Abstract
Background:
Organ preservation protocols have increasingly been applied for the treatment of head and neck cancers, including hypopharyngeal squamous cell carcinoma (HSCC). We sought to evaluate whether patients treated with primary surgery followed by adjuvant therapy had survival benefit over patients treated with initial nonsurgical modalities.
Methods:
We retrospectively reviewed patients with new diagnosis of HSCC at the University of Pittsburgh (1994-2014) treated with either primary total laryngectomy with pharyngectomy or organ preservation.
Results:
One hundred thirty-seven patients were identified. Surgical cases were more likely to be of advanced T stage. Initial surgery was more likely to be performed in the earlier years of the cohort. After adjusting for this imbalance using a propensity score, primary surgery was associated with improved survival compared with nonoperative therapy (P = 0.02).
Conclusions:
Due to its survival advantage, primary surgery followed by adjuvant treatment should be considered as a viable treatment of HSCC.
Keywords: hypopharyngeal cancer, laryngopharyngectomy, organ preservation, outcomes, survival
1 |. INTRODUCTION
Hypopharyngeal squamous cell carcinoma (HSCC) carries with it an unfavorable prognosis despite current aggressive treatment modalities. Tumors of this region often remain clinically silent until disease has reached an advanced stage. Approximately 80% of HSCC is stage III or IV at presentation, with locally advanced disease present in the majority of patients.1–3 Moreover, clinical and pathologic evaluation of the extent of disease can be difficult as tumors in this location demonstrate submucosal spread, infiltrating the rich lymphatic network of the hypopharynx. Recurrence is unfortunately common despite aggressive therapy with both local and distant metastasis occurring with roughly equal frequency.3
The success of organ-preservation protocols in the treatment of laryngeal cancer has led to an increase in the use of primary radiation (RT) and chemoradiation (CRT) to treat tumors of other head and neck subsites. These efforts originated with the landmark clinical trial led by the Veterans Affairs (VA) Laryngeal Cancer Study Group published in 1991. This study demonstrated equivalent survival in patients with advanced laryngeal cancer treated with induction chemotherapy followed by definitive RT for chemotherapy responsive tumors as compared to patients treated with laryngectomy and postoperative RT.4 Results from the Radiation Therapy Oncology Group (RTOG) trial 91-11 showed improved local control rates with concurrent CRT.5,6 These results demonstrated that concurrent CRT regimens allow for organ preservation without adversely impacting survival rates in properly selected patients with squamous cell carcinoma of the larynx.
While organ preservation strategies are viable for advanced laryngeal cancer, the results for HSCC are less encouraging. Attempts at laryngeal preservation in HSCC have resulted in higher loco-regional recurrence rates than in patients with advanced laryngeal cancer. In those patients failing organ preservation protocols, laryngopharyngectomy is often the only option. Diagnosis of persistent or recurrent tumor in HSCC patients may be delayed secondary to local treatment effects and submucosal tumor growth, which make surveillance challenging.7,8 Further, the extent of surgery and complication rates in patients failing primary CRT are established and well described.9,10 Overall survival comparisons between primary surgical treatment and initial organ preservation protocols have revealed mixed results with larger series demonstrating no difference in overall survival.11
Most studies on the treatment of HSCC are retrospective in nature, as is ours. One common finding among these studies is the typically poor prognosis associated with HSCC. In this context, the current study sought to revisit the role of primary surgery in the treatment of HSCC by reviewing our experience over a 20-year period. We examined whether primary surgery conferred a stage-independent survival advantage over primary RT or CRT in our effort to maximize survival.
2 |. METHODS
We performed a retrospective review of all patients with a primary diagnosis of HSCC from the University of Pittsburgh Head and Neck Cancer Database between the years of 1994 and 2014. Patients were excluded for diagnoses other than squamous cell carcinoma (primarily minor salivary tumors and basosquamous variant of squamous cell carcinoma), follow-up of <1 year, and for surgical resection of less than total laryngectomy. All patient data was deidentified and IRB approval was obtained.
Patients (n = 137) were grouped into two cohorts based on their initial treatment modality (laryngopharyngectomy or CRT) and their outcomes compared. Prior to 2000, the majority of HSCC patients in our cohort received primary surgery (23 primary surgery patients vs 1 CRT patient). Furthermore, those subjects with T4 HSCC were more likely to have been treated surgically. Because both factors may affect survival, a propensity score analysis was undertaken to balance the patient distribution in the study population background covariates. The propensity score for an individual is the probability of being treated conditional on the individual’s covariate values.12 Therefore, subjects in treatment and control groups with equal (or nearly equal) propensity scores will tend to have the same (or nearly the same) distributions on their background covariates. The propensity score as applied to this study is the probability of treatment by surgery. The covariates selected for construction of the propensity score were (1) year of treatment and (2) clinical T stage. The covariates considered, but not selected, were age, sex, smoking history, alcohol history, grade, and clinical N stage. The primary endpoint was overall survival. The cohorts were compared with proportional hazards regression with adjustment as needed for clinical and demographic factors. The assumption of proportional hazards was verified by examination of Schoenfeld residuals. A secondary endpoint, disease-specific survival (DSS) (death due to cancer) was analyzed by the Kaplan-Meier method with a log rank test, censoring deaths from other causes. The treatment groups were further compared by comparing cumulative incidence with Gray’s test.13
3 |. RESULTS
3.1 |. Patient characteristics
A total of 137 patients with primary, untreated HSCC were identified. Patient characteristics are displayed in Table 1. Of the 137 patients identified, 81 (59%) patients were treated with initial surgical resection, with the majority (58/81, 72%) receiving postoperative RT. The remaining 56 (41%) patients underwent initial RT, with the majority (52/56, 93%) receiving concurrent CRT. Clinical T stage was higher in the surgery group due to a preponderance of T4 tumors (58% vs 23% for the CRT cohort. Within the surgical group, 74% had American Joint Committee on Cancer (AJCC) stage IV disease; the CRT cohort contained 61% stage IV patients. While number of surgical cases were fairly constant over the observation period, averaging 3.8 cases per year, an increase in patients undergoing organ preservation therapy was noted beginning in 2001. Of note, within the CRT arm, 15 of 56 (27%) patients underwent salvage laryngopharyngectomy for persistent or recurrent disease. Other patient variables, including age, sex, history of tobacco use (both 93%) and alcohol use (86% vs 89%), and clinical N stage did not differ between the primary surgical vs CRT arms.
TABLE 1.
Patient characteristics
| Patient characteristic |
Surgery |
Nonsurgery |
|
|---|---|---|---|
| (Col percent in parentheses) | N = 81 (59%) | N = 56 (41%) | Test of equalitya |
| Age | (W) | ||
| Median | 61 | 61 | P = 0.59 |
| Range | 37-83 | 56-86 | |
| Sex | (F) | ||
| Male | 69 (85) | 44 (79) | P = 0.36 |
| Female | 12 (15) | 12 (21) | |
| Smoking historyb | (F) | ||
| No | 5 (7) | 4 (7) | P = 1.0 |
| Yes | 71 (93) | 51 (93) | |
| Alcohol historyc | (F) | ||
| No | 11 (14) | 6 (11) | P = 0.79 |
| Yes | 65 (86) | 47 (89) | |
| Year of treatment | (CA) | ||
| 1988-2000 | 31 (38) | 1 (2) | P = 0.002 |
| 2001-2007 | 22 (7) | 33 (59) | |
| 2008-2014 | 28 (35) | 22 (39) | |
| Clinical T stage | (CA) | ||
| 1 | 1 (1) | 3 (5) | P < 0.0001 |
| 2 | 10 (12) | 16 (29) | |
| 3 | 23 (28) | 24 (43) | |
| 4 | 47 (58) | 13 (23) | |
| Clinical N stage | (CA) | ||
| 0 | 22 (27) | 19 (34) | P = 1.0 |
| 1 | 21 (26) | 9 (16) | |
| 2 | 33 (41) | 23 (41) | |
| 3 | 5 (6) | 5 (9) | |
W, Wilcoxon Test; F, Fishers Exact Test; CA, Cochran-Armitage Trend Test.
Six cases were missing smoking history.
Eight cases were missing alcohol history.
3.2 |. Survival analysis
Median follow-up for the 55 patients alive at last follow-up was 38 months (range 11-171 months) with longer median follow-up for the surgery cohort (45 vs 35 months). Initially overall survival analysis revealed no difference between the primary surgery cohort and the organ preservation arm. Kaplan-Meier estimates of overall survival showed median survival for both groups was identical and that for the organ preservation/CRT group of patients, the overall was 28% (95%: 17%-47%) while primary surgical resection 5-year overall survival was 48.0% (95%: 38%-61%), and log rank test P = 0.25 (Figure 1). Moreover, proportional hazards regression estimated the hazard ratio for surgery vs CRT was 0.77 (95% CI, 0.49-1.20) verifying that there that there is no apparent indication of benefit for initial surgery.
FIGURE 1.
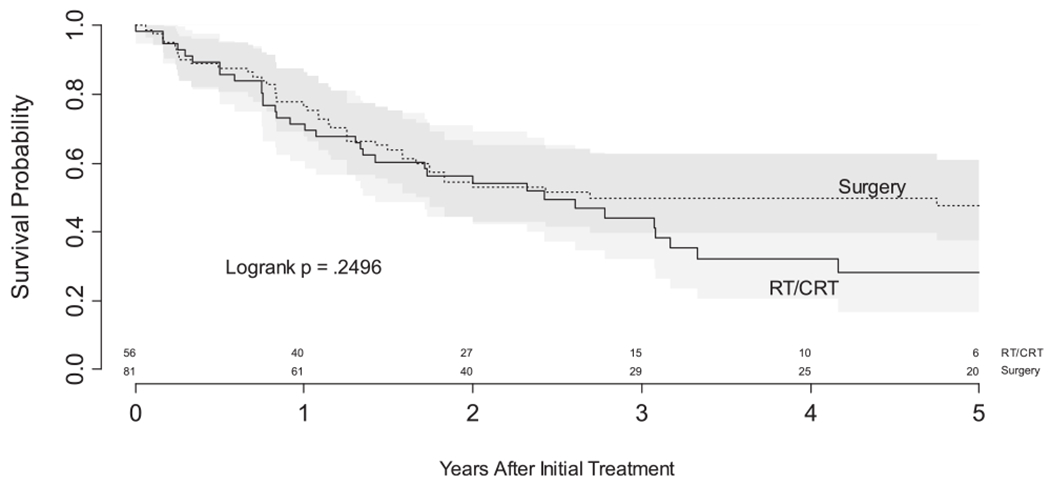
Overall survival by treatment (unadjusted)
3.3 |. Survival analysis with propensity score considerations
Differences between the retrospective cohorts may be confounded by selection bias. Accordingly, we evaluated imbalance in cohort characteristics that may influence survival. As seen on Table 1, imbalance between treatment modalities was evident in year of treatment and clinical T stage. The relative influence of year of treatment and clinical T stage is shown in Figure 2. The probability of surgical treatment can be seen to increase with an earlier year of treatment and with the presence of a T4 tumor.
FIGURE 2.
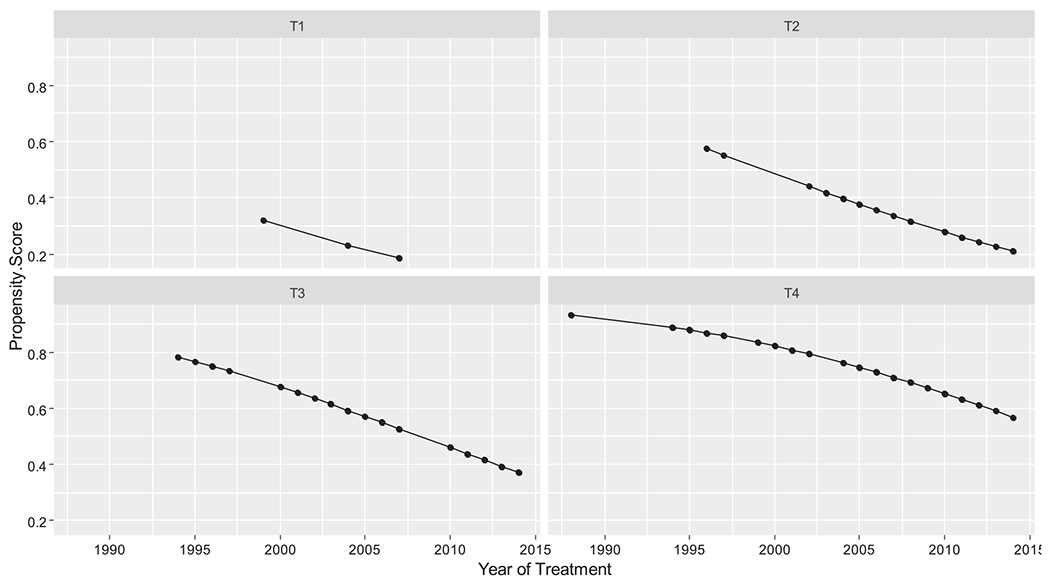
Propensity scores by year of surgery and clinical T stage
A reanalysis of overall survival including propensity score demonstrated a survival benefit in the primary surgery cohort. This analysis was a multivariate proportional hazards model with four covariates: propensity score, clinical N stage, age, and treatment modality. The contributions of each covariate is shown in Table 2. This analysis revealed that primary surgical intervention resulted in a reduction in the risk of death by 48% (hazard ratio, 0.52; 95% CI, 0.31-0.89; P = 0.02) when compared to organ-preservation strategies in the setting of HSCC (Figure 3).
TABLE 2.
Multivariate proportional hazards for overall survival
| Covariate | Reference | Hazard ratio | 95% CI | P |
|---|---|---|---|---|
| Age | 54-71 | 1.41 | 0.99-2.01 | 0.06 |
| N stage | 0-2 | 1.68 | 1.06-2.69 | 0.03 |
| Propensity score | 0.41-0.75 | 2.11 | 1.33-3.34 | 0.001 |
| Treatment modality | Surgery: RT | 0.52 | 0.31-0.89 | 0.02 |
FIGURE 3.
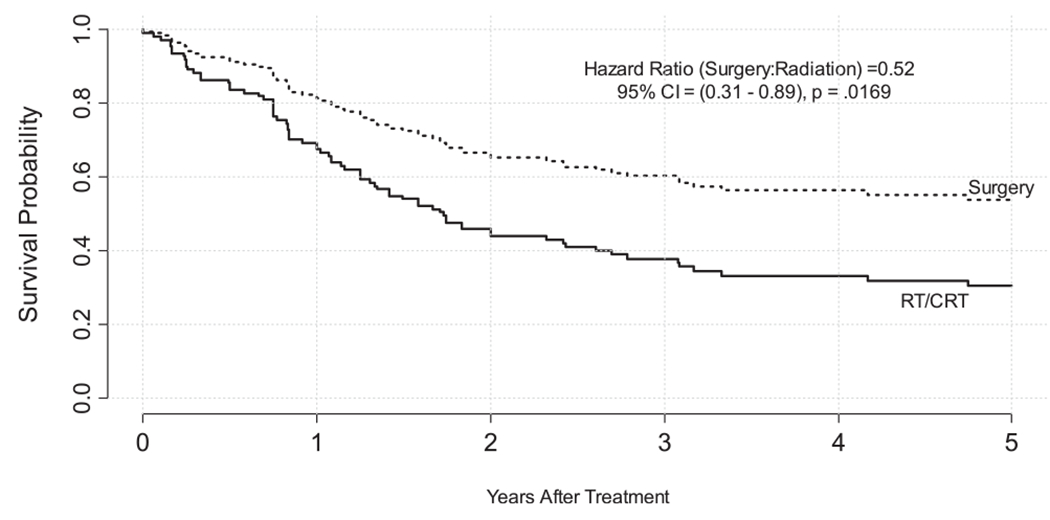
Overall survival by treatment (adjusted by propensity score, age, and pNStage)
3.4 |. Cause of death and DSS
Among 82 deaths, 69 could be attributed to either disease or other causes. In particular, 16 of 36 deaths among the surgery cohort were ascribed to other causes, leaving 20 of 36 due to cancer. To complement analysis of overall survival we examined DSS by excluding deaths attributed to causes other than cancer. Figure 4A, a Kaplan-Meier plot of DSS (censoring death due to other causes), shows a survival advantage for the surgery group. Median survival was 2.8 years for the RT/CRT group but not reached for the surgery group. This contrasts with overall survival for which the subgroup medians were nearly identical at 2.4 and 2.7 months for RT/CRT and surgery, respectively. This difference due among survival endpoints also suggest that death due to causes other than cancer may constitute a competing risk for death due to disease. Figure 4B shows cumulative incidence of death due to disease when unaffected by the competing risk of death due to other causes. Both the log rank test (Figure 4A) and Gray’s test (Figure 4B) are significant (P = 0.02 and P = 0.006, respectively), indicating that death due to disease was higher with organ preservation therapy than with upfront surgery.
FIGURE 4.
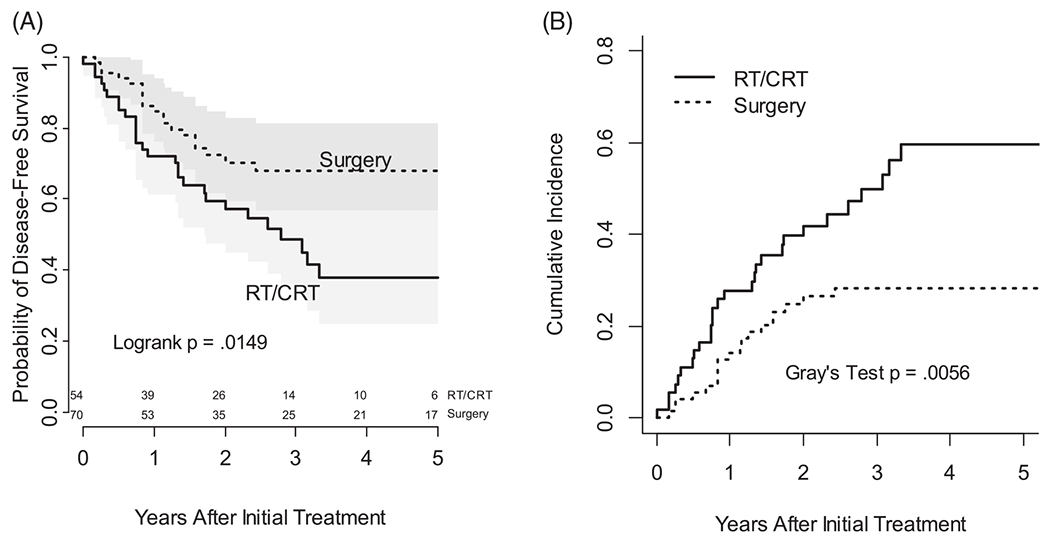
(A) Kaplan-Meier plot of disease specific survival (censoring death due to causes other than cancer). (B) Cumulative incidence of death due to disease (unaffected by competing risk of death due to other causes)
4 |. DISCUSSION
Our current study addresses the appropriate use of organ preservation strategies in the setting of HSCC. An initial retrospective review of the University of Pittsburgh experience revealed no statistically significant survival advantage to primary surgical intervention in the treatment of HSCC. However, this comparison was confounded by imbalance in cohort characteristics. Adjusting a prognostic model for these imbalances using a propensity score revealed that, indeed, primary surgical treatment conferred a survival advantage over organ preservation (CRT) and that surgery yielded a 50% risk reduction in mortality (hazard ratio, 0.52; 95% CI, 0.31-0.89; P = 0.02). A further analysis of death due to cancer using DSS and cumulative incidence confirmed the survival advantage of surgery over RT/CRT.
These results suggest that radical resection followed by postoperative RT may provide improved survival as compared to primary CRT in the treatment of advanced HSCC.
Despite the fact that our current study focuses entirely on survival, a discussion of quality of life and function for patients with HSCC is important given the radical surgery needed for patients with advanced disease. The function of a patient’s larynx at presentation is of significant importance not only in terms of tumor stage, but also in regard to initial treatment decisions. The functional outcomes of nonoperative treatment must be considered as well. While the potential perioperative complications as well as permanent changes of a laryngopharyngectomy are known, the long-term sequela of RT and, more often, CRT can be significant as well.14 It is our philosophy and experience that a larynx which is dysfunctional at presentation is very unlikely to regain function from treatment with CRT, particularly for HSCC. If a patient is dependent on either a gastrostomy tube, tracheostomy tube, or both we feel these are strong indications for primary surgical treatment. Surgery followed by adjuvant therapy is likely to provide such patients with better long-term swallowing function, the ability to rehabilitate their speech, and, as our data suggest, may also afford improved survival.
Quality of life indicators further support primary surgery for the treatment of HSCC. An analysis of the RTOG Trials 91-11, 97-03, and 99-14 indicates that nonoperative treatments resulted in late toxicity, including laryngeal dysfunction and feeding tube dependence, in 43% of the assessed patients.15
Our RT/CRT cohort may exhibit adverse long-term sequela of RT. In Figure 1, we present a (unadjusted) Kaplan-Meier analysis of survival by cohort. While the survivor functions are not statistically dissimilar and have equivalent medians, the survivor functions diverge beginning at 3 years. Importantly, despite 10 more months of follow-up for the surgery cohort, there were no deaths among the 20-29 surgery patients surviving at least 3 years, whereas additional deaths were seen among the RT/CRT cohort. This observation is consistent with the hypothesis that surgery, with or without adjuvant therapy, is more likely to be curative and that complete resection minimizes the risk of recurrence after 3 years, whereas primary organ preservation is less likely to be definitive. A larger study with careful surveillance for disease recurrence would be needed to verify this phenomenon and establish whether a cure is feasible by 3 years after primary surgery (±adjuvant therapy) but that risk of death persists beyond 3 years for primary organ-preserving therapy. This observation suggests future research of primary hypopharynx treatment needs a minimum of 5 years of follow-up.
Few randomized trials regarding the management of HSCC exist. The European Organization for the Research and Treatment of Cancer’s (EORTC) phase III trial compared initial surgical resection followed by postoperative RT with induction chemotherapy followed by definitive RT for patients with complete or partial response based on endoscopic exam. Greater than 90% of the patients in the study had advanced (stages III or IV) HSCC with no prior treatment.16
The EORTC trial randomized 194 HSCC patients to receive either primary surgical resection followed by RT (50-70 Gy) or induction chemotherapy with Cisplatin and 5-FU. After each cycle of chemotherapy, patients in the nonsurgical arm were evaluated endoscopically and only those patients with either complete or partial response continued on with a third cycle of chemotherapy. Final endoscopic evaluation was performed after the third cycle and those patients without a complete response underwent surgical resection followed by RT. Those with a complete response as judged by endoscopy were then treated with definitive RT.
Their Head and Neck Cancer Cooperative Group demonstrated that the 3-year and 5-year survival rates of patients treated with the CRT protocol, alive, with a functional larynx were 28% (95% confidence interval = 17%-37%) and 17% (95% confidence interval = 8%-26%), respectively.17 These figures are significantly below the 3-year and 5-year survival rates with a functional larynx reported in early laryngeal preservation protocols (67% and 58%, respectively)4 and indicate that the two disease processes, though in close anatomic proximity, are not the same entity. In our series, 26.8% of the patients treated with primary CRT required salvage surgery to control residual or recurrent disease. In addition to the problems of malnutrition and poor wound healing, the incidence and extent of submucosal tumor spread is higher in patients who have undergone previous RT, with submucosal spread present in 82% of studied patients.7 Diagnosis of persistence or recurrence in this patient population can be a challenge, as can obtaining negative surgical margins in a radiated field with submucosal tumor. Importantly, the RTOG Trial 91-11, which included only patients with laryngeal cancer, demonstrated that patient’s undergoing salvage laryngectomy experienced a worse outcome as there was a 10% decrement in survival in those patients receiving salvage laryngectomy compared to those who did not.6
Retrospective and population-based reviews of HSCC treatment offer a mixed picture in terms of the best therapeutic strategy for this disease. Hall et al. performed a population-based review of HSCC treatment in Ontario from 1990 to 1999. They studied nearly 600 patients in both cohort and case match fashion and found no differences in survival based on initial treatment strategy. Interestingly, they also studied their population based on “natural experiment,” referring to the treatment traditions and preferences of different areas within Ontario. They noted large differences in regional treatment modalities but did not demonstrate significant differences in overall or DSS based on these differences. Based on the dates of their retrospective study, wide spread adoption of concurrent CRT had not occurred for a significant percentage of their study population.11
In a matched pair analysis of 254 patients, Iwae et al. demonstrated no difference in 5-year or DSS between surgical and nonsurgical patients although they did note a statistically significant improvement in local control for the surgical group. When subanalyzed for T4a tumors, they noted a trend toward improved survival but this did not reach statistical significance.18
Harris et al. reviewed their experience with HSCC over a 14-year period. Of their 76 patients, the majority (63%) were treated nonsurgically. A significant portion of both their surgical (46%) and nonsurgical (48%) patients had T4 primary tumors. They demonstrated superior 5-year overall survival and recurrence free survival in their surgical cohort although the results did not reach statistical significance19; they concluded that up-front surgery may confer improved overall and recurrence free survival for advanced HSCC.
Tsou et al. retrospectively compared 202 patients with HSCC treated either with initial surgery (72/202 or 35.6%) or with initial CRT (130/202, 64.4%).20 Treatment preference was based on patient choice. The majority of patients (179/202, 88.6%) had either stages III or IV disease with no significant differences between the two cohorts. Of the 72 patients treated with initial surgery, 47/72 (65%) received adjuvant postoperative treatment (RT or CRT). Sixty-nine of the 130 patients (53.1%) treated with initial nonsurgical therapy eventually underwent salvage surgery for persistence or recurrence of disease.
In similar fashion to our review, Tsou et al. compared their cohorts based on initial treatment strategy regardless of whether patients treated initially with CRT eventually underwent salvage surgery. For patients with stage III HSCC they reported a 5-year DSS of 51.1% for those treated with surgery followed by adjuvant treatment, compared with a rate of 38.3% for those treated initially with CRT (P = 0.03); patients with stage IV disease achieved a DSS of 23.1% in the primary surgical group vs 11.4% in the CRT arm (P = 0.05). They concluded that for advanced HSCC, primary surgical resection followed by RT or CRT provided superior results in terms of survival.
Emerging data, including the current study, indicate that the extent of disease and the specific anatomic subsite within the head and neck play important roles in determining the success of organ preservation paradigms. In fact, the landmark trial by the VA Laryngeal Cancer Study Group in 1991 demonstrated that CRT offered a worse outcome than primary surgical intervention in those patients with large T4 lesions.4,21 With propensity score adjustment, our current study reveals a survival advantage conferred by primary surgery in the treatment of advanced HSCC. Despite higher overall stage, our surgical cohort experienced better survival than those treated with primary CRT. The clinical stage of disease, however, may underestimate the true pathologic stage, which is, of course, only available in the primary surgery cohort. With the increased accuracy and access to modern CT imaging and Positron Emission Tomography (PET), future studies will likely be able to focus on survival outcomes with an increasingly precise idea of a patient’s true tumor burden at presentation.
Limitations of our current study include the retrospective nature of the study with the inherent selection bias present in any nonrandomized study comparing surgical and nonsurgical outcomes. Despite all cases coming from a single institution, we were unable to describe how treatment decisions were historically made. It is unknown whether a consistent rationale was applied or whether the choice of treatment was due to case management favored by a surgeon, medical oncologist, or a RT oncologist or whether there was an institutional shift in treatment paradigm during the observation period. Further, given the 20 year range of our study, significant changes and improvements have occurred in the administration of both surgical and nonsurgical treatment for head and neck cancer including widespread adoption of Intensity-modulated Radiation Therapy (IMRT) and readily available free tissue transfer reconstruction. Given these limitations, as well as the relative “front loading” of our surgical cohort earlier in the series, we feel the improved survival of the patients treated with upfront surgery is notable.
With propensity score taken into account, the findings of our series demonstrate improved survival with primary laryngopharyngectomy followed by adjuvant therapy as compared to initial treatment with chemoradiotherapy with surgery reserved for salvage. With a larger cohort, we believe our results could achieve significance even in unadjusted data; a multicenter collaboration is likely the most expeditious manner to obtain such a cohort.
Our retrospective study was designed to assess overall survival which while it does represent a sensitive measure of oncologic outcome did not consider surgical outcomes, functional status, or quality of life. In addition, we did not assess patients’ comorbidities and their relationship to outcomes (surgical, functional, or oncologic). Comorbidities were not factored into the construction of our propensity score. These are weaknesses of our initial approach focused on survival for this deadly disease and in future comparisons between surgical and nonsurgical options we will consider including additional data and endpoints.
HSCC represents the most aggressive head and neck squamous cell cancer subsite. Despite aggressive treatment regimens, survival in this patient population remains poor. Perhaps due to the advanced disease at the time of diagnosis as well as the poor prognosis, nonsurgical treatment with attempted laryngeal preservation has been advocated for advanced, but resectable disease. Injudicious use of organ sparing treatments can result in preservation of a dysfunctional organ and, based on our data, possibly worse survival. Likely due to the rich lymphatics of the hypopharynx as well as the lack of the anatomic barriers seen in the larynx, results of organ preservation protocols for HSCC have been poor and treatment toxicity is common. While 5-year survival for those undergoing primary surgical resection remains at or below 50% in most series, we feel this likely represents the best current therapeutic strategy for this lethal disease.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
REFERENCES
- 1.Hoffman HT, Karnell LH, Shah JP, et al. Hypopharyngeal cancer patient care evaluation. Laryngoscope. 1997;107(8):1005–1017. [DOI] [PubMed] [Google Scholar]
- 2.Kraus D, Zelefsky M, Brock H, Huo J, Harrison L, Shah J. Combined surgery and radiation therapy for squamous cell carcinoma of the hypopharynx. Otolaryngol Head Neck Surg. 1997;116(6 Pt 1):637–641. [DOI] [PubMed] [Google Scholar]
- 3.Hall SF, Groome PA, Irish J, O’Sullivan B. The natural history of patients with squamous cell carcinoma of the hypopharynx. Laryngoscope. 2008;118 (8):1362–1371. [DOI] [PubMed] [Google Scholar]
- 4.The Department of Veterans Affairs Laryngeal Cancer Study Group, Wolf G, Fisher S, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. New Engl J Med. 1991;324(24):1685–1690. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. New Engl J Med. 2003;349(22):2091–2098. [DOI] [PubMed] [Google Scholar]
- 6.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy. Arch Otolaryngol Head Neck Surg. 2003;129(1):44–49. [DOI] [PubMed] [Google Scholar]
- 7.Ho CM, Ng WF, Lam KH, Wei WI, Yuen APW. Submucosal tumor extension in hypopharyngeal cancer. Arch Otolaryngol Head Neck Surg. 1997; 123(9):959–965. [DOI] [PubMed] [Google Scholar]
- 8.Wei WI. The dilemma of treating hypopharyngeal carcinoma: more or less. Arch Otolaryngol Head Neck Surg. 2002;128(3):229–232. [DOI] [PubMed] [Google Scholar]
- 9.Godballe C, Jorgensen K, Hansen O, Bastholt L. Hypopharyngeal cancer: results of treatment based on radiation therapy and salvage surgery. Laryngoscope. 2002;112(5):834–838. [DOI] [PubMed] [Google Scholar]
- 10.Stoeckli SJ, Pawlik AB, Lipp M, Huber A, Schmid S. Salvage surgery after failure of nonsurgical therapy for carcinoma of the larynx and hypopharynx. Arch Otolaryngol Head Neck Surg. 2000;126(12):1473–1477. [DOI] [PubMed] [Google Scholar]
- 11.Hall SF, Groome PA, Irish J, O’Sullivan B. Radiotherapy or surgery for head and neck squamous cell cancer. Cancer. 2009;115(24):5711–5722. [DOI] [PubMed] [Google Scholar]
- 12.Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg. 2002;123(1):8–15. [DOI] [PubMed] [Google Scholar]
- 13.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 14.Lee NY, O’Meara W, Chan K, et al. Concurrent chemotherapy and intensity-modulated radiotherapy for locoregionally advanced laryngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2007;69(2):459–468. [DOI] [PubMed] [Google Scholar]
- 15.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefebvre J-L, Bonneterre J. Current status of larynx preservation trials. Curr Opin Oncol. 1996;8(3):209–214. [DOI] [PubMed] [Google Scholar]
- 17.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: Preliminary results of a European organization for research and treatment of cancer phase III trial. J Natl Cancer Inst. 1996;88(13):890–899. [DOI] [PubMed] [Google Scholar]
- 18.Iwae S, Fujii M, Hayashi R, et al. Matched-pair analysis of patients with advanced hypopharyngeal cancer: Surgery versus concomitant chemoradiotherapy. Int J Clin Oncol. 2017;22(6):1001–1008. [DOI] [PubMed] [Google Scholar]
- 19.Harris BN, Biron VL, Donald P, et al. Primary surgery vs chemoradiation treatment of advanced-stage hypopharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(7):636–640. [DOI] [PubMed] [Google Scholar]
- 20.Tsou Y-A, Lin M-H, Hua C-H, et al. Survival outcome by early chemoradiation therapy salvage or early surgical salvage for the treatment of hypopharyngeal cancer. Otolaryngol Head Neck Surg. 2007;137(5):711–716. [DOI] [PubMed] [Google Scholar]
- 21.Forastiere AA. Larynx preservation trials: a critical appraisal. Semin Radiat Oncol. 1998;8(4):254–261. [DOI] [PubMed] [Google Scholar]


