Abstract
Background
Botswana has a large burden of disease from injury, but no trauma registry. This study sought to design and pilot test a trauma registry at two hospitals.
Methods
A cross sectional study was piloted at a tertiary hospital and a secondary level hospital in Botswana. The study consisted of two stages: stage 1 – stakeholders' consultation and trauma registry prototype was designed. Stage 2 consisted of two phases: Phase I involved retrospective collection of existing data from existing data collection tools and Phase II collected data prospectively using the proposed trauma registry prototype.
Results
The pre-hospital road traffic accident data are collected using hard copy forms and some of these data were transferred to a stand-alone electronic registry. The hospital phase of road traffic accident data all goes into hard copy files then stored in institutional registry departments. The post-hospital data were also partially stored as hard copies and some data are stored in a stand-alone electronic registry. The demographics, pre-hospital, triage, diagnosis, management and disposition had a high percent variable completion rate with no significant difference between phases I and II. However, the primary survey variables in Phase I had a low percent variable completion rate which was significantly different from the high completion rates in phase II at both hospitals. A similar picture was observed for the secondary survey at both hospitals.
Conclusion
Electronic trauma registries are feasible and data completion rate is high when using the electronic data registry as opposed to data collected using the existing paper-based data collection tools.
Keywords: Trauma registry, Injury registry, Road traffic crushes registry, Road traffic accident trauma registry, Road accident registry
African relevance
-
•
Addresses trauma care systems and resource limitations as it pertains to the African continent
-
•
Demonstrate the feasibility of using available and affordable eHealth platform to develop trauma registries in resource limited context.
-
•
Provides a recommendation of a trauma registry prototype for adoption and implementation in a resource limited setting.
Introduction
Injury is a major public health problem causing almost 6 million deaths worldwide each year; which is one third more than HIV/AIDS, Malaria and TB combined [1]. Road traffic accidents are the world's leading cause of death for individuals aged 15 to 29, and among the top three in the age range 15–44 years [2]. It is estimated that road traffic accidents will be the world's fifth leading cause of death by 2030 [1]. Compared to other continents, deaths rate per motorized vehicle in Africa (50/10,000) is disproportionally high [3].
A recent systematic analysis of African injury data reported an increasing injury rate overall but an official death rate reduction [2]. However, these figures represented only 15 countries on the continent and most data were police or traffic reports, which do not reflect accurate morbidity and mortality rates [2,3]. These figures reflect pre-hospital morbidity and mortalities and lack the in-hospital and post-hospital trauma related morbidity and mortality data. The Southern African nation of Botswana was not included in the analysis and researchers in this region reported significant morbidity and mortality figures from injury studies at regional hospitals [3]. To accurately measure this large burden of disease, researchers advocate for immediate changes in data registration [4]. The lack of trauma data is known to negatively impact on the ability to meaningfully respond to the burden of trauma in low- and middle-income countries (LMICs) [5].
Trauma care systems have been shown to save lives in developed countries [6], but assessing their impact in developing countries requires a sound data capture system. Many global health experts recommend improved injury surveillance to identify gaps and develop further management and prevention strategies [3,5].
An epidemiological review of emergency presentations to Botswana's largest referral hospital-Princess Marina Hospital (PMH)- in 2011 revealed that trauma was the second most common presenting problem in all age groups [7]. Trauma care in Botswana is currently inconsistent and not delivered in a systematic fashion and the country does not have a trauma registry or formal system for reporting trauma statistics in the health system [8,9]. Missing data variables and patient file unavailability are frequently encountered problems affecting trauma data retrieval [[8], [9], [10]]. Manually searching and retrieval of these paper-based patient files from the registry department is a daunting process. The existing data collection tools, which are paper-based, make data entry inconsistent and data retrieval difficult. Legibility and lack of consistency in documentation of clinical information in patient files contribute to the difficulty in data acquisition. The development of well-structured electronic trauma registries may facilitate higher data completion rates, consistency in data entry and easy data retrieval. Trauma registries are a source of evidence necessary to guide interventions to reduce trauma related morbidity and morbidities.
The current study set out to develop and pilot test a trauma registry prototype at a secondary and a tertiary level hospital. The structure of the trauma registry prototype is informed by the Advanced Trauma Life Support (ATLS) principles of initial assessment and management of the injured patient. These principles (ATLS principles) emphasis the attendance of life-threatening injuries first in the order of priority to reduce mortality, morbidity and disability associated with injuries.
Methods
Study design
This cross-sectional study took place between August 2017 and May 2018 at one tertiary hospital, Princess Marina Hospital (PMH), and one secondary level hospital, Scottish Livingstone Hospital (SLH). The study consisted of two stages. The first stage (stage1) of the study entailed stakeholder consultations to identify existing data collection tools. During this stage, the stakeholders also agreed on the platform for designing the trauma registry prototype based on the recommendations from their respective Information Technology and Communication (ITC) units. The platform was chosen based on duration of development process, cost of development, ease of use, offline and online accessibility, and technical support availability. The stakeholders identified core data variables (mandatory variables) which must always be completed on all trauma patients whether their findings are positive or negative. The trauma registry prototype was designed during stage 1 and its structure followed the standard ATLS principles of initial assessment and management of the injured patient. The second stage (stage 2) consisted of two phases: Phase I is the retrospective collection of old data from patient files (Pre-intervention data) and phase II is the collection data prospectively using the proposed trauma registry prototype (Intervention data). The intervention is the use of the proposed trauma registry prototype to prospectively collect data with the intent of improving percent variable completion rate (PVCR). The PVCR for the pre-intervention data was compared to that of the intervention data. Fig. 1 shows a flow chart of the study design.
Fig. 1.
Study design flow chart.
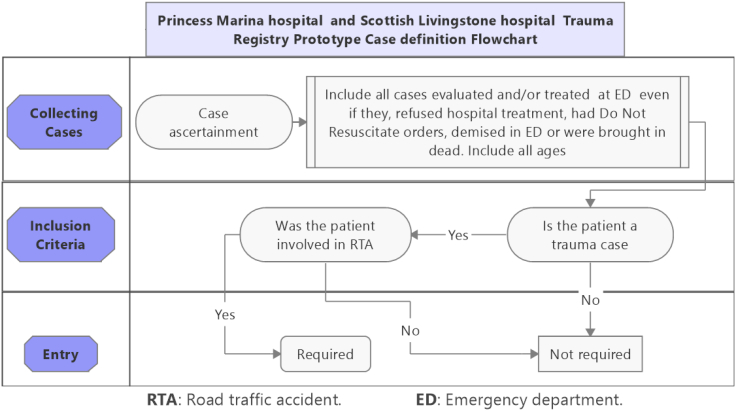
Most of our research assistants were surgical department doctors and nursing staff who had RedCap accounts created for them. They used the electronic trauma registry to directly enter the data. However, the registry had a printable pdf version which was also used as a hardcopy option to capture data, and latter entered into the registry. This form was used especially by research assistants who were not members of the surgical department at the participating hospital. A list of enrolled participants was kept and updated continually with regards to whether the patients were still under hospital care or were discharged. Patients were followed using either the paper form or the electronic registry until they left the hospital. Fig. 2 shows a case definition flowchart that was used during data collection.
Fig. 2.
Case definition flowchart.
The data registry did not have a built-in logic to force or remind completion of missing/incorrect mandatory variables. This functionality was deliberately omitted to avoid bias in rate of variable completion. The RedCap form however had an option of saving the form as ‘incomplete’ if the patient data collection was still ongoing, but not because a mandatory variable is not completed/is missing. This was an option chosen by the research assistant upon saving the record to indicate whether the patient care is complete or still ongoing. If the patient care was complete the form would be saved as ‘complete’.
Stakeholders
Stakeholders were departments or institutions involved in the care of trauma patients in the pre-hospital, in-hospital and post-hospital phase. The involved stakeholders were the Department of Traffic and Road Safety (DTRS), Motor Vehicle Accident Fund, Ministry of Health and Wellness (MOHW), and the University of Botswana. These institutions were identified because of their history of engagement in RTA and research on RTA.
Personnel and training
The research assistants consisted of two nursing staffs with the experience of working in the emergency departments and one medical officer who worked in the surgical department. They attended one-day training on ethical code of conduct and standards of practice. Site surveys were conducted with the team lead at both sites. The research assistants had a support group consisting of senior clinicians, data acquisition and processing team, finance, and management teams. The data collection team was coordinated and supervised closely by a General Surgeon with special interest in trauma. This ensured that data were accurately extracted, cleaned and recorded by the research assistants. Interval meetings were scheduled for feedback and needs assessment.
Analysis
The PVCR of mandatory variables was used to assess data completeness of pre-intervention data and the intervention data.
Paired sample t-test statistics with significance set at p < 0.05 was used to assess the significance of the difference in the mean PVCR in pre-intervention data vs intervention data.
This was a minimal risk study. Data was anonymized by de-identification. The study however received ethical clearance from the University of Botswana, Princess Marina Hospital and Scottish Livingstone Hospital Institutional Review Boards while permission to carry out the study in the hospital was granted by the Ministry of Health and Wellness.
Results
Stage 1 (consultations): existing data collection tools
During the stage 1 of the study (consultations), the existing data collection tools were identified from the Department of Traffic and Road Safety (DTRS), Motor Vehicle Accident Fund (MVAF) and Ministry of Health and Wellness. There were few tools, most of which were paper-based and only a few were electronic data collection tools. A list of the existing data collection tools and their formats is shown on Table 1. Two pre-hospital forms were identified: The Police BP.68 Accident Scene Form and the Ministry of Health Ambulance Patient Report Forms, which are both paper-based data collection tools. Similarly, Form v2, 2012 is an in-hospital paper-based data collection tool used for all acute medical and surgical presentation at PMH and most secondary level hospitals including SLH. The form however, is not structured to facilitate data entry in line with the standard principles of initial assessment and management of trauma patients.
Table 1.
Existing data collection tools and their formats.
| Stakeholders | Existing data collection tools | Type/format |
|---|---|---|
| Department of Traffic and Road Safety (DTRS) | Police BP.68 Accident Scene Form | Paper-based |
| Microcomputer Accident Analysis Package (MAAP) | Electronic system | |
| Motor Vehicle Accident Fund (MVAF) | MVAF Forms | Paper-based |
| MVAF standalone database | Electronic system | |
| Ministry of Health and Wellness | Ambulance Patient Report Forms | Paper-based |
| Form v2, 2012 | Paper-based | |
| Patient Admission Forms | Paper-based |
The demographics information on the Form v2, 2012 is completed by the registry department and the pre-hospital information including vital signs, triage information, past medical history and allergies information is completed by the nursing staff. The Form has a blank space where the doctor documents his/her findings and it is at the doctor's discretion to structure his/her notes according the ATLS principles. The in-hospital trauma clinical information is ultimately filed and stored as hardcopies in the departmental records/registry. Retrieving data and data analysis to inform clinical practice was complicated because of the types of data collection and storage methods.
The pre-hospital road traffic accident data from the BP.68 Police Form are ultimately entered into the Microcomputer Accident Analysis Package (MAAP) by the Traffic Police Division. MAAP is used to collect and analyse road accident data in a systematic manner. The MAAP system has two distinct sections including the input of accident data from police road traffic accident report forms and the analysis of the accident database.
The MVAF standalone database is a post-hospital trauma database which hosts road traffic accident information concerning rehabilitation and accident fund claims information. MVAF database is not integrated with the MAAP system although stakeholders highlighted that there are plans for that. However, during the stakeholder consultations, it became apparent that the hospital phase trauma data are mainly collected and stored in hard copy format which makes it difficult to pull out and analyse the data and will not be easy to integrate it to create a comprehensive road traffic accident registry that would inform clinical practice and policy making. Fig. 3 summaries the road traffic accidents data flow map.
Fig. 3.
Diagrammatic representation of road traffic accident data flow.
The ICT units from respective stakeholder departments were engaged to enlighten the team on the available options of platforms for designing the trauma registry prototype. The MOHW uses the Integrated Patient Management System (IPMS). Though the systems are freely available in government hospitals and have technical support team, the process of designing and incorporating a new module needed to go through a lengthy approval and validation process. IPMS is also available online only with potential challenges during network failures. The Botswana Harvard Partnership ICT department offered the possibility of designing the registry for us. However, this entailed some costs, a potential lengthy process to get it up and running. The offline availability and an easy to use user interface were a possibility. The University of Botswana however had the REDCap (Research Electronic Data Capture) platform which was freely available, team members had experience using it in their previous projects, was available online and offline. It also had available technical support. REDCap was therefore chosen as a viable option for a six months pilot study. Table 2 shows the available platforms for designing the trauma registry prototype and how they were assessed.
Table 2.
Available platforms for designing the trauma registry prototype and their assessment.
| Ministry of Health and Welfare |
University of Botswana |
Harvard and Botswana Partnership |
|
|---|---|---|---|
| Integrated Patient Management System (IPMS) | REDCap | Designing a database | |
| Choice determining factors | |||
| Duration of development process | Lengthy process | Quick | Lengthy process |
| Cost of development | Free | Free | Not free |
| Ease to use | Complex interface | Yes | Possibly |
| Technical support availability | Yes | Yes | Yes |
| Offline & online availability | No | Yes | Possible |
Development of the trauma registry prototype
The structure of the trauma registry was informed by the ATLS principles of approach to the injured patient. The structure is outline in Fig. 4 and mandatory variables are highlighted with a tick. A REDCap account was created and the trauma registry designed. A mobile App was also available to access the account and collect data online and offline.
Fig. 4.
The structure of the trauma registry prototype.
Stage 2: phase 1 and 2 study results
At PMH, 3 months' data were collected for each Phase (September–November 2017 retrospective data collection from patient files and March–May 2018 prospective data collection using the trauma registry prototype). However, due to distance and administrative constraints at SLH, 2 months' data for each phase was collected (September–October 2017 retrospective data collection and April–May 2018 prospective data collection).
Phase I data collection from PMH and SLH yielded 153 and 65 records respectively while the Phase II data from PMH and SLH yielded 139 and 57 records respectively. PMH had a higher number of road traffic accident cases. The PVCR of each mandatory variable for both phases of the study at PMH and SLH is shown in Table 3. Table 3 also contains data bars for visual illustration of the percentage rate of completion trends.
Table 3.
Mandatory variable percent variable completion rate (PVCR) for phase I & II at SLH and PMH.
Comparison of the mandatory variables PVCR between phase I and phase II
The significance of the observed overall differences in the mandatory variables PVCR in Phases I and II at SLH and PMH are presented in Table 4. The Phase I and Phase II differences of mean PVCR for SLH and PMH were 11.6% (p = 0.004) and 16.8% (p < 0.0001) respectively.
Table 4.
Paired t-test results for overall mean differences in the PVCR in phase I and II at SLH and PMH.
| Paired samples statistics |
Paired samples t-test |
||||
|---|---|---|---|---|---|
| Trauma registry component | Phase I mean PVCR | Phase II mean PVCR | Phase II – phase I mean PVCR difference | STD error (SE) | Significance (p-value) |
| PMH overall mandatory variable PVCR | 76.9 | 93.7 | 16.77 | 3.87 | >0.0001a |
| SLH overall mandatory variable PVCR | 77.7 | 89.3 | 11.62 | 3.72 | 0.004a |
Indicates significant difference.
Table 5 shows a statistically significant difference in the mean PVCR of mandatory variables in the “Primary Survey” and “AMPLE History” components: 28.3% (p = 0.001) and 27.7% (p = 0.014) respectively at PMH. The differences in PVCR of mandatory variables in other components were not statistically significant. At SLH, the only significant difference in the mean PVCR was observed in the “Primary Survey” component: 22.9% (p = 0.01) as shown on Table 6.
Table 5.
Paired t-test results for differences in the mean PVCR of mandatory variables at PMH.
| Paired samples statistics |
Paired samples t-test |
|||||
|---|---|---|---|---|---|---|
| Trauma registry component | Mandatory variables | PMH phase I mean completion rate | PMH phase II mean completion rate | Phase II - phase I mean difference | STD error (SE) | Significance (p-value) |
| Demographics and registration information | Sex | 95.9 | 94.3 | −1.56 | 0.91 | 0.163 |
| DOB | ||||||
| Hosp No (PM) | ||||||
| Reg No (PA) | ||||||
| Referring Institution | ||||||
| Pre-hospital and triage information | Injury datetime | 87.7 | 97.4 | 9.67 | 9.34 | 0.348 |
| Triage datetime | ||||||
| Triage code | ||||||
| Datetime seen by Doctor | ||||||
| Presentation History | ||||||
| Mode of injury | ||||||
| Primary survey: airway breathing circulation disability exposure | Airway Status | 66.5 | 94.8 | 28.33 | 6.91 | 0.001a |
| C-Spine Status | ||||||
| C-spine immobilization | ||||||
| Inspection | ||||||
| Resp rate | ||||||
| SO2 (%) | ||||||
| Oxygen delivery mode | ||||||
| Chest Examination | ||||||
| Heart Rate | ||||||
| Systolic BP | ||||||
| Diastolic BP | ||||||
| Source of blood loss | ||||||
| Normal Pupil Size and Reactivity | ||||||
| GCS | ||||||
| Exposure & Temperature | ||||||
| Secondary survey: ample history | Allergies | 60.7 | 88.3 | 27.69 | 6.66 | 0.014a |
| Medications | ||||||
| Past Medical history | ||||||
| Last meal | ||||||
| Events Surrounding the incident | ||||||
| Diagnosis, management and disposition | Final Diagnosis | 98.5 | 95.7 | −2.79 | 1.57 | 0.217 |
| Management | ||||||
| Disposition | ||||||
| Discharge summary | Date of discharge | 81.0 | 86.1 | 5.05 | 6.92 | 0.542 |
| Status at Discharge | ||||||
| Management Plan at discharge | ||||||
Indicates significant difference.
Table 6.
Paired t-test results for differences in the mean PVCR of mandatory variables at SLH.
| Paired samples statistics |
Paired samples t-test |
|||||
|---|---|---|---|---|---|---|
| Trauma registry component | Mandatory variables | SLH phase I mean completion rate | SLH phase II mean completion rate | Phase II - phase I mean difference | STD error (SE) | Significance (p-value) |
| Demographics and registration information | Sex | 84.6 | 89.1 | 4.51 | 2.12 | 0.101 |
| DOB | ||||||
| Hosp No (PM) | ||||||
| Reg No (PA) | ||||||
| Referring Institution | ||||||
| Pre-hospital and triage information | Injury datetime | 90.5 | 95.6 | 5.1 | 3.90 | 0.248 |
| Triage datetime | ||||||
| Triage code | ||||||
| Datetime seen by Doctor | ||||||
| Presentation History | ||||||
| Mode of injury | ||||||
| Primary survey: airway breathing circulation disability exposure | Airway Status | 66.4 | 89.2 | 22.88 | 7.69 | 0.01a |
| C-Spine Status | ||||||
| C-spine immobilization | ||||||
| Inspection | ||||||
| Resp rate | ||||||
| SO2 (%) | ||||||
| Oxygen delivery mode | ||||||
| Chest Examination | ||||||
| Heart Rate | ||||||
| Systolic BP | ||||||
| Diastolic BP | ||||||
| Source of blood loss | ||||||
| Normal Pupil Size and Reactivity | ||||||
| GCS | ||||||
| Exposure & Temperature | ||||||
| Secondary survey: ample history | Allergies | 66.7 | 74 | 7.27 | 9.94 | 0.505 |
| Medications | ||||||
| Past Medical history | ||||||
| Last meal | ||||||
| Events Surrounding the incident | ||||||
| Diagnosis, management and disposition | Final Diagnosis | 98.5 | 95.3 | 3.14 | 3.03 | 0.409 |
| Management | ||||||
| Disposition | ||||||
| Discharge summary | Date of discharge | 94.9 | 97.1 | 2.20 | 1.54 | 0.288 |
| Status at Discharge | ||||||
| Management Plan at discharge | ||||||
Indicates significant difference.
Discussion
The global burden of injury is enormous, especially in low- and middle-income countries. Trauma registry therefore is crucial for monitoring the epidemiology, processes, and outcomes of trauma care and informing policy-making. Among other things, resource limitations are a challenge for the development of trauma registries in low- and middle-income countries [[1], [2], 3., [4], [5], [6], [7],11].
From the stakeholder consultations we observed that road traffic accidents data from prehospital, hospital and post-hospital phases of road traffic accident are largely collected and stored in hard copy files in the institutional registry departments. There are also some standalone electronic registries (MAAP, MVAF) which are not integrated. Trauma data from the Ministry of Health and Wellness Ambulance Patient Report Forms are not recorded in the MAAP. At the time of this study there are ongoing developments attempting to upgrade the MAAP software to enable integration of the prehospital trauma data. This would be a great step towards integrating prehospital and hospital data. The current trauma registry practices result in fragmented data that cannot be used for quality assurance, research, trauma care improvement and policy-making. Hard copy registries make data accessibility, data filtering and manipulation difficult, and the need to establish an integrated trauma registry that is compressive was identified during stakeholder consultations. Several studies have highlighted this need particularly in low- and middle-income countries where the impact of consequences of injuries is disproportionately high [[11], 12., [13], [14], [15], [16], [17], [18]].
The completeness of collected data is a key component of a comprehensive trauma registry [[11], 12., [13], [14]]. Studies have shown high rates of data completeness and correctness when using electronic trauma registries [12]. The overall percent variable completion rate of mandatory variables in this study was higher in phase II (intervention phase) than in phase I (Pre-intervention phase) at both at PMH and SLH (p < 0.0001 and p = 0.004 respectively). These findings can be attributed to several factors including the design of the registry which had built in logic which guided data entry. Certain variables would appear on the electronic form depending on the responses to preceding variables. In addition, the use of data validation methods such as drop-down menus, bullet list, and auto-calculation minimised the need to type hence make the electronic data capture more user-friendly. Similarly, the low percent completion rate of variables during phase I of the study could be explained by the existing data collection tools which were designed for general use and not with trauma patient care in mind. The approach to the initial assessment and management of the injured patient is unique. It was therefore upon the attending doctor to follow the structure of documentation peculiar to standard care of the injured.
Demographics & registration, and pre-hospital & triage variables were completed by the registration personnel and nursing staff. The mandatory variables of these data categories showed no significant difference in the percent completion rate for phase I and II at PMH and SLH (p = 0.163, 0.348 and p = 0.101, 0.248 respectively). These variable categories also had higher variable completion rates for both phases of the study at both sites. Studies in Blantyre, Malawi and Cape Town, South Africa reported that the data categories indicated above were completed by data clerks. They also found the higher completion rate for variables completed by data clerks compared to variables completed by clinicians [11,19]. The researchers concluded that this was partially because the demographic information is relevant to open a hard copy file for the patients so that they can be attended by the clinician. The prehospital information and the triage information is documented by the nursing staff and the higher percent completion rate may be partially because part of the information is necessary for triaging patients before they can be attended by the doctor. Both this condition may compel the registry personnel and triage nurses to always complete the variables.
The highest mean difference in percent variable completion rates between phase I and II was observed in the primary survey category at both PMH and SLH (p = 0.001 and p = 0.010 respectively). This finding suggests that clinical data collected previously using existing data collection tools were deficient in primary survey information when compared to the data collected prospectively using the proposed trauma registry. A similar observation was made in some regional studies [11,20]. It is unclear whether these observations indicated lack of proper training in trauma care of the clinicians or simply a recurrent omission in documentation of a critical component of the initial assessment. The poor documentation of this component is concerning and may be a reflection of the need for constant training and awareness improvement of clinicians with regard to standard trauma care.
A significant mean difference in percent variable completion rates between phase I and II was also observed in the secondary survey category of variables at PMH (p = 0.014). However, this difference was not significant at SLH (p = 0.505). The differences in the mean percent variable completion rate of phase I and II variables for the diagnosis, management, disposition and discharge were not statistically significant at both sites. However, it is also worth noting that these variable categories had very high completion rates (mostly over 90%) for both phases of study at both sites.
The two hospitals road traffic accident trauma registries were successfully piloted though with some challenges. Overseeing data collection at the two institutions was a challenge due to distance (50 km apart) and administrative demands. We lost two research assistants during the data collection phase of the research and we had to recruit new research assistants and train them which delayed the data collection process.
The doctors and nurses who used the electronic registry found the platform to be user-friendly. Some research assistants highlighted that using a laptop or electronic device while caring for the patient was challenging and therefore, they sometimes resorted to using the hardcopy forms and transferring the data immediately after finishing. This is a concern as it is workload duplication. The offline system availability was also reported as helpful particularly during network instabilities. Considering all lessons learnt during this study we consider an electronic multi-centre trauma registry feasible and sustainable in Botswana. There are freely available low maintenance platforms such as Redcap which are freely available both online and offline, a desirable feature in LMICs.
The authors acknowledge that there might have been some bias towards completion of the patient clinical information during phase II of the study. There are cases at both study sites, where names of patients appeared on the accident and emergency registry books but their files could not be found at the registry departments. We also acknowledge that the trauma registry in this study was developed using only MVA data.
Conclusion
We developed and successfully piloted the road traffic accident trauma registry at SLH and PMH in Botswana. The study demonstrated that electronic trauma registries are possible and there is high data completion rate when using the electronic data registry as opposed to data collected using the paper-based/hardcopy existing data collection tools. We recommend the adoption and implementation of the trauma registry prototype in Botswana's health system.
We recommend further testing of the trauma registry prototype at more health care facilities across the country with the aim of eventually producing a robust trauma registry prototype and recommending it for adoption. The country needs a compressive trauma registry which would generate information necessary to improve trauma care and inform policy making.
Dissemination of results
The results from this study was shared were shared with staff members at the data collection site through an informal presentation. The results were also presented at two local surgical conferences.
Authors' contribution
Authors contributed as follows to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content: MJM contributed 45%; YM 25%; and MS, AHF, PM, LM, MM and TM contributed 5% each. All authors approved the version to be published and agreed to be accountable for all aspects of the work.
Declaration of competing interest
The authors declared no conflicts of interest.
Acknowledgments
We acknowledge funding support from the National Institutes of Health grant, P20CA210283-01 that supported the larger project - Planning for NCDs Research Centre of Excellence in Southern Africa- from which this paper grew under a sub-project. This work would not have been possible without the grant support.
References
- 1.WHO . 2014. WHO library cataloguing-in-publication data injuries and violence: the facts. [Google Scholar]
- 2.Adeloye D., Thompson J.Y., Akanbi M.A., Azuh D., Samuel V., Omoregbe N. The burden of road traffic crashes, injuries and deaths in Africa: a systematic review and meta-analysis. Bull World Health Organ. 2016;94(7):510–521a. doi: 10.2471/BLT.15.163121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . 2015. Global status report on road safety. [Google Scholar]
- 4.Parkinson F., Kent S., Aldous C., Oosthuizen G., Clarke D. Road traffic crashes in South Africa: the burden of injury to a regional trauma centre. S Afr Med J. 2013;103(11):850–852. doi: 10.7196/samj.6914. [DOI] [PubMed] [Google Scholar]
- 5.Hofman K., Primack A., Keusch G., Hrynkow S. Addressing the growing burden of trauma and injury in low- and middle-income countries. Am J Public Health. 2005;95(1):13–17. doi: 10.2105/AJPH.2004.039354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron P.A., Gabbe B.J., Cooper D.J., Walker T., Judson R., McNeil J. A statewide system of trauma care in Victoria: effect on patient survival. Med J Aust. 2008;189(10):546–550. doi: 10.5694/j.1326-5377.2008.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 7.Chandra A., Mullan P., Ho-Foster A., Langeveldt A., Caruso N., Motsumi J. Epidemiology of patients presenting to the emergency centre of Princess Marina Hospital in Gaborone, Botswana. Afr J Emerg Med. 2014;4(3):109–114. [Google Scholar]
- 8.Cox M., Becker T.D., Motsumi M. Head injury burden in a major referral hospital emergency centre in Botswana. Afr J Emerg Med. 2018;8(3):100–105. doi: 10.1016/j.afjem.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox M., Becker T., Motsumi M. Head trauma: a significant public health concern among young men in Botswana. Etiology referral patterns and opportunities for interventions. J Public Health Afr. 2018;9(2):798. doi: 10.4081/jphia.2018.798. Published 2018 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manwana M.E., Mokone G., Kebaetse M., Young T. Epidemiology of traumatic orthopaedic injuries at Princess Marina Hospital, Botswana. SA Orthop J. 2018;17(1) doi: 10.17159/2309-8309/2018/v17n1a6. [DOI] [Google Scholar]
- 11.Chokotho L.C., Mulwafu W., Nyirenda M., Mbomuwa F.J., Pandit H.G., Le G. Establishment of trauma registry at Queen Elizabeth Central Hospital (QECH), Blantyre, Malawi and mapping of high risk geographic areas for trauma. World J Emerg Med. 2019;10(1):33–41. doi: 10.5847/wjem.j.1920-8642.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali Ali B, Lefering R, Belzunegui Otano T. Quality assessment of Major Trauma Registry of Navarra: completeness and correctness. Int J Inj Contr Saf Promot. 2018:1–8. doi: 10.1080/17457300. [DOI] [PubMed]
- 13.O’Reilly G., Fitzgerald M. Integrating trauma registry data into real-time patient care. Emerg Med Australas. 2019;31(1):138–140. doi: 10.1111/1742-6723.13217. [DOI] [PubMed] [Google Scholar]
- 14.Mehmood A., Razzak J.A., Kabir S., Mackenzie E.J., Hyder A.A. Development and pilot implementation of a locally developed Trauma Registry: lessons learnt in a low-income country. BMC Emerg Med. 2013;13:4. doi: 10.1186/1471-227X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz C.R., Ford H.R., Cassidy L.D., Shultz B.L., Blanc C., King-Schultz L.W. Development of a hospital-based trauma registry in Haiti: an approach for improving injury surveillance in developing and resource-poor settings. J Trauma. 2007;63(5):1143–1154. doi: 10.1097/TA.0b013e31815688e3. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie K., Walker S., Besenyei A., Aitken L.M., Allison B. Assessing the concordance of trauma registry data and hospital records. Health Inf Manag. 2005;34(1):3–7. doi: 10.1177/183335830503400103. [DOI] [PubMed] [Google Scholar]
- 17.Bommakanti K., Feldhaus I., Motwani G., Dicker R.A., Juillard C. Trauma registry implementation in low- and middle-income countries: challenges and opportunities. J Surg Res. 2018;223:72–86. doi: 10.1016/j.jss.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 18.Paradis T., St-Louis E., Landry T., Poenaru D. Strategies for successful trauma registry implementation in low- and middle-income countries-protocol for a systematic review. Syst Rev. 2018;7(1):33. doi: 10.1186/s13643-018-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicol A., Knowlton L.M., Schuurman N., Matzopoulos R., Zargaran E., Cinnamon J. Trauma surveillance in Cape Town, South Africa: an analysis of 9236 consecutive trauma center admissions. JAMA Surg. 2014;149(6):549–556. doi: 10.1001/jamasurg.2013.5267. [DOI] [PubMed] [Google Scholar]