Pneumococcal survival in the host and capacity to transition from a commensal to a pathogenic lifestyle are closely linked to the organism’s ability to utilize specific nutrients in distinct niches. Galactose is a major carbon source for pneumococci in the upper respiratory tract. We have shown that both the Leloir and tagatose 6-phosphate pathways are necessary for pneumococcal growth in galactose and demonstrated GalR-mediated interplay between the two pathways. Moreover, the three putative phosphorylation sites in the transcriptional regulator GalR play a critical role in galactose metabolism and are important for pneumococcal colonization of the nasopharynx, middle ear, and lungs.
KEYWORDS: GalR, Streptococcus pneumoniae, carbon metabolism, galactose, pneumococcus, protein phosphorylation, virulence
ABSTRACT
Streptococcus pneumoniae (the pneumococcus) is a formidable human pathogen that is capable of asymptomatically colonizing the nasopharynx. Progression from colonization to invasive disease involves adaptation to distinct host niches, which vary markedly in the availability of key nutrients such as sugars. We previously reported that cell-cell signaling via the autoinducer 2 (AI-2)/LuxS quorum-sensing system boosts the capacity of S. pneumoniae to utilize galactose as a carbon source by upregulation of the Leloir pathway. This resulted in increased capsular polysaccharide production and a hypervirulent phenotype. We hypothesized that this effect was mediated by phosphorylation of GalR, the transcriptional activator of the Leloir pathway. GalR is known to possess three putative phosphorylation sites, S317, T319, and T323. In the present study, derivatives of S. pneumoniae D39 with putative phosphorylation-blocking alanine substitution mutations at each of these GalR sites (singly or in combination) were constructed. Growth assays and transcriptional analyses revealed complex phenotypes for these GalR mutants, with impacts on the regulation of both the Leloir and tagatose 6-phosphate pathways. The alanine substitution mutations significantly reduced the capacity of pneumococci to colonize the nasopharynx, middle ear, and lungs in a murine intranasal challenge model.
IMPORTANCE Pneumococcal survival in the host and capacity to transition from a commensal to a pathogenic lifestyle are closely linked to the organism’s ability to utilize specific nutrients in distinct niches. Galactose is a major carbon source for pneumococci in the upper respiratory tract. We have shown that both the Leloir and tagatose 6-phosphate pathways are necessary for pneumococcal growth in galactose and demonstrated GalR-mediated interplay between the two pathways. Moreover, the three putative phosphorylation sites in the transcriptional regulator GalR play a critical role in galactose metabolism and are important for pneumococcal colonization of the nasopharynx, middle ear, and lungs.
INTRODUCTION
Streptococcus pneumoniae is a human-adapted bacterium often carried asymptomatically in the nasopharynx. However, in a proportion of carriers, it can spread to other sites of the body and cause a wide range of illnesses, including otitis media and sinusitis, as well as severe diseases such as bacteremia, pneumonia, and meningitis (1, 2). Globally, S. pneumoniae infections account for 1 million to 2 million deaths every year, making it one of the world’s foremost bacterial pathogens (3, 4). Colonization of the upper respiratory tract (URT) is an essential prerequisite for invasive disease. However, in this environment, carbon sources are scarce and the host actively eliminates glucose (Glc) to help maintain airway sterility (5). Galactose (Gal) is the most abundant sugar in the URT (6), so it follows that the ability to metabolize it may be a crucial factor for pneumococcal survival within host niches.
In a previous study, we discovered a direct link between carbohydrate utilization and virulence (7). In particular, we found that the quorum-sensing signaling molecule autoinducer 2 (AI-2) promotes transition of the pneumococcus from colonizer to pathogen. Importantly, AI-2 signaling via the fructose-specific phosphoenolpyruvate phosphotransferase system (PTS) component FruA enables the bacterium to utilize Gal as a carbon source and upregulates the Leloir pathway, thereby leading to increased production of capsular polysaccharide (CPS) and a hypervirulent phenotype (7). CPS precursors are synthesized from Glc 1-phosphate, which can be produced via either the Glc 6-phosphate pathway in the presence of Glc (as occurs in the blood) or the Leloir pathway when Gal is the predominant sugar (as occurs in the URT).
S. pneumoniae possesses two pathways for galactose metabolism, the Leloir pathway and the tagatose 6-phosphate (T6P) pathway. In the T6P pathway, extracellular Gal is transported into the cell and phosphorylated through a PTS (unrelated to FruA) (8). The resultant Gal 6-phosphate is then converted into T6P by the enzyme Gal 6-phosphate isomerase (encoded by lacAB). The T6P kinase (encoded by lacC) then converts T6P into tagatose 1,6-bP. Finally, lacD codes for the tagatose 1,6-bP aldolase, which converts tagatose 1,6-bP into dihydroxyacetone-P and d-glyceraldehyde-3-P (9, 10). In the Leloir pathway, Gal enters the cell via a proposed ABC transporter (8). It is then phosphorylated intracellularly at the C1 position by a specific kinase (encoded by galK) to yield Gal 1-phosphate, which is then converted into Glc 1-phosphate by hexose 1-phosphate uridyltransferase (encoded by galT) and UDP-glucose epimerase (encoded by galE). The transcriptional regulator of this pathway is GalR, which is believed to possess three putative phosphorylation sites, S317, T319, and T323 (11). We previously proposed that phosphorylated AI-2 imported via FruA facilitates phosphorylation of GalR at these sites, thereby activating (or relieving repression of) the gal operon (7). In the present study, we conducted mutational analysis of the putative GalR phosphorylation sites and examined the impact on expression of Leloir and T6P pathway genes and Gal metabolism.
RESULTS
The location of the GalR putative phosphorylation sites.
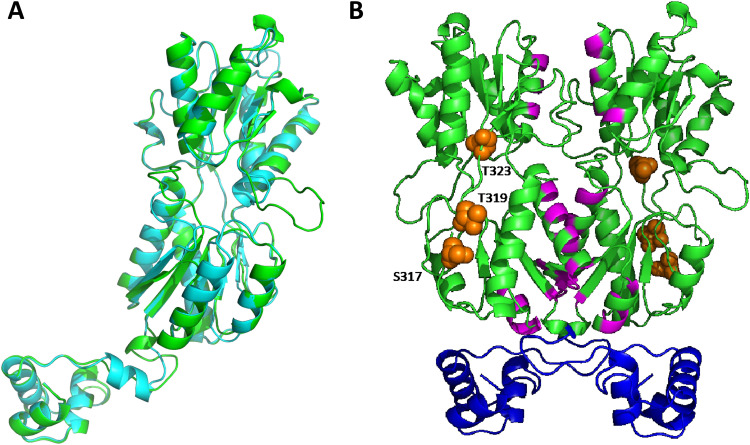
In the absence of structural information for GalR, we sought to examine the location of the putative phosphorylated residues (S317, T319, and T323) by generating a structural homology model. A homology model of GalR was constructed using SWISS-MODEL based on the homodimeric 2.4-Å structure (PDB: 1JFS) of the Escherichia coli PurR W147F mutant (35% sequence similarity, 93% sequence coverage) (12). Alignment of the GalR model (green) with the template (cyan) revealed a moderate level of variation (root mean square deviation [RMSD], 2.868 Å), with an additional loop present in the GalR model that was absent in PurR, corresponding to residues 183 to 191 (Fig. 1A). To complement these studies, we performed a conserved domain search to investigate whether any of the putative phosphorylated residues were located within regions of possible functional importance (Fig. 1B). The putative galactose binding residues (magenta) are situated at the putative dimer interface of GalR, suggesting a role in protein dimer stabilization upon sugar binding, while the N-terminal region of GalR harbors the helix-turn-helix domain (blue) responsible for the interaction with DNA. All of the putative phosphorylated residues (orange spheres) were situated in a region distinct from the residues proposed to be involved in galactose binding and DNA binding (Fig. 1B). As any functional impact of S317, T319, or T323 phosphorylation is more likely a consequence of allosteric changes rather than a direct impact on sugar or DNA binding, we investigated the contribution of each putative phosphorylated residue to GalR function. We constructed a series of GalR amino acid substitution mutants in S. pneumoniae D39 in which S317, T319, and T323 were replaced, either singly or in combination, with the nonphosphorylatable residue alanine (A), using the Janus cassette system (see Materials and Methods). A total of 7 substitution mutants were generated (designated D39AAA, D39ATT, D39SAT, D39STA, D39AAT, D39ATA, and D39SAA), as well as a galR deletion mutant (D39ΔgalR) (see Table 1).
FIG 1.
Structural homology model of S. pneumoniae GalR. (A) Cartoon representation of the protomeric homology model of S. pneumoniae GalR (green) based on the 2.9-Å structure of the Escherichia coli PurR W147F mutant (cyan; RMSD, 2.868 Å). (B) Cartoon representation of the dimeric homology model of GalR. The DNA binding helix-turn-helix domain is shown in blue, and the putative sugar binding regions are highlighted in magenta. The serine (S317) and threonine (T319 and T323) residues hypothesized to be phosphorylated are depicted as orange spheres.
TABLE 1.
Strains used in this study
| Strain | Descriptiona | Source |
|---|---|---|
| D39 | Capsular serotype 2 | NCTC 7466 |
| D39ΔgalR | galR deletion-replacement mutant, erythromycinr | This study |
| D39ΔgalK | galK deletion-replacement mutant, spectinomycinr | This study |
| D39ΔlacAB | lacAB deletion-replacement mutant, spectinomycinr | This study |
| D39ΔlacD | lacD deletion-replacement mutant, erythromycinr | This study |
| D39ΔgalRΔlacD | galR, lacD deletion-replacement mutant, erythromycinr and spectinomycinr | This study |
| D39ΔgalR Janus | galR deletion-replacement mutant utilizing Janus cassette, kanamycin, streptomycins | This study |
| D39AAA | galR S317A, T319A, T323A amino acid substitution mutant, kanamycins, streptomycins | This study |
| D39ATT | galR S317A amino acid substitution mutant, kanamycins, streptomycinr | This study |
| D39SAT | galR T319A amino acid substitution mutant, kanamycins, streptomycinr | This study |
| D39STA | galR T323A amino acid substitution mutant, kanamycins, streptomycinr | This study |
| D39AAT | galR S317A, T319A amino acid substitution mutant, kanamycins, streptomycinr | This study |
| D39ATA | galR S317A, T323A amino acid substitution mutant, kanamycins, streptomycinr | This study |
| D39SAA | galR T319A, T323A amino acid substitution mutant, kanamycins, streptomycinr | This study |
Superscript “r” and “s” following antibiotic names indicate resistance and sensitivity, respectively.
Impact of GalR putative phosphorylation sites on galactose metabolism.
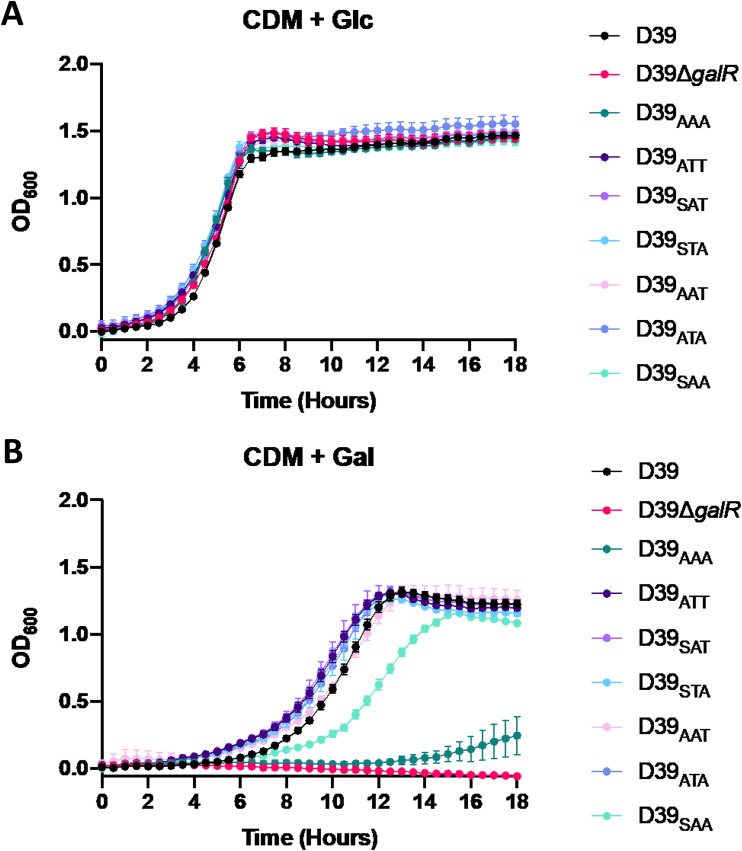
Growth in chemically defined medium with Glc as the sole carbon source (CDM + Glc) revealed that each mutant grew comparably to the D39 wild-type strain (Fig. 2A). Conversely, when grown in chemically defined medium with Gal as the sole carbon source (CDM + Gal), significant growth differences between strains became apparent (Fig. 2B). First, in comparison to D39, the D39ΔgalR strain displayed complete abrogation of growth in the presence of Gal, indicating an essential role for galR in the ability to utilize galactose, as previously shown (13). The D39AAA strain, where the three putative phosphorylation sites are nonphosphorylatable, showed almost a complete inability to grow in galactose. D39SAA showed a delay in growth, with a slower generation time and a decrease in final cell density compared to D39. The remaining GalR substitution mutants, D39ATT, D39SAT, D39STA, D39AAT, and D39ATA, had a capacity to utilize Gal similar to that of D39. This indicates that mutation of any one of the three GalR phosphorylation sites alone does not significantly impact the capacity to grow in CDM + Gal. However, mutation of both T319 and T323, as occurs in D39SAA, reduced the capacity of the strain to grow in this medium. Thus, the first GalR phosphorylation site (S317) on its own is insufficient to fully sustain growth in Gal.
FIG 2.
Impact of GalR mutations on bacterial growth. D39, D39ΔgalR, D39AAA, D39ATT, D39SAT, D39STA, D39AAT, D39ATA, and D39SAA were grown in CDM + Glc (A) or CDM + Gal (B). Growth was monitored by measuring the OD600 every 30 min for a total of 18 h. Data points are the mean OD600 from triplicate assays.
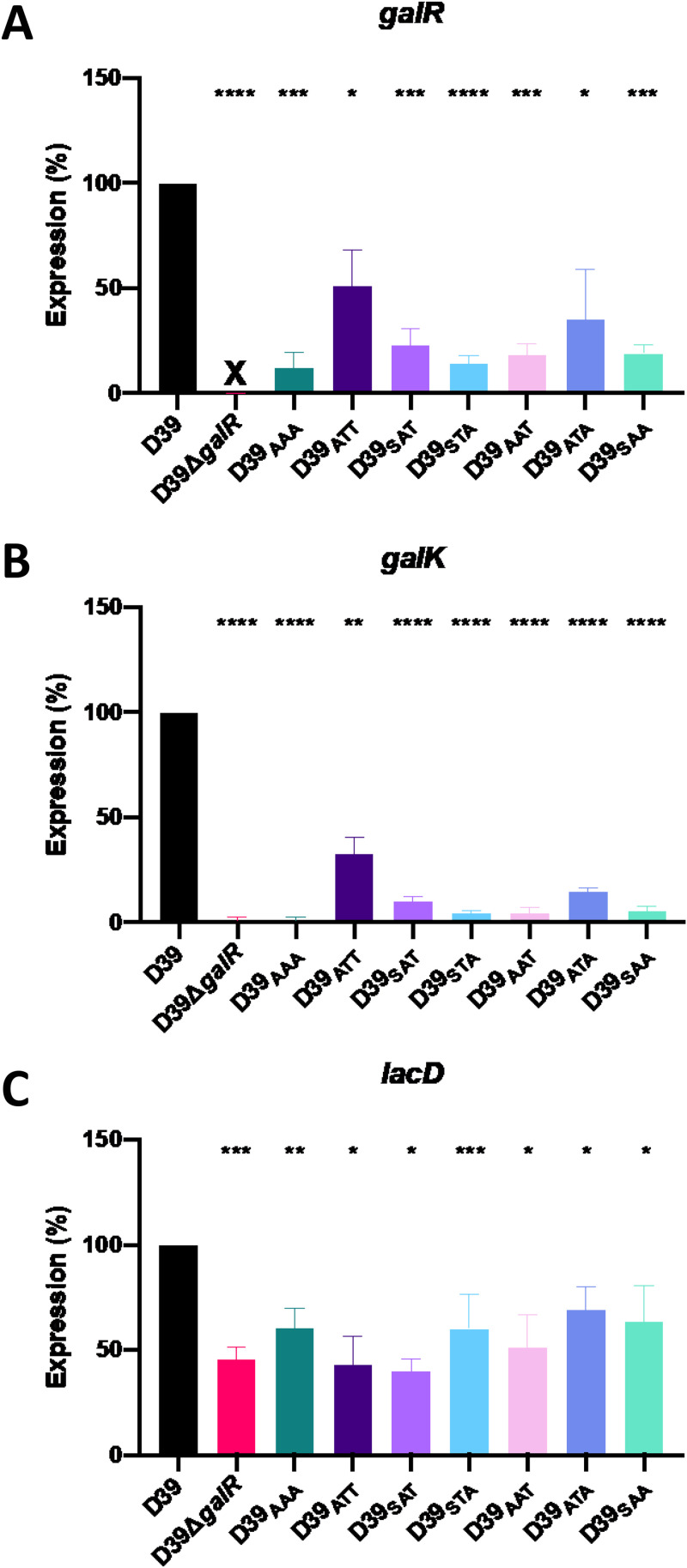
To complement these data, gene expression analyses were conducted on all the GalR mutants to assess the impact on expression of both Leloir and T6P pathway genes. Strains were grown overnight on blood agar, washed and resuspended in CDM + Gal, and then incubated for 30 min. RNA was then extracted, and expression of galR, galK, and lacD was quantitated by reverse transcription-qualitative PCR (qRT-PCR) (see Materials and Methods) (Fig. 3). Expression of galR itself was, as expected, undetectable in D39ΔgalR. galR expression was also significantly downregulated in all the mutants tested, with D39AAA being the most affected, showing an 88% reduction in expression (Fig. 3A).
FIG 3.
Differential gene expression in GalR mutants. D39, D39ΔgalR, D39AAA, D39ATT, D39SAT, D39STA, D39AAT, D39ATA, and D39SAA were cultured overnight on blood agar plates, washed, and resuspended to a final OD600 of 0.25 in CDM + Gal and incubated for 30 min. RNA was then extracted and the levels of galR (A), galK (B), and lacD (C) mRNA were quantitated by qRT-PCR using gyrA as an internal control. Data presented are the mean ± standard deviation from three independent experiments, expressed as a percentage of the result for D39. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, unpaired t test (relative to D39); ns, not significant; X, transcript absent due to gene deletion.
It was previously shown that galR regulates the galKT operon (14). Here, there was significantly decreased expression of galK in all GalR mutants compared to wild-type D39 (Fig. 3B). In particular, the D39ΔgalR and D39AAA strains showed similarly low levels of galK expression (≥98% reduction). These findings are largely consistent with the effects of the mutations on the expression of galR itself (Fig. 3A). It is worth noting that only those mutants with >98% reduction in galK expression (D39ΔgalR and D39AAA) exhibited severe growth defects in CDM + Gal (Fig. 2B).
We also assessed whether the absence of functional Leloir pathway expression had any effect on the expression of the T6P pathway by examining lacD expression. lacD encodes the last enzyme of the T6P pathway and is responsible for the conversion of tagatose 1,6-bP to dihydroxyacetone-P and d-glyceraldehyde-3-P, which can then feed into the glycolytic pathway. Interestingly, lacD expression was significantly (30 to 60%) lower in all GalR mutants than in D39 (Fig. 3C), indicating a direct or indirect role for GalR phosphorylation in the expression of T6P pathway genes. However, there was no apparent association between reduced lacD expression and the relative ability of the various strains to grow in CDM + Gal (Fig. 2B). Collectively, these analyses indicate that all three putative GalR phosphorylation sites, S317, T319, and T323, are essential for galactose metabolism in S. pneumoniae and are required for the activation of both the Leloir and T6P pathways.
Both the Leloir and T6P pathways are required for growth in galactose.
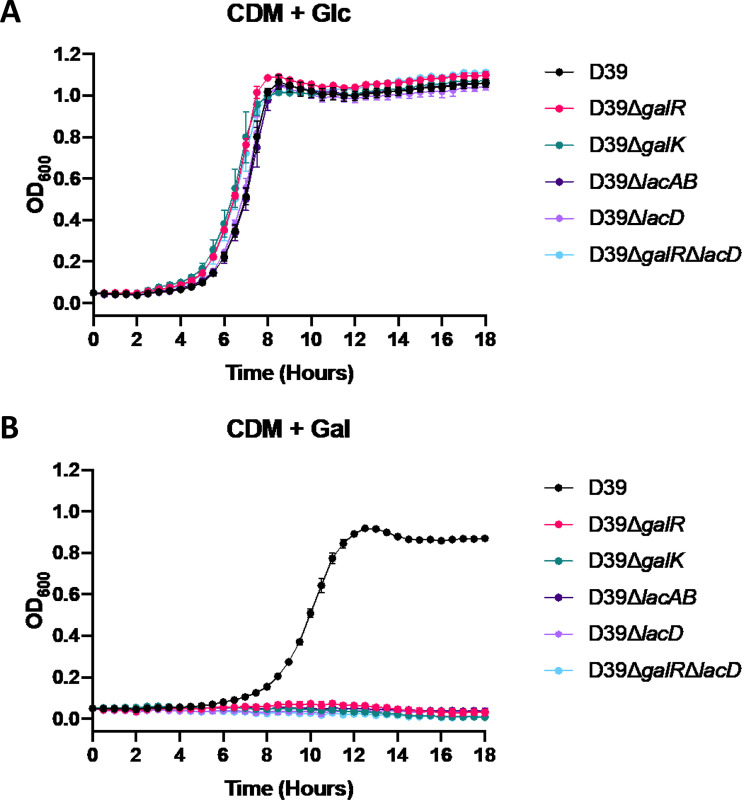
In order to assess the contribution of the Leloir and T6P pathways to Gal metabolism, growth of D39, D39ΔgalR, D39ΔgalK, D39ΔlacAB, D39ΔlacD, and D39ΔgalRΔlacD was analyzed in both CDM + Glc and CDM + Gal (Fig. 4). All mutant strains grew as well as D39 when Glc was the only carbon source (Fig. 4A). In contrast, in CDM + Gal, all mutant strains displayed complete abrogation of growth (Fig. 4B). Thus, the presence of both the functional Leloir and T6P pathways is required for growth in Gal, indicating potential interplay between these two pathways.
FIG 4.
Differential growth of Leloir and T6P pathway mutants. D39, D39ΔgalR, D39ΔgalK, D39ΔlacAB, D39ΔlacD, and D39ΔgalRΔlacD were grown in CDM + Glc (A) or CDM + Gal (B). Growth was monitored by measuring the OD600 every 30 min for 18 h. Data points are the mean OD600 from triplicate assays.
Contribution of GalR and its putative phosphorylation sites to regulation of Gal metabolism.
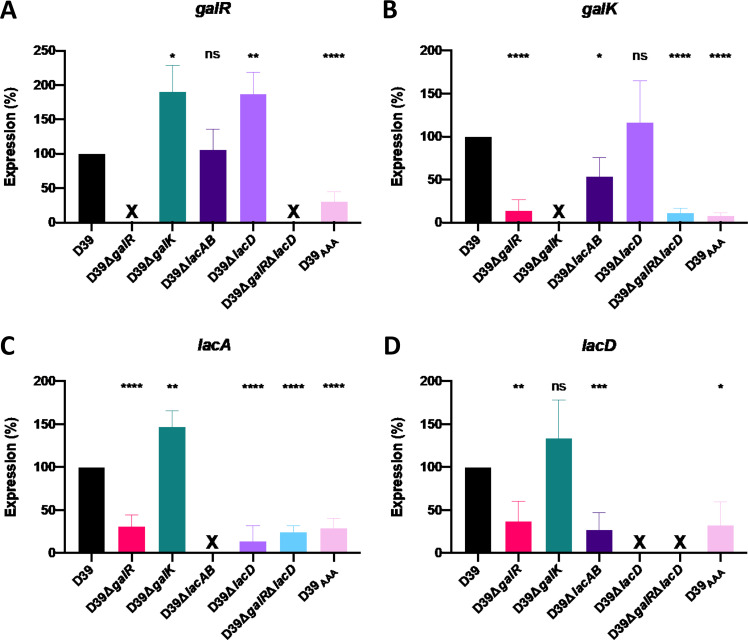
To further understand the apparent cross talk between the Leloir and T6P pathways, we analyzed the expression of galR, galK, lacA, and lacD in D39, D39ΔgalR, D39ΔgalK, D39ΔlacAB, D39ΔlacD, D39ΔgalRΔlacD, and D39AAA (Fig. 5). The expression of galR was significantly upregulated in both D39ΔgalK and D39ΔlacD relative to D39, whereas it was unaffected in D39ΔlacAB (Fig. 5A). galK expression was unaffected in D39ΔlacD but was significantly downregulated relative to D39 in D39ΔlacAB (Fig. 5B). Both galR and galK expression were also largely abrogated in D39ΔgalR and D39AAA, confirming the requirement for functional GalR for activation of the Leloir pathway, as previously shown in Fig. 3B. Unsurprisingly, the expression of these two genes was also abrogated in D39ΔgalRΔlacD.
FIG 5.
Differential gene expression in Leloir and T6P pathway mutants. D39, D39ΔgalR, D39ΔgalK, D39ΔlacAB, D39ΔlacD, D39ΔgalRΔlacD, and D39AAA were cultured overnight on blood agar, washed, and resuspended to a final OD600 of 0.25 in CDM + Gal and incubated for 30 min. RNA was then extracted, and qRT-PCR was used to assess levels of galR, galK, lacAB, and lacD mRNA, using gyrA as an internal control. Data presented are the mean ± standard deviation from three independent experiments, expressed as a percentage of that for D39. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by unpaired t test (relative to D39); ns, not significant; X, transcript absent due to gene deletion.
Interestingly, lacA expression (Fig. 5C) was significantly upregulated relative to D39 in D39ΔgalK. In the remaining mutants, the expression of both lacA and lacD was downregulated relative to D39 (Fig. 5C and D). Collectively, these findings underscore the requirement for GalR (and putative phosphorylation sites therein) for upregulation of the T6P pathway when the Leloir pathway is blocked. This could be due to feedback inhibition from accumulation of either Leloir or T6P intermediates. We also assessed expression of lacR2, the repressor of the lacI operon. In the presence of Gal, there was a significant decrease in expression of lacR2 in D39ΔlacD relative to D39, implying derepression of the operon to promote upregulation of the T6P pathway genes. However, there was no significant difference in lacR2 expression between D39 and D39ΔgalR, indicating that effects of GalR on T6P pathway gene expression are not mediated via lacR2 (data not shown).
Impact of putative GalR phosphorylation in a mouse model of pneumococcal infection.
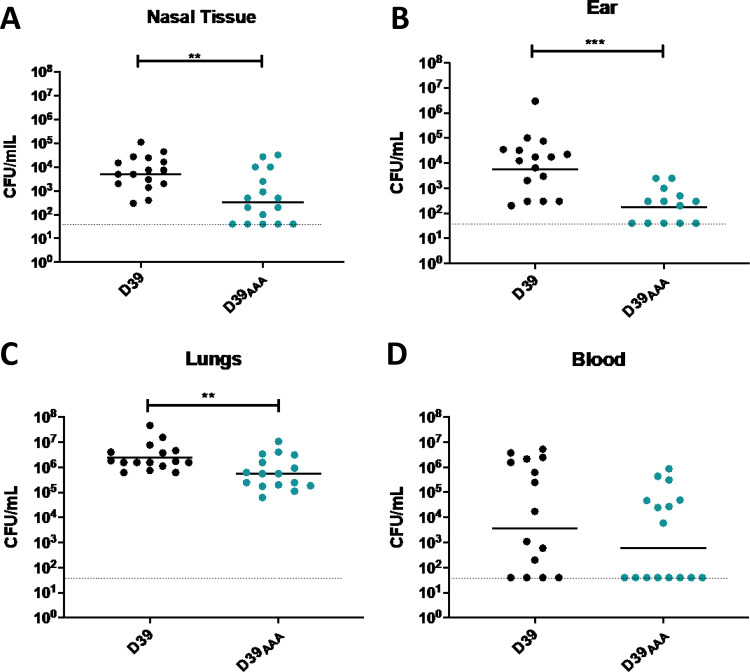
To determine the role of putative GalR phosphorylation during pneumococcal infection, the virulence of the S. pneumoniae D39 and D39AAA strains was assessed in a murine model of infection. Groups of mice were challenged intranasally with 1 × 107 CFU of each strain, and the numbers of pneumococci present in various niches (nasal tissue, ear, lungs, and blood) were determined at 24 h postchallenge (Fig. 6). The D39AAA strain exhibited a significantly attenuated virulence phenotype, with reduced bacterial loads relative to D39 in nasal tissue (Fig. 6A), ears (Fig. 6B), and lungs (Fig. 6C). No significant difference in bacterial numbers between D39 and D39AAA was observed in the blood (Fig. 6D).
FIG 6.
Virulence phenotypes of D39 and D39AAA. Groups of 16 mice were infected intranasally with 107 CFU of the indicated strain. At 24 h, mice were euthanized, and the numbers of pneumococci isolated from the nasal tissue (A), ears (B), lungs (C), and blood (D) were enumerated. Viable counts (total CFU per tissue or CFU per ml blood) are shown for each mouse in each niche; the horizontal bars indicate the geometric mean (GM) CFU for each group; the dotted line indicates the threshold of detection. The significance of the differences in GM bacterial load between groups was analyzed using unpaired t tests; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
The results of this study support previous findings that GalR is important for galactose metabolism in S. pneumoniae (14). Here, we demonstrated that the putative GalR phosphorylation sites (S317, T319, and T323) are required for growth in a defined medium with Gal as the sole carbon source. Mutation of all putative phosphorylation sites to alanine (D39AAA) completely abrogated growth in Gal and reduced expression of galR and galK to levels comparable to those of the D39ΔgalR strain (Fig. 2B and Fig. 3). Moreover, the substitution of these amino acids with alanine appears to alter the interaction of GalR with the galR operator sequence; as a result, the defects observed in galK expression may be at least partially attributable to a reduction in GalR abundance, rather than a direct alteration in binding to the galK operator. The precise mechanism behind these defects remains unknown but may be due to effects on folding, dimerization, or binding of effector molecules rather than by directly preventing phosphorylation. However, structural modeling of GalR shows that these residues are positioned in a distinct location to the putative galactose and DNA binding regions (Fig. 1B). Additional studies exploring the interaction of purified GalR and GalRAAA with each operator DNA sequence may provide greater insight into the regulation of the Leloir pathway genes.
In S. pneumoniae, the kinase responsible for phosphorylation of serine and threonine residues is StkP (15, 16). A previous proteomic study performed in medium containing Glc failed to identify GalR as a target of StkP, but LacA was identified as a possible target for StkP-mediated phosphorylation (17). Preliminary data have revealed that a D39ΔstkP strain was unable to grow in Gal (data not shown), but this may be a consequence of the defect in LacA phosphorylation rather than GalR. As a result, additional studies to directly examine the role of StkP in GalR phosphorylation are required.
Gene expression studies demonstrated that of the various GalR mutants constructed in this study, those with the greatest defects in growth in CDM + Gal, namely, D39ΔgalR and D39AAA, exhibited virtually undetectable levels of galK expression (>98% reduction) (Fig. 3B), showing a direct link between Leloir pathway gene expression and growth in Gal. The single and double point mutants also showed significantly reduced expression of galR and galK, but this level of expression still enabled sufficient Leloir pathway activity to sustain growth in CDM + Gal (Fig. 2B). Interestingly, within the single or double point mutants, the D39SAA mutant was the only one to show a defect in growth in Gal compared to D39 (Fig. 2B), but gene expression was similar to that in the other mutants that grew comparably to the wild type (Fig. 2B and Fig. 3). Therefore, growth in Gal can occur even at low levels of galK expression. Thus, the effects on Leloir pathway gene expression and growth in CDM + Gal could be attributable to inadequate levels of GalR in the cell, reduced capacity of the respective GalR phosphorylation site mutants to activate Leloir pathway genes such as galK, or a combination of both.
An unexpected finding of the current study was that all the investigated GalR mutants exhibited significantly reduced expression of the T6P pathway gene lacD (Fig. 3C), indicating a direct or indirect effect of GalR on the T6P pathway. This may provide a mechanistic basis for a previously proposed subtle regulatory link between the Leloir and T6P pathways (6). This was further examined by comparing the growth and gene expression phenotypes of D39 mutants with deletions in galR, galK, lacAB, lacD, and galR plus lacD. Unlike growth in CDM + Glc, none of these mutants was capable of growth in CDM + Gal (Fig. 4), indicating that both the Leloir and T6P pathways are essential for growth under these conditions. A previous study of a similar D39 galK deletion mutant (14) reported that growth in the presence of Gal was reduced relative to wild-type D39 but not completely abrogated as shown in the present study. This discrepancy may be attributable to the use of a more nutrient-rich M17 medium in that study (14). A separate study reported complete abrogation of growth of a D39 galK deletion mutant in Gal, whereas a lacD deletion mutant was able to grow logarithmically in Gal, albeit after a long lag phase (6).
In the present study, gene expression analyses have shown that maximal expression of the T6P pathway genes lacA and lacD require functional GalR and that all three putative phosphorylation sites of GalR are necessary to achieve this function (Fig. 5). Additionally, there appears to be a link between galK and lacAB gene expression. Deletion of galK upregulated lacA expression, perhaps as a consequence of the upregulation of galR in this mutant. On the other hand, deletion of lacAB significantly reduced galK expression but did not impact expression of galR. Furthermore, galR expression was significantly elevated in the lacD mutant. Thus, there is a complex interplay between the Leloir and T6P pathways in the various mutants, presumably mediated by intracellular concentrations of intermediates or end products of either pathway.
Notwithstanding the above-described complexities, the present study has shown that the GalR putative phosphorylation sites play a significant role in pneumococcal infection. Mice infected with D39AAA displayed significantly reduced bacterial loads in the nasopharynx, middle ear, and lungs relative to those infected with wild-type D39 (Fig. 6). These findings are compatible with previous studies showing reduced nasopharyngeal colonization and reduced systemic virulence of D39 galK and lacD deletion mutants after intranasal, but not intravenous, challenge of mice (6). However, the impact of the putative GalR phosphorylation sites has not previously been investigated. Clearly, the capacity to metabolize Gal is important for survival and proliferation in the upper respiratory tract and the middle ear, where it is an important carbon source (6). Moreover, metabolism of Gal by pneumococci in vitro is known to lead to increased production of CPS relative to cells growing on Glc, which may be the basis for the altered virulence profiles (7). We previously showed that treatment with the quorum-sensing molecule AI-2 upregulates Leloir pathway gene expression and CPS production in the presence of Gal in vitro, as well as virulence in an intranasal challenge model (7). This upregulation was dependent on the PTS component FruA, which is presumed to be the bacterial surface receptor for AI-2. This signaling molecule is a di-ketopentose and may structurally mimic the natural cargo of FruA, namely, fructose, and if AI-2 is capable of internalization via the FruA PTS system, then it would be expected to be phosphorylated during import. It is tempting to speculate that such phosphorylated AI-2 may play a direct or indirect role in GalR phosphorylation, perhaps acting as a phosphate donor, thereby mediating upregulation of the Leloir pathway. This study has shown that, collectively, the GalR putative phosphorylation sites play a key role in virulence and the ability to metabolize Gal and has revealed a complex interplay between the Gal metabolic pathways in S. pneumoniae.
MATERIALS AND METHODS
Structural modeling of GalR.
The GalR amino acid sequence (SPD_1635) was obtained from the NCBI database and input into SWISS-MODEL (18). A homology model was generated based on the 2.4-Å structure (PDB: 1JFS) of the Escherichia coli purine repressor (PurR) W147F mutant (12). The cartoon representation of the GalR homology model and the aligned PurR template were generated in PyMOL version 2.3.3 (Schrödinger). The root mean square deviation (RMSD) between the GalR model and the PurR template was determined by alignment in PyMOL. The DNA binding domain and putative sugar binding residues were identified by the NCBI conserved domain search (19), and the locations of the putative phosphorylated residues were determined based on the phosphoproteomic findings (11).
Bacterial strains and growth conditions.
The S. pneumoniae strains used in this study are listed in Table 1. Pneumococci were routinely cultured on Columbia blood agar base supplemented with 5% sterile horse blood overnight at 37°C with 5% CO2. In order to select for mutant strains, blood agar plates were supplemented with 0.2 μg/ml erythromycin, 200 μg/ml spectinomycin, 200 μg/ml kanamycin, or 200 μg/ml streptomycin, as appropriate. Growth experiments were performed with pneumococci grown in chemically defined medium (CDM) supplemented with vitamins, amino acids, choline, and catalase, as previously described (20), and either 0.5% glucose (CDM + Glc) or galactose (CDM + Gal).
Construction of mutants.
Genes were deleted from S. pneumoniae using overlap extension PCR using the primers listed in Table 2, followed by transformation, essentially as previously described (21). For strains harboring single amino acid substitutions within galR, allelic exchange mutagenesis was performed through use of the Janus cassette system, as described previously (20, 22). Mutants were confirmed by PCR and Sanger sequencing using the primers listed in Table 2 (AGRF, Adelaide, Australia).
TABLE 2.
Primers used in this study
| Primer | Sequence (5′–3′) |
|---|---|
| GalR F | AAGACAAGCCAGAACCATTTGGG |
| GalR Ery F | CGGGAGGAAATAATTCTATGAGGAAAGTACCCTAAATCAAGAATAG |
| GalR R | GACAAGGTTGTTGTTATCGGTGAT |
| GalR Ery R | TTGTTCATGTAATCACTCCTTCTGTGCAATGTCTTTTAAGGTAGCC |
| LacD F | CTGTATCGCTATTCTCCACG |
| LacD Ery F | CGGGAGGAAATAATTCTATGAGGGTATCATCTCAGCTCTTGC |
| LacD R | TGATCTGCTAGCTTCTGAC |
| LacD Ery R | TTGTTCATGTAATCACTCCTTCGCAAGAGCTGAGATGATACC |
| Ery F | GAAGGAGTGATTACATGAACAA |
| Ery R | CTCATAGAATTATTTCCTCCCG |
| GalK F | CCTTCATTAAGTCATAGCCAGA |
| GalK Spec F | AAATAACAGATTGAAGAAGGTATAAGAAGTAGTTGGATACGCTCC |
| GalK R | GTAGGACAGACATTGGCCA |
| GalK Spec R | TATGTATTCATATATATCCTCCTCCAAATTGATACGACCTGGTG |
| LacAB F | AATATCGGACAAGCTGGT |
| LacAB Spec F | AAATAACAGATTGAAGAAGGTATAAGCTCAACAAACAGACGC |
| LacAB R | TTCGTCTGAGCTATCTACATC |
| LacAB Spec R | TATGTATTCATATATATCCTCCTCATCTCAAACCTGCAGCATC |
| LacD F | CTGTATCGCTATTCTCCACG |
| LacD Spec F | AAATAACAGATTGAAGAAGGTATAATGAAGCAGCAGCTCGCGAAT |
| LacD R | TATGTATTCATATATATCCTCCTCGCAAGAGCTGAGATGATACC |
| LacD Spec R | GTTGCGTAATCACATCATAGAT |
| J253 | GAGGAGGATATATATGAATACATACG |
| J254 | TTATACCTTCTTCAATCTGTTATTTAAATAGTTTATAGTTA |
| AAA F | ACTCCACGGTCGCAAAATTCCTGCGCTGGCCATGCTGGGAGCCAGACTGACATTAAGA |
| AAA R | TCTTAATGTCAGTCTGGCTCCCAGCATGGCCAGCGCAGGAATTTTGCGACCGTGGAGT |
| ATT F | AGTACTCCACGGTCGCAAAATTCCTGCCCTGACCATGCTGGGAACCAGACTGACATTAAGAGAAAGTACCC |
| ATT R | GGGTACTTTCTCTTAATGTCAGTCTGGTTCCCAGCATGGTCAGGGCAGGAATTTTGCGACCGTGGAGTACT |
| SAT F | AGTACTCCACGGTCGCAAAATTCCTAGCCTGGCCATGCTGGGAACCAGACTGACATTAAGAGAAAGTACCC |
| SAT R | GGGTACTTTCTCTTAATGTCAGTCTGGTTCCCAGCATGGCCAGGCTAGGAATTTTGCGACCGTGGAGTACT |
| STA F | AGTACTCCACGGTCGCAAAATTCCTAGCCTGACCATGCTGGGAGCCAGACTGACATTAAGAGAAAGTACCC |
| STA R | GGGTACTTTCTCTTAATGTCAGTCTGGCTCCCAGCATGGTCAGGCTAGGAATTTTGCGACCGTGGAGTACT |
| AAT F | AGTACTCCACGGTCGCAAAATTCCTGCCCTGGCCATGCTGGGAACCAGACTGACATTAAGAGAAAGTACCC |
| AAT R | GGGTACTTTCTCTTAATGTCAGTCTGGTTCCCAGCATGGCCAGGGCAGGAATTTTGCGACCGTGGAGTACT |
| ATA F | AGTACTCCACGGTCGCAAAATTCCTGCCCTGACCATGCTGGGAGCCAGACTGACATTAAGAGAAAGTACCC |
| ATA R | GGGTACTTTCTCTTAATGTCAGTCTGGCTCCCAGCATGGTCAGGGCAGGAATTTTGCGACCGTGGAGTACT |
| SAA R | AGTACTCCACGGTCGCAAAATTCCTAGCCTGGCCATGCTGGGAGCCAGACTGACATTAAGAGAAAGTACCC |
| SAA R | GGGTACTTTCTCTTAATGTCAGTCTGGCTCCCAGCATGGCCAGGCTAGGAATTTTGCGACCGTGGAGTACT |
| gyr-RT-F | ACTGGTATCGCGGTTGGGAT |
| gyr-RT-R | ACCTGATTTCCCCATGCAA |
| galR-RT-F | TCTCTATCGCCGACCGTATCC |
| galR-RT-R | GGTGTAGCCCAGCTCTTCAG |
| galK-RT-F | CACGTTTCTCTGGAGCATGA |
| galK-RT-R | ATGGCACAGCCACTAAAACC |
| galT-RT-F | GTGGGAGAAGGTGTTTTGGA |
| galT-RT-R | ACGCGCAGTCTGACTATCCT |
| lacA-RT-F | CGTGATTGATGCTTATGGAG |
| lacA-RT-R | AGCCAATTCATCACCAACAAG |
| lacD-RT-F | CATCGGTTCTGAGTGTGTGG |
| lacD-RT-R | AAAGCGTGGGTCTGAAAAGA |
| galR–Seq F | AATCTATCATGATGAACTGGTC |
| galR–Seq R | CATAATGGAGGGCGTATGG |
Growth assays.
Growth assays were performed in flat-bottom 96-well microtiter plates with a final volume of 200 μl. Cells were inoculated at a starting optical density at 600 nm (OD600) of 0.05 in either CDM + Glc or CDM + Gal and then incubated at 37°C with 5% CO2 (20). The OD600 was measured every 30 min for a total of 18 h in a SpectroSTAR Omega spectrophotometer (BMG Labtech). Assays were performed in triplicate with a minimum of two independent experiments.
Bacterial RNA extraction and real-time qRT-PCR.
S. pneumoniae strains were first cultured overnight on blood agar plates at 37°C with 5% CO2. Cells were then harvested, washed, and resuspended in 1 ml of CDM + Gal to a final OD600 of 0.25. Cells were then incubated for 30 min at 37°C with 5% CO2, after which RNA was extracted using a Qiagen RNeasy minikit as per the manufacturer’s instructions. Gene expression was analyzed using one-step relative real-time qRT-PCR in a Roche LC480 real-time cycler, as described previously (20). The primers used to amplify target genes (listed in Table 2) were used at a final concentration of 200 nM. Amplification data were analyzed using the comparative critical threshold (2ΔΔCT) method (23) and are presented as a percentage of total expression relative to that for D39 for each gene. Assays were performed in triplicate with a minimum of two independent experiments. Statistical analyses were performed using two-tailed Student’s t test; P values of <0.05 were deemed statistically significant.
Murine infection model.
All animal experiments were approved by the University of Adelaide Animal Ethics Committee. Female outbred 4- to 6-week-old CD-1 (Swiss) mice were anaesthetized by intraperitoneal injection with ketamine and xylazine before being intranasally inoculated with 1 × 107 CFU of D39 or D39AAA in a total of 50 μl, as previously described (24). The challenge dose was retrospectively confirmed by serial dilution and plating on blood agar plates. At 24 h postinfection, mice were euthanized by CO2 asphyxiation before harvesting the blood, lungs, nasal tissue, and ears. Pneumococci were enumerated from homogenized tissue as described previously by serial dilution and plating on Columbia blood agar plates supplemented with 40 μg/ml gentamicin (20). Statistical analyses of log-transformed CFU data were performed using two-tailed Student’s t test; P values of <0.05 were deemed statistically significant.
ACKNOWLEDGMENTS
We acknowledge the contributions of Patrick R. Andreassen, Shannon C. David, and Hannah N. Agnew for assistance with mutant generation and animal experiments.
This work was supported by the Australian Research Council (ARC) Discovery Project DP190102980 to C.T. and J.C.P., National Health and Medical Research Council (NHMRC) Program grant 1071659, and NHMRC Investigator grant 1174876 to J.C.P., as well as a University of Adelaide Beacon Fellowship to C.T.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.2010. Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children: use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 59:1–18. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5911a1.htm. [PubMed] [Google Scholar]
- 2.Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, Craig AS, Schaffner W, Thomas A, Lewis MM, Scallan E, Schuchat A, Emerging Infections Programs N . 2011. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 364:2016–2025. doi: 10.1056/NEJMoa1005384. [DOI] [PubMed] [Google Scholar]
- 3.WHO. 2007. Pneumococcal conjugate vaccine for childhood immunization: WHO position paper. Wkly Epidemiol Rec 82:93–104. [PubMed] [Google Scholar]
- 4.Gray BM, Converse GM III, Dillon HC Jr.. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 5.Pezzulo AA, Gutierrez J, Duschner KS, McConnell KS, Taft PJ, Ernst SE, Yahr TL, Rahmouni K, Klesney-Tait J, Stoltz DA, Zabner J. 2011. Glucose depletion in the airway surface liquid is essential for sterility of the airways. PLoS One 6:e16166. doi: 10.1371/journal.pone.0016166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paixao L, Oliveira J, Verissimo A, Vinga S, Lourenco EC, Ventura MR, Kjos M, Veening JW, Fernandes VE, Andrew PW, Yesilkaya H, Neves AR. 2015. Host glycan sugar-specific pathways in Streptococcus pneumoniae: galactose as a key sugar in colonisation and infection [corrected]. PLoS One 10:e0121042. doi: 10.1371/journal.pone.0121042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trappetti C, McAllister LJ, Chen A, Wang H, Paton AW, Oggioni MR, McDevitt CA, Paton JC. 2017. Autoinducer 2 signaling via the phosphotransferase FruA drives galactose utilization by Streptococcus pneumoniae, resulting in hypervirulence. mBio 8:e02269-16. doi: 10.1128/mBio.02269-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bidossi A, Mulas L, Decorosi F, Colomba L, Ricci S, Pozzi G, Deutscher J, Viti C, Oggioni MR. 2012. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PLoS One 7:e33320. doi: 10.1371/journal.pone.0033320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afzal M, Shafeeq S, Kuipers OP. 2014. LacR is a repressor of lacABCD and LacT is an activator of lacTFEG, constituting the lac gene cluster in Streptococcus pneumoniae. Appl Environ Microbiol 80:5349–5358. doi: 10.1128/AEM.01370-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng L, Das S, Burne RA. 2010. Utilization of lactose and galactose by Streptococcus mutans: transport, toxicity, and carbon catabolite repression. J Bacteriol 192:2434–2444. doi: 10.1128/JB.01624-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Ge F, Xiao CL, Yin XF, Ge R, Zhang LH, He QY. 2010. Phosphoproteomic analysis reveals the multiple roles of phosphorylation in pathogenic bacterium Streptococcus pneumoniae. J Proteome Res 9:275–282. doi: 10.1021/pr900612v. [DOI] [PubMed] [Google Scholar]
- 12.Huffman JL, Lu F, Zalkin H, Brennan RG. 2002. Role of residue 147 in the gene regulatory function of the Escherichia coli purine repressor. Biochemistry 41:511–520. doi: 10.1021/bi0156660. [DOI] [PubMed] [Google Scholar]
- 13.Fleming E, Lazinski DW, Camilli A. 2015. Carbon catabolite repression by seryl phosphorylated HPr is essential to Streptococcus pneumoniae in carbohydrate-rich environments. Mol Microbiol 97:360–380. doi: 10.1111/mmi.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afzal M, Shafeeq S, Manzoor I, Kuipers OP. 2015. GalR Acts as a transcriptional activator of galKT in the presence of galactose in Streptococcus pneumoniae. J Mol Microbiol Biotechnol 25:363–371. doi: 10.1159/000439429. [DOI] [PubMed] [Google Scholar]
- 15.Echenique J, Kadioglu A, Romao S, Andrew PW, Trombe MC. 2004. Protein serine/threonine kinase StkP positively controls virulence and competence in Streptococcus pneumoniae. Infect Immun 72:2434–2437. doi: 10.1128/iai.72.4.2434-2437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novakova L, Saskova L, Pallova P, Janecek J, Novotna J, Ulrych A, Echenique J, Trombe MC, Branny P. 2005. Characterization of a eukaryotic type serine/threonine protein kinase and protein phosphatase of Streptococcus pneumoniae and identification of kinase substrates. FEBS J 272:1243–1254. doi: 10.1111/j.1742-4658.2005.04560.x. [DOI] [PubMed] [Google Scholar]
- 17.Hirschfeld C, Gómez-Mejia A, Bartel J, Hentschker C, Rohde M, Maaß S, Hammerschmidt S, Becher D. 2019. Proteomic investigation uncovers potential targets and target sites of pneumococcal serine-threonine kinase StkP and phosphatase PhpP. Front Microbiol 10:3101. doi: 10.3389/fmicb.2019.03101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T. 2014. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A. 2020. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res 48:D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minhas V, Harvey RM, McAllister LJ, Seemann T, Syme AE, Baines SL, Paton JC, Trappetti C. 2019. Capacity to utilize raffinose dictates pneumococcal disease phenotype. mBio 10:e02596-18. doi: 10.1128/mBio.02596-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannelli F, Pozzi G. 2004. Method for introducing specific and unmarked mutations into the chromosome of Streptococcus pneumoniae. Mol Biotechnol 26:81–86. doi: 10.1385/MB:26:1:81. [DOI] [PubMed] [Google Scholar]
- 22.Sung CK, Li H, Claverys JP, Morrison DA. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol 67:5190–5196. doi: 10.1128/AEM.67.11.5190-5196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Tikhomirova A, Trappetti C, Standish AJ, Zhou Y, Breen J, Pederson S, Zilm PS, Paton JC, Kidd SP. 2018. Specific growth conditions induce a Streptococcus pneumoniae non-mucoidal, small colony variant and determine the outcome of its co-culture with Haemophilus influenzae. Pathog Dis 76:fty074. doi: 10.1093/femspd/fty074. [DOI] [PubMed] [Google Scholar]