Abstract
Objectives:
Many patients with squamous cell carcinoma of the head and neck (SCCHN) are ≥65 years old; comorbidities and other age-related factors may affect their ability to tolerate traditional chemotherapy. Nivolumab is the only immunotherapy to significantly improve overall survival (OS) versus investigator’s choice (IC) of single-agent chemotherapy at primary analysis in a phase 3 trial (CheckMate 141) in patients with recurrent/metastatic SCCHN post-platinum therapy. In this post hoc analysis, we report efficacy and safety by age.
Patients and methods:
Eligible patients were randomized 2:1 to nivolumab 3 mg/kg every 2 weeks (n = 240) or IC (methotrexate, docetaxel, or cetuximab n = 121). The primary endpoint of the trial was OS. For this analysis, outcomes were analyzed by age < 65 and ≥65 years. The data cut-off date was September 2017 (minimum follow-up 24.2 months).
Results:
At baseline, 68 patients (28.3%) receiving nivolumab and 45 patients (37.2%) receiving IC were ≥65 years. Baseline characteristics were generally similar across age groups. OS and tumor response benefits with nivolumab versus IC were maintained regardless of age. The 30-month OS rates of 11.2% (< 65 years) and 13.0% (≥65 years) with nivolumab were more than tripled versus corresponding IC rates of 1.4% and 3.3%, respectively. The nivolumab arm had a lower rate of treatment-related adverse events versus IC regardless of age, consistent with the overall patient population.
Conclusion:
In CheckMate 141, nivolumab resulted in a higher survival versus IC in patients < 65 and ≥65 years, with a manageable safety profile in both age groups.
ClinicalTrials.gov:
Keywords: Biomarkers, Nivolumab, Squamous cell carcinoma of the head and neck, Age, Phase 3 clinical trial
Introduction
Over half of the 500,000 new cases of squamous cell carcinoma of the head and neck (SCCHN) worldwide occur in patients 65 years of age and older [1,2], and this is expected to increase as the population ages [3,4]. A high proportion of cases will go on to develop recurrent/metastatic disease [5,6], for which platinum-based chemotherapy with or without cetuximab or pembrolizumab can be used as first-line therapy for patients able to tolerate treatment [7-9].
Immune checkpoint inhibitors are a recent treatment strategy for patients with SCCHN and offer an opportunity for durable responses with a manageable safety profile [2]. Two programmed death-1 (PD-1) inhibitors, nivolumab and pembrolizumab, are currently approved for the treatment of patients with recurrent/metastatic SCCHN who experienced disease progression after platinum-based therapy. However, there are concerns that age-related decline in immune function may impact the activity of checkpoint inhibitors [10,11]. Some data have been reported for these agents in elderly patients with other solid tumors [11,12], and a recent publication of pembrolizumab in recurrent/metastatic SCCHN post-platinum therapy included limited data on efficacy by age [13].
At the primary analysis of the randomized, open-label, phase 3 CheckMate 141 trial (NCT02105636), nivolumab significantly improved overall survival (OS) versus investigator’s choice (IC) of therapy in patients with recurrent/metastatic SCCHN who experienced tumor progression or recurrence within 6 months of platinum-based therapy administered in the adjuvant, primary (i.e. with radiation), recurrent, or metastatic setting; survival benefit was maintained at 1 and 2 years of follow-up irrespective of tumor programmed death ligand 1 (PD-L1) expression and human papillomavirus (HPV) status [14-16]. The safety profile of nivolumab was manageable, with fewer grade 3–4 treatment-related adverse events (TRAEs) compared with IC [15]. Here, we report a post hoc analysis of the efficacy and safety of nivolumab by age (< 65 and ≥65 years old) in patients with recurrent/metastatic SCCHN from CheckMate 141.
Patients and methods
Study design and patients
CheckMate 141 is a randomized, open-label, phase 3 trial; the detailed study design has been described previously [14]. Briefly, eligible patients were 18 years of age or older, had histologically confirmed, recurrent/metastatic SCCHN of the oral cavity, oropharynx, hypo-pharynx, or larynx, and had tumor progression on or within 6 months after the last dose of platinum-based chemotherapy administered in the locally advanced, recurrent, or metastatic disease setting. Patients were randomized 2:1 to receive nivolumab (3 mg/kg every 2 weeks) or standard single agent of IC (methotrexate 40–60 mg/m2 weekly, docetaxel 30–10 mg/m2 weekly, or cetuximab 400 mg/m2 once, then 250 mg/m2 weekly) and stratified by prior cetuximab treatment. Treatment continued until tumor progression or unacceptable toxicity. Patients in the nivolumab arm were allowed to continue nivolumab treatment beyond tumor progression if they met predefined, protocol-specified criteria [15].
CheckMate 141 was conducted in accordance with the ethical principles in the Declaration of Helsinki. Written informed consent was obtained from all patients prior to enrollment. The study was approved by the institutional review board or independent ethics committee at each center and was conducted in accordance with Good Clinical Practice guidelines defined by the International Conference on Harmonisation.
Outcomes
The primary endpoint was OS, defined as the time from randomization to death due to any cause. Progression-free survival (PFS), defined as the time from randomization to first date of investigator-assessed progression, and objective response rate (ORR), defined as the proportion of randomized patients who achieved a best response of complete or partial response as per investigator assessment, were secondary endpoints; duration of objective response, defined as time from objective response until a progression event, was an exploratory endpoint. Tumor responses were evaluated every 6 weeks from week 9 until disease progression or treatment discontinuation using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 [17]. Safety was evaluated using the Common Terminology Criteria for Adverse Events, version 4.0, at each treatment visit and for 100 days after receipt of last dose. Adverse events with potential immunologic cause were characterized as select adverse events. Tumor PD-L1 expression and HPV status were assessed as previously described [14].
For this post hoc subgroup analysis, outcomes were analyzed by age (< 65 years and ≥65 years). This analysis is based on a September 2017 data cutoff, representing a minimum follow-up of 24.2 months (Supplementary Fig 1.).
Statistical analyses
Efficacy analyses were conducted in patients < 65 and ≥65 years old in the intent-to-treat population; safety analyses were conducted in the same subgroups among patients who received at least one dose of treatment.
OS and PFS were estimated by the Kaplan-Meier method [18]; Cox proportional hazards models were used to estimate hazard ratios and corresponding two-sided 95% confidence intervals (CIs). A generalization of the Brookmeyer and Crowley method with a log-log transformation was used to compute CIs for median survival times [19], and a two-sided Cochran-Mantel-Haenszel test was used to compute the odds ratio and corresponding CIs for tumor response [20,21].
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Results
Patients and treatment
Of the 361 patients who underwent randomization, 68 of 240 patients in the nivolumab arm (28.3%) and 45 of 121 in the IC arm (37.2%) were ≥65 years old; 31 (12.9%) and 23 patients (19.0%), respectively, were ≥70 years old. Baseline characteristics were generally similar between patients < 65 years and ≥65 years old; notably, percentages of prior systemic therapy received were evenly matched between groups (Table 1).
Table 1.
Baseline characteristics.
| Patients, n (%) | < 65 Years | ≥65 Years | ||
|---|---|---|---|---|
| Nivolumab (n = 172) |
IC (n = 76) | Nivolumab (n = 68) |
IC (n = 45) | |
| Sex, male | 134 (77.9) | 67 (88.2) | 63 (92.6) | 36 (80.0) |
| Race | ||||
| White | 139 (80.8) | 66 (86.8) | 57 (83.8) | 38 (84.4) |
| Black or African American | 8 (4.7) | 2 (2.6) | 2 (2.9) | 1 (2.2) |
| Asian | 20 (11.6) | 8 (10.5) | 9 (13.2) | 6 (13.3) |
| Other | 5 (2.9) | 0 | 0 | 0 |
| ECOG performance status | ||||
| 0 | 40 (23.3) | 16 (21.1) | 9 (13.2) | 7 (15.6) |
| 1 | 132 (76.7) | 56 (73.7) | 57 (83.8) | 38 (84.4) |
| ≥2 | 0 | 3 (3.9) | 1 (1.5) | 0 |
| Not reported | 0 | 1 (1.3) | 1 (1.5) | 0 |
| Region | ||||
| North America | 76 (44.2) | 28 (36.8) | 25 (36.8) | 16 (35.6) |
| Europe | 75 (43.6) | 39 (51.3) | 34 (50.0) | 23 (51.1) |
| Rest of world | 21 (12.2) | 9 (11.8) | 9 (13.2) | 6 (13.3) |
| Tobacco use | ||||
| Current/former | 135 (78.5) | 57 (75.0) | 56 (82.4) | 29 (64.4) |
| Never | 28 (16.3) | 17 (22.4) | 11 (16.2) | 14 (31.1) |
| Unknown | 9 (5.2) | 2 (2.6) | 1 (1.5) | 2 (4.4) |
| Site of primary tumor | ||||
| Oral cavity | 85 (49.4) | 42 (55.3) | 23 (33.8) | 25 (55.6) |
| Pharynx | 66 (38.4) | 24 (31.6) | 26 (38.2) | 13 (28.9) |
| Larynx | 18 (10.5) | 8 (10.5) | 16 (23.5) | 6 (13.3) |
| Other | 3 (1.7) | 2 (2.6) | 3 (4.4) | 1 (2.2) |
| HPV status | ||||
| Positive | 45 (26.2) | 18 (23.7) | 19 (27.9) | 11 (24.4) |
| Negative | 38 (22.1) | 23 (30.3) | 18 (26.5) | 14 (31.1) |
| Unknown/not reported | 89 (51.7) | 35 (46.1) | 31 (45.6) | 20 (44.4) |
| PD-L1 expression | ||||
| ≥1% | 71 (41.3) | 37 (48.7) | 25 (36.8) | 26 (57.8) |
| < 1% | 49 (28.5) | 31 (40.8) | 27 (39.7) | 9 (20.0) |
| Lines of prior systemic cancer therapy | ||||
| 1 | 73 (42.4) | 33 (43.4) | 33 (48.5) | 25 (55.6) |
| 2 | 62 (36.0) | 31 (40.8) | 18 (26.5) | 13 (28.9) |
| ≥3 | 37 (21.5) | 12 (15.8) | 17 (25.0) | 7 (15.6) |
| Prior systemic therapy receiveda | ||||
| Platinum-based therapy | 172 (100.0) | 76 (100.0) | 68 (100.0) | 45 (100.0) |
| Monoclonal antibody | 113 (65.7) | 45 (59.2) | 40 (58.8) | 28 (62.2) |
| Folic acid analog | 5 (2.9) | 3 (3.9) | 2 (2.9) | 0 |
| Taxane | 97 (56.4) | 41 (53.9) | 35 (51.5) | 21 (46.7) |
| Other – approved | 107 (62.2) | 51 (67.1) | 33 (48.5) | 18 (40.0) |
| Other – experimental | 19 (11.0) | 7 (9.2) | 4 (5.9) | 7 (15.6) |
Some patients may have been treated with more than one type of therapy. Abbreviations: ECOG = Eastern Cooperative Oncology Group; HPV = human papillomavirus; IC = investigator’s choice; PD-L1 = programmed death ligand 1.
Relative dosing intensity was comparable between the two age groups (< 65 and ≥65 years old) in the nivolumab arm (Supplementary Table 1). Median duration of therapy was similar between age groups in both the nivolumab and IC arms (1.6–1.9 months).
Survival
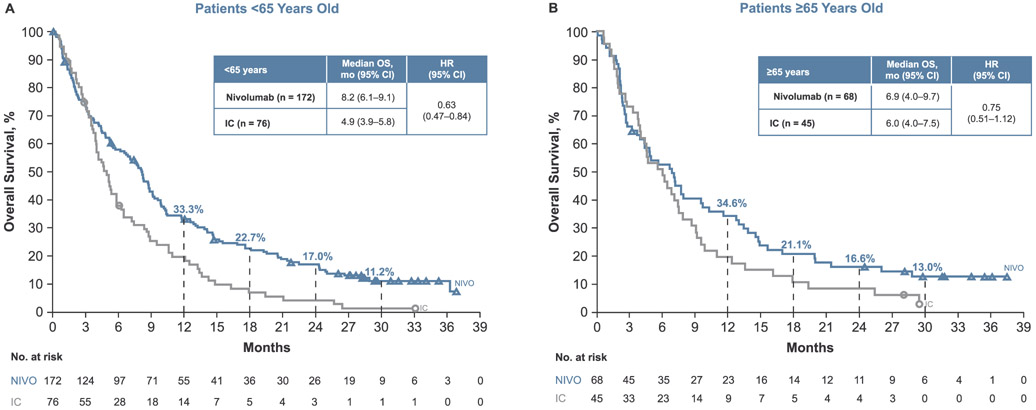
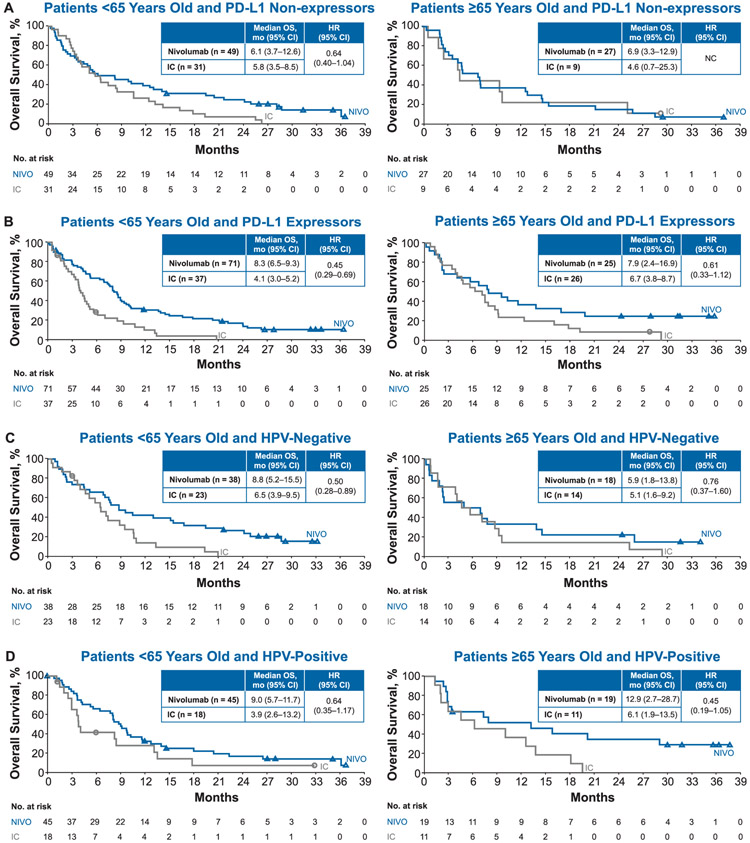
Nivolumab resulted in a higher median OS compared with IC in patients < 65 years old (8.2 vs. 4.9 months, HR 0.63; 95% CI 0.47–0.84) and in patients ≥65 years old (6.9 vs. 6.0 months; HR 0.75; 95% CI 0.51–1.12; Fig. 1). Estimated OS rates at 12 months were higher in the nivolumab arms compared with IC in both age groups: 33.3% (95% CI 26.2–40.4) versus 19.7% (95% CI 11.5–29.6) in patients < 65 years old and 34.6% (95% CI 23.5–45.9) versus 20.0% (95% CI 9.9–32.6) in patients ≥65 years old. Similar results were observed at 30 months: OS rates with nivolumab were 11.2% (95% CI 6.7–16.9) in patients < 65 years old and 13.0% (95% CI 6.2–22.5) in patients ≥65 years old; corresponding rates with IC were 1.4% (95% CI 0.1–6.7) and 3.3% (95% CI 0.3–13.2), respectively. OS benefit with nivolumab was maintained irrespective of tumor PD-L1 expression levels and HPV status (Fig. 2) across both age groups.
Fig. 1.
Overall survival (OS) in patients (A) < 65 years old and (B) ≥65 years old. Abbreviations: CI = confidence interval; HR = hazard ratio; IC = investigator’s choice; NIVO = nivolumab.
Fig. 2.
Overall survival (OS) by (A, B) tumor programmed death ligand 1 (PD-L1) expression and (C, D) human papillomavirus (HPV) status.
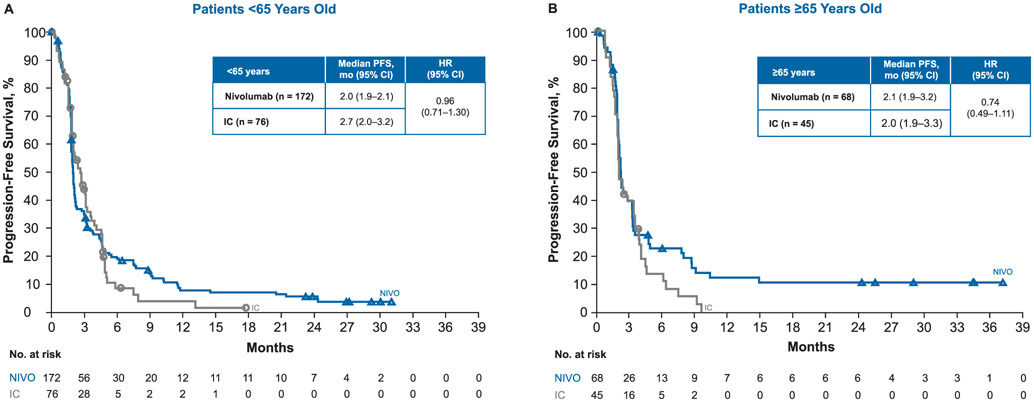
Median PFS was similar in both treatment arms for patients < 65 years old (nivolumab, 2.0 months; IC, 2.7 months; HR 0.96; 95% CI 0.71–1.30) and for patients ≥65 years old (nivolumab, 2.1 months; IC, 2.0 months; HR 0.74; 95% CI 0.49–1.11), consistent with results in the overall study population (Fig. 3).
Fig. 3.
Progression-free survival (PFS) in patients (A) < 65 years old and (B) ≥65 years old. Abbreviations: CI = confidence interval; HR = hazard ratio; IC = investigator’s choice; NIVO = nivolumab.
Best overall response
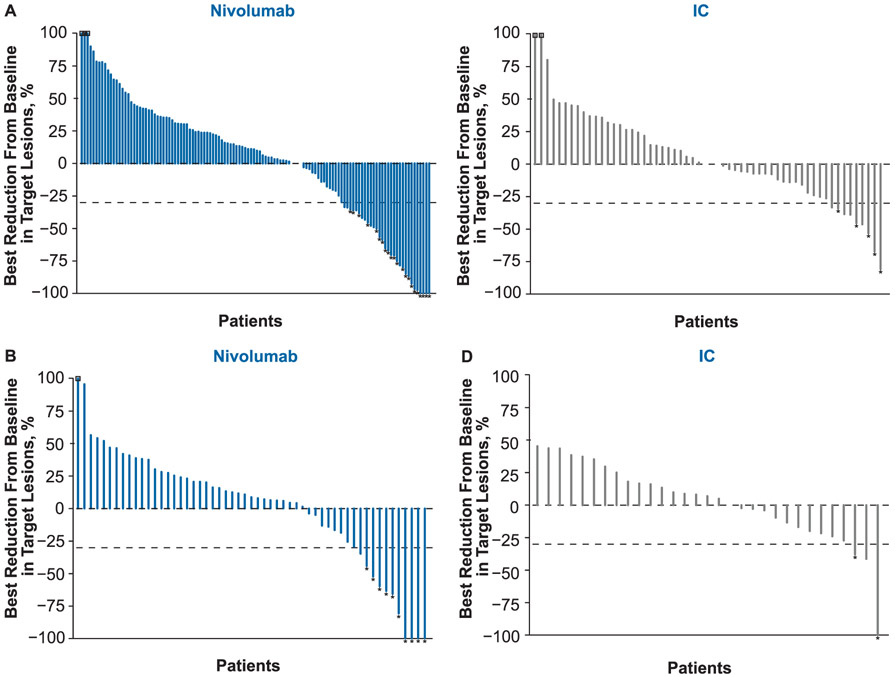
Treatment with nivolumab resulted in higher ORR versus IC in both age groups (Table 2). In patients < 65 years old, ORRs for the nivolumab and IC arms were 12.8% and 6.6%, respectively; in patients ≥65 years old, ORRs were 14.7% and 4.4%, respectively, including three patients (4.4%) with complete responses and seven patients (10.3%) with partial responses in the nivolumab arm (Fig. 4). Among patients ≥65 years old receiving IC, 1 patient (2.2%) had a complete response and 1 patient (2.2%) had a partial response. Median duration of objective response in the nivolumab arm was 8.5 months for patients < 65 years old and was not reached for patients ≥65 years old.
Table 2.
Best overall response per investigator. Abbreviations: + = censored value; CI = confidence interval; IC = investigator’s choice; NR = not reached; ORR = objective resDonse rate.
| < 65 Years | ≥65 Years | |||
|---|---|---|---|---|
| Nivolumab (n = 172) | IC (n = 76) | Nivolumab (n = 68) | IC (n = 45) | |
| Best overall response, n (%) | ||||
| Complete response | 4 (2.3) | 0 | 3 (4.4) | 1 (2.2) |
| Partial response | 18 (10.5) | 5 (6.6) | 7 (10.3) | 1 (2.2) |
| Stable disease | 36 (20.9) | 31 (40.8) | 19 (27.9) | 12 (26.7) |
| Progressive disease | 72 (41.9) | 25 (32.9) | 27 (39.7) | 17 (37.8) |
| Unable to determine | 42 (24.4) | 15 (19.7) | 12 (17.6) | 14 (31.1) |
| ORR, n (%) | 22 (12.8) | 5 (6.6) | 10 (14.7) | 2 (4.4) |
| [95% CI] | [8.2–18.7] | [2.2–14.7] | [7.3–25.4] | [0.5–15.1] |
| Time to objective response among responders, median (range), mo | 2.1 (1.8–7.4) | 2.0 (1.9–2.0) | 2.1 (1.8–6.3) | 3.5 (2.3–4.6) |
| Duration of response among responders, median (range), mo | 8.5 (2.8 to 28.0+) | 3.0 (2.7+ to 11.3) | NR (2.8 to 32.8+) | 4.9 (1.5+to 4.9) |
Fig. 4.
Best reduction from baseline in target lesions in patients (A) < 65 years old and (B) ≥65 years old. Abbreviation: * = responders; square symbol = % change truncated to 100%; IC = investigator’s choice.
Safety
TRAEs are summarized in Table 3. Among patients < 65 years old, any grade and grade 3–4 TRAEs were reported in 63.7% and 16.1% of patients receiving nivolumab, respectively, and in 77.5% and 31.0% of patients receiving IC, respectively. Among patients who were ≥65 years old, any grade and grade 3–4 events were reported in 57.4% and 13.2%, respectively, of patients receiving nivolumab, and in 82.5% and 47.5%, respectively, of patients receiving IC. The most common select TRAEs in patients ≥65 years old in the nivolumab arm were skin-related (14 patients; 20.6%); no grade 3–4 select TRAEs were reported in this patient subgroup (Supplementary Table 2).
Table 3.
Treatment-related adverse events (TRAEs) in ≥10% of patients. Abbreviation: IC = investigator’s choice.
| < 65 Years | ≥65 Years | |||||||
|---|---|---|---|---|---|---|---|---|
| Nivolumab (n = 168) | IC (n = 71) | Nivolumab (n = 68) | IC (n = 40) | |||||
| Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | |
| Any TRAE, n (%) | 107 (63.7) | 27 (16.1) | 55 (77.5) | 22 (31.0) | 39 (57.4) | 9 (13.2) | 33 (82.5) | 19 (47.5) |
| Fatigue | 24 (14.3) | 3 (1.8) | 14 (19.7) | 1 (1.4) | 13 (19.1) | 2 (2.9) | 6 (15.0) | 2 (5.0) |
| Nausea | 17 (10.1) | 0 | 16 (22.5) | 1 (1.4) | 5 (7.4) | 0 | 7 (17.5) | 0 |
| Rash | 14 (8.3) | 0 | 1 (1.4) | 0 | 5 (7.4) | 0 | 4 (10.0) | 1 (2.5) |
| Diarrhea | 13 (7.7) | 1 (0.6) | 7 (9.9) | 1 (1.4) | 7 (10.3) | 0 | 9 (22.5) | 1 (2.5) |
| Decreased appetite | 11 (6.5) | 0 | 6 (8.5) | 0 | 8 (11.8) | 0 | 2 (5.0) | 0 |
| Anemia | 8 (4.8) | 2 (1.2) | 13 (18.3) | 3 (4.2) | 4 (5.9) | 1 (1.5) | 6 (15.0) | 3 (7.5) |
| Asthenia | 8 (4.8) | 0 | 12 (16.9) | 2 (2.8) | 2 (2.9) | 1 (1.5) | 5 (12.5) | 0 |
| Vomiting | 6 (3.6) | 0 | 4 (5.6) | 0 | 2 (2.9) | 0 | 4 (10.0) | 0 |
| Mucosal inflammation | 4 (2.4) | 0 | 9 (12.7) | 1 (1.4) | 0 | 0 | 6 (15.0) | 1 (2.5) |
| Stomatitis | 2 (1.2) | 1 (0.6) | 8 (11.3) | 2 (2.8) | 4 (5.9) | 0 | 4 (10.0) | 1 (2.5) |
| Alopecia | 0 | 0 | 10 (14.1) | 0 | 0 | 0 | 4 (10.0) | 0 |
Efficacy and safety among patients ≥70 years old
In patients ≥70 years old (nivolumab, n = 31; IC, n = 23), the efficacy of nivolumab treatment was consistent with that seen in patients ≥65 years old and in the overall patient population; median OS in this group was 4.8 months in the nivolumab arm compared with 4.6 months in the IC arm (HR 0.91; 95% CI 0.52–1.60). Median PFS was 2.1 months in the nivolumab arm compared with 2.3 months in the IC arm (HR 1.0; 95% CI 0.57–1.75). Among patients who were ≥70 years old, ORRs in the nivolumab and IC arms were 6.5% and 8.7%, respectively. Any grade and grade 3–4 TRAEs were reported in 48.4% and 9.7%, respectively, of patients receiving nivolumab, and in 81.0% and 52.4%, respectively, of patients receiving IC.
Discussion
In this post hoc analysis from CheckMate 141, the threshold of 65 years of age was chosen to maintain an optimal number of patients in each treatment arm; importantly, this cutoff also corresponds with established age classifications applied to patients with cancer [22,23]. While 70 years is also often used as the threshold for clinical trials in elderly patients [24,25], in CheckMate 141, patient numbers with this cutoff were too small for meaningful analyses. Nivolumab therapy resulted in an OS benefit compared with IC and responses were durable in patients with recurrent/metastatic SCCHN across both age groups of < 65 and ≥65 years old at a minimum follow-up of 2 years. Among patients ≥65 years old in the nivolumab arm, ORR was 14.7% with 10 patients experiencing complete or partial responses. The median OS for this subgroup was 6.9 months and 13.0% of patients were still alive at 30 months, nearly triple that of patients in the IC arm. Although the number of patients ≥70 years old was limited (31 in the nivolumab arm and 23 in the IC arm), the efficacy and safety of nivolumab in these patients were consistent with results seen in patients ≥65 years old.
These data in the elderly population of CheckMate 141 are comparable to those previously reported in the primary analysis and 2-year update to the study [14,15]. At 2 years, the ORR for patients receiving nivolumab was 13.3%; median OS was 7.7 months with 16.9% of patients still alive at 24 months [15]. OS benefit was maintained with nivolumab irrespective of tumor PD-L1 expression levels and HPV status, and this finding is consistent with results presented in the current analysis across age groups. Even though no clear difference could be observed in patients ≥65 years without PD-L1–expressing tumors, the number of patients in this subgroup was small. Results were recently published from the phase 3 KEYNOTE-040 study comparing pembrolizumab versus methotrexate, docetaxel or cetuximab [13].
Age-related immune dysfunction, attributed to both cellular components of the immune system as well as changes in the tumor microenvironment, is a key challenge in developing effective immunotherapies for elderly patients [26,27]. Immunosenescence is thought to impair the antitumor immune response through reduced tumor antigen release, altered antigen-uptake and antigen-presenting functions, and impaired T-cell activation and trafficking, all of which can hinder the elimination of tumor cells [28]. However, tumor mutational burden, which has been shown to increase with age, among other factors, leads to increased neoantigen formation and subsequent increased immune response [29]. The greater immunogenicity of tumors with high tumor mutational burden makes them a target for immuno-oncology therapies [30,31]. Advanced age may also exacerbate treatment-related toxicities due to metabolic changes, polypharmacy, and comorbidities [32], thereby limiting therapeutic options. A higher prevalence of adverse events such as nephrotoxicity, thrombocytopenia, and myelosuppression is associated with chemotherapy in elderly patients [33,34]. In contrast, nivolumab was well tolerated in the current study, and the incidence and severity of TRAEs were comparable among patients in both age groups. The most common associated adverse events in patients ≥65 years old were fatigue, decreased appetite, and diarrhea, similar to those reported in the overall study population [14,15].
A meta-analysis was recently published using random-effects estimates to evaluate the efficacy of PD-1 and PD-L1 inhibitors in patients ≥65 years old with advanced solid tumors, including clinical studies of nivolumab in non-small cell lung cancer, renal cell carcinoma, and melanoma [35]. Similar to the results of the current analysis, a consistent improvement in survival associated with the use of these therapies across groups of younger and older patients was reported. A retrospective study of PD-1 and PD-L1 inhibitors in patients with melanoma also found no significant differences in toxicity between younger and older age groups, although there was a trend toward a higher rate of endocrine-related events in elderly patients [11].
The current analysis addresses an unmet need for data among elderly patients with SCCHN. Elderly patients are often underrepresented in clinical trials [2,36,37], which contributes to this increasing need given the changing demographics of head and neck cancer. In clinical trials specific to elderly patients, treatment selection is often based on fitness or geriatric evaluation as frailty can vary among patients considered to be elderly [24,37-39]. The current study did not assess frailty before enrollment; however, patients randomized to the IC arm of this study were considered able and fit to undergo chemotherapy (including taxane) or treatment with cetuximab. While limited by the post hoc nature of the analysis, our results highlight the opportunity for new interventions to improve outcomes and minimize toxicities in elderly patients with SCCHN.
Conclusion
Here, we show that nivolumab therapy demonstrated efficacy benefit, durable response, and a manageable safety profile in patients ≥65 years old with SCCHN, suggesting that age should not be a critical factor when selecting second-line immunotherapy. These data support the use of nivolumab in younger and older patients with recurrent/metastatic SCCHN post-platinum therapy.
Supplementary Material
Acknowledgements
The authors thank the patients and their families as well as the clinical study teams for making this study possible. All authors contributed to and approved the manuscript; writing and editorial assistance was provided by Brooke Middlebrook, BS, of Evidence Scientific Solutions, funded by Bristol-Myers Squibb. K. J. Harrington acknowledges support from the Royal Marsden/the Institute of Cancer Research, National Institute of Health Research Biomedical Research Centre, and Oracle Cancer Trust. R. L. Ferris is supported by the P50 CA097190-14, P30 CA047904-28, and R01 CA206517 grants. The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant P30 CA016672).
Sources of funding
This study was sponsored by Bristol-Myers Squibb (Princeton, NJ, USA) and ONO Pharmaceutical Company, Ltd. (Osaka, Japan).
Role of the funding source
Bristol-Myers Squibb was involved in the study concept and design, quality control of data, data analysis and interpretation, statistical analysis, and manuscript preparation, editing, and review.
Abbreviations:
- CI
confidence interval
- ECOG
Eastern Cooperative Oncology Group
- HPV
human papillomavirus
- HR
hazard ratio
- IC
investigator’s choice
- NR
not reached
- ORR
objective response rate
- OS
overall survival
- PD-1
programmed death-1
- PD-L1
programmed death ligand 1
- PFS
progression-free survival
- SCCHN
squamous cell carcinoma of the head and neck
- TRAE
treatment-related adverse event
Footnotes
Declaration of Competing Interest
N. F. S. reports funding for research from Bristol-Myers Squibb and Exelexis, and other from Aduro, Bristol-Myers Squibb, GSK, Lilly, Merck, and Pfizer. G. B. reports grants from Adaptimmune, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Exelixis, Genentech, GlaxoSmithKline, Hoffman-La Roche, Immatics, Immunocore, Incyte, Kite Pharma, Macrogenics, Medimmune, Merck, Novartis, Torque, and Xcovery; and other from AbbVie, Adicet, Amgen, Ariad, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Clovis, Genentech, Hoffman-La Roche, Merck, Novartis, and Xcovery. J. G. reports grants from Bristol-Myers Squibb and Merck KGaA; advisory board fees from AstraZeneca, Bristol-Myers Squibb, Innate Pharma, Nanobiotix, and Merck KGaA; and other from Bristol-Myers Squibb and Merck KGaA. L. Licitra received grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene International, Eisai, Exelixis, Inc., Hoffmann–La Roche Ltd., IRX Therapeutics, MedPace, Inc., Merck Serono, MSD, Novartis, Pfizer, and Roche; honoraria/consultation fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Debiopharm, Doxa Pharma SRL, Eisai, Health & Life SRL, Immuno-Oncology Hub, Incyte Biosciences Italy SRL, Ipsen Innovation, Kura Oncology, Merck Serono, MSD, Nanobiotics SA, Novartis, Roche, and SOBI; and other from Bayer, Bristol-Myers Squibb, Debiopharm, Merck Serono, MSD, and SOBI. J. F. reports grants from AstraZeneca and Bristol-Myers Squibb; honoraria/consultation fees from AstraZeneca, Biogen, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, Innate Pharma; personal fees from AstraZeneca, Bristol-Myers Squibb, and Merck Sharpe & Dohme. K. J. H. reports grants from AstraZeneca and Merck Sharp & Dohme; personal fees from AstraZeneca, Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, and Pfizer. M. L. G. reports grants from AstraZeneca, Bristol-Myers Squibb, Kyowa, and Merck; and personal fees from Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Lilly, and Merck. R. L. F. reports grants and clinical trial support from AstaZeneca/MedImmune, Bristol-Myers Squibb, Merck, Tesaro, and VentiRx Pharmaceuticals; advisory board/consultation fees from Amgen, AstraZeneca/MedImmune, Bain Capital Life Sciences, Bristol-Myers Squibb, EMD Serono, GlaxoSmithKline, Iovance Biotherapeutics, Inc., Lilly, Merck, Numab Therapeutics AG, Oncorus, Inc., Ono Pharmaceutical Co. Ltd., Pfizer, PPD (Benitec, Immunicum), Regeneron Pharmaceuticals, Inc., Tesaro, Torque Therapeutics Inc., and TTMS. N. K. reports grants from ONO, AstraZeneca, and Pfizer; honoraria from ONO, Bristol-Myers Squibb, Merck Serono, Eisai, and Bayer. L. Li is an employee of and holds stock in Bristol-Myers Squibb. V. J. is an employee of Bristol-Myers Squibb and a former employee of AstraZeneca. All other authors report no conflict of interest.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.oraloncology.2019.06.017.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Szturz P, Vermorken JB. Treatment of elderly patients with squamous cell carcinoma of the head and neck. Front Oncol 2016;6:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–65. [DOI] [PubMed] [Google Scholar]
- [4].Gugic J, Strojan P. Squamous cell carcinoma of the head and neck in the elderly. Rep Pract Oncol Radiother 2012;18:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–52. [DOI] [PubMed] [Google Scholar]
- [6].Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–44. [DOI] [PubMed] [Google Scholar]
- [7].Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, De Castro G Jr, et al. KEYNOTE-048: phase 3 study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Ann Oncol 2018;29(mdy424):045. [Google Scholar]
- [8].National Comprehensive Cancer Network. Head and Neck Cancer (Version 2.2018) [Available from: < https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf > ].
- [9].Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. New Engl J Med 2008;359:1116–27. [DOI] [PubMed] [Google Scholar]
- [10].Elias R, Karantanos T, Sira E, Hartshorn KL. Immunotherapy comes of age: immune aging and checkpoint inhibitors. J Geriatr Oncol 2017;8:229–35. [DOI] [PubMed] [Google Scholar]
- [11].Betof AS, Nipp RD, Giobbie-Hurder A, Johnpulle RAN, Rubin K, Rubinstein SM, et al. Impact of age on outcomes with immunotherapy for patients with melanoma. Oncologist 2017;22:963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Elias R, Morales J, Rehman Y, Khurshid H. Immune checkpoint inhibitors in older adults. Curr Oncol Rep 2016;18:47. [DOI] [PubMed] [Google Scholar]
- [13].Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–67. [DOI] [PubMed] [Google Scholar]
- [14].Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Haddad R, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Treatment beyond progression with nivolumab in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) in the phase 3 CheckMate 141 study: a biomarker analysis and updated clinical outcomes [abstract]. Ann Oncol 2017;28(Suppl. 5):1043O. [Google Scholar]
- [16].Gillison ML, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. CheckMate 141: 1-year update and subgroup analysis of nivolumab as first-line therapy in patients with recurrent/metastatic head and neck cancer. Oncologist 2018;23:1079–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [18].Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- [19].Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics 1982;38:29–41. [Google Scholar]
- [20].Cochran WG. Some methods for strengthening the common X2 tests. Biometrics 1954;10:417–51. [Google Scholar]
- [21].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [22].Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Balducci L Management of cancer in the elderly. Oncology (Williston Park) 2006;20:135–43. discussion 44, 46, 51-2. [PubMed] [Google Scholar]
- [24].Argiris A, Li Y, Murphy BA, Langer CJ, Forastiere AA. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. J Clin Oncol 2004;22:262–8. [DOI] [PubMed] [Google Scholar]
- [25].Balducci L Geriatric oncology: challenges for the new century. Eur J Cancer 2000;36:1741–54. [DOI] [PubMed] [Google Scholar]
- [26].Tomihara K, Curiel TJ, Zhang B. Optimization of immunotherapy in elderly cancer patients. Crit Rev Oncog 2013;18:573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hurez V, Padron AS, Svatek RS, Curiel TJ. Considerations for successful cancer immunotherapy in aged hosts. Clin Exp Immunol 2017;187:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Daste A, Domblides C, Gross-Goupil M, Chakiba C, Quivy A, Cochin V, et al. Immune checkpoint inhibitors and elderly people: a review. Eur J Cancer 2017;82:155–66. [DOI] [PubMed] [Google Scholar]
- [29].Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019;30:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Conway JR, Kofman E, Mo SS, Elmarakeby H, Van Allen E. Genomics of response to immune checkpoint therapies for cancer: implications for precision medicine. Genome Med 2018;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kimmick GG, Fleming R, Muss HB, Balducci L. Cancer chemotherapy in older adults. A tolerability perspective. Drugs Aging 1997;10:34–49. [DOI] [PubMed] [Google Scholar]
- [33].Mountzios G Optimal management of the elderly patient with head and neck cancer: issues regarding surgery, irradiation and chemotherapy. World J Clin Oncol 2015;6:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Balducci L, Hardy CL, Lyman GH. Hemopoietic reserve in the older cancer patient: clinical and economic considerations. Cancer Control 2000;7:539–47. [DOI] [PubMed] [Google Scholar]
- [35].Elias R, Giobbie-Hurder A, McCleary NJ, Ott P, Hodi FS, Rahma O. Efficacy of PD-1 and PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer 2018;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 2003;21:1383–9. [DOI] [PubMed] [Google Scholar]
- [37].Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol 2015;33:3305–13. [DOI] [PubMed] [Google Scholar]
- [38].Sarris EG, Harrington KJ, Saif MW, Syrigos KN. Multimodal treatment strategies for elderly patients with head and neck cancer. Cancer Treat Rev 2014;40:465–75. [DOI] [PubMed] [Google Scholar]
- [39].Syrigos KN, Karachalios D, Karapanagiotou EM, Nutting CM, Manolopoulos L, Harrington KJ. Head and neck cancer in the elderly: an overview on the treatment modalities. Cancer Treat Rev 2009;35:237–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.