008-5472.CAN-08-0951. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y., Guo L., Li Y., Feng G.H., Teng F., Li W., Zhou Q. MicroRNA-494 promotes cancer progression and targets adenomatous polyposis coli in colorectal cancer. Mol. Cancer. 2018;17:1. doi: 10.1186/s12943-017-0753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y., Lv Z., He G., Wang J., Zhang X., Lu G., Ren X., Wang F., Zhu X., Ding Y. The SOX17/miR-371-5p/SOX2 axis inhibits EMT, stem cell properties and metastasis in colorectal cancer. Oncotarget. 2015;6:9099–9112. doi: 10.18632/oncotarget.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang W., Xiao X., Chen X., Huo Y., Xi W.J., Lin Z.F., Zhang D., Li Y.F., Yang F., Wen W.H. Tumor-suppressive miR-145 co-repressed by TCF4-β-catenin and PRC2 complexes forms double-negative regulation loops with its negative regulators in colorectal cancer. Int. J. Cancer. 2018;142:308–321. doi: 10.1002/ijc.31056. [DOI] [PubMed] [Google Scholar]
- 70.Luo Y., Chen J.J., Lv Q., Qin J., Huang Y.Z., Yu M.H., Zhong M. Long non-coding RNA NEAT1 promotes colorectal cancer progression by competitively binding miR-34a with SIRT1 and enhancing the Wnt/β-catenin signaling pathway. Cancer Lett. 2019;440-441:11–22. doi: 10.1016/j.canlet.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Zeng M., Zhu L., Li L., Kang C. miR-378 suppresses the proliferation, migration and invasion of colon cancer cells by inhibiting SDAD1. Cell. Mol. Biol. Lett. 2017;22:12. doi: 10.1186/s11658-017-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang B., Yang H., Cheng X., Wang D., Fu S., Shen W., Zhang Q., Zhang L., Xue Z., Li Y. tRF/miR-1280 suppresses stem cell-like cells and metastasis in colorectal cancer. Cancer Res. 2017;77:3194–3206. doi: 10.1158/0008-5472.CAN-16-3146. [DOI] [PubMed] [Google Scholar]
- 73.Li P., Zhang X., Wang H., Wang L., Liu T., Du L., Yang Y., Wang C. MALAT1 is associated with poor response to oxaliplatin-based chemotherapy in colorectal cancer patients and promotes chemoresistance through EZH2. Mol. Cancer Ther. 2017;16:739–751. doi: 10.1158/1535-7163.MCT-16-0591. [DOI] [PubMed] [Google Scholar]
- 74.Zhuang M., Zhao S., Jiang Z., Wang S., Sun P., Quan J., Yan D., Wang X. MALAT1 sponges miR-106b-5p to promote the invasion and metastasis of colorectal cancer via SLAIN2 enhanced microtubules mobility. EBioMedicine. 2019;41:286–298. doi: 10.1016/j.ebiom.2018.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M., Wang Q., Xue F., Wu Y. lncRNA-CYTOR works as an oncogene through the CYTOR/miR-3679-5p/MACC1 axis in colorectal cancer. DNA Cell Biol. 2019;38:572–582. doi: 10.1089/dna.2018.4548. [DOI] [PubMed] [Google Scholar]
- 76.Zhu H., He G., Wang Y., Hu Y., Zhang Z., Qian X., Wang Y. Long intergenic noncoding RNA 00707 promotes colorectal cancer cell proliferation and metastasis by sponging miR-206. OncoTargets Ther. 2019;12:4331–4340. doi: 10.2147/OTT.S198140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y., Lu Z., Wang N., Feng J., Zhang J., Luan L., Zhao W., Zeng X. Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Exp. Mol. Med. 2018;50:1–17. doi: 10.1038/s12276-018-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen D.L., Lu Y.X., Zhang J.X., Wei X.L., Wang F., Zeng Z.L., Pan Z.Z., Yuan Y.F., Wang F.H., Pelicano H. Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression. Theranostics. 2017;7:4836–4849. doi: 10.7150/thno.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He G.Y., Hu J.L., Zhou L., Zhu X.H., Xin S.N., Zhang D., Lu G.F., Liao W.T., Ding Y.Q., Liang L. The FOXD3/miR-214/MED19 axis suppresses tumour growth and metastasis in human colorectal cancer. Br. J. Cancer. 2016;115:1367–1378. doi: 10.1038/bjc.2016.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gu J., Wang G., Liu H., Xiong C. SATB2 targeted by methylated miR-34c-5p suppresses proliferation and metastasis attenuating the epithelial-mesenchymal transition in colorectal cancer. Cell Prolif. 2018;51:e12455. doi: 10.1111/cpr.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng W., Li J., Chen R., Gu Q., Yang P., Qian W., Ji D., Wang Q., Zhang Z., Tang J., Sun Y. Upregulated METTL3 promotes metastasis of colorectal cancer via miR-1246/SPRED2/MAPK signaling pathway. J. Exp. Clin. Cancer Res. 2019;38:393. doi: 10.1186/s13046-019-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim S.W. [The role of microRNAs in colorectal cancer] Korean J. Gastroenterol. 2017;69:206–211. doi: 10.4166/kjg.2017.69.4.206. [DOI] [PubMed] [Google Scholar]
- 83.Lièvre A., Bachet J.B., Boige V., Cayre A., Le Corre D., Buc E., Ychou M., Bouché O., Landi B., Louvet C. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 84.Velho S., Oliveira C., Ferreira A., Ferreira A.C., Suriano G., Schwartz S., Jr., Duval A., Carneiro F., Machado J.C., Hamelin R. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur. J. Cancer. 2005;41:1649–1654. doi: 10.1016/j.ejca.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 85.Principe D.R., Doll J.A., Bauer J., Jung B., Munshi H.G., Bartholin L., Pasche B., Lee C., Grippo P.J. TGF-β: duality of function between tumor prevention and carcinogenesis. J. Natl. Cancer Inst. 2014;106:djt369. doi: 10.1093/jnci/djt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.To K.K., Tong C.W., Wu M., Cho W.C. MicroRNAs in the prognosis and therapy of colorectal cancer: from bench to bedside. World J. Gastroenterol. 2018;24:2949–2973. doi: 10.3748/wjg.v24.i27.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grady W.M., Myeroff L.L., Swinler S.E., Rajput A., Thiagalingam S., Lutterbaugh J.D., Neumann A., Brattain M.G., Chang J., Kim S.J. Mutational inactivation of transforming growth factor β receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 88.Zhang F., Luo Y., Shao Z., Xu L., Liu X., Niu Y., Shi J., Sun X., Liu Y., Ding Y., Zhao L. MicroRNA-187, a downstream effector of TGFβ pathway, suppresses Smad-mediated epithelial-mesenchymal transition in colorectal cancer. Cancer Lett. 2016;373:203–213. doi: 10.1016/j.canlet.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 89.Ramadan F., Fahs A., Ghayad S.E., Saab R. Signaling pathways in Rhabdomyosarcoma invasion and metastasis. Cancer Metastasis Rev. 2020;39:287–301. doi: 10.1007/s10555-020-09860-3. [DOI] [PubMed] [Google Scholar]
- 90.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 91.Ørom U.A., Derrien T., Beringer M., Gumireddy K., Gardini A., Bussotti G., Lai F., Zytnicki M., Notredame C., Huang Q. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu Y., Nangia-Makker P., Farhana L., Majumdar A.P.N. A novel mechanism of lncRNA and miRNA interaction: CCAT2 regulates miR-145 expression by suppressing its maturation process in colon cancer cells. Mol. Cancer. 2017;16:155. doi: 10.1186/s12943-017-0725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yoon J.H., Abdelmohsen K., Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 2014;34:9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.López-Urrutia E., Bustamante Montes L.P., Ladrón de Guevara Cervantes D., Pérez-Plasencia C., Campos-Parra A.D. Crosstalk between long non-coding RNAs, micro-RNAs and mRNAs: deciphering molecular mechanisms of master regulators in cancer. Front. Oncol. 2019;9:669. doi: 10.3389/fonc.2019.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tse J.W.T., Jenkins L.J., Chionh F., Mariadason J.M. Aberrant DNA methylation in colorectal cancer: what should we target? Trends Cancer. 2017;3:698–712. doi: 10.1016/j.trecan.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 97.Ozsolak F., Poling L.L., Wang Z., Liu H., Liu X.S., Roeder R.G., Zhang X., Song J.S., Fisher D.E. Chromatin structure analyses identify miRNA promoters. Genes Dev. 2008;22:3172–3183. doi: 10.1101/gad.1706508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Puccini A., Berger M.D., Naseem M., Tokunaga R., Battaglin F., Cao S., Hanna D.L., McSkane M., Soni S., Zhang W., Lenz H.J. Colorectal cancer: epigenetic alterations and their clinical implications. Biochim. Biophys. Acta Rev. Cancer. 2017;1868:439–448. doi: 10.1016/j.bbcan.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Issa I.A., Noureddine M. Colorectal cancer screening: an updated review of the available options. World J. Gastroenterol. 2017;23:5086–5096. doi: 10.3748/wjg.v23.i28.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu C.W., Ng S.S., Dong Y.J., Ng S.C., Leung W.W., Lee C.W., Wong Y.N., Chan F.K., Yu J., Sung J.J. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61:739–745. doi: 10.1136/gut.2011.239236. [DOI] [PubMed] [Google Scholar]
- 101.Duffy M.J. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin. Chem. 2001;47:624–630. [PubMed] [Google Scholar]
- 102.Chen B., Xia Z., Deng Y.N., Yang Y., Zhang P., Zhu H., Xu N., Liang S. Emerging microRNA biomarkers for colorectal cancer diagnosis and prognosis. Open Biol. 2019;9:180212. doi: 10.1098/rsob.180212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kijima T., Hazama S., Tsunedomi R., Tanaka H., Takenouchi H., Kanekiyo S., Inoue Y., Nakashima M., Iida M., Sakamoto K. MicroRNA-6826 and -6875 in plasma are valuable non-invasive biomarkers that predict the efficacy of vaccine treatment against metastatic colorectal cancer. Oncol. Rep. 2017;37:23–30. doi: 10.3892/or.2016.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L., Peterson A., Noteboom J., O’Briant K.C., Allen A. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwarzenbach H., Hoon D.S., Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 106.Maierthaler M., Benner A., Hoffmeister M., Surowy H., Jansen L., Knebel P., Chang-Claude J., Brenner H., Burwinkel B. Plasma miR-122 and miR-200 family are prognostic markers in colorectal cancer. Int. J. Cancer. 2017;140:176–187. doi: 10.1002/ijc.30433. [DOI] [PubMed] [Google Scholar]
- 107.Hur K., Toiyama Y., Okugawa Y., Ide S., Imaoka H., Boland C.R., Goel A. Circulating microRNA-203 predicts prognosis and metastasis in human colorectal cancer. Gut. 2017;66:654–665. doi: 10.1136/gutjnl-2014-308737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Okugawa Y., Toiyama Y., Hur K., Yamamoto A., Yin C., Ide S., Kitajima T., Fujikawa H., Yasuda H., Koike Y. Circulating miR-203 derived from metastatic tissues promotes myopenia in colorectal cancer patients. J. Cachexia Sarcopenia Muscle. 2019;10:536–548. doi: 10.1002/jcsm.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sabry D., El-Deek S.E.M., Maher M., El-Baz M.A.H., El-Bader H.M., Amer E., Hassan E.A., Fathy W., El-Deek H.E.M. Role of miRNA-210, miRNA-21 and miRNA-126 as diagnostic biomarkers in colorectal carcinoma: impact of HIF-1α-VEGF signaling pathway. Mol. Cell. Biochem. 2019;454:177–189. doi: 10.1007/s11010-018-3462-1. [DOI] [PubMed] [Google Scholar]
- 110.Xuan Y., Yang H., Zhao L., Lau W.B., Lau B., Ren N., Hu Y., Yi T., Zhao X., Zhou S., Wei Y. MicroRNAs in colorectal cancer: small molecules with big functions. Cancer Lett. 2015;360:89–105. doi: 10.1016/j.canlet.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 111.Kosaka N., Iguchi H., Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–2092. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li J., Chen Y., Guo X., Zhou L., Jia Z., Peng Z., Tang Y., Liu W., Zhu B., Wang L., Ren C. GPC1 exosome and its regulatory miRNAs are specific markers for the detection and target therapy of colorectal cancer. J. Cell. Mol. Med. 2017;21:838–847. doi: 10.1111/jcmm.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fu F., Jiang W., Zhou L., Chen Z. Circulating exosomal miR-17-5p and miR-92a-3p predict pathologic stage and grade of colorectal cancer. Transl. Oncol. 2018;11:221–232. doi: 10.1016/j.tranon.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahmed F.E., Jeffries C.D., Vos P.W., Flake G., Nuovo G.J., Sinar D.R., Naziri W., Marcuard S.P. Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue. Cancer Genomics Proteomics. 2009;6:281–295. [PubMed] [Google Scholar]
- 115.Ghanbari R., Rezasoltani S., Hashemi J., Mohamadkhani A., Tahmasebifar A., Arefian E., Mobarra N., Asadi J., Nazemalhosseini Mojarad E., Yazdani Y. Expression analysis of previously verified fecal and plasma dow-regulated microRNAs (miR-4478, 1295-3p, 142-3p and 26a-5p), in FFPE tissue samples of CRC patients. Arch. Iran Med. 2017;20:92–95. [PubMed] [Google Scholar]
- 116.Ghanbari R., Mosakhani N., Asadi J., Nouraee N., Mowla S.J., Poustchi H., Malekzadeh R., Knuutila S. Decreased expression of fecal miR-4478 and miR-1295b-3p in early-stage colorectal cancer. Cancer Biomark. 2015;15:189–195. doi: 10.3233/CBM-140453. [DOI] [PubMed] [Google Scholar]
- 117.Dong Y., Wu W.K., Wu C.W., Sung J.J., Yu J., Ng S.S. MicroRNA dysregulation in colorectal cancer: a clinical perspective. Br. J. Cancer. 2011;104:893–898. doi: 10.1038/bjc.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Drusco A., Nuovo G.J., Zanesi N., Di Leva G., Pichiorri F., Volinia S., Fernandez C., Antenucci A., Costinean S., Bottoni A. MicroRNA profiles discriminate among colon cancer metastasis. PLoS ONE. 2014;9:e96670. doi: 10.1371/journal.pone.0096670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Coebergh van den Braak R.R.J., Sieuwerts A.M., Lalmahomed Z.S., Smid M., Wilting S.M., Bril S.I., Xiang S., van der Vlugt-Daane M., de Weerd V., van Galen A., MATCH study group∗ Confirmation of a metastasis-specific microRNA signature in primary colon cancer. Sci. Rep. 2018;8:5242. doi: 10.1038/s41598-018-22532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsukamoto M., Iinuma H., Yagi T., Matsuda K., Hashiguchi Y. Circulating exosomal MicroRNA-21 as a biomarker in each tumor stage of colorectal cancer. Oncology. 2017;92:360–370. doi: 10.1159/000463387. [DOI] [PubMed] [Google Scholar]
- 121.Peng Z.Y., Gu R.H., Yan B. Downregulation of exosome-encapsulated miR-548c-5p is associated with poor prognosis in colorectal cancer. J. Cell. Biochem. 2019;120:1457–1463. doi: 10.1002/jcb.27291. [DOI] [PubMed] [Google Scholar]
- 122.Chi Y., Zhou D. MicroRNAs in colorectal carcinoma—from pathogenesis to therapy. J. Exp. Clin. Cancer Res. 2016;35:43. doi: 10.1186/s13046-016-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu C.W., Ng S.C., Dong Y., Tian L., Ng S.S., Leung W.W., Law W.T., Yau T.O., Chan F.K., Sung J.J. Identification of microRNA-135b in stool as a potential noninvasive biomarker for colorectal cancer and adenoma. Clin. Cancer Res. 2014;20:2994–3002. doi: 10.1158/1078-0432.CCR-13-1750. [DOI] [PubMed] [Google Scholar]
- 124.Chen E., Li Q., Wang H., Yang F., Min L., Yang J. miR-92a promotes tumorigenesis of colorectal cancer, a transcriptomic and functional based study. Biomed. Pharmacother. 2018;106:1370–1377. doi: 10.1016/j.biopha.2018.07.098. [DOI] [PubMed] [Google Scholar]
- 125.Humphreys K.J., McKinnon R.A., Michael M.Z. miR-18a inhibits CDC42 and plays a tumour suppressor role in colorectal cancer cells. PLoS ONE. 2014;9:e112288. doi: 10.1371/journal.pone.0112288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moody L., He H., Pan Y.X., Chen H. Methods and novel technology for microRNA quantification in colorectal cancer screening. Clin. Epigenetics. 2017;9:119. doi: 10.1186/s13148-017-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang N., Shi X.M., Guo H.Q., Zhao X.Z., Zhao W.W., Xu J.J., Chen H.Y. Gold nanoparticle couples with entropy-driven toehold-mediated DNA strand displacement reaction on magnetic beads: toward ultrasensitive energy-transfer-based photoelectrochemical detection of miRNA-141 in real blood sample. Anal. Chem. 2018;90:11892–11898. doi: 10.1021/acs.analchem.8b01966. [DOI] [PubMed] [Google Scholar]
- 128.Liu Q., Fan J., Zhou C., Wang L., Zhao B., Zhang H., Liu B., Tong C. Quantitative detection of miRNA-21 expression in tumor cells and tissues based on molecular beacon. Int. J. Anal. Chem. 2018;2018:3625823. doi: 10.1155/2018/3625823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fujii S., Kamiya K., Osaki T., Misawa N., Hayakawa M., Takeuchi S. Purification-free microRNA detection by using magnetically immobilized nanopores on liposome membrane. Anal. Chem. 2018;90:10217–10222. doi: 10.1021/acs.analchem.8b01443. [DOI] [PubMed] [Google Scholar]
- 130.Huang Z., Huang D., Ni S., Peng Z., Sheng W., Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int. J. Cancer. 2010;127:118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 131.Huang S., Tan X., Huang Z., Chen Z., Lin P., Fu S.W. MicroRNA biomarkers in colorectal cancer liver metastasis. J. Cancer. 2018;9:3867–3873. doi: 10.7150/jca.28588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gentner B., Schira G., Giustacchini A., Amendola M., Brown B.D., Ponzoni M., Naldini L. Stable knockdown of microRNA in vivo by lentiviral vectors. Nat. Methods. 2009;6:63–66. doi: 10.1038/nmeth.1277. [DOI] [PubMed] [Google Scholar]
- 133.De Guire V., Caron M., Scott N., Ménard C., Gaumont-Leclerc M.F., Chartrand P., Major F., Ferbeyre G. Designing small multiple-target artificial RNAs. Nucleic Acids Res. 2010;38:e140. doi: 10.1093/nar/gkq354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beg M.S., Brenner A.J., Sachdev J., Borad M., Kang Y.K., Stoudemire J., Smith S., Bader A.G., Kim S., Hong D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest. New Drugs. 2017;35:180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gandellini P., Doldi V., Zaffaroni N. MicroRNAs as players and signals in the metastatic cascade: implications for the development of novel anti-metastatic therapies. Semin. Cancer Biol. 2017;44:132–140. doi: 10.1016/j.semcancer.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 136.Janssen H.L., Reesink H.W., Lawitz E.J., Zeuzem S., Rodriguez-Torres M., Patel K., van der Meer A.J., Patick A.K., Chen A., Zhou Y. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y., Costanza F., Li C., Nimmagadda A., Song D., Cai J. PEG-poly(amino acid)s/microRNA complex nanoparticles effectively arrest the growth and metastasis of colorectal cancer. J. Biomed. Nanotechnol. 2016;12:1510–1519. doi: 10.1166/jbn.2016.2253. [DOI] [PubMed] [Google Scholar]
- 138.Sun R.P., Xi Q.Y., Sun J.J., Cheng X., Zhu Y.L., Ye D.Z., Chen T., Wei L.M., Ye R.S., Jiang Q.Y., Zhang Y.L. In low protein diets, microRNA-19b regulates urea synthesis by targeting SIRT5. Sci. Rep. 2016;6:33291. doi: 10.1038/srep33291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jarry J., Schadendorf D., Greenwood C., Spatz A., van Kempen L.C. The validity of circulating microRNAs in oncology: five years of challenges and contradictions. Mol. Oncol. 2014;8:819–829. doi: 10.1016/j.molonc.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Normanno N., Cervantes A., Ciardiello F., De Luca A., Pinto C. The liquid biopsy in the management of colorectal cancer patients: current applications and future scenarios. Cancer Treat. Rev. 2018;70:1–8. doi: 10.1016/j.ctrv.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 141.Yang W., Ma J., Zhou W., Zhou X., Cao B., Zhang H., Zhao Q., Fan D., Hong L. Molecular mechanisms and clinical implications of miRNAs in drug resistance of esophageal cancer. Expert Rev. Gastroenterol. Hepatol. 2017;11:1151–1163. doi: 10.1080/17474124.2017.1372189. [DOI] [PubMed] [Google Scholar]