Abstract
GABAergic dysfunctions have been implicated in the pathogenesis of schizophrenia, especially the associated cognitive impairments. The GABA synthetic enzyme glutamate decarboxylase 67-kDa isoform (GAD67) encoded by the GAD1 gene is downregulated in the brains of patients with schizophrenia. Furthermore, a patient with schizophrenia harboring a homozygous mutation of GAD1 has recently been discovered. However, it remains unclear whether loss of function of GAD1 leads to the symptoms observed in schizophrenia, including cognitive impairment. One of the obstacles faced in experimental studies to address this issue is the perinatal lethality of Gad1 knockout (KO) mice, which precluded characterization at the adult stage. In the present study, we successfully generated Gad1 KO rats using CRISPR/Cas9 genome editing technology. Surprisingly, 33% of Gad1 KO rats survived to adulthood and could be subjected to further characterization. The GABA concentration in the Gad1 KO cerebrum was reduced to ~52% of the level in wild-type rats. Gad1 KO rats exhibited impairments in both spatial reference and working memory without affecting adult neurogenesis in the hippocampus. In addition, Gad1 KO rats showed a wide range of behavioral alterations, such as enhanced sensitivity to an NMDA receptor antagonist, hypoactivity in a novel environment, and decreased preference for social novelty. Taken together, the results suggest that Gad1 KO rats could provide a novel model covering not only cognitive deficits but also other aspects of the disorder. Furthermore, the present study teaches an important lesson: differences between species should be considered when developing animal models of human diseases.
Subject terms: Schizophrenia, Molecular neuroscience
Introduction
γ-Aminobutyric acid (GABA) is a major inhibitory neurotransmitter in the mammalian central nervous system. GABA is synthesized from glutamate by two glutamate decarboxylases (GADs), 67 and 65-kDa isoforms (GAD67 and GAD65) encoded by Gad1 and Gad2 genes, respectively1–3. While GAD67 is constitutively active and accounts for basal GABA synthesis in the soma of neurons, GAD65 is transiently activated and responsible for on-demand production in the axonal terminal4,5. The difference in phenotypes between knockout (KO) mice of the two GADs also has indicated distinct physiological roles: the Gad1 KO mice displayed cleft palate accompanied by perinatal lethality6, while the Gad2 KO mice developed epileptic seizures in adulthood7.
Deficits in GABA production could also have a large impact on brain function in humans. In the cerebral cortex of subjects with schizophrenia, GAD67 mRNA8–11 and proteins12 are decreased. Conversely, GAD65 expression is unaffected10 or slightly reduced13. Interestingly, a study reported that GAD65 expression levels were reduced in schizoaffective disorder, but not in schizophrenia14. GAD67 downregulation is likely brain-wide because it was observed in the prefrontal cortex, parietal cortex, visual cortex15, and hippocampus16. In schizophrenia, cognitive impairments such as working memory deficits are key symptoms affecting the functional outcome of patients17. Because the GABAergic system plays an important role in performing working memory tasks by synchronizing neuronal activity by generating gamma oscillation18, GAD67 reduction is hypothesized to be a cause of cognitive impairment in schizophrenia. Accordingly, GABA concentrations in the dorsolateral prefrontal cortex have been correlated with working memory performance in healthy humans19.
Genetic evidence has also supported the link between the GAD1 gene and schizophrenia. Some single nucleotide polymorphisms (SNPs) surrounding the GAD1 locus have been associated with childhood-onset schizophrenia in a North American cohort20 and in ordinary schizophrenia in a Chinese cohort21. Among these SNPs, one was significantly associated with lower levels of GAD67 expression in postmortem analysis22. Some SNP variations in GAD1 were also associated with the poorer performance of attention and working memory23. Furthermore, homozygous missense mutations in the GAD1 gene were identified in a family of schizophrenia patients by whole-exome sequencing in Italy24,25. This mutation prevents the homodimerization of GAD67 proteins, which is necessary for their sufficient activity25. Based on these genetic findings combined with accumulated evidence from postmortem brain studies, we can assume that loss-of-function mutations of Gad1 have a causal effect on the symptoms of schizophrenia, particularly on cognitive impairment. However, because the above studies are observational studies, it is unclear whether the Gad1 mutation is the cause of the disorder.
The development of a Gad1 knockout (KO) animal and characterization of its behavior is a reasonable strategy to test this hypothesis. However, since global Gad1 KO is lethal in mice on the first postnatal day6,26, they cannot undergo behavioral testing. Researchers have also developed several conditional KO mice or knockdown (KD) mice in which GAD67 was disrupted in a restricted subpopulation of GABAergic neurons to avoid lethality27–29. However, these mice never exhibited any working memory impairment despite showing a few schizophrenia-related phenotypes. Do these results ultimately refute the “GAD67 hypothesis” of cognitive impairments in schizophrenia?
Animal species used for KO experiments in biomedical research have been largely restricted to mice due to technical limitations. Recently, genome editing techniques such as the clustered regularly interspaced short palindrome repeat (CRISPR)/Cas9 system30 have allowed researchers to develop KO or transgenic animals in various species other than mice, such as rats31,32, ferrets33, and marmosets34. In this situation, careful examination is necessary when selecting a species suitable for experiments35. Although both mice and rats belong to the order Rodentia, there are significant differences that can affect behavioral testing. First, most behavioral tests were originally developed in rats, and some of these tests are more suitable for rats than mice. For instance, in the Morris water maze test used for the assessment of spatial memory, mice have greater difficulty in learning the location of the platform compared to rats because mice tend to avoid swimming as a habit35. Second, rats are suggested to have a much abundant repertoire of behavioral tests for investigating cognitive functions, which is advantageous for research on psychiatric disorders31. This can make a difference in detecting possible cognitive impairments of Gad1 knockout animals. In addition, with respect to GAD67 and GAD65 expression levels, a species difference has already been reported. We have previously shown that the expression levels of GAD67 and GAD65 are similar in the adult mouse brain28. Conversely, although the biological significance is unclear, the expression level of GAD67 is lower than that of GAD65 in rat and human brains36,37. Thus, the GAD67/GAD65 expression ratio in human brains is closer to that in rat brains than in mouse brains, suggesting that the rat is an invaluable experimental animal for studying the roles of GAD67 in brain function and dysfunction, in particular, the pathophysiology of schizophrenia.
Based on the above, we planned to re-examine the phenotypes caused by global and conditional Gad1 KO in rats rather than mice. Surprisingly, we found that some global Gad1 KO rats can survive to adulthood. Therefore, herein, we focused on the behavioral characterization of global Gad1 KO rats to provide evidence supporting the cause-effect relationship between loss of function of GAD67 and schizophrenia-related phenotypes, including cognitive impairment. Our data will provide insight into not only the pathophysiology of the patient with the ultrarare mutation of GAD1 but also that of other ordinary patients.
Materials and methods
All experiments were approved by the Animal Care and Experimentation Committees of Gunma University, the Animal Research Committee of Osaka University, and the Institutional Laboratory Animal Care and Use Committee of Tohoku University. Every effort was made to minimize the number of animals used and their suffering.
Animals
A rat line with exon-6 deletion of Gad1 (Gene ID: 24379) was generated on a Long-Evans background (Japan SLC, Inc., Hamamatsu, Shizuoka, Japan) using previously described methods38. It is noteworthy that rat Gad1 exon-6 corresponds to mouse Gad1 exon-539,40. Briefly, two CRISPR guide RNAs (gRNAs) were designed to target exon-6 of Gad1 (Fig. 1a). To obtain both Gad1 KO and Gad1-flox lines, a long single-strand DNA (lssDNA) composed of exon-6 flanked by two loxP sequences was electroporated with the two gRNAs and Cas9 mRNA into pronuclear stage embryos. The Gad1-flox line will be described elsewhere; herein, we focused on the Gad1 KO line. Embryos developing into the two-cell stage after the introduction of RNAs and lssDNA were transplanted into oviducts of foster mothers. We successfully acquired an F0 rat with a 291 bp deletion, which includes exon-6 (Fig. 1a). The F0 rat was crossed with wild-type (WT) Long-Evans rat to obtain F1 heterozygous KO rats. For further experiments, we crossed heterozygous male and female rats to obtain WT (Gad1+/+), heterozygous (Gad1+/−), and homozygous (Gad1−/−) animals. The rats were housed in a room maintained at 22 ± 3 °C with a 12-h light-dark cycle (lights on at 6:00, lights off at 18:00). Food (CLEA Rodent Diet CE-2, Clea Japan, Meguro, Tokyo, Japan) and water were provided ad libitum.
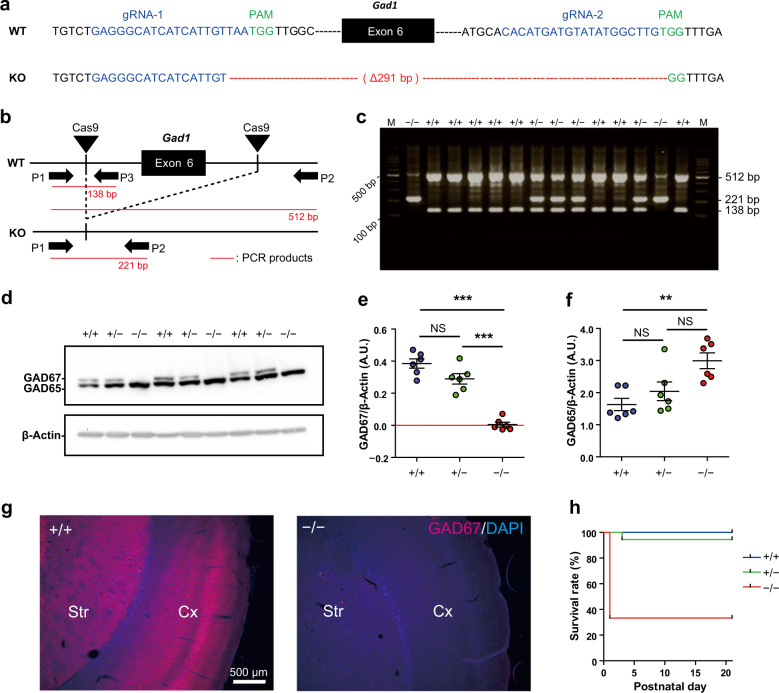
Fig. 1. Generation of Gad1 knockout rats by the CRISPR/Cas9 system.
a Targeted site of Gad1 knockout rats. WT and KO represent wild-type and knockout alleles, respectively. The two sequences highlighted in blue denote the sites recognized by the complex of Cas9 and guide RNAs (gRNA-1 and gRNA-2). The protospacer adjacent motif (PAM) sequences next to the two sites are highlighted in green. The KO allele has a 291-bp deletion, including exon-6 of the Gad1 gene. b Schematic representation of CRISPR/Cas9-mediated deletion of exon-6 and the approximate locations of primers for genotyping PCR (P1, P2, and P3). c A representative result of genotyping PCR. M: DNA molecular weight marker. d Protein levels of GAD67 in the adult brain taken from each genotype (n = 6 in each genotype). e Western blot analysis using an antibody that binds both GAD65 and GAD67 revealed the loss of GAD67 protein in Gad1−/− rats (F(2, 15) = 55.372, p < 0.001). f GAD65 was increased in Gad1−/− rats (F(2, 15) = 8.0857, p < 0.01). β-Actin was used as an internal control in d–f. g The loss of GAD67 immunoreactivity in the Gad1−/− rat was also observed in the immunohistochemical analysis. Cx cerebral cortex, Sr striatum. The nuclei were stained using DAPI. Scale bar = 500 μm. h Gad1−/− rats showed a significantly lower survival rate than Gad1+/+ and Gad1+/− rats (log-rank test, p < 0.001). The data were analyzed using one-way ANOVA and post hoc test adjusted by Holm’s method for multiple comparisons (e, f) and log-rank test (h). The results are presented as the average ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Genotyping PCR
The genotype of each obtained pup was determined using genomic DNA extracted from the tail tissue at P0 (to determine the survival curve) or P21–P28 (for other experiments). PCR genotyping was performed using the following primers: 5′-ACTGGGCCATTGTTCCAGCTCCA-3′ (primer 1), 5′-GCTCTCTCACGAGTATGCCCTTGCT-3′ (primer 2), and 5′-CGAGCTGGAGAAGGGGGAAGAAGAT-3′ (primer 3). Two DNA fragments of 512 bp (primer 1 and primer 2) and 138 bp (primer 1 and primer 3) were amplified from the WT allele, and a fragment of 221 bp (primer 1 and primer 2) was amplified from the KO allele (Fig. 1b, c).
Quantification of GAD proteins and GABA in rat brain tissue
Elimination of GAD67 in Gad1 KO rats was confirmed in cerebral cortex tissue samples taken from adult (three months old) or juvenile rats (P20–P24). GAD67 or GAD65 protein was quantified by western blot analysis as described previously28. Concentrations of GABA and glutamate were measured by high-pressure liquid chromatography (HPLC)41 in the cerebral cortex and whole cerebellum samples of rats at P20–P24 (Supplementary Information).
Immunohistochemistry
Perfusion with 4% paraformaldehyde and immunohistochemistry were performed as described in the Supplementary materials using mouse anti-GAD67 (1:1000; Merck Millipore, Burlington, MA, USA) and anti-doublecortin (DCX) antibodies (1:1000; Merck Millipore). See Supplementary Information for details.
Behavioral analysis
Before the behavioral tests, rats were habituated to the experimenters by a 3-day handling period. All rats were male and older than 10 weeks at the start of the behavioral analyses. We performed the Morris water maze, eight-arm radial maze, open field, novel object recognition, social interaction, Y-maze, elevated plus maze, acoustic startle response, prepulse inhibition (PPI), and forced swim tests. The eight-arm radial maze test was performed as described previously with minor modifications42. For the Morris water maze test, we modified the method of Nunez43. All other behavioral tests were carried out using a modified version of Fujihara et al.28 for rats. Except in the Morris water maze and radial maze tests, the genotypes of each rat were blinded to the experimenter. See the Supplementary Information for detailed procedures.
Statistical analysis
The sample sizes of each experiment were determined based on our previous study28. No randomization was performed. For comparison between genotypes, we employed Welch’s t-test, Wilcoxon rank-sum test, one-way or two-way ANOVA with post hoc Holm’s method. Although the variances of each group were similar in most of the experiments, we applied the Welch’s t-test rather than Student’s t-test because the former is more robust to unequal variances. If the Shapiro–Wilk normality test showed a lack of normality of the data, we applied the Wilcoxon rank-sum test for group comparison. To analyze the correlations between these parameters, we calculated Spearman’s rank correlation coefficient (rho). For survival analysis, we used the Kaplan–Meier method with a log-rank test. We also conducted an analysis of covariance (ANCOVA) to compare the number of DCX-positive cells between the groups to adjust for age effects. p-values < 0.05 were considered statistically significant.
Results
Verification of the Gad1 knockout
We successfully obtained a Gad1 KO rat line, as shown in Fig. 1a–c and Supplementary Fig. 1a, b. To verify that Gad1−/− rats expressed no GAD67 protein, we carried out Western blot analysis for the brain extract using two different antibodies (Fig. 1d and Supplementary Fig. 1b). GAD67 protein was undetectable in the Gad1−/− rats, whereas the amount of GAD65 protein in Gad1−/− rats was significantly upregulated to 183.5% of that in Gad1+/+ rats (Fig. 1f). A slight reduction in GAD67 protein in Gad1+/− rats was observed but did not reach statistical significance. The elimination of GAD67 was further confirmed by immunohistochemistry in the cerebral cortex and striatum. No immunoreactivity was detected in Gad1−/− rats (Fig. 1g).
Lower survival rate and growth delay of Gad1−/− rats
Each genotype of the rats was obtained at a Mendelian frequency (Supplementary Fig. 1a). The survival rate of Gad1−/− rats was significantly lower than that of Gad1+/+ littermates, but 33% of Gad1−/− rats grew to adulthood (Fig. 1h). The survival rate of Gad1+/− rats was not significantly different from that of Gad1+/+ rats. This result was in contrast to cases of Gad1−/− mice with 100% lethality at P06. Furthermore, no apparent malformations, such as the cleft palate or omphalocele, were observed in Gad1−/− mice6,26. Gad1−/− rats did not show any hindlimb clasping, suggesting that they had no ataxic-like motor dysfunction (Supplementary Fig. 1c). Conversely, the body size and body weight of Gad1−/− rats were smaller than those of Gad1+/+ rats after birth to approximately two months of age (P28: Gad1+/+, 92.5 ± 2.96 g; Gad1−/−, 59.65 ± 10.23 g; Supplementary Fig. 2), although the growth curve of homozygous Gad1−/− rats caught up to adult rats (Supplementary Fig. 2c, d). Furthermore, the brains of Gad1−/− rats had no obvious abnormality in gross morphology at the adult stage compared with Gad1+/+ rats (data not shown).
Decrease in GABA concentration in Gad1−/− rats
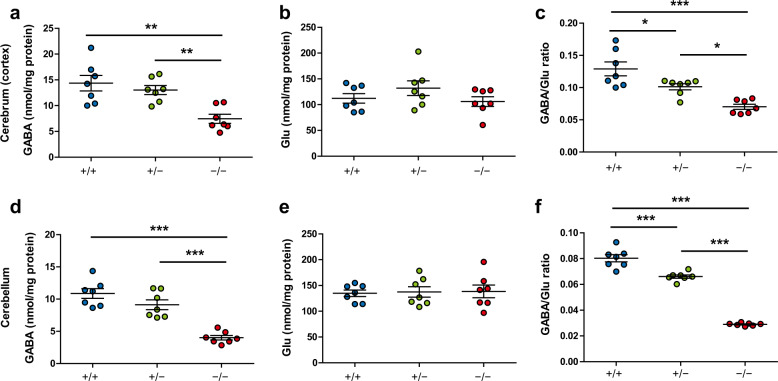
The functional impact of GAD67 deletion in Gad1−/− rats was measured by the concentration of GABA in brain tissue (Fig. 2). GABA concentrations in both the cerebral cortex and cerebellum were significantly reduced in homozygous Gad1−/− rats (Fig. 2a, d), while Gad1+/− rats showed no significant reductions. Concentrations of glutamate (Glu), the precursor of GABA, showed no differences among the three genotypes in either region (Fig. 2b, e). The GABA/Glu ratio differed among all genotypes in both the cerebral cortex and cerebellum (Fig. 2c, f).
Fig. 2. GABA and glutamate concentrations in the brains of Gad1 knockout rats.
Brain samples were collected from P20 to P24. a In the cerebral cortex, the GABA concentration in Gad1−/− rats was reduced to 51.88% of that of Gad1+/+ (F(2, 18) = 16.748, p < 0.001). b There were no differences in the glutamate (Glu) concentrations among the three genotypes in the cerebral cortex (F(2, 18) = 1.4734, p = 0.2555). c The GABA/Glu ratio was reduced in both Gad1+/− and Gad1−/− rats in the cerebral cortex (F(2, 18) = 10.578, p < 0.001). d GABA concentrations in the cerebellum (F(2, 18) = 29.646, p < 0.001). e Glu concentrations in the cerebellum (F(2, 18) = 0.0372, p = 0.9636). f GABA/Glu ratios in the cerebellum (F(2, 18) = 218.79, p < 0.001). n = 7 in each genotype. The results are presented as the average ± SEM. The data were analyzed using one-way ANOVA and post hoc test adjusted by Holm’s method for multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001.
Reduced locomotion and rearing in Gad1−/− rats
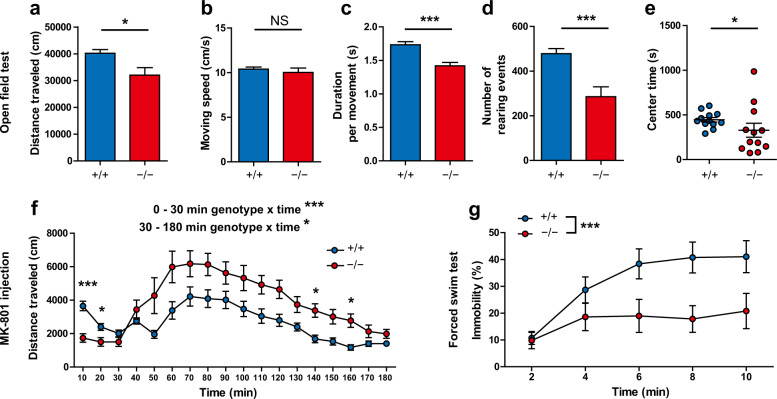
Behavioral consequences of Gad1 deletion were determined by behavioral testing in Gad1−/− rats and Gad1+/+ littermates. In the open field test for seven days, Gad1−/− rats showed reduced distance traveled compared with Gad1+/+ rats (Fig. 3a), although their moving speed was comparable to Gad1+/+ rats (Fig. 3b). The duration per movement and the number of rearing events were also reduced in Gad1−/− rats (Fig. 3c, d). Figure 3 reports the 7-day summations for each parameter. The time course of each parameter is shown in Supplementary Fig. 3. As a whole, Gad1−/− rats were characterized by hypoactive behavior.
Fig. 3. Task-dependent alterations of activities in Gad1−/− rats.
a–e Open field test (n = 12 for each genotype). Gad1−/− rats exhibited decreased distance traveled compared with Gad1+/+ (t(16.051) = –2.6272, p < 0.05) (a), but their moving speed was unaltered (t(15.717) = −0.6772, p = 0.5081) (b). Duration per movement (t(21.82) = –4.541, p < 0.001) (c) and number of rearing events (t(16.456) = −3.8561, p < 0.01) (d) were also decreased in Gad1−/− rats, suggesting hypoactivity in the open field. Gad1−/− rats stayed less time in the center region (W = 37, p < 0.05) (e). f Response to NMDA receptor antagonist (Gad1+/+, n = 12; Gad1−/−, n = 11). Gad1−/− rats showed hypoactivity at baseline (0–30 min; genotype × time, F(2, 40) = 9.3185, p < 0.001) but showed enhanced hyperactivity after MK-801 injection (0.2 mg/kg, intraperitoneal injection) (30–180 min; genotype × time, F(14, 280) = 1.9404, p < 0.05; simple main effect of genotype, adjusted by Holm’s method for multiple comparisons; 130–140 min, p < 0.05; 150–160 min, p < 0.05). g Forced swim test (Gad1+/+, n = 12; Gad1−/−, n = 11). Gad1−/− rats showed a significant decrease in immobility (genotype main effect, F(1, 20) = 6.4689, p < 0.05). The results are presented as the average ± SEM. The data were analyzed using the Wilcoxon rank-sum test (e), two-way repeated measures ANOVA (f–h), and Welch’s t-test (others). *p < 0.05, **p < 0.01, ***p < 0.001; NS not significant.
The exploration time in the center region (center time) and number of fecal boli in the open field were measured. Because the data of the exploration time in the center region (center time) in Gad1−/− rats showed neither normality (W = 0.845, p < 0.05) nor equality of variance to Gad1+/+ rats (F(11, 11) = 9.1871, p < 0.001), a nonparametric test was used for comparisons. There was a significant decrease in the center time in Gad1−/− rats (Fig. 3e; Supplementary Fig. 4a). The total number of fecal boli in the open field session also increased in Gad1−/− rats (Supplementary Fig. 4b) and negatively correlated with the center time and distance traveled only in Gad1−/− rats (Supplementary Fig. 4c, d).
Gad1−/− rats showed no alterations in the elevated plus-maze test
We assessed the behavior of Gad1−/− rats on an elevated plus-maze. However, we did not detect any alterations, such as reduced exploration of the open arms of the maze (Supplementary Fig. 5).
Enhanced sensitivity to NMDA receptor antagonists in Gad1−/− rats
Treatment with NMDA receptors can elicit schizophrenia-like symptoms in humans and schizophrenia-like behaviors in rodents28,44. Therefore, we analyzed the sensitivity to the NMDA antagonist MK-801 (0.2 mg/kg, intraperitoneal injection) (Fig. 3f). During the habituation phase of the open field arena before administration of MK-801, Gad1−/− rats showed significantly lower locomotor activity. Following MK-801 injection, the moving distance in both genotypes increased from baseline. Meanwhile, the distance traveled by Gad1−/− rats was significantly higher than that traveled by Gad1+/+ rats. Subsequent post hoc tests confirmed the enhanced hyperlocomotion induced by MK-801 in Gad1−/− rats compared with Gad1+/+ rats.
Gad1−/− rats exhibited reduced immobility in the forced swim test
Gad1−/− rats also showed enhanced activity in the forced swim test. In the test session, Gad1−/− rats became significantly less immobile than Gad1+/+ rats (Fig. 3g).
Normal acoustic startle response and sensorimotor gating in Gad1−/− rats
Deficits in sensorimotor gating are a translatable endophenotype of schizophrenia28. We tested possible deficits in the startle response and sensorimotor gating using an acoustic startle response, yet there were no differences between Gad1−/− and Gad1+/+ rats (Supplementary Fig. 6).
Reduced social novelty preference in Gad1−/− rats with intact object recognition
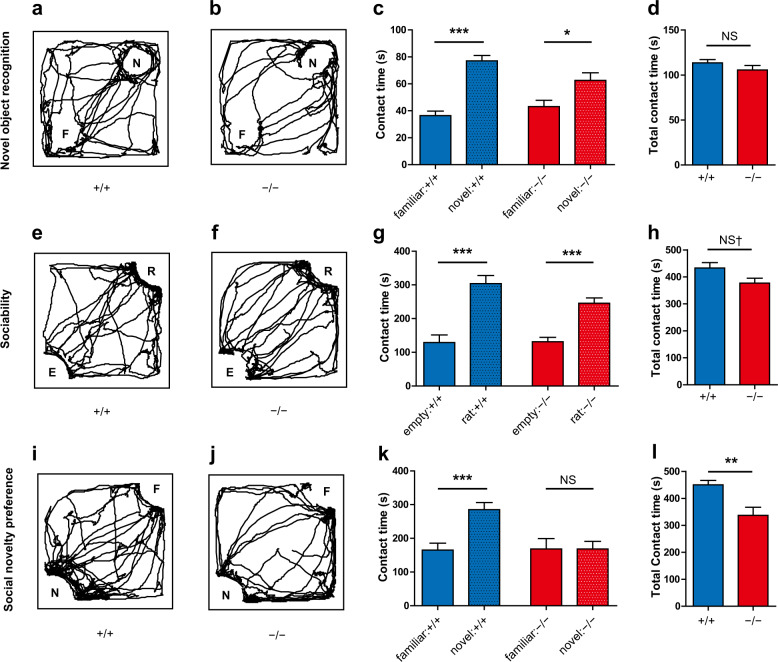
We assessed the short-term recognition memory of Gad1−/− rats using the novel object recognition task45. Both Gad1−/− and Gad1+/+ rats explored the novel object longer than the familiar object (Fig. 4a–c). The total contact time to the objects (novel object + familiar object) was also comparable between the two genotypes (Fig. 4d). Thus, Gad1−/− rats had no obvious deficit in recognition memory, at least discriminating between two objects.
Fig. 4. Gad1−/− rats showed a deficit in social novelty preference with intact novel object recognition.
a–d Novel object recognition task (Gad1+/+, n = 12; Gad1−/−, n = 11). Representative trajectories of Gad1+/+ (a) and Gad1+/+ (b) rats in the arena. N: novel object, F: familiar object. Both Gad1+/+ and Gad1−/− rats showed longer contact with the novel object than with the familiar object (Gad1+/+, t(21.351) = –7.696, p < 0.001; Gad1−/−, t(19.277) = –2.6428, p < 0.05) (c). Total contact time (novel + familiar) was similar between the two genotypes (t(18.949) = 1.257, p = 0.2240) (d). e–h Sociability test (Gad1+/+, n = 12; Gad1−/−, n = 11). Representative trajectories of Gad1+/+ (e) and Gad1−/− (f) rats are shown. R: rat, E: empty. Gad1−/− rats preferred the cage enclosing another rat to the empty cage, similar to Gad1+/+ rats (Gad1+/+, t(21.908) = 5.2842, p < 0.001; Gad1−/−, t(19.447) = –5.5212, p < 0.001) (g). However, the total contact time (rat-occupied cage + empty cage) of Gad1−/− rats decreased slightly (t (20.92) = 2.0506, p = 0.05304) (h). i–l Social novelty preference test (Gad1+/+, n = 12; Gad1−/−, n = 11). The trajectory of Gad1+/+ rats accumulated around the novel stranger rat (i). N: novel rat, F: familiar rat. This trend diminished in Gad1−/− rats (j). Gad1−/− rats spent significantly less time interacting with the novel rat than Gad1+/+ rats (Gad1+/+, t(21.991) = −4.1077, p < 0.001; Gad1−/−, t(18.266) = –0.0005, p = 0.9996) (k). In addition, the total contact time (novel rat + familiar rat) was significantly lower in Gad1−/− rats (t(15.604) = 3.3153, p < 0.01) (l). The results are presented as the average ± SEM. Data were analyzed using Welch’s t-test. †p < 0.1, *p < 0.05, **p < 0.01, ***p < 0.001; NS not significant.
We also tested rats for sociability and social novelty preference using the same apparatus as the novel object recognition test. In the sociability test for social vs. empty preferences, both genotypes of rats spent more time interacting with the rat-occupied cage than with the empty cage (Fig. 4e–g). Gad1−/− rats showed slightly reduced total contact times (rat-occupied cage + empty cage) but at a subthreshold level (Fig. 4h). Subsequently, we examined social novelty preferences, which are relevant to social memory. While Gad1+/+ rats spent more time interacting with the novel rat than with the familiar rat (Fig. 3i, k), Gad1−/− rats did not display such a preference (Fig. 4j–l). Furthermore, Gad1−/− rats showed significantly reduced total contact times (novel rats + familiar rats) (Fig. 4l).
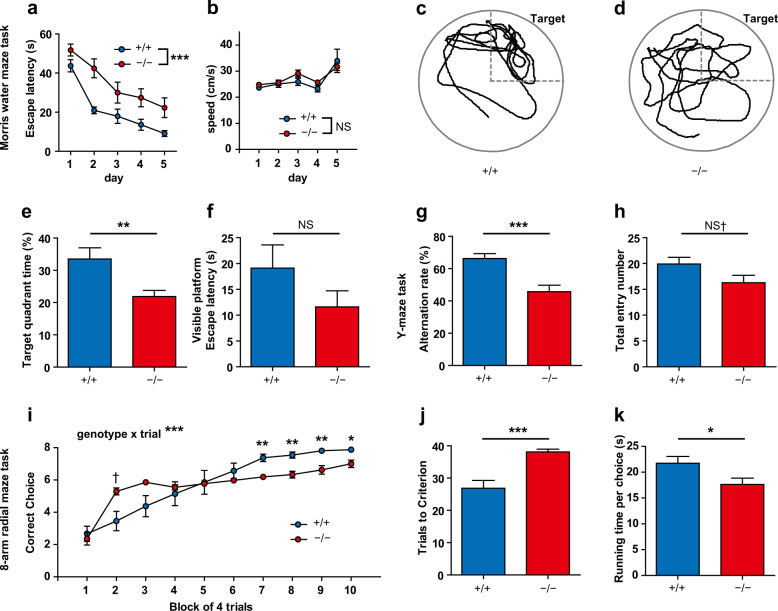
Gad1−/− rats exhibited spatial cognitive impairments and aberrant hyperactivity
To assess the spatial reference memory of Gad1−/− rats, the hidden platform task in the Morris water maze was used. During task training, the latency to escape of Gad1−/− rats was significantly longer than that of Gad1+/+ rats (Fig. 5a). However, the swimming speed of Gad1−/− rats was unaltered (Fig. 5b). Furthermore, in the probe test the day after the end of the training, Gad1−/− rats stayed less in the target quadrant (Fig. 5c–e). In the visible platform test, however, there was no significant difference in the escape latency between the two genotypes (Fig. 5f).
Fig. 5. Spatial cognitive impairments in Gad1−/− rats.
Gad1−/− rats displayed significant cognitive impairments in multiple tasks. a–f Morris water maze task (Gad1+/+, n = 12; Gad1+/+, n = 12). Gad1−/− rats required a longer time to reach the hidden platform during the 5-day training period (main effect of genotype, F(1, 22) = 20.4574, p < 0.001) (a). However, the swimming speed of the Gad1−/− rat was very similar to that of the Gad1+/+ rat (main effect of genotype, F(1, 22) = 0.5307, p = 0.4740) (b). Representative trajectories of Gad1+/+ (c) and Gad1−/− (d) rats in the probe test. The target quadrant is shown in the panels. In Gad1−/− rats, the accumulation of the trajectory to the target was decreased. The percentage of time spent within the target quadrant was significantly reduced in Gad1−/− rats compared with Gad1+/+ rats (t(17.033) = −2.9599, p < 0.01) (e). There was no significant difference between genotypes in the visible platform task (t(19.591) = −1.3764, p = 0.1842) (f). g–h Y-maze test for working memory (Gad1+/+, n = 12; Gad1+/+ n = 11). Gad1−/− rats showed less alternation behavior than Gad1+/+ rats (t(18.877) = –4.0583, p < 0.001) (g). The number of entries to arms was also slightly decreased in Gad1−/− rats (t(20.453) = –1.8815, p = 0.07422) (h). i–k Eight-arm radial maze task for working memory (Gad1+/+, n = 12; Gad1−/−, n = 12). Gad1−/− rats showed a different pattern in the learning curve from that of Gad1+/+ rats (genotype × trials, F(9, 198) = 9.1844, p < 0.001). At the end of the training, the number of correct choices was less in Gad1−/− rats than in Gad1+/+ rats (simple main effects of genotype, adjusted by Holm’s method; 7th to 9th, p < 0.01; 10th, p < 0.05). However, in the second block, Gad1−/− rats transiently scored higher than WT rats (2nd, p = 0.05227) (i). Gad1−/− rats also needed more trials to reach the learning criterion of the task (t(13.78) = 4.3409, p < 0.001) (j). Running time per choice was decreased in Gad1−/− rats (t(21.868) = 2.3065, p < 0.05) (k). The results are presented as the average ± SEM. Data were analyzed using two-way repeated measures ANOVA (a, b, i) and Welch’s t-test (e–h, j, k). †p < 0.1, p < 0.05, **p < 0.01, ***p < 0.001; NS, not significant.
Spatial working memory was measured using the Y-maze task (Fig. 5g, h) and the eight-arm radial maze task (Fig. 5i–k). Gad1−/− rats showed reduced spontaneous alternation behavior in the Y-maze task (Fig. 5g). Total entry number to arms, an indicator of locomotor activity on the maze, showed a trend of reduction in Gad1−/− rats, albeit the difference was not statistically significant (Fig. 5h).
In the acquisition curve of the eight-arm radial maze task, there was a significant genotype×trial interaction on the number of correct choices (Fig. 5i). The post hoc test revealed that the performance of Gad1−/− rats was significantly poorer than that of WT rats during the 7th to 10th block of training. The number of trials needed to meet the learning criterion (correct choice ≥7 for five consecutive trials) was also prolonged (Fig. 5j). Conversely, Gad1−/− rats showed slightly higher scores on the 2nd block (Fig. 5i). However, this did not indicate that Gad1−/− rats performed well because their score was similar to the chance-level value of the radial maze task (~5.3)46. This was caused by increased running speed from the early stage of training in Gad1−/− rats (see below). A similar acquisition curve was observed in a pharmacological model of schizophrenia in our previous study42. The running time per choice42, which reflects the running speed of rats during the task, was significantly shortened in Gad1−/− rats (Fig. 5k). The faster a rat runs on the maze, the smaller the running time becomes. Therefore, the results indicate that Gad1−/− rats moved faster than Gad1+/+ rats in the radial maze task, unlike in the open field test and the Y-maze task.
Adult neurogenesis in the dentate gyrus was not altered in Gad1−/− rats
The results from the behavioral tests strongly suggest that Gad1−/− rats had spatial cognitive deficits, which can be affected by abnormalities in adult neurogenesis. GABAergic transmission plays a pivotal role in regulating adult neurogenesis47. Furthermore, alterations in adult neurogenesis have been reported in some animal models of schizophrenia48. DCX is known as one of the molecular markers of neurogenesis48. Therefore, we investigated possible impairments of adult neurogenesis in the hippocampus, where we counted DCX-positive cells in the dentate gyrus of Gad1−/− and Gad1+/+ rats (Supplementary Fig. 7a, b). Both genotypes showed an age-dependent reduction in the number of DCX-positive cells. After adjustment for age, there was no difference in the number of DCX-positive cells (Supplementary Fig. 7c).
Discussion
To test the hypothesis that loss-of-function mutation in the GAD1 gene leads to cognitive impairments observed in schizophrenia, we generated Gad1 KO rats using genome editing. Despite the relatively high neonatal mortality and transient growth retardation during the developmental stage, behavioral tests were feasible in adulthood, unlike Gad1−/− mice6. In line with our hypothesis, we found significant impairments in both spatial reference and working memory in adult male Gad1−/− rats. To our knowledge, this is the first direct evidence that elimination or decrease in GAD67 expression can cause distinct impairments in spatial memory in particular. Since we also identified behavioral alterations alongside cognitive impairments, Gad1−/− rats may recapitulate a broader range of symptoms of schizophrenia.
How does loss of GAD67 expression lead to spatial cognitive impairments? The Morris water maze, eight-arm radial maze, and Y-maze tasks are known as hippocampus-dependent tasks49–51. Although adult neurogenesis plays distinct roles in hippocampus-dependent functions such as cognition52, we did not detect any reduction in adult-born neurons in the Gad1−/− hippocampus. Thus, the poorer performance observed in these tasks must be independent of hippocampal adult neurogenesis. CA1 and hilar GABAergic neurons are required for spatial working memory and spatial reference memory, respectively53,54. Therefore, cognitive impairments in Gad1−/− rats may be partially explained by loss of GAD67 and subsequent impaired GABAergic transmission. However, conditional KO or KD (cKO/KD) of Gad1, which mainly targets parvalbumin (PV)-positive GABAergic neurons, causes no impairment of spatial working memory in mice27–29,55. PV neurons are the largest population of GABAergic neurons56,57 and are preferentially impaired in schizophrenia12. In mice, acute optogenetic suppression of PV neurons in the hippocampus impairs working memory53, which is consistent with the present study but is inconsistent with the results of cKO/KD mice. The optogenetic technique probably can suppress GABAergic transmission more strongly than the genetic reduction of GAD67 because the release of GABA produced by GAD65 is also inhibited. Different effects on working memory between our global Gad1 KO rats and the cKO/KD mice also may be attributed to differences in the severity of cognitive impairment. All previous studies of cKO/KD mice employed only spontaneous alternation behavior on the Y-maze for evaluation of spatial working memory27–29,55. However, the number of arms to be memorized is considerably fewer in the Y-maze than in the eight-arm radial maze. In addition, spontaneous alternation is suggested to be less sensitive for detecting working memory impairment than the eight-arm radial maze task in some cases58. Compared to global KO rats, GAD67 reduction in cKO/KD mice is considered to be mild. If the working memory impairment in the mice was also moderate, it would be difficult to detect it merely by spontaneous alternation. Furthermore, species differences in the functional role of GAD67 between mice and rats should also be considered as the cause of the discrepancy, although the significance of species differences in the GAD67/GAD65 ratio has yet to be determined. Gad1 cKO/KD rats are needed in the future to explore species differences more directly. Since we have generated a Gad1-flox rat line, this will be our focus in subsequent studies.
In addition to cognitive impairments, Gad1−/− rats showed characteristic alterations in activity. In the open field test, spontaneous locomotion and rearing were significantly reduced, while NMDA receptor antagonist-induced hyperlocomotion was enhanced compared with Gad1+/+ rats. We must carefully interpret the reduced activity in the context of schizophrenia research using animal models. One possible interpretation is that hypoactivity is a model of negative symptoms. Patients with chronic schizophrenia display increased resting time during daily activities, and the extent of their activity is negatively correlated with the severity of negative symptoms59. Even after the 7-day habituation period in the open field, Gad1−/− rats still displayed a shorter duration of movement, i.e., a longer resting period. Therefore, the lower baseline activity of Gad1−/− rats may represent a negative symptom-like phenotype. Gad1−/− rats also displayed reduced social novelty preference, which is considered indicative of negative symptoms or impaired social recognition memory28. Because Gad1−/− rats could perform normally in the novel object recognition task, this alteration is specific to the social context. Another speculation for hypoactivity is that it is a manifestation of increased anxiety in Gad1−/− rats. We observed significant correlations among the center time, number of fecal boli, and locomotor activity in Gad1−/− rats. The decrease in exploration time of the center region of the open field60 and increased number of fecal boli61 are considered anxiety-like behaviors in rodents. The enhanced anxiety-like phenotype is partially consistent with somatostatin neuron-specific Gad1 KO mice, which showed a decrease in the center time during the open field test62. Conversely, no differences between the two genotypes were noted in the elevated plus-maze test. Therefore, caution is warranted when making conclusions about anxiety levels at present.
In contrast to hypoactivity in the open field test, hypersensitivity to NMDA receptor antagonists is relatively easy to interpret as a schizophrenia symptom. This phenotype is considered a hallmark of animal models of positive symptoms28 and is suggested to be mediated by the dopaminergic system63. Therefore, our results suggest that the decrease in GAD67 levels also influences the positive symptom-like phenotype in rats. It will be of interest to determine whether dopamine levels are altered following the administration of an NMDA receptor antagonist or amphetamine in Gad1−/− rats. Because this phenotype is shared with PV neuron-specific Gad1 heterozygous KO mice28, as we have reported previously, it is also likely to be mediated by dysfunction of this subtype of GABAergic neurons.
Gad1−/− rats also share some phenotypes with pharmacological models of schizophrenia. Neonatal repetitive administration of NMDA receptor antagonists, such as MK-801 and ketamine, are pharmacological models of schizophrenia42,58,64,65. MK-801-treated rats showed significantly decreased rearing or a trend-level reduction in locomotor activity as adults, similar to Gad1−/− rats42,58,65. Surprisingly, these rats displayed hyperactivity in the eight-arm radial maze task42 and decreased immobility in the forced swim test after stress58. Although the neurobiological basis of these behavioral manifestations in NMDA receptor antagonist-treated rats remains to be determined, some common mechanisms may exist between Gad1−/− rats and NMDA receptor antagonist-treated rats. Notably, blockade of NMDA receptors during the postnatal period induces a marked reduction in PV-positive GABAergic neurons66 and GAD67 protein67. Assuming that GABAergic dysfunction itself is the common pathway of the behavioral alterations, the Gad1−/− rat model represents a helpful tool to reveal the downstream phenomena of repetitive postnatal NMDA receptor blockade.
This study also indicated that Gad1−/− rats failed to show deficits in PPI, an intermediate phenotype in models of schizophrenia widely assessed in animal studies28,65. Because GAD65 elimination causes a robust deficit of PPI in mice68, GABAergic transmissions have been implicated in normal sensorimotor gating. However, interestingly, GAD67 elimination showed no alterations in PPI in rats. According to postmortem brain studies, GAD65 expression is unchanged in schizophrenia patients11, although we observed that GAD65 expression was markedly upregulated in Gad1−/− rat brains. The intact PPI may be attributed to this molecular discrepancy between the patients and Gad1−/− rats.
Combined with studies on Gad1−/− mice7, the present study revealed evident differences between mice and rats with loss-of-function mutations in Gad1. In terms of mortality, the Gad1−/− phenotype in rats appears to be less severe than that of Gad1−/− mice. Although the biological significance of the species difference in the GAD67/GAD65 ratio remains elusive, the reduced dependence on GAD67 in rats would enable them to survive to adulthood even if this isoform was lost. Furthermore, the cleft palate observed in Gad1−/− mice was absent in Gad1−/− rats, which implies that the lower GAD67/GAD65 ratio in rats prevented palate malformation as well. Meanwhile, we unexpectedly discovered a reduced body weight of Gad1−/− rats only during development. Although some evidence suggests that GABA has important roles in controlling food intake and secretion of growth hormone69–74, the precise mechanisms of growth retardation in Gad1−/− rats should be addressed to exclude potential effects on brain development and behavior. Notably, GAD67 elimination reduced GABA levels to almost half those in Gad1+/+ rat brains (Fig. 2), although the GAD67 protein amount accounted for only 23% of the total GAD proteins in the Gad1+/+ rat brain36, and GAD65 showed compensatory upregulation in Gad1−/− rats (Fig. 1f). These findings indicate that GAD67 is important for maintaining baseline GABA levels in rats. Asada et al.6 speculated that the cause of death in Gad1−/− mice was respiratory failure rather than cleft palate. The lethality without cleft palate observed in a subpopulation of Gad1−/− rats also supports their hypothesis.
We also need to acknowledge some limitations of the current study. First, the Gad1−/− rats are just a model of an ideal situation wherein GAD67 is completely eliminated. As reported in the postmortem brain studies, GAD67 is not completely lost in schizophrenia. GAD67 mRNA levels are only reduced by 15–35% when compared to the unaffected population9,11,12. Moreover, this reduction occurs largely in the PV-positive GABAergic neurons12. The effect of milder reduction and cell type-specific knockout of GAD67, as well as their interaction with environmental factors, should be analyzed in future studies. Second, although some studies have discovered the association between GAD1 and schizophrenia, a recent genome-wide association study in a larger population did not report GAD1 as a susceptibility gene75. Therefore, it should be noted that the evidence of an association between the genetic variation of GAD1 and schizophrenia is limited. We also would like to emphasize that the Gad1−/− rats are a model for revealing the effects of GAD67 reduction and not a model that exactly mimics the genetic variation of GAD1 discovered in patients.
In conclusion, the loss-of-function mutation in the Gad1 gene causes not only cognitive impairments but also several behavioral alterations possibly relevant to positive and negative symptoms of schizophrenia. Gad1−/− rats will represent a novel tool to study pathophysiology and to develop treatments for cognitive impairment in schizophrenia. Furthermore, the results of our study warn researchers to pay closer attention to potential species differences between mice and rats when developing animal models of human disorders.
Supplementary information
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP26290002 (Y.Y.), JP15H05872 (Y.Y.), JP16H06276 [Platform of Advanced Animal Model Support, AdAMS] (Y.Y.), JP17H05550 (Y.Y.), JP17K17628 (K.F.), JP19K06881 (Y.Y.), JP15H05879 (H.M.), and AMED under Grant Number JP19dm0207001 (H.M.). This project was also partly supported by the Takeda Science Foundation (Y.Y.), the Life Science Foundation of Japan (K.F.), and the Collaborative Research Project of the Brain Research Institute, Niigata University (Y.Y.). We are grateful to Takumi Sato, Misuzu Umehara, Shunsuke Teshima, Kazuya Higeta, Yugo Nakajima, Sota Abe, and Yuki Fukuda for technical assistance. We thank Dr. Ryosuke Kaneko for his helpful comments on the analysis of data and the members of our laboratory for their support and encouragement. We also thank the staff at the Institute of Experimental Animal Research, Gunma University Graduate School of Medicine. Finally, we thank Dr. Takako Fujihara for her encouragement and critical comments as a clinical psychiatrist.
Author contributions
K.F. and Y.Y. designed the research and wrote the manuscript. Y.M. and T.M. generated Gad1 KO rats. With help from T.K., S.M., T.S., D.K., S.S., T.O., H.M., M.W., and H.Y., Y.Y., K.F., W.J., K.Y., Y.I., and Y.K. performed all the other experiments. T.K. and M.W. produced antibodies. K.F., K.Y., Y.K., Y.I., and Y.Y. analyzed the data. All authors approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kazuyuki Fujihara, Email: psy_fujihara@gunma-u.ac.jp.
Yuchio Yanagawa, Email: yuchio@gunma-u.ac.jp.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-01108-6).
References
- 1.Obata K. Synaptic inhibition and γ-aminobutyric acid in the mammalian central nervous system. Proc. Jpn. Acad. Ser. B. 2013;89:139–156. doi: 10.2183/pjab.89.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bu DF, et al. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc. Natl Acad. Sci. USA. 1992;89:2115–2119. doi: 10.1073/pnas.89.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soghomonian J, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol. Sci. 1998;19:500–505. doi: 10.1016/S0165-6147(98)01270-X. [DOI] [PubMed] [Google Scholar]
- 4.Patel AB, De Graaf RA, Martin DL, Battaglioli G, Behar KL. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J. Neurochem. 2006;97:385–396. doi: 10.1111/j.1471-4159.2006.03741.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman DL, Houser CR, Tobin AJ. Two forms of the γ-aminobutyricacid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J. Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asada H, et al. Cleft palate and decreased brain γ-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl Acad. Sci. USA. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kash SF, et al. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc. Natl Acad. Sci. USA. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto T, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidotti A, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch. Gen. Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 11.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch. Gen. Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 12.Curley AA, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: Clinical, protein, and cell type-specific features. Am. J. Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto T, et al. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am. J. Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glausier JR, Kimoto S, Fish KN, Lewis DA. Lower glutamic acid decarboxylase 65-kDa isoform messenger RNA and protein levels in the prefrontal cortex in schizoaffective disorder but not schizophrenia. Biol. psychiatry. 2015;77:167–176. doi: 10.1016/j.biopsych.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsubomoto M, et al. Expression of transcripts selective for GABA neuron subpopulations across the cortical visuospatial working memory network in the healthy state and schizophrenia. Cereb. Cortex. 2019;29:3540–3550. doi: 10.1093/cercor/bhy227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J. Psychiatry Neurosci. 2011;36:195–203. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keefe, R.S. & Harvey, P.D. in Novel Antischizophrenia Treatments, Handbook of Experimental Pharmacology (eds. Geyer, M. & Gross, G.) (Springer, Berlin, Heidelberg, 2012).
- 18.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon JH, Grandelis A, Maddock RJ. Dorsolateral prefrontal cortex GABA concentration in humans predicts working memory load processing capacity. J. Neurosci. 2016;36:11788–11794. doi: 10.1523/JNEUROSCI.1970-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Addington AM, et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD 67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol. Psychiatry. 2005;10:581–588. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- 21.Du J, et al. Comprehensive analysis of polymorphisms throughout GAD1 gene: A family-based association study in schizophrenia. J. Neural Transm. 2008;115:513–519. doi: 10.1007/s00702-007-0844-z. [DOI] [PubMed] [Google Scholar]
- 22.Hyde TM, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J. Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straub RE, et al. Allelic variation in GAD1 (GAD 67) is associated with schizophrenia and influences cortical function and gene expression. Mol. Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 24.Giacopuzzi E, et al. Exome sequencing in schizophrenic patients with high levels of homozygosity identifies novel and extremely rare mutations in the GABA/glutamatergic pathways. PLoS ONE. 2017;12:e0182778. doi: 10.1371/journal.pone.0182778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magri C, et al. A novel homozygous mutation in GAD1 gene described in a schizophrenic patient impairs activity and dimerization of GAD67 enzyme. Sci. Rep. 2018;8:15470. doi: 10.1038/s41598-018-33924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakizaki T, Oriuchi N, Yanagawa Y. GAD65/GAD67 double knockout mice exhibit intermediate severity in both cleft palate and omphalocele compared with GAD67 knockout and VGAT knockout mice. Neuroscience. 2015;288:86–93. doi: 10.1016/j.neuroscience.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Brown JA, et al. Inhibition of parvalbumin-expressing interneurons results in complex behavioral changes. Mol. Psychiatry. 2015;20:1499–1507. doi: 10.1038/mp.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujihara K, et al. Glutamate decarboxylase 67 deficiency in a subset of GABAergic neurons induces schizophrenia-related phenotypes. Neuropsychopharmacolog. 2015;40:2475–2486. doi: 10.1038/npp.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolata SM, et al. Neuropsychiatric phenotypes produced by GABA reduction in mouse cortex and hippocampus. Neuropsychopharmacology. 2018;43:1445–1456. doi: 10.1038/npp.2017.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leo D, et al. Pronounced hyperactivity, cognitive dysfunctions, and BDNF dysregulation in dopamine transporter knock-out rats. J. Neurosci. 2018;38:1959–1972. doi: 10.1523/JNEUROSCI.1931-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bao D, et al. Preliminary characterization of a leptin receptor knockout rat created by CRISPR/Cas9 system. Sci. Rep. 2015;5:15942. doi: 10.1038/srep15942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson MB, et al. Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size. Nature. 2018;556:370–375. doi: 10.1038/s41586-018-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato K, et al. Generation of a nonhuman primate model of severe combined immunodeficiency using highly efficient genome editing. Cell Stem Cell. 2016;19:127–138. doi: 10.1016/j.stem.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis. Model Mech. 2016;9:1079–1087. doi: 10.1242/dmm.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheikh SN, Martin SB, Martin DL. Regional distribution and relative amounts of glutamate decarboxylase isoforms in rat and mouse brain. Neurochem. Int. 1999;35:73–80. doi: 10.1016/S0197-0186(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 37.Xu G, et al. Late development of the GABAergic system in the human cerebral cortex and white matter. J. Neuropathol. Exp. Neurol. 2011;70:841–858. doi: 10.1097/NEN.0b013e31822f471c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyasaka Y, et al. CLICK: One-step generation of conditional knockout mice. BMC Genomics. 2018;19:318. doi: 10.1186/s12864-018-4713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagawa Y, et al. Structure and alternative promoters of the mouse glutamic acid decarboxylase 67 gene. Biochem. J. 1997;326:573–578. doi: 10.1042/bj3260573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, et al. Utilization of an intron located polyadenlyation site resulted in four novel glutamate decarboxylase transcripts. Mol. Biol. Rep. 2009;36:1469–1474. doi: 10.1007/s11033-008-9337-x. [DOI] [PubMed] [Google Scholar]
- 41.Suto T, Kato D, Obata H, Saito S. Tropomyosin receptor kinase B receptor activation in the locus coeruleus restores impairment of endogenous analgesia at a late stage following nerve injury in rats. J. Pain. 2019;20:600–609. doi: 10.1016/j.jpain.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Kawabe K, Iwasaki T, Ichitani Y. Repeated treatment with N-methyl-D-aspartate antagonists in neonatal, but not adult, rats causes long-term deficits of radial-arm maze learning. Brain Res. 2007;1169:77–86. doi: 10.1016/j.brainres.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 43.Nunez J. Morris water maze experiment. J. Vis. Exp. 2008;19:pii 897. doi: 10.3791/897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bubeníková-Valešová V, Horáček J, Vrajová M, Höschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci. Biobehav. Rev. 2008;32:1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Antunes M, Biala G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckerman DA. Monte Carlo estimation of chance performance for the radial arm maze. Bull. Psychon. Soc. 1980;15:93–95. doi: 10.3758/BF03334476. [DOI] [Google Scholar]
- 47.Pallotto M, Deprez F. Regulation of adult neurogenesis by GABAergic transmission: signaling beyond GABAA-receptors. Front. Cell Neurosci. 2014;8:166. doi: 10.3389/fncel.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang E, Wen Z, Song H, Christian KM, Ming GL. Adult neurogenesis and psychiatric disorders. Cold Spring Harb. Perspect. Biol. 2016;8:a019026. doi: 10.1101/cshperspect.a019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 50.Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- 51.Richman CL, Dember WN, Kim P. Spontaneous alternation behavior in animals: A review. Curr. Psychol. 1986;5:358–391. doi: 10.1007/BF02686603. [DOI] [Google Scholar]
- 52.Toda T, Parylak SL, Linker SB, Gage FH. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry. 2019;24:67–87. doi: 10.1038/s41380-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray AJ, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat. Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrews-Zwilling Y, et al. Hilar GABAergic interneuron activity controls spatial learning and memory retrieval. PLoS ONE. 2012;7:e40555. doi: 10.1371/journal.pone.0040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Georgiev D, et al. Cortical gene expression after a conditional knockout of 67 kDa glutamic acid decarboxylase in parvalbumin neurons. Schizophr. Bull. 2016;42:992–1002. doi: 10.1093/schbul/sbw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamamaki N, et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 57.Uematsu M, et al. Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb. Cortex. 2008;18:315–330. doi: 10.1093/cercor/bhm056. [DOI] [PubMed] [Google Scholar]
- 58.Kawabe K. Effects of chronic forced-swim stress on behavioral properties in rats with neonatal repeated MK-801 treatment. Pharmacol. Biochem. Behav. 2017;159:48–54. doi: 10.1016/j.pbb.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 59.Sano W, et al. Enhanced persistency of resting and active periods of locomotor activity in schizophrenia. PLoS ONE. 2012;7:e43539. doi: 10.1371/journal.pone.0043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc. Natl Acad. Sci. USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seibenhener ML, Wooten MC. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015;96:e52434. doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miyata S, et al. Loss of glutamate decarboxylase 67 in somatostatin-expressing neurons leads to anxiety-like behavior and alteration in the akt/gsk3β signaling pathway. Front. Behav. Neurosci. 2019;13:131. doi: 10.3389/fnbeh.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bubser M, Keseberg U, Notz PK, Schmidt WJ. Differential behavioural and neurochemical effects of competitive and non-competitive NMDA receptor antagonists in rats. Eur. J. Pharmacol. 1992;229:75–82. doi: 10.1016/0014-2999(92)90288-F. [DOI] [PubMed] [Google Scholar]
- 64.Kawabe K, Miyamoto E. Effects of neonatal repeated MK-801 treatment on delayed nonmatching-to-position responses in rats. Neuroreport. 2008;19:969–973. doi: 10.1097/WNR.0b013e328302ee31. [DOI] [PubMed] [Google Scholar]
- 65.Uehara T, et al. Neonatal exposure to MK-801, an N-methyl-D-aspartate receptor antagonist, enhances methamphetamine-induced locomotion and disrupts sensorimotor gating in pre- and postpubertal rats. Brain Res. 2010;1352:223–230. doi: 10.1016/j.brainres.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 66.Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- 67.Lema Tomé CM, et al. Decline in age-dependent, MK801-induced injury coincides with developmental switch in parvalbumin expression: Somatosensory and motor cortex. Dev. Psychobiol. 2008;50:665–679. doi: 10.1002/dev.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heldt SA, Green A, Ressler KJ. Prepulse inhibition deficits in GAD65 knockout mice and the effect of antipsychotic treatment. Neuropsychopharmacology. 2004;29:1610–1619. doi: 10.1038/sj.npp.1300468. [DOI] [PubMed] [Google Scholar]
- 69.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim ER, et al. Hypothalamic Non-AgRP, Non-POMC GABAergic neurons are required for postweaning feeding and NPY hyperphagia. J. Neurosci. 2015;35:10440–10450. doi: 10.1523/JNEUROSCI.1110-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsuji M, et al. A new case of GABA transaminase deficiency facilitated by proton MR spectroscopy. J. Inherit. Metab. Dis. 2010;33:85–90. doi: 10.1007/s10545-009-9022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Louro P, et al. Phenotyping GABA transaminase deficiency: a case description and literature review. J. Inherit. Metab. Dis. 2016;39:743–747. doi: 10.1007/s10545-016-9951-z. [DOI] [PubMed] [Google Scholar]
- 73.Koenig MK, et al. Phenotype of GABA-transaminase deficiency. Neurology. 2017;88:1919–1924. doi: 10.1212/WNL.0000000000003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson RA, Mitchell R. Effects of γ-aminobutyric acid receptor agonists on the secretion of growth hormone, luteinizing hormone, adrenocorticotrophic hormone and thyroid-stimulating hormone from the rat pituitary gland in vitro. J. Endocrinol. 1986;108:1–8. doi: 10.1677/joe.0.1080001. [DOI] [PubMed] [Google Scholar]
- 75.Ripke S, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.