Abstract
Given the widespread concern but general lack of information over the possibility of SARS-CoV-2 infection in public transport, key issues such as passenger personal hygiene, efficient air circulation systems, and the effective disinfection of frequently touched surfaces need to be evaluated to educate the public and diminish the risk of viral transmission as we learn to live with the ongoing pandemic. In this context we report on a study involving the collection of 99 samples taken from inside Barcelona buses and subway trains in May to July 2020. From this sample group 82 (58 surface swabs, 9 air conditioning (a/c) filters, 3 a/c dust, 12 ambient air) were selected to be analysed by RT-PCR for traces of the SARS-CoV-2 virus. Thirty of these selected samples showed evidence for one or more of 3 target RNA gene regions specific for this virus (IP2, IP4, E). Most (24) of these 30 samples showed positivity for only 1 of the 3 RNA targets, 4 samples yielded 2 targets, and 2 samples provided evidence for all 3 targets. RNA remnants were more common in surface swabs from support bars (23 out of 58) than in ambient air inside the vehicles (3 out of 12), with relatively higher concentrations of viral RNA fragments in buses rather than in trains. Whereas subway train a/c filters examined were all virus-free, 4 of the 9 bus a/c filter/dust samples yielded evidence for viral RNA. After nocturnal maintenance and cleaning most buses initially yielding positive results subsequently showed elimination of the RT-PCR signal, although signs of viral RNA remained in 4 of 13 initially positive samples. The presence of such remnant viral traces however does not demonstrate infectivity, which in the present study is considered unlikely given the fragmentary nature of the gene targets detected. Nevertheless, best practice demands that close attention to ventilation systems and regular vehicle disinfection in public transport worldwide need to be rigorously applied to be effective at eliminating traces of the virus throughout the vehicle, especially at times when COVID-19 cases are peaking. Additionally, infectivity tests should be implemented to evaluate the efficiency of disinfection procedures to complement the information resulting from RT-PCR analysis. Modelling the probability of infection whilst travelling in buses under different scenarios indicates that forced ventilation greatly reduces the risk.
Keywords: SARS-CoV-2, COVID-19, Disinfection, Public transport, Ozone, RT-qPCR
1. Introduction
The possibility of SARS-CoV-2 infection while travelling in public transport is an obvious concern for the millions of commuters around the world using this transportation means. As the current COVID-19 pandemic spread across the world this concern has led to many people choosing to avoid public transportation systems, which consequently have been hit especially hard (Tan and Ma, 2020, Tirachini and Cats, 2020). Those in favour of using public transportation and its environmental benefits emphasise the likely efficacy of current protocols on mask wearing, personal hygiene, improved ventilation and avoiding rush hour travel, and argue that available data on COVID-19 clusters suggest the risk of infection in such transport microenvironments is relatively low (Normile, 2020, Sadik-Khan and Solomonow, 2020; Santé Publique France, 2020). To date, however, published scientific evidence related to possible COVID-19 transmission on city buses, trams and subways is very limited.
Our understanding of the spread of COVID-19 in public transportation is based on the knowledge that the most important routes for the transmission of respiratory viral diseases are via small airborne micro-droplets (aerosols), larger respiratory droplets (which fall close to where they are expired), and through contact with contaminated surfaces (fomites) (Jones, 2020, Morawska et al., 2020, Ren et al., 2020, Yao et al., 2020). After initial focus on the role of fomites and larger droplets, aerosol transmission of the disease is now increasingly recognised to be important (e.g. Miller et al., 2020, Morawska and Milton, 2020). In a report published in July 2020 the World Health Organisation moved forward in its recognition of the likely role of aerosols in spreading COVID-19, concluding that Current evidence suggests that the main way the virus spreads is by respiratory droplets among people who are in close contact with each other. Aerosol transmission can occur in specific settings, particularly in indoor, crowded and inadequately ventilated spaces, where infected person(s) spend long periods of time with others, such as restaurants, choir practices, fitness classes, nightclubs, offices and/or places of worship (WHO, 2020a, WHO, 2020).
When a person harbouring SARS-CoV-2 is breathing, speaking, shouting, sneezing or coughing, potentially infectious particles are emitted into the surrounding atmosphere (Allen and Marr, 2020). These particles can remain suspended for hours, with complex movements and thermodynamic transformations (e.g. dehydration and evaporation) affecting the droplets as soon as they are emitted depending on factors such as air flow, temperature and humidity. It has been shown that SARS-CoV-2 present in experimentally-generated aerosols can remain biologically active in the air for 3 h (van Doremalen et al., 2020), and possibly considerably more (Fears et al., 2020, Moriarty et al., 2020). Likewise, it is known that SARS-CoV-2 can remain viable for hours or even days after its deposition on surfaces (Bar-On et al., 2020, Biryukov et al., 2020, Chin et al., 2020, Ren et al., 2020). However, it is important to emphasise that the presence of fragments of the viral genome able to yield positive RT-PCR signals is not an indication of the occurrence of infectious viruses. In this context, despite RNA SARS-CoV-2 traces having been detected in outdoor air in routine surveillance of air quality, sampling industrial and urban background atmospheric particulate matter (PM), their infectivity was not tested so that it remains unclear whether they were biologically viable (Setti et al., 2020). Of particular relevance here is the fact that outdoor exposure to UV light works to inactivate the virus. Decay rates in simulated saliva, under simulated sunlight levels representative of late winter/early fall and summer have been reported as 0.121 ± 0.017 min−1 (90% loss: 19 min) and 0.379 ± 0.072 min−1 (90% loss: 6 min), respectively (Schuit et al., 2020). Thus the extrapolation of data produced from artificially-generated aerosols to actual-life transmission of the SARS-CoV-2 infection needs to be interpreted carefully.
Most of the relevant studies on the current pandemic published to date have reported on the presence of the virus within hospitals. In such environments nosocomial transmission is known to have taken place because SARS-CoV-2 can be widely distributed both in fomites (that is, any passive vector capable of contaminating and transmitting the disease) and in air (e.g. Guo et al., 2020, Jiang et al., 2020, Kampf et al., 2020, Liu et al., 2020, Ong et al., 2020, Rahmani et al., 2020, Sommerstein et al., 2020, Tran et al., 2012; Zhou et al., 2020, Morawska and Milton, 2020). Particularly implicated in contamination on surfaces in hospital rooms are floors, air vents, bedrails, bathroom areas, patient masks, hand sanitizer dispensers, door handles and personal protective equipment (Chia et al., 2020, Guo et al., 2020, Razzini et al., 2020, Hirota, 2020). Viruses deposited on these surfaces have the potential to be picked up by susceptible persons touching these surfaces. They also have the potential to be resuspended in the air, adding an additional complicating factor to the challenge of preventing their transmission, and emphasising the importance of cleaning protocols. Airborne virus can be inhaled and cause infection if the dose is high enough.
Regarding urban public transport settings by road, rail and air, although there is little scientific evidence on relevant contributions to the total SARS-CoV-2 infections, it is reasonable to assume that crowding, poor air circulation, and/or lack of sanitation and personal protection in these environments will increase the risk of microbial infection (Coleman et al., 2018, Furuya, 2007, Goscé and Johansson, 2018, Ikonen et al., 2018, Lei et al., 2018, Morawska and Cao, 2020, Morawska and Milton, 2020, Musselwhite et al., 2020, Nishiura et al., 2020, Qian et al., 2020). This is well demonstrated by a case of COVID-19 infection between private bus passengers during a day trip to a religious event in January 2020 (Shen et al., 2020), although this occurred just before widespread pandemic awareness and so the passengers travelled unprotected by masks or disinfection measures. By now, however, the entire human population is well aware of the problem and attention has inevitably turned to the possibility of transmission of COVID-19 due to the proximity of passengers even wearing masks passing on the infection during crowding and sometimes prolonged time inhalation exposure (Lou et al., 2020), thus highlighting the importance of ventilation systems, personal hygiene, and effective disinfection of frequently contacted surfaces (Morawska et al., 2020, SAGE, 2020). Mechanical escalator handrails for example are known to be capable of carrying a high microbial load and can therefore be the focus of pathogen transmission (Afshinnekoo et al., 2015), and similar arguments presumably apply to the bars, buttons and handles in public transport vehicles which are regularly touched by passengers. Obvious ways of reducing the risk of infection in the closed spaces typical of public transport vehicles involve increasing the ventilation (uptake of external air, Morawska et al., 2020), developing best practice disinfection protocols, and insisting on personal mask protection (Chu et al., 2020, Dzisi and Dei, 2020, Eikenberry et al., 2020, Greenhalgh et al., 2020, Javid et al., 2020, Leung et al., 2020, Prather et al., 2020). Such improved hygiene practices are already widely employed in our city transport systems (e.g. Iolov, 2020) but would benefit from scientific information on the presence or otherwise of viral traces within these public vehicles.
In this paper therefore we present the results of a study carried out from May to July 2020 in public buses and subway trains in the city of Barcelona, Spain, specifically looking for the presence of traces of SARS-CoV-2 RNA on surfaces, in the air, and in the air conditioning systems. The samples from buses included collecting before and after the nocturnal maintenance period. Additionally, we have modelled the effect of increasing ventilation rate inside a bus in reducing the individual infection risk. The study provides an example of a close collaborative effort between academic research teams working together with the local transport company to produce peer-reviewed scientific data published and freely available as an open access paper.
2. Methodology
The study involved a total of 75 samples from buses and 24 from subway trains, collected from surfaces using swabs (78 samples), from ambient air (12 samples), and from air-conditioning filters (9 samples). In the case of the buses, the sampling was done in the bus depot in the early morning before (Sample Group A) and after (Sample Group B) the night time maintenance period, which included cleaning operations using bleach or ozone (O3), whereas subway sampling was done during late working hours. The samples were then analyzed for the occurrence of SARS-CoV-2 RNA.
2.1. Subway
Sampling of surfaces inside carriages of 15 subway trains took place on the night of June 11, 2020 between 21:00 and 22:15 at the end of lines 1, 3 and 5 (L1, L3 and L5, Table S1), as chosen by Transports Metropolitans of Barcelona (TMB) for the study. The sampling was carried out in the first (front) carriage after the train had been vacated by passengers and was in the process of changing tracks for the return journey. Polyester swabs (ref 300265, Deltalab, Barcelona) were used to sample all the vertical support bars in the carriage. Around 300 cm2 located around 1.00–1.25 m from the floor (a height deduced to be an area of maximum hand contact by passengers, Fig. 1 a) were scrubbed per bar. In addition, on L3, where door opening is manual, door handles (around 25 cm2) were also sampled. Swabs were immediately placed in tubes with 1 ml of transport medium (ref 304103, Deltalab).
Fig. 1.
Sampling locations in the subway train surfaces (a) and air (b), and buses surfaces (c) and air (d).
Air sampling in the subway took place the following week (June 17–19, 2020) in lines L1, L3, and L5 on three consecutive days. Six samples of particulate matter with a diameter of<2.5 µm (PM2.5) were collected inside 6 trains using 47 mm Teflon filters with PEM (Personal Environmental Monitor) equipment (Table S1). The instruments operated at 10 L/min with Leland pumps placed inside the driver's cabin and connected to filters placed above the closed access door to the driver area on the passenger side (Fig. 1b). Sampling was carried out simultaneously on two trains on the same line for 9–10 h of train running (Table S1). Finally, TMB supplied three samples of 3 air conditioning filters (one of which had been treated with a virucidal agent before testing) used in wagons of the same lines (L1, L3 and L5).
2.2. Buses
The sampling of the buses for the possible presence of SARS-CoV-2 took place between 20:00 and 03:00 on the night of May 25–26, 2020 in one of the four main bus depots in Barcelona. For the sampling performed before the nightly maintenance and cleaning, polyester swabs were wiped across the left side of call buttons (around 10 cm2) and plastic/aluminium holding bars (250 cm2, 20 cm above and below each call button: Samples A Fig. 1c). After sampling, the bus was driven away for maintenance and disinfection, a procedure that in most cases took between 2 and 5 h. The disinfection was done using either bleach (manual cleaning with 5% sodium hypochlorite) or ozone (O3 cannon inside closed bus for 20 min). In the latter case, O3 concentrations, in the range of 1 to 1000 ppb (2 to 2000 µg/m3) were measured, using POM instruments (2B-USA, based on ultraviolet radiation absorption method) in five buses. After each bus had been cleaned and prepared, the sampling procedure was repeated but on the right side of the same call buttons and bars (Samples B). Table S1 summarises the sample numbers, timings and type of disinfection applied.
With regard to the O3 disinfection procedures, an in-vitro study to test the inactivation capacity of different O3 treatments using a surrogate for SARS-CoV-2 (Coronavirus 229E, hereinafter CoV 229E) on bus bar surfaces was also performed. To this end bus bars were removed from the buses and transported to the Enteric Virus Laboratory facilities and 100 µl of a viral suspension of CoV 229E (105 TCID50) was spiked on each of nine surface (around 5 cm2) in a BSL-2 cabinet. After allowing the suspension to dry for 30 min, 3 untreated control samples were left standing aside, and O3 treatments of 300 and 600 ppb for 20 min at 60% relative humidity were each applied to 3 experimental surfaces. O3 concentrations were monitored using a POM O3 monitor (2B Technologies). After the O3 treatments, the virus was recovered from untreated and treated surfaces using swabs which were subsequently placed in tubes with 1 ml of transport medium for infectivity determination.
Additionally, three samples from each air conditioning (a/c) filter in six buses were collected. In three of these six buses, samples of dust from immediately behind the a/c filter were also collected (Table S1).
Finally, ambient air particles (PM2.5) were collected during the entire bus work day from further six buses operating on the June 3 to 5, 2020 (two buses per day; routes selected by TMB) using the same protocol described to this end for the subway (Fig. 1d, Table S1).
2.3. Real-Time RT-qPCR
Virus detection on surfaces was carried out by analysing the presence of viral RNA with Real-Time Reverse-Transcription PCR (Real-Time RT-qPCR) using three target gene regions: two targets from the RNA-dependent RNA polymerase (IP2 and IP4 designed by Institute Pasteur; Protocol: Real-time RT-PCR assays for the detection of SARS-CoV-2: WHO 2020b) and a fragment from the envelope gene (E designed by Charité, Berlin; Schoeman and Fielding, 2019, Corman et al., 2020). Three one-step RT-qPCR assays were performed using the RNA UltraSense™ One-Step Quantitative RT-PCR System (Invitrogen, Life Technologies). The standard curve was constructed using the Twist Synthetic SARS-CoV-2 RNA Control 2 (MN908947.3) (Twist Bioscience) and their parameters are shown in Table S2 of the Supplementary Information.
Quality control and quality assurance were ascertained using negative and positive controls to determine any potential contamination and/or inhibition, respectively. The positive controls consisted of the addition of two distilled water samples containing 5x103 copies of the Twist RNA, which were run in parallel in each RT-PCR plate. Additionally, all samples were assayed undiluted and diluted 1/10 to further elucidate the action of inhibitors, which in the 1/10 dilution usually disappears. The negative controls comprised five distilled water samples per run: two from the beginning of the assay, to control any potential contamination during the RNA extraction, and three in the RT-PCR, to control any potential contamination during nucleic acids amplification.
Two or three strongly positive target regions suggest a relatively abundant and/or relatively recent virus contamination, whereas a single positive target indicates weak contamination. In whichever case, however, none of the RT-PCR results prove infectivity, since only fragments of the genome are detected. With regard to the bus surface samples, in group B only those that gave a positive result in group A (before cleaning) were subjected to PCR analysis.
2.4. Infectivity assays
Infectious CoV 229E numbers were determined after infecting Huh-7AI cell monolayers (Pinto et al., 2018) as TCID50 units (50% tissue culture infectious dose in 96 well multiplates (Costafreda et al., 2014). All titrations of untreated (3) and treated (6) samples were performed in duplicate. Ninety-six well multiplates were used, with an inoculum of 20 µl / well, at a rate of 8 replicates per dilution, following a 1/5 dilution series. Cells inoculated with culture medium were used as “negative controls”. Each titration had its corresponding “positive control”, specifically cells inoculated with a viral suspension of CoV 229E of known titer. The formula used to calculate the TCID50 was applied following the SOP 0,350,300 DAD/001 of the Enteric Virus Group of the Department of Genetics, Microbiology and Statistics from the University of Barcelona.
2.5. Modelling of the risks of potential infection by increasing ventilation rate in a bus
In the prospective assessment of the individual infection risk and the basic reproduction number for bus commuters due to the airborne transmission of SARS-CoV-2 in the presence of a SARS-CoV-2 infected subject without wearing a mask the following methodology was adopted:
-
-
Definition of different exposure scenarios (in terms of bus geometry, ventilation, number of commuters, activity levels and inhalation rates of the commuters). The exposure scenarios considered in the assessment are summarized in Table 1:
-
-
Calculation of the probability of infection and the basic reproduction number starting from the quanta emission rates estimated by Buonanno et al. (2020a).
-
-
Calculation of the individual infection risk.
Table 1.
Details of the exposure scenarios simulated.
| Scenario | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| bus | small | small | small | small | large | large |
| journey time (min) | 90 | 90 | 90 | 90 | 90 | 90 |
| exposure scenario | 2 groups of 50 people | 2 groups of 50 people | 6 groups of 20 people | 6 groups of 20 people | 2 groups of 65 people | 2 groups of 65 people |
| exposure time per group (min) | 45 | 45 | 15 | 15 | 45 | 45 |
| Notes | 50 people (including 1 infectious subject) get-on the bus simultaneously and stay on-board for 45 min, then they get-off the bus and other 50 people get-on simultaneously and stay on– board for 45 min | 20 people (including 1 infectious subject) get-on the bus simultaneously and stay on-board for 15 min, then they get-off the bus and other 20 people get on simultaneously and stay on– board for 15 min. Thus every 15 min a new 20-people group of people get-on the bus (6 groups in total) | 65 people (including 1 infectious subject) get-on the bus simultaneously and stay on-board for 45 min, then they get-off the bus and other 65 people get-on simultaneously and stay on– board for 45 min | |||
| fresh air | no fresh air (3400 m3/h are just recirculated) | fresh air (25% of the 3400 m3/h) | no fresh air (3400 m3/h are just recirculated) | fresh air (25% of the 3400 m3/h) | no fresh air (3400 m3/h are just recirculated) | fresh air (25% of the 3400 m3/h) |
| AER (h−1) | 0.2 | 12.6 | 0.2 | 12.6 | 0.2 | 8.4 |
| Quanta emission rate (quanta/h) | For each scenario, 2 sub-scenarios were tested considering 2 different expiratory activities while standing: a) speaking = 3.8 quanta/h; b) oral breathing = 0.8 quanta/h | |||||
| IR exposed subjects (m3/h) | 0.54 | 0.54 | 0.54 | 0.54 | 0.54 | 0.54 |
The following definitions should be considered:
-
-
Probability of infection (PI) = ratio between infected cases (C) and the number of susceptible subject exposed (S): PI = C/S.
-
-
Basic reproduction number (R0) = ratio between the number of infected cases (C) and the number of infected subject (I): R0 = C/I.
-
-
Individual infection risk (R(ERq)) = risk of an exposed subject to be infected as it takes into account how likely the calculated probability of infection occurs, i.e. how likely the rate of quanta emitted by the infected subject occurs: R(ERq) = PI(ERq)·PERq. For the purposes of this report, a value of R < 0.1% will be considered acceptable.
-
-
PERq = probability of occurrence of a certain rate of quanta (ERq) emitted by the infected subject.
Thus, in order to calculate the probability of infection and, then, the individual infection risk, a Monte Carlo simulation taking into account all possible emission rates of the infected subject should be run as described in Buonanno et al. (2020b). The authors point out that the quanta emission rate is a key parameter to evaluate the individual infection risk. The quanta emission rates here considered were calculated on the basis of a novel approach proposed in a previous paper (Buonanno et al., 2020a) which allows the estimation of the quanta emission rate of SARS-CoV-2 as a function of different viral loads, infectivity, respiratory activities, and activity levels. This approach represented an important step forward in the scientific literature, because until this advance simplified backward calculations were used to estimate the emission of an infected subject based on retrospective assessments of infectious outbreaks only at the end of an epidemic (Dai and Zhao, 2020). A simplified estimate of R (instead of the Monte Carlo method) can be carried out adopting the ERq value assumed with a probability of occurrence PERq = 1 and inducing a PI(ERq) equal to the risk R evaluated through the Monte Carlo method. Such emission rate at PERq = 1 resulted as the ERq at the 66th percentile (Buonanno et al., 2020b). Thus, adopting such simplified calculation (ERq at 66th percentile), the probability of infection matches the individual risk.
The a-priori (prospective) assessment of the probability of infection was performed considering the following steps:
-
•
calculation of the quanta concentration n(t) in the bus through the equation:
where IVRR (h−1) represents the infectious virus removal rate in the space investigated, n0 represents the initial quanta concentration in the bus, I is the number of infectious subjects (1 in our simulations), V is the volume of the buses considered, and ERq is the quanta emission rate, 66th percentile, (quanta/h) of the SARS-CoV-2 infected subject. The quanta concentration calculation adopted here is based on the following hypotheses: the quanta emission rate is considered to be constant, the latent period of the disease is longer than the time scale of the model, and the emitted particles are instantaneously and evenly distributed in the room (Gammaitoni and Nucci, 1997). The infectious virus removal rate, IVRR, is the sum of three contributions (Yang and Marr, 2011): the air exchange rate (AER) via ventilation, the particle deposition on surfaces (k, e.g. via gravitational settling), and the viral inactivation (λ). The deposition rate was evaluated as the ratio between the settling velocity of super-micrometric particles (roughly 1.0 × 10-4 m/s as measured by Chatoutsidou and Lazaridis, 2019) and the height of the emission source (1.5 m); thus, k was 0.24/h. The viral inactivation was evaluated on the basis of the SARS-CoV-2 half-life (1.1 h) detected by (van Doremalen et al., 2020), thus λ was 0.63/h. As regards the quanta emission rate (ERq), for each scenario, two sub-scenarios were tested considering two different expiratory activities while standing: a): speaking (66th percentile equal to 3.8 quanta/h), b) oral breathing (66th percentile equal to 0.8 quanta/h).
-
•
evaluation of the dose of quanta (Dq) of the exposed subjects as:
where T is the total exposure time of the exposed subjects and IR is their inhalation rate (IR) which is affected by their activity level (here considered IR = 0.54 m3/h for oral breathing while standing)
-
•
evaluation of the probability of infection through a well-known exponential dose–response model:
3. Results
3.1. Subway samples
Of a total of 15 swab surface samples, 6 gave a positive RT-qPCR signal (Table 2 ), but for only one of the 3 targets analysed, either IP4 (3 cases) or E (3 cases). Genome count (GC) values ranged between 20 and 443/m2 for IP4 and 49–714/m2 for E.
Table 2.
Results of the analysis of determination of SARS-CoV-2 by Real-Time RT-PCR in the subway samples using three targets of the virus genome. - negative detection result, + to ++++, positive from traces to very abundant. In brackets the quantification cycle (Cq) values obtained in the positive dilution rendering the highest positivity. D: direct (not diluted). GC: Counts of Genome. *Virucidal filter.
| SUBWAY SURFACES | ||||||
|---|---|---|---|---|---|---|
| Sample | IP2 | IP2 GC/m2 | IP4 | IP4 GC/m2 | E | E GC/m2 |
| L1-1 | – | – | + (41 1/10) |
49 |
||
| L1-2 | – | + (39 D) |
20 |
– | ||
| L1-3 | – | – | – | |||
| L1-4 | – | – | ++ (39 1/10) |
132 |
||
| L1-5 | – | – | – | |||
| L3-1 | – | + (41 1/10) |
66 |
– | ||
| L3-2 | – | – | +++ (37 1/10) |
714 |
||
| L3-3 | – | – | – | |||
| L3-4 | – | – | – | |||
| L3-5 | – | – | – | |||
| L5-1 | – | – | – | |||
| L5-2 | – | ++ (38 1/10) |
443 |
– | ||
| L5-3 | – | – | – | |||
| L5-4 | – | – | – | |||
| L5-5 | – | – | – | |||
| SUBWAY AIR | ||||||
| Sample: Line-wagoon | IP2 | IP2 GC/m3 | IP4 | IP4 GC/m3 | E | E GC/m3 |
| L1-6 | – | – | – | |||
| L1-7 | + (40 1/10) |
23.4 |
– | – | ||
| L3-6 | + (40 1/10) |
18.8 |
– | + (37 D) |
5.6 |
|
| L3-7 | – | – | – | |||
| L5-6 | – | – | – | |||
| L5-7 | – | – | – | |||
| SUBWAY FILTERS AIR CONDITIONING | ||||||
| Filter AC | IP2 | IP4 | E | |||
| L1 AC | – | – | – | |||
| L3 AC* | – | – | – | |||
| L5 AC | – | – | – | |||
With regard to the 6 PM2.5 samples collected in ambient air inside the wagons, 2 gave a positive RT-qPCR signal in one (train L1; sample L1-7) or two (train L3; sample L3-6) targets (Table 2). In the first case (L1-7) the target gene region identified was IP2, with a figured viral load of 23.4 GC/m3. In the second case (L3-6) the amplified target gene regions were IP2 (18.8 GC/m3) and the envelope protein E (5.6 GC/m3). We estimated that, in the worst case situation considering that the RNA load would represent equivalent virus with an infectivity potential (which is likely to be far from reality), with an infective virion concentration of 23 RNA copies/m3, a person would inhale on average 1.5 copies of the virus in a journey lasting 11 min (average length of a subway journey in Barcelona, TMB own data). However, once again we emphasise that in our study only fragments of one or two gene targets were identified, not an infective virion, and furthermore, even if they were infectious viruses, it is estimated that only one particle in 10 million would be able to produce an infectious cycle. The remaining 4 trains showed no contamination (Table 2). In the case of the three air conditioning filters analysed by RT-qPCR, none was positive in any target.
3.2. Bus surface samples before disinfection (group A samples)
Of the total of 30 swab surface samples taken from call buttons and support bars inside buses prior to disinfection (Sample group A), 13 detected some evidence for the presence of the RNA traces (Table 3 ). In the majority of these cases (9 of 13) only 1 of the 3 target gene regions (either IP2 or E) produced a positive result. Of these 9 cases, 4 were IP2 positives and 5 E positives, but all indicated low or trace amounts of the virus (samples A, Table 3). Of the remaining results 3 were positive for 2 of the targets (IP2 and IP4 or IP4 and E), and one (sample B29) gave positive for all three targets (Table 3). Genome count values ranged between 14 and 446/m2 for IP2, 9–490/m2 for IP4 and 5–378/m2 for E.
Table 3.
Results of the analysis of determination of SARS-CoV-2 by RT-PCR in the bus samples using three targets of the virus genome with some positive result. - negative detection result, + to ++++, positive from traces to very abundant. In brackets de Cq values obtained in the positive dilution rendering the highest positivity. D: direct (not diluted). GC: Counts of Genome. NA: Not applicable.
| BUS SURFACES | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bus | Cleaning method | IP2 | IP2 GC/m2 | IP4 | IP4 GC/m2 | E | E2 GC/m2 | ||||||
| A | B | A | B | A | B | A | B | A | B | A | B | ||
| B2 | O3 | – | – | – | – | ++ (38 1/10) |
++ (38 1/10) |
338 |
342 |
||||
| B3 | NaClO | – | – | – | – | ++ (39 1/10) |
– |
208 |
|||||
| B5 | O3 | – | – | – | – | + (41 D) |
– |
5 |
|||||
| B6 | O3 | ++ (39 1/10) |
– |
408 |
– | – | – | – | |||||
| B8 | NaClO | + (39 D) |
– |
38 |
– | – | – | – | |||||
| B10 | NaClO | ++ (39 1/10) |
– |
446 |
+ (40 D) |
– |
13 |
– | – | ||||
| B13 | O3 | + (41 1/10) |
– |
86 |
– | – | – | – | |||||
| B19 | O3 | – | – | ++ (38 1/10) |
– |
490 |
++ (38 1/10) |
– |
344 |
||||
| B20 | O3 | + (42 1/10) |
+ (40 1/10) |
48 |
236 |
– | ++ (38 1/10) |
745 |
– | ++ (39 1/10) |
314 |
||
| B22 | O3 | – | – | – | – | ++ (38 1/10) |
++ (39 1/10) |
378 |
270 |
||||
| B24 | O3 | – | – | + (41 D) |
– |
9 |
++ (39 1/10) |
++ (38 1/10) |
210 |
633 |
|||
| B29 | O3 | + (41 D) |
– |
14 |
+ (40 D) |
– |
11 |
++ (38 D) |
– |
37 |
|||
| B30 | NaClO | – | – | – | – | + 39 (D) |
27 |
||||||
| BUS AIR | ||||||
|---|---|---|---|---|---|---|
| Sample | IP2 | IP2 GC/m3 | IP4 | E | ||
| B32 3.6 | + (40 D) |
1.4 |
– | – | ||
| B32 4.6 | – | – | – | |||
| B32 5.6 | – | – | – | |||
| B31 3.6 | – | – | – | |||
| B31 4.6 | – | – | – | |||
| B31 5.6 | – | – | – | |||
| BUS FILTERS AIR CONDITIONING | ||||||
| Sample | IP2 | IP4 | E | E GC/m2 | ||
| Dust behind B33 | – | – | ++ 38 (1/10) |
NA |
||
| Dust behind B36 | – | – | – | |||
| Dust behind B38 | – | – | – | |||
| B33 AC | – | – | ++ (38 1/10) |
9500 |
||
| B36 AC | – | – | – | |||
| B38 AC | – | – | ++ (39 1/10) |
7333 |
||
| B23 AC | – | – | + (38 D) |
989 |
||
| B24 AC | – | – | – | |||
| B25 AC | – | – | – | |||
3.3. Bus surface samples after disinfection (Group B samples)
The 13 surface swab group B samples taken from buses paired with positive group A samples were also selected for RT-PCR analysis. Four of these 13 buses had been cleaned using bleach, with the remaining 9 having been treated with O3, and there had been a time gap of between around 2 and 5 h between initial sampling (A) and the second sample group (B). The majority of these group B samples (9 out of 13) showed total elimination of RNA traces (Table 3). In three samples in group B, traces of RNA were still observed (B2, B22, and B24), and in the anomalous case of sample B20 a signal for the IP4 and E targets emerged, this highlighting the methodological limitation of taking samples A and B from adjacent areas (Table 3).
The fact that a minority of the previously RNA positive samples still showed some RNA traces suggests that the disinfection/cleaning procedure was not totally effective in removing the RNA. In this context, it is also possible that the time elapsed between the sampling events before and after cleaning/maintenance could have played a role in weakening the RNA signals but evidence for this is unclear: Fig. 2 plots the 12 samples (excluding the anomalous B20 sample) showing time elapsed between sampling and type of cleaning employed in each case. Of these 12 samples the only ones retaining some evidence for the viral RNA had been cleaned using ozone.
Fig. 2.
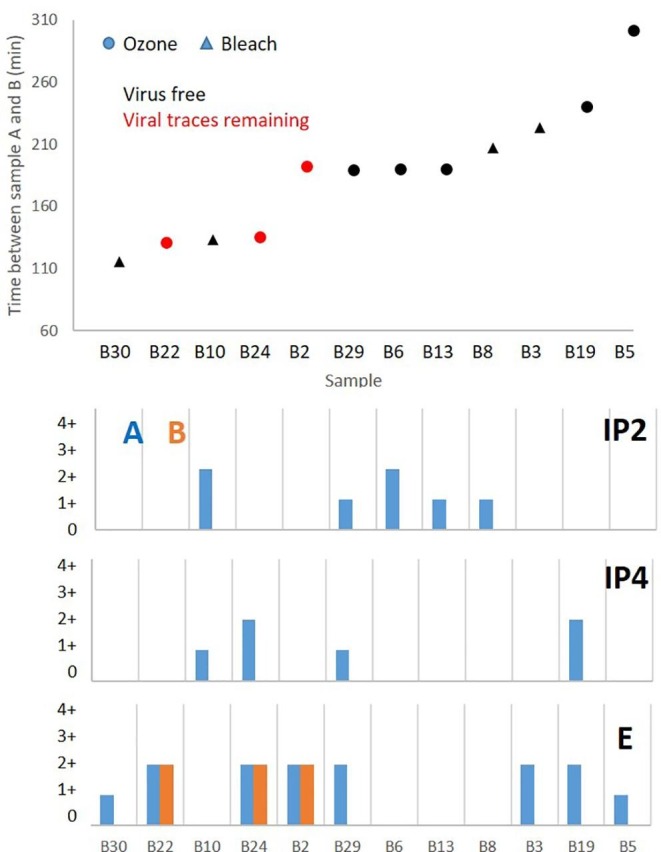
Top: Time difference between collection of group samples A and B in bus surfaces indicating if the viral traces were removed, and whether the cleaning protocol used bleach or ozone. Bottom: Differences between the number of positives (indicating proportion of traces of the virus, as shown in Table 3) between group samples A and B for the 3 target gene regions. Sample B20 not included in the figure.
As stated above, two methods were utilised for bus disinfection, 8 applying bleach manually and the remaining 22 using O3 cannons. Of the 8 bleach-cleaned buses 4 of them had proved RNA positive prior to cleaning, whereas after cleaning all 4 (B3, B8, B10, B30; A to B time difference 1 h 55 min to 3 h 43 min) were RNA-free. Of the 22 buses cleaned using O3, 9 had proved RNA positive in sample group A taken before cleaning. After cleaning only 5 of these 9 were RNA free (samples B5, B6, B13, B19, B29). Genome count values ranged between 236/m2 for IP2, 745/m2 for IP4 and 270–633/m2 for E.
3.4. Ozone treatment
A closer examination of the disinfection method using O3 revealed room for improvement in the cleaning protocol. The first problem encountered was that the cannons themselves did not display O3 levels in the bus during operation, so that the operator had no way of verifying that the equipment was functioning efficiently. We therefore took the precaution of placing two personal O3 monitors (POM) inside the bus to verify O3 levels during treatment. One POM was positioned immediately behind the O3 cannon inside the driver compartment, while the other either in the passenger area just the other side of a plastic screen fitted across the width of the bus to protect the driver during the pandemic, or at the back of the bus. In two cases O3 levels remained far too low due to inefficient functioning of the cannon (Table 4 : buses A and D, neither used for swab sampling).
Table 4.
Buses and mean concentration values (ppb) of O3 measured by the 3 measurement equipments by ultraviolet absorption (POM) for the 20 min at each location.
| Cleaning period | O3 (ppb) | ||||
|---|---|---|---|---|---|
| BUS | ON | OFF | Driver POM-1 |
Gate 2 POM-2 |
Last row POM-3 |
| A | 23:10 | 23:30 | 9 | 0.7 | |
| B | 23:35 | 0:05 | 149 | 0.7 | |
| C | 0:23 | 0:43 | 132 | 0.6 | |
| D | 1:49 | 1:55 | 10 | 0.7 | |
| E | 1:57 | 2:17 | 155 | 2 | |
Another problem emerging from these observations was that even if the O3 cannon was functioning correctly O3 levels within the main body of the bus remained far lower than in the driver area. The likely main reason for this non-uniform ozone distribution is insufficient air circulation and consequent trapping of the gas behind the plastic screen protecting the driver. Furthermore, within the transport microenvironment of the bus depot one would expect elevated levels of O3 -consuming substances such as volatile organic compounds (VOCs) and nitrous oxide (NO) from vehicle exhaust, hydrocarbon evaporation and interior degassing of plastics and paints. The latter effect was evident from the drastic reduction in O3 as soon as the cannon was turned off, with O3 dropping to < 1 ppb in just 2 min.
The TCID50/ml results of the inactivation capacity study of ozone treatments using CoV 229E on bus bar surfaces are presented in Table 5 . The TCID50/ml represents the concentration of infectious viral particles of an inoculum that causes the destruction of the cell monolayer in half of the replicates of inoculated cultures or with a probability of 0.5. The ozone inactivation capacity against CoV 229E was determined by the reduction factor, that is, the difference in infectious titer (log10 TCID50/ml) of the control virus not exposed to ozone treatment and the infectious titer obtained after exposure to ozone. The treatment tested must demonstrate a decimal logarithmic reduction of at least 4 to verify that it has disinfection capacity. Based on the results in Table 5, it is concluded that the ozone had no disinfection activity against CoV 229E on bus bar surfaces at concentrations of 300 ppb and 600 ppb under the conditions tested (exposure time of 20 min at a relative humidity of 60% ± 2). Levels of 300 ppb O3 were not reached in the 5 buses studied during the nocturnal disinfection (Table 4).
Table 5.
Test of the possible antiviral capacity of ozone on CoV 229E after a 20-minute exposure to concentrations of 300 and 600 ppb of ozone and a relative humidity of 60% ± 2.
| Tested condition | Replicate | log10TCID50/ml ± IC 95% | Mean log10TCID50/ml ± IC 95% |
Reduction factor ± IC 95% |
|---|---|---|---|---|
| 1 | 4.55 ± 0.26 | |||
| Control | 2 | 4.50 ± 0.35 | 4.49 ± 0.32 | |
| 3 | 4.41 ± 0.35 | |||
| 1 | 4.41 ± 0.19 | |||
| 300 ppb O3 | 2 | 4.24 ± 0.30 | 4.37 ± 0.26 | 0.12 ± 0.41 |
| 3 | 4.46 ± 0.29 | |||
| 1 | 4.50 ± 0.51 | |||
| 600 ppb O3 | 2 | 4.67 ± 0.30 | 4.53 ± 0.37 | −0.04 ± 0.49 |
| 3 | 4.41 ± 0.30 |
3.5. Bus ambient air and air conditioning samples
Of the 6 air samples collected in the buses, one (B32 3.6) gave a weak positive result in one of the target genes (IP2), with a calculated viral load (again supposing that RNA represents the unlikely worst situation of being representative of infective virus load) of 1.44 GC/m3 (Table 3). In the case of the six air conditioning filter samples analysed by RT-PCR, 3 of these (B33, B38, B23) revealed evidence for the presence of the gene target E (Table 3). Genome count values ranged between 989 and 9500/m2 for E. The same RNA target was also detected in one of the swab dust samples taken from behind the air conditioning filter (bus B33).
3.6. Modelling reduction of risk of infection by increasing ventilation in a bus
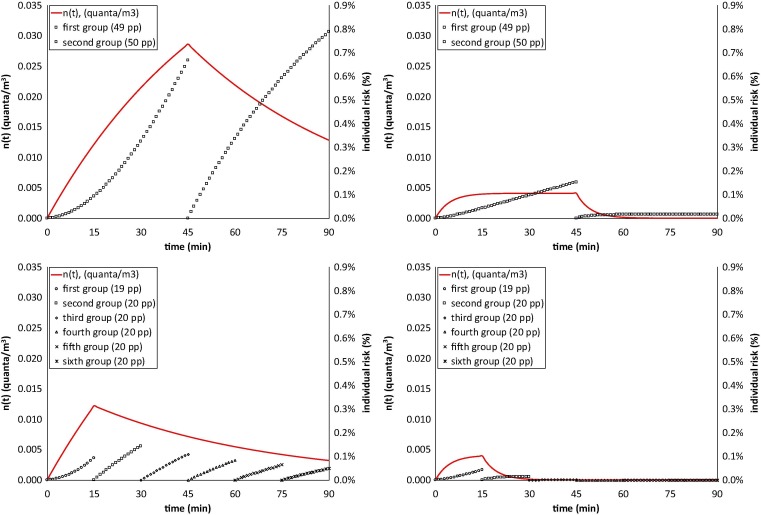
Illustrative examples of quanta concentration in the bus due to the presence of an infectious person without wearing a mask and probabilities of infection of the groups of people simultaneously travelling in the bus without wearing a mask are reported for all scenarios in Fig. 3 . The trends clearly highlight that the scenarios with fresh air produce significant reduction in the quanta concentrations experienced by the travellers and, consequently, their probability of infection.
Fig. 3.
Quanta concentration in a bus and risk of infection (%) of the groups of exposed people: scenarios 1a-4a (speaking; Table 1).
The average probability of infection, individual infectious risk, and basic reproduction for people exposed in the six scenarios (and corresponding sub-scenarios) simulated are shown in Table 6 . The simulations performed showed that for both small and large buses with an infectious person speaking a basic reproduction number, R0 > 0.5 was estimated when the buses are simultaneously used by large groups of people (50 and 65 people for small and large buses, respectively) and no fresh air is provided, i.e. when the AER just relies upon the bus air leakages and the door opening periods, 0.2/h in our simulations (scenario 1a and 5a, Table 1). For the same scenarios, a R0 < 0.2 can be reached when the infectious person is just oral breathing. In fact, for scenarios 1 and 5 the individual risks resulted higher than the acceptable individual risk (0.1%), whereas it was lower for all the other scenarios simulated.
Table 6.
Average probability of infection, basic reproduction number, and individual infectious risk for people susceptible people exposed in the six scenarios simulated. a) speaking; b) oral breathing.
| Scenario |
1 | 2 | 3 | 4 | 5 | 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | a | b | a | b | a | b | a | b | a | b | |
| Individual infectious risk, R (%) | 0.72% | 0.15% | 0.09% | 0.02% | 0.09% | 0.02% | 0.01% | 0.00% | 0.48% | 0.10% | 0.08% | 0.02% |
| Basic reproduction number, R0 | 0.85 | 0.18 | 0.11 | 0.02 | 0.11 | 0.02 | 0.01 | 0.00 | 0.62 | 0.13 | 0.10 | 0.02 |
When fresh air is provided (850 m3/h) high AER values are reached both in the small and large buses, thus significantly reducing the R0, including where an infectious person is speaking (R0 = 0.11 and 0.10 for small and large buses, respectively; scenario 2a and 6a).
For less crowded conditions, tested for a small bus, a significant reduction of R0 was obtained for exposure scenarios with no fresh air provided (i.e. scenario 3a, R0 = 0.11 and individual risk equal to 0.09%). These results offer insight into how to reduce the risk of airborne infection not just for SARS-CoV-2, but also for other airborne infectious agents.
4. Conclusions, recommendations and limitations of the study
From a total of 82 bus and subway train samples analysed using RT-qPCR during this study, 30 showed evidence for the presence of one or more of the 3 target RNA gene regions (IP2, IP4, E) of the SARS-CoV-2 virus. It is important to again emphasise that the detection of fragments of RNA belonging to the SARS-CoV-2 virus does not imply infectivity of this pathogen, especially in the context of this study in which almost all samples showed positivity only for one or two of the three targets under investigation. Most (24) of the 30 positive samples yielded evidence for only 1 of the 3 RNA targets. Of the remaining minority, 4 samples yielded 2 targets, and 2 samples yielded evidence for all 3 of IP2, IP4, and E.
Positive results were more common in surface swab samples from support bars (23 out of 58) than in ambient air inside the vehicles (3 out of 12).
Whereas no evidence for RNA traces was found in the small number of subway train a/c filters examined, this was not the case for the bus a/c filter and dust samples, which showed evidence for SARS-CoV-2 RNA in 4 out of 9 samples.
There was a notable decrease in RNA traces detected after the bus cleaning/maintenance period. All samples disinfected using bleach proved free of the viral genome after cleaning, but this was not the case with all buses disinfected using ozone. A limitation of the study was that there was significant delay before each bus was returned to the sampling team after cleaning and maintenance. Thus, it remains unclear whether this decrease or absence of RNA traces was entirely due to the cleaning procedures or in some measure to the decay of recently deposited viral material over time (in this case 2–5 h between samplings). Our results on the effectivity of cleaning with ozone in the laboratory suggest that the aforementioned decrease or absence of RNA may have been due to decay rather that to the cleaning procedure. Future studies need to investigate both possibilities. Another methodological limitation of the study was demonstrated by the one sample that showed higher RNA contamination after cleaning, raising the possibility of inhomogeneous contamination across adjacent sampling surfaces.
Our findings demonstrate that traces of the SARS-CoV-2 viral genome can be detected within public transport vehicles, both on surfaces inside the vehicle and in the ambient air. Although in the case of this study evidence for concentrations of the viral genome was fragmentary, generally weak, and the chances of infectivity considered to be extremely low, our data identified a need for tightening up cleaning procedures.
Cleaning procedures in public transport worldwide need to be rigorously validated to be effective for virus disinfection/removal, especially at times when COVID-19 cases are peaking. If O3 is used, concentrations need to be monitored and held at levels known to eliminate viral infectivity (not reached in this study). Manual cleaning using bleach or other disinfectant agents needs to be done extremely thoroughly. The disinfection procedure needs to be not only concentrated on the driver area but also include the entire bus. The need for this is indicated by the fact that of the 30 buses sampled in our study before and after cleaning procedures 4 still yielded traces of the RNA segments after cleaning. However, for testing the disinfection efficiency, RNA analysis is not enough because this only detects the remains of viral genetic material but cannot determine infective potential. Thus cleaning procedures should be validated in virology laboratories using cultivated coronaviruses and registering any residual infectivity of these after the cleaning protocol has being applied. Using this approach, for example, our pilot study on disinfection capacity suggests that under O3 concentrations of 600 ppb at 60% relative humidity, disinfection of surfaces was very inefficient.
Regarding the evaluation of the risk by modelling there is an unacceptable risk for both small and large buses with full occupancy in the case of natural ventilation. In the case of reduced occupancy (20 people for exposure time of 15 min) the risk can be calculated as acceptable. The best risk reduction is achieved by applying forced ventilation which in our model succeeded in producing acceptable results for both small and large buses with full occupancy and even with an infected subject speaking. The use of masks has not been modelled, but they are known to lower the risk as they decrease the emitted quanta (e.g. Leung et al., 2020) from the potentially infectious passenger.
As the COVID-19 pandemic has progressed, so urban commuter behaviour has changed as individuals adapt to the new reality and learn how best to minimise their exposure to viral infection. Correctly fitted face masks are by now obligatory on public transport, and people are increasingly aware that remaining silent whilst travelling is likely to reduce the risk of aerosol transmission. Transport companies are similarly developing new strategies to protect their passengers and staff, and in this context we encourage them to widen the availability of hydroalcoholic gels, operate remote door opening so that fewer fomite surfaces need to be touched by passengers, work to maintain a high frequency of service to reduce crowding, and encourage avoidance of peak hour travel. Most of all, we recommend close attention to be paid to ventilation systems, increasing the forced ventilation rate and the introduction of external air wherever possible (evaluating the result by using CO2 sensors) and improving filtration systems. Such approaches, combined with the application of rigorously effective cleaning and disinfection protocols to reduce the risk of fomite transmission, will not only act to suppress potential infection but also help to rebuild confidence in the general public regarding the use of urban transport in times of viral pandemic.
CRediT authorship contribution statement
Teresa Moreno: Conceptualization, Writing, Sampling, Supervision. Rosa María Pintó: Viral studies design, Writing, Supervision. Albert Bosch: Viral studies design, Editing. Natalia Moreno: Sampling methodology, Editing. Andrés Alastuey: Sampling methodology, Editing. María Cruz Minguillón: Sampling methodology, Editing. Eduard Anfruns-Estrada: Viral methodology. Susana Guix: Viral methodology. Cristina Fuentes: Viral methodology. Giorgio Buonanno: Modelling, Writing. Luca Stabile: Modelling, Writing. Lidia Morawska: Methodology, Editing. Xavier Querol: Conceptualization, Editing, Supervision.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgement
This study was supported by the Spanish Research Council (CSIC, Project COVID-19 CSIC 202030E226), Transports Metropolitans de Barcelona (TMB) and the Generalitat de Catalunya (SGR41). IDAEA-CSIC is a Centre of Excellence Severo Ochoa (Spanish Ministry of Science and Innovation, Project CEX2018-000794-S).
Handling Editor: Olga Kalantzi
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.106326.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Afshinnekoo E., Meydan C., Chowdhury S., Jaroudi D., Boyer C., Bernstein N., Maritz J.M., Reeves D., Gandara J., Chhangawala S. Geospatial Resolution of Human and Bacterial Diversity with City-Scale Metagenomics. Cell Systems. 2015;1:72–87. doi: 10.1016/j.cels.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J., Marr L. Recognizing and controlling airborne transmission of SARS-CoV-2 in indoor environments. Indoor Air. 2020;30:557–558. doi: 10.1111/ina.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On Y, Flamholz A, Phillips R, Milo R. 2020. SARS-CoV-2 (COVID-19) by the numbers. eLife Sciences 9.DOI: 10.7554/eLife.57309. [DOI] [PMC free article] [PubMed]
- Biryukov J, Boydston JA, Dunning RA. 2020. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. mSphere 5:e00441-20. Doi: 10.1128/mSphere.00441-20. [DOI] [PMC free article] [PubMed]
- Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020 doi: 10.1101/2020.04.12.20062828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno G., Morawska L., Stabile L. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: prospective and retrospective applications. Environ. Int. 2020 doi: 10.1016/j.envint.2020.106112. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatoutsidou S.E., Lazaridis M. Assessment of the impact of particulate dry deposition on soiling of indoor cultural heritage objects found in churches and museums/libraries. J. Cult. Heritage. 2019;39:221–228. doi: 10.1016/j.culher.2019.02.017. [DOI] [Google Scholar]
- Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, Lim XF, Lim AS, Sutjipto S, Lee PH, Son TT, Young BE, Milton DK, Gray GC, Schuster S, Barkham T, De PP, Vasoo S, Chan M, Ang BSP, Tan BH, Leo YS, Ng OT, Wong MSY, Marimuthu K, Singapore Novel Coronavirus Outbreak Research Team. 2020. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun 11(1):2800. [DOI] [PMC free article] [PubMed]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L. Stability of SARS-CoV-2 in different environmental conditions. Lancet. 2020;1 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. The Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K.K., Nguyen T.T., Yadana S., Hansen-Estruch C., Lindsley W.G., Gray G.C. Bioaerosol Sampling for Respiratory Viruses in Singapore's Mass Rapid Transit Network. Sci. Rep. 2018;30:8(1):17476. doi: 10.1038/s41598-018-35896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu D, Bleicker T, Brünink S, Schneider J, Schmidt M, Mulders D, Haagmans B, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans M, Drosten C. 2020. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro surveillance. 25: 2000045. ttps://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed]
- Costafreda M.I., Pérez-Rodriguez F.J., D'Andrea L., Guix S., Ribes E., Bosch A. Hepatitis A Virus Adaptation to Cellular Shutoff Is Driven by Dynamic Adjustments of Codon Usage and Results in the Selection of Populations with Altered Capsids. J. Virol. 2014;88:5029–5041. doi: 10.1128/JVI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H., Zhao B. Association of the infection probability of COVID-19 with ventilation rates in confined spaces. Build. Simul. 2020;13:1321–1327. doi: 10.1007/s12273-020-0703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzisi Jr.EK, Dei OA. 2020. Adherence to social distancing and wearing of masks within public transportation during the COVID 19 pandemic. Transportation Research Interdisciplinary Perspectives, 7 (2020), p. 100191, 10.1016/j.trip.2020.100191. [DOI] [PMC free article] [PubMed]
- Eikenberry S.E., Mancuso M., Iboi E., Phan T., Eikenberry K., Kuang Y., Kostelich E., Gumel A.B. To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infectious Disease Modelling. 2020;5(2020):293–308. doi: 10.1016/j.idm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears AC, Klimstra WB, Duprex P, Hartman A, Weaver SC, Plante KS, Mirchandani D, Plante J, Aguilar PV, Fernandez D, Nalca A, Totura A, Dyer D, Kearney B, Lackemeyer M, Bohannon JK, Johnson R, Garry RF, Reed DS, Roy CJ.2020. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv; 2020. doi:10.1101/2020.04.13.20063784. PPR152437.
- Furuya H, 2007. Risk of transmission of airborne infection during train commute based on mathematical model. Environ Health Prev Med 12, 78–83 (2007). Doi: 10.1007/BF02898153. [DOI] [PMC free article] [PubMed]
- Gammaitoni L., Nucci M.C. Using a mathematical model to evaluate the efficacy of TB control measures. Emerg. Infect. Dis. 1997:335–342. doi: 10.3201/eid0303.970310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goscé L, Johansson A. 2018. Analysing the link between public transport use and airborne transmission: mobility and contagion in the London underground. Environ. Health 17(1):84. Doi: 10.1186/s12940-018-0427-5. [DOI] [PMC free article] [PubMed]
- Greenhalgh T., Schmid M.B., Czypionka T., Bassler D., Gruer L. Face masks for the public during the covid-19 crisis. BMJ (Clinical research ed) 2020;369 doi: 10.1136/bmj.m1435. [DOI] [PubMed] [Google Scholar]
- Guo Z.-D., Wang Z.-Y., Zhang S.-F., Li X., Li L., Li C., Cui Y., Fu R.-B., Dong Y.-Z., Chi X.-Y., Zhang M.-Y., Liu K., Cao C., Liu B., Zhang K., Gao Y.-W., Lu B., Chen W. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan. China. Emerging Infectious Diseases. 2020;26(7):1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K. Air contamination with SARS-CoV-2 in the operating room. J Anesth. 2020 doi: 10.1007/s00540-020-02814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen N., Savolainen-Kopra C., Enstone J.E. Deposition of respiratory virus pathogens on frequently touched surfaces at airports. BMC Infect. Dis. 2018;18:437. doi: 10.1186/s12879-018-3150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iolov TV. October 31 2020. Train ventilation in Barcelona subway lowers risk of COVID-19 contagion. The Mayor.eu. https://www.themayor.eu/en/train-ventilation-in-barcelona-subway-lowers-risk-of-covid-19-contagion.
- Javid B., Weekes M.P., Matheson N.J. COVID-19: should the public wear face masks? BMJ. 2020;369 doi: 10.1136/bmj.m1442. [DOI] [PubMed] [Google Scholar]
- Jiang FC., Jiang XL, Wang ZG, Meng ZH, Shao SF, Anderson BD, Ma MJ. 2020. Detection of Severe Acut Respiratory Syndrome Coronavirus 2 RNA on Surfaces in Quarantine Rooms.“ Emerging Infectious Diseases 26 (9): 2162-2164. doi:10.3201/eid2609.201435. [DOI] [PMC free article] [PubMed]
- Kampf G, Todt D, Pfaender S, Steinmann E. 2020. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. Journal of Hospital Infection, 104(3), 246-251. Doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed]
- Jones R.M. Relative contributions of transmission routes for COVID-19 among healthcare personnel providing patient care. Journal of Occupational and Environmental Hygiene. 2020 doi: 10.1080/15459624.2020.1784427. [DOI] [PubMed] [Google Scholar]
- Lei H., Li Y., Xiao S., Lin C.H., Norris S.L., Wei D., Hu Z., Ji S. Routes of transmission of influenza A H1N1, SARS CoV, and norovirus in air cabin: Comparative analyses. Indoor Air. 2018;28(3):394–403. doi: 10.1111/ina.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, N.H.L., Chu, D.K.W., Shiu, E.Y.C. et al., 2020. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature Medicine 26, 676–680 (2020). Doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K.F., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Lou J., Tian S.J., Niu S.M., Kang X.-Q., Lian H.X., Zhang L.X., Zhang J.J. Coronavirus disease 2019: a bibliometric analysis and review. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3411–3421. doi: 10.26355/eurrev_202003_20712. [DOI] [PubMed] [Google Scholar]
- Miller S.L., Nazaroff W.W., Jimenez J.L., Boerstra A., Buonanno G., Dancer S.J., Kurnitsk J., Marr L.C., Morawska L., Noakes C. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2020 doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L, Milton DK, 2020. It is Time to Address Airborne Transmission of COVID-19. Clinical Infectious Diseases, ciaa939, Doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed]
- Moriarty L.F., Plucinski M.M., Marston B.J., Kurbatova E.V., Knust B. Public health responses to COVID-19 outbreaks on cruise ships — Worldwide, February–March 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:347–352. doi: 10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselwhite C., Avineri E., Susilo Y. Editorial JTH 16 –the coronavirus disease COVID-19 and implications for transport and health. J. Transp. Health. 2020;16(2020) doi: 10.1016/j.jth.2020.100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H, Oshitani H, Kobayashi T, Saito T, Sunagawa T, Matsui T, Wakita T, Suzuki M. 2020. Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19). medRxiv. Preprint, April 16, 2020. doi:10.1101/2020.02.28.20029272.
- Normile D. May 26 2020. Japan ends its COVID-19 state of emergency. Science. doi:10.1126/science.abd0092.
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto RM, Perez-Rodriguez FJ, D'Andrea L, de Castellarnau M, Guix S, Bosch A. Hepatitis A Virus Codon Usage: Implications for Translation Kinetics and Capsid Folding. Cold Spring Harbor perspectives in medicine 2018; 8. [DOI] [PMC free article] [PubMed]
- Prather K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science. 2020;368(6498):1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- Qian H, Miao T, Liu L, Zheng X, Luo D, Li Y. 31 October 2020. Indoor transmission of SARS-CoV-2. Indoor Air. Doi: 10.1111/ina.12766. [DOI] [PubMed]
- Rahmani A.R., Leili M., Azarian G., Poormohammadi A. Sampling and detection of corona viruses in air: A mini review. Sci Tot Env. 2020;740 doi: 10.1016/j.scitotenv.2020.140207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzini K., Castrica M., Menchetti L., Maggi L., Negroni L., Orfeo N., Pizzoccheri A., Stocco M., Muttini S., Balzaretti C.M. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci Tot Env. 2020;742 doi: 10.1016/j.scitotenv.2020.140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S., Wang W., Hao Y., Zhang H., Wang Z., Chen Y., Gao R. Stability and infectivity of coronaviruses in inanimate environments. World J Clin Cases. 2020;8(8):1391–1399. doi: 10.12998/wjcc.v8.i8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadik-Khan J., Solomonow S. Fear of public transit got ahead of the evidence. The Atlantic. 2020 https://www.theatlantic.com/ideas/archive/2020/06/fear-transit-bad-cities/612979/ [Google Scholar]
- SAGE. 2020. Transmission and Control of SARS-CoV-2 on Public Transport. Paper prepared by the UK Environmental and Modelling group (EMG) for the Scientific Advisory Group for Emergencies (SAGE). https://www.gov.uk/government/publications/emg-transmission-and-control-of-sars-cov-2-on-public-transport-18-may-2020.
- Santé Publique France. COVID-19: point épidémiologique du 29 octobre 2020 [Internet]. Paris: Santé Publique France, 2020 [cited 6 November 2020]. Available from: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-29-octobre-2020#:~:text=Points%20cl%C3%A9s&text=Forte%20acc%C3%A9l%C3%A9ration%20de%20l%27%C3%A9pid%C3%A9mie,Augmentation%20du%20nombre%20de%20d%C3%A9c%C3%A8s.
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virology Journal. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit M, Ratnesar-Shumate S, Yolitz J, Williams G, Weaver W Green, B, Miller D, Krause M, Beck K, Wood S, Holland B, Bohannon J, Freeburger D, Hooper I, Biryukov J, Altamura L A, Wahl V, Hevey M Dabisch P. 2020. Airborne SARS-CoV-2 Is Rapidly Inactivated by Simulated Sunlight. The Journal of Infectious Diseases, 2020:222, 564-71. DOI: 10.1093/infdis/jiaa334. [DOI] [PMC free article] [PubMed]
- Setti L, Passarini F, De Gennaro G, Baribieri P, Perrone MG, Borelli M, Palmisani J, Di Gilio A, Torboli V, Pallavicini A, Ruscio M, Piscitelli P, Miani A. 2020. SARS-Cov-2 RNA Found on Particulate Matter of Bergamo in Northern Italy: First Preliminary Evidence. Doi: 10.1101/2020.04.15.20065995. [DOI] [PMC free article] [PubMed]
- Shen Y., Li Ch., Dong H. Community Outbreak Investigation of SARS-CoV-2 Transmission Among Bus Riders in Eastern China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerstein R., Fux C., Vuichard-Gysin D., Abbas M., Marschall J., Balmelli C., Troillet N., Harbarth S., Schlegel M., Widmer A., Swissnoso Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100. doi: 10.1186/s13756-020-00763-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Ma C. 2020. Choice behavior of commuters’ rail transit mode during the COVID-19 pandemic based on a logistic model. Journal of Traffic and Transportation Engineering (English Edition) (2020), 10.1016/j.jtte.2020.07.002.
- Tirachini A, O Cats. 2020. COVID-19 and public transportation: current assessment, prospects, and research needs. J. Public Transp., 22 (1) (2020), 10.5038/2375-0901.22.1.1. [DOI] [PMC free article] [PubMed]
- Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. The New England Journal of Medicine. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020a. Scientific briefing: Transmission of SARS-CoV-2: implications for infection prevention precautions. 9 July 2020. Geneva: World Health Organization; 2020. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-forinfection-prevention-precautions.
- WHO. 2020b. Protocol: Real-time RT-PCR assays for the detection of SARS-CoV-2 Institut Pasteur, Paris. https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2.
- Yang W., Marr L.C. Dynamics of Airborne Influenza A Viruses Indoors and Dependence on Humidity. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Zhang L Ma J, Zhou L. 2020. On airborne transmission and control of SARS-CoV-2. Sci Tot Env 731, 139178. [DOI] [PMC free article] [PubMed]
- Zhou J., Otter J., Price J., Cimpeanu C., Meno Garcia D., Kinross J., Boshier P., Mason S., Bolt F., Holmes A., Barclay W. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin. Infect. Dis. 2020;ciaa905 doi: 10.1093/cid/ciaa905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.