Abstract
Normal aging is associated with declines in sensorimotor function. Previous studies have linked age-related behavioral declines to decreases in neural differentiation (i.e., dedifferentiation), including decreases in the distinctiveness of neural activation patterns and in the segregation of large-scale neural networks at rest. However, no studies to date have explored the relationship between these two neural measures and whether they explain the same aspects of behavior. To investigate these issues, we collected a battery of sensorimotor behavioral measures in older and younger adults and estimated (a) the distinctiveness of neural representations in sensorimotor cortex and (b) sensorimotor network segregation in the same participants. Consistent with prior findings, sensorimotor representations were less distinct and sensorimotor resting state networks were less segregated in older compared to younger adults. We also found that participants with the most distinct sensorimotor representations exhibited the most segregated sensorimotor networks. However, only sensorimotor network segregation was associated with individual differences in sensorimotor performance, particularly in older adults. These novel findings link network segregation to neural distinctiveness, but also suggest that network segregation may play a larger role in maintaining sensorimotor performance with age.
Keywords: aging, dedifferentiation, resting-state, sensorimotor, task activity
Introduction
Aging is associated with extensive declines in sensorimotor function including fine motor control, gait, and balance (Seidler et al., 2010). Although these declines can reflect changes in the peripheral sensorimotor system (Cole et al., 1999), they also likely reflect changes in the central nervous system, such as alterations in brain structure and function (Seidler et al., 2010). A central challenge, then, is to advance our understanding of the neural mechanisms that underlie age-related declines in sensorimotor function. Doing so could lead to new interventions to extend work productivity and facilitate a variety of daily life activities in older adults.
Previous research has found that neural representations are less selective, or distinctive, in older compared with younger adults. This phenomenon is often referred to as age-related neural dedifferentiation (Li and Lindenberger, 1999), reflecting the fact that neural activity in response to different stimulus categories is less differentiated in older adults. For example, in young adults, the neural activation patterns evoked by looking at pictures of faces are quite different from those evoked by looking at pictures of houses. However, these activation patterns are more similar (i.e., less distinct or less differentiated) in older adults (Park et al., 2004). Furthermore, older adults who exhibit less distinct neural representations often perform significantly worse on a range of cognitive (Park et al., 2010) and motor tasks (Bernard and Seidler, 2012) than older adults whose neural representations are more distinct.
Accumulating evidence also points to age differences in the organization of functional brain networks (Damoiseaux, 2017). This type of organization is most frequently investigated using resting-state functional connectivity (Biswal et al., 1995) and more recently, using graph theoretical analyses (Bullmore and Sporns, 2009). In graph theory, a brain network is treated as a set of nodes (corresponding to brain regions) with edges (corresponding to functional connections) between them. Using this framework, one can calculate multiple measures of brain networks. One common measure is network segregation, defined as the scaled difference between within-network connectivity and between-network connectivity. A number of studies have demonstrated that older age is associated with less segregated (i.e., dedifferentiated) networks (Damoiseaux, 2017). Further, less segregated networks predict poorer cognitive (Chan et al., 2014) and sensorimotor (King et al., 2018) performance.
Given that both neural distinctiveness and network segregation decline with age and that both are associated with behavior, a natural question is whether these two measures of neural dedifferentiation are actually measuring the same underlying construct and whether they predict similar aspects of behavior. One reason to suspect that they do is that previous work has found a relationship between the brain’s functional connectivity at rest and activity during task performance. For instance, Langan and colleagues found that reduced interhemispheric resting state connectivity in older adults was associated with greater activity in the non-dominant hemisphere during unimanual motor performance (Langan et al., 2010). Chan et al. recruited participants across the adult life span (from ages 20–89 years), and demonstrated that reduced differentiation between network-specific connector and non-connector nodes measured at rest correlated with reduced differentiation of connector vs. non-connector nodes during visual and semantic task performance (Chan et al., 2017). These findings suggest that network topology observed at rest may constrain functional activity of brain areas during motor, visual and semantic task performance, and that network segregation might be closely related to neural distinctiveness.
In this study, we investigated the relationship between neural distinctiveness and network segregation in the sensorimotor domain, by measuring both in the same participants. We also collected a battery of sensorimotor behavioral measures to examine the relationship between the neural measures and performance.
We explored three questions. First, do older adults exhibit reduced sensorimotor distinctiveness and sensorimotor network segregation relative to young adults? Second, are less distinct sensorimotor representations associated with less segregated sensorimotor networks? Third, are both neural distinctiveness and network segregation associated with sensorimotor performance and does either explain significant behavioral variance over and above the other?
Materials and methods
Participants
We collected data from 25 younger adults (age range 19 to 29 years; 16 females) and 46 older adults (age range 65 to 81 years; 28 females), as part of the larger Michigan Neural Distinctiveness study (Gagnon et al., 2019). Some of these participants (22 younger adults and 23 older adults) were also included in our previous study, in which we examined sensorimotor network segregation and its relationship with GABA levels and sensorimotor behavior (Cassady et al., 2019). All participants were right-handed, native English speakers. They were screened to ensure they had no history of stroke, were not taking any medications with psychotropic effects, and were free of any MRI safety contraindications. Participants were also screened for cognitive impairments using the Montreal Cognitive Assessment (Brenkel et al., 2017), and only those with scores ≥23 were included in this study. Two younger adult participants were excluded from further task-based fMRI analyses because of excessive head motion in the MRI scanner (more than 3 mm or 3 degrees in any axis). An additional three older adult participants did not finish their fMRI task-based session and therefore were excluded from this analysis. A detailed explanation of the study was provided, and written informed consent was obtained from all participants. The study was approved by the Institutional Review Board of the University of Michigan.
Experimental Design and Statistical Analysis
All participants completed two separate test sessions: an imaging session during which we collected task-based and resting-state fMRI data and a behavioral session during which we collected sensorimotor behavioral data. The order of the fMRI and behavioral sessions was counterbalanced across participants. All data was obtained within an average period of 24 days.
To examine age differences in neural distinctiveness, network segregation and behavior between young and older adults, we performed independent sample t-tests. To assess the relationship between distinctiveness and segregation, we performed partial correlation analysis (controlling for age, GM volume, motion, and univariate activation). To examine the relationship between distinctiveness, segregation, and behavior, we performed multiple regressions across all participants (including the same covariates). For all statistical analyses, data points greater than three standard deviations above or below the group mean were excluded. SPSS software was used for all statistical analyses (SPSS Inc., Chicago IL).
Sensorimotor assessments
We used a National Institute of Health sensorimotor test battery that includes tests of fine motor dexterity (tested with the 9-hole pegboard dexterity test), grip strength, and endurance (measured with a 2-minute walk endurance test). Please refer to Cassady et al. (2019) and Gagnon et al., (2019) for details of the sensorimotor assessments. A summary of age-group means (and standard errors) for each behavioral measure is included in Table 1. Participants’ scores for all tests were submitted to an exploratory factor analysis in order reduce the dimensionality of the data. Please refer to the Table 21 for factor analysis model coefficients across all participants.
Table 1.
Mean and standard error of demographics and behavioral measures across all participants, and just in the older and younger adult groups.
| All participants | Old adults | Young adults | |
|---|---|---|---|
| Age | 53.4 ± 2.8 | 70.4 ± 0.6 | 22.9 ± 0.6 |
| MoCA | 27.6 ± 0.2 | 27.3 ± 0.3 | 28.2 ± 0.3 |
| Dexterity | 102.1 ± 1.1 | 98.4 ± 1.2 | 109 ± 1.1 |
| GS | 99 ± 1.4 | 97.2 ± 1.7 | 102.4 ± 2.5 |
| Endurance | 90.6 ± 1.5 | 86 ± 1.7 | 99.3 ± 2 |
GS = Grip strength.
Table 2.
Model coefficients from factor analysis across all participants.
| Behavioral measure | Factor 1 | Factor 2 |
|---|---|---|
| Dexterity dominant | −0.04 | 0.63 |
| Dexterity non-dominant | −0.11 | 1.03 |
| GS dominant | 1.03 | −0.1 |
| GS non-dominant | 0.96 | −0.08 |
| Endurance | 0.52 | 0.35 |
GS = Grip strength.
MRI data acquisition
Structural and functional brain images were obtained using a GE Signa 3-Tesla MRI scanner, located at the University of Michigan Functional Magnetic Resonance Imaging Laboratory. A 16-rod bird cage head coil was used for all participants, and movement was minimized by using head cushions and Velcro straps. During each participant’s scanning session, we acquired T1-weighted structural images, high-resolution structural images using spoiled 3D gradient-echo acquisition (SPGR), and T2*-weighted functional images (one resting state scan including 240 volumes and one task-based scan including 180 volumes). Functional images were obtained using a single-shot gradient-echo (GRE) reverse spiral pulse sequence. The field of view was 220 × 200 mm, the voxel size was 3 × 3 × 4 mm (40 axial slices), the TR (repetition time) was 2 seconds, and the TE (echo time) was 30 ms. Respiratory and cardiac data were collected for both resting state and task scans, and were subsequently controlled for in first-level analyses.
Resting state fMRI preprocessing and analysis
Preprocessing of the resting state fMRI data was performed with the Statistical Parametric Mapping software (SPM; www.fil.ion.ucl.ac.uk/spm). Preprocessing steps included slice-time correction, realignment, segmentation of structural images, normalization into standard Montreal Neurological Institute (MNI) space, and spatial smoothing using a Gaussian kernel of 8mm full width at half-maximum. To detect and reject head motion artifacts in the scanner, we used the Artifact Detection Toolbox (ART; https://www.nitrc.org/projects/artifact_detect), which included “scrubbing” scans with excessive motion using a threshold of .2mm frame-wise displacement (FWD). Outliers in the global mean signal intensity and motion were subsequently included as nuisance covariates in the first level general linear model (GLM). There were a total of 40 outlier volumes from 6 participants during preprocessing for the resting state data (14 volumes in the older adult group and 26 volumes in the younger adult group). The difference between the age groups was not statistically significant, t(69) = 0.87, p = .39. There were a total of 74 outlier volumes from 13 participants during preprocessing for the task data (28 volumes from the older adult group and 46 volumes from the younger adult group). The difference between the age groups was not statistically significant, t(69) = 1, p = .33. Including both this measure of motion and the motion measure from the resting state data in the regression analyses did not alter the results.
We performed additional denoising on the resting state data with the CONN toolbox. The data were first filtered using a temporal band-pass filter of 0.008 to 0.09 Hz to examine the frequency band of interest and to exclude higher frequency sources of noise. For additional noise reduction, the anatomical component-based noise correction method, aCompCor (Behzadi et al., 2007), was used. This method models the influence of noise as a voxel-specific linear combination of multiple empirically estimated noise sources by acquiring principal components from noise ROIs and subsequently including them as nuisance parameters in the first-level GLM. In particular, each participant’s structural image was segmented into white matter (WM), gray (GM) matter, and cerebrospinal fluid (CSF) masks. Next, the WM and CSF masks were eroded by one voxel in order to minimize partial voluming effects. Finally, these eroded WM and CF masks were used as nuisance ROIs. The signals from all ROIs were extracted from the unsmoothed functional images to avoid potential “spillage” of the BOLD signal from nearby regions. Residual head motion parameters (three rotations, three translations, and six parameters representing their first-order temporal derivatives) and signals from WM and CSF were regressed out during the calculation of functional connectivity maps. To test whether there were age differences in the percentage of variance removed from preprocessing of the resting state data, we calculated the variance in the signal before and after preprocessing for all participants. We found that, on average, 81% of the variance was removed for older adult and 82% was removed for younger adults. There was no significant age group difference in this relationship, t(69) = 1.1, p=.27.
We performed an ROI-to-ROI first-level functional connectivity analysis using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012). To do so, we first created (5mm radius spheres; each sphere has a volume of about 524 mm3) ROIs using MNI coordinates published in Power et al. (Power et al., 2011). We used all coordinates from this previous study except for those that belonged to “subcortical” and “undefined” networks, leaving us with 214 ROIs. Each ROI was labeled according to this published functional network map, which included ten networks (Hand sensorimotor, Visual, Mouth sensorimotor, Auditory, Default, Frontal-parietal control, Ventral attention, Cingulo-opercular control, Dorsal attention, and Salience networks). For each participant, the resting state time series within each ROI was then extracted from the unsmoothed functional images and the mean time series was computed. Next, the cross-correlation between each ROI’s time course with every other ROI’s time course was calculated, producing a 214 × 214 correlation matrix for each participant. Fisher’s r-to-z transformation was then used to convert correlation coefficients (i.e., graph edges) into z-values. Last, in keeping with previous functional connectivity studies, negatively-weighted edges were set to zero in each participant’s correlation matrix to avoid potential misinterpretation of negative edge weights2.
Network segregation was calculated to examine within-network correlations in relation to between-network correlations. As introduced in Chan et al. (2014), network segregation was defined as the difference in mean within-network connectivity and mean between-network connectivity as a proportion of mean within-network connectivity:
where is the mean Fisher z-transformed correlation between ROIs within the same network and is the mean Fisher z-transformed correlation between ROIs of one network with all ROIs in other networks (Chan et al., 2014).
Task-based fMRI design, preprocessing and analysis
During the task-based fMRI session, participants performed one (6-minute) run of a sensorimotor task while blood oxygenation-level dependent (BOLD) data were collected. For this task, participants were instructed to tap their left thumb (six blocks per run), right thumb (six blocks per run), or to fixate at a crosshair stimulus in the center of the visual display (rest blocks; twelve blocks per run). The left/right tapping conditions were cued by flashing arrows that pointed to the left and to the right of the visual display. Each block consisted of the stimulus presented for 500ms with a 500ms inter-stimulus interval. The order of the experimental blocks was randomized and interleaved with rest blocks. Each experimental block lasted 20 seconds; each rest block lasted 10 seconds. Stimuli were presented using E-Prime (Psychology Software Tools, Pittsburgh, PA) and displayed using a back-projection system. Participant responses were collected via a Celeritas 5-button fiber optic response unit so that we could ensure that participants were following the instructions of the task. Subsequent analyses assessed the number of hits, misses, and reaction time for all participants.
To examine the specificity of the relationship between distinctiveness and segregation in the sensorimotor system, we also assessed the association between sensorimotor neural distinctiveness and network segregation outside of sensorimotor cortex (i.e., visual and auditory network segregation; refer to Chamberlain et al. (Chamberlain et al., 2019) and Lalwani et al (Lalwani et al., 2019) for further details about the visual and auditory tasks, respectively).
The visual task consisted of one six-minute run with six 20-second blocks of faces and six 20-second blocks of houses, presented in a pseudorandom order. All experimental blocks were interleaved with 10-second fixation blocks. During the face blocks, participants viewed greyscale images of male faces. During the house blocks, participants viewed greyscale images of houses. Each stimulus appeared for 500ms, after which there was a 500ms inter-stimulus interval. To ensure that participants were attending to the visual stimuli, they were instructed to press a button with their right index finger whenever they saw a female face during the face blocks and whenever they saw an apartment building during the house blocks. These “target” trials occurred about once per minute. Stimuli were presented using E-Prime 2.0 on a back-projection system. Participants’ responses were recorded using a Lumina response pad (Cedrus).
The auditory task consisted of six 20-second blocks of foreign speech clips, six 20-second blocks of instrumental music clips, and twelve 10-second blocks of fixation between each pair of auditory blocks. The order of speech and music blocks was pseudorandomized. Specifically, each speech block consisted of 20-second news segments in one of the following foreign languages: Creole, Macedonian, Marathi, Persian, Swahili and Ukrainian. Each music block consisted of 20-second segments of instrumental music from one of the following pieces: Bach Sinfonia No. 5, Smokey by Mountain, Bamboula by L.M Gottschalk, Spagnoletta Nuova by Fabritio Caroso, Kuhlau: Fantaisie for Solo Flute in D major (Op. 38, No. 3), and a violin adaptation of the country song “When the right one comes along”. To ensure that participants were attending to the auditory stimuli, target trials (consisting of guitar plucks) occurred randomly about once per minute during the task. Participants were instructed to press a button with their right index finger every time a target trial was presented. Auditory stimuli were presented using E-Prime 2.0 via an MRI-compatible Avotec Conformal Headset.
FreeSurfer and FSFAST were used to perform the preprocessing and first-level analyses of the task-based fMRI data (http://surfer.nmr.mgh.harvard.edu/). Surface-based methods as implemented in the FreeSurfer environment were used to reconstruct the cortical surface from the T1-weighted anatomical image. Preprocessing procedures included slice-timing correction, motion correction, and spatial smoothing using a Gaussian kernel with full width at half maximum of 5 mm. Given that the resting state data were preprocessed in MNI space (see above), we also analyzed the task-based data in MNI space to allow direct comparison with the volume-based resting state data. For this procedure, we performed the same preprocessing steps (except for the denoising steps) for the task-based data as we did for the resting state data.
Neural distinctiveness was measured using multi-voxel pattern analysis (MVPA) in both anatomically- and functionally-defined regions of interest (ROIs). Neural responses were first estimated by fitting a General Linear Model, implemented in FSFAST. The model included separate regressors for each of the experimental blocks convolved with a canonical hemodynamic response function.
Using FreeSurfer’s Cortical Parcellation technique (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2004), we created bilateral anatomical masks in each participant that included precentral gyrus, postcentral gyrus, and supramarginal gyrus. Estimates of gray matter volume were also computed in each of these anatomical regions to account for age differences in brain structure. Next, we used custom MATLAB code to define each participant’s functional ROI. To do so, we first sorted the vertices within each participant’s anatomical mask based on activation level for left hand tapping (experimental condition 1) vs. rest. We then sorted the vertices within the anatomical ROI for right hand tapping (experimental condition 2) vs. rest. Finally, the functional ROI was defined by alternating between the two sorted lists, adding the most active voxel for condition 1 that had not already been included to the functional ROI, then adding the most active voxel for condition 2 that had not already been included, then the next most active voxel for condition 1, and so on. This procedure was continued until we reached our target functional ROI size of 2000 vertices (see Figure 1), which corresponds to a surface area of approximately 600 mm2.
Figure 1.
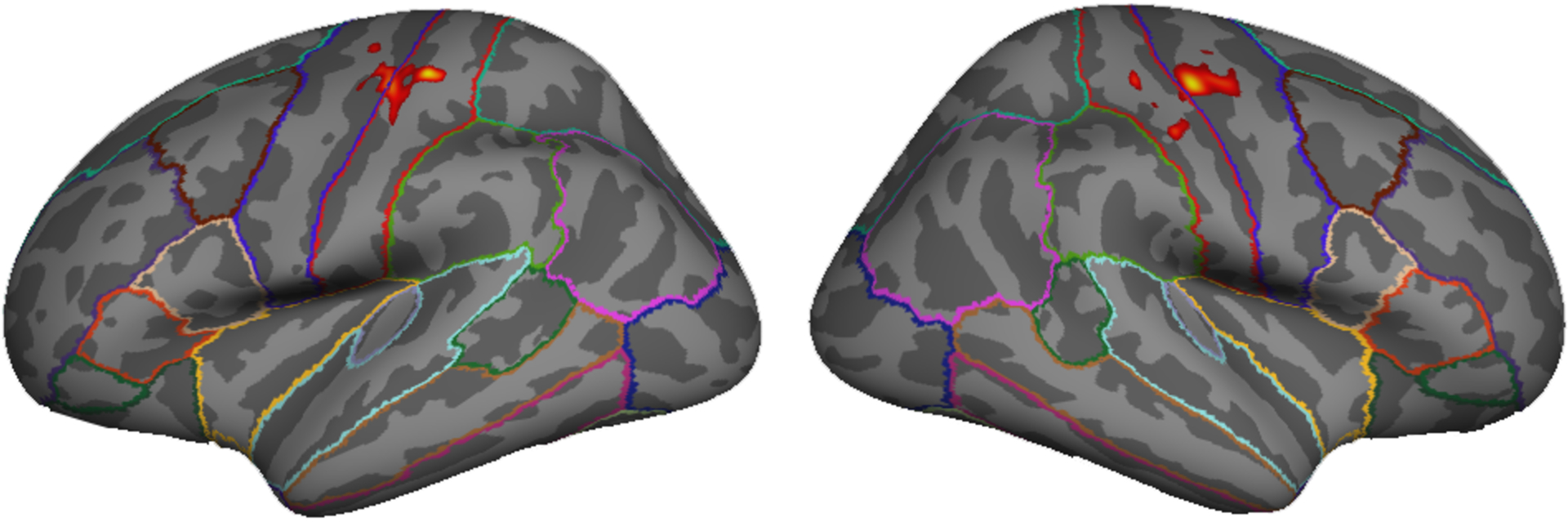
Functional mask (size = 1000 vertices in each hemisphere) from a representative older adult participant used for calculating neural distinctiveness of sensorimotor representations evoked from right vs. left hand finger tapping.
It is also important to note that this ROI-defining procedure was orthogonal to the hypotheses tested later (involving age and behavior). The region-defining analyses were based on two within-subject comparisons: left vs. fixation and right vs. fixation. Specifically, the most active vertices for each of these contrasts in each participant were included in that participant’s functional ROI. Neural distinctiveness between the left and right conditions was then computed within this functional ROI. In contrast, the hypothesis testing involved two orthogonal between-subject contrasts, one involving age (is neural distinctiveness significantly different in young vs. older participants?) and one involving behavior (do participants exhibiting lower neural distinctiveness also exhibit worse sensorimotor performance?).
After defining each participant’s functional ROI, we used the activation estimates within each participant’s functional ROI to measure the distinctiveness of multi-voxel representations for the two experimental tasks (i.e., left vs. right hand tapping). Inspired by Haxby and colleagues (Haxby et al., 2001), neural distinctiveness was defined as the difference between the average within-condition Pearson correlation (i.e., the average of the correlations between left hand patterns and the correlations between right hand patterns) and between-conditions (i.e., the average of the correlations between left-hand and right-hand patterns). Higher scores indicate greater distinctiveness whereas lower scores indicate less distinctiveness. This approach was used rather than alternative classification methods (i.e., support vector machines) to avoid ceiling effects in classifier accuracy (Carp et al., 2011a) (a linear SVM classifier was 100% accurate in classifying the activation patterns for 28/46 older adults and 18/25 younger adults).
To allow direct comparison with the volume-based resting state data, we also analyzed the task-based data in MNI space using the Power et al. (2011) sensorimotor hand ROIs as an anatomical mask (which includes about 435 voxels) for each subject. We used each participant’s activation estimates within this mask to measure the distinctiveness of multi-voxel activation patterns associated with the experimental conditions (i.e., left vs. right hand tapping).
We also investigated main effects of age on univariate activation, using second-level t-tests with SPM. In these models, age effects on three first-level contrasts (1. right hand movement, 2. left hand movement, and 3. right vs. left hand movement) were examined and motion was included as a covariate. Statistical significance was determined by applying a family wise error (FWE) of p<.05 at the peak level to correct for multiple comparisons.
Results
Age differences in sensorimotor performance
There were no age differences in performance during the sensorimotor fMRI task, either in hits (t(65)=.11, p=.92), misses (t(65)=.66, p.51), or reaction time (t(65)=.044, p=.97).
The factor analysis of all sensorimotor behavioral measures identified two factors: (1) grip strength and endurance and (2) fine motor dexterity. These two sensorimotor factors were used in all further statistical analyses. Given that grip strength and endurance reflect gross motor performance whereas dexterity reflects fine motor performance, we refer to the grip strength/endurance factor as “gross motor performance” and to the dexterity factor as “fine motor performance”.
Significant age differences were observed in both gross (t(68)=2.69, p=.009; Figure 2A) and fine (t(68)=5.01, p<.001; Figure 2B) motor performance, with older adults exhibiting worse performance than younger adults. To test whether sex differences influenced these results, we performed follow-up ANCOVAs using age group as the independent variable, behavior as the dependent variable, and sex as a covariate. After controlling for sex, we still observed significant age differences in both gross (F(1, 67)=14, p<.001) and fine (F(1, 67)=24.74, p<.001) motor performance. Moreover, there were no significant sex by age group interactions.
Figure 2.
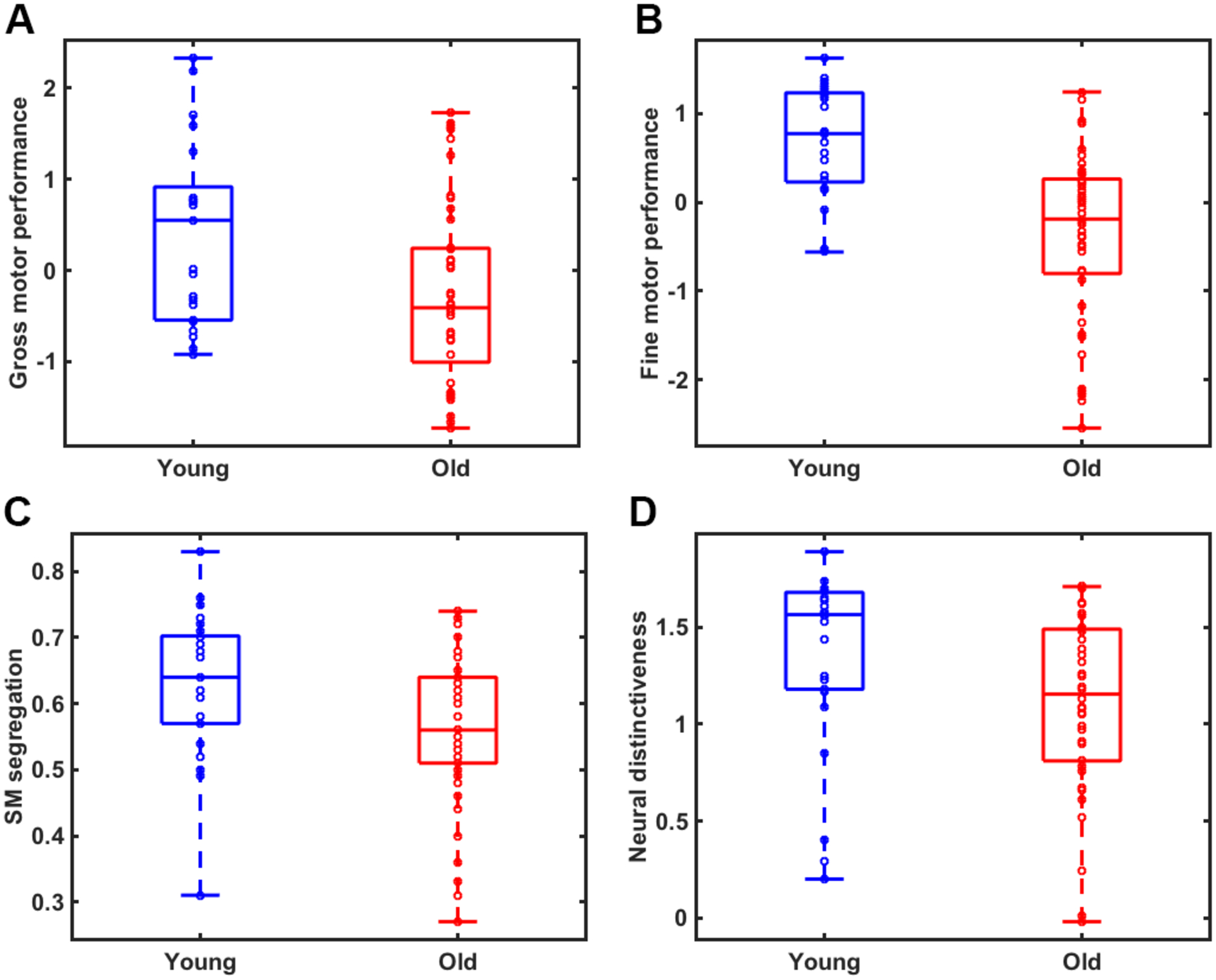
Significant age differences were observed in A) a summary measure of gross motor performance (t=2.69, p=.009); B) fine motor performance (t=5.01, p<.001); C) sensorimotor network segregation (t=2.55, p=.013); and D) neural distinctiveness of sensorimotor representations (t=2.29, p=.027) between young (blue) and older (red) adults.
Age differences in network segregation
Consistent with our previous findings (Cassady et al., 2019), we found that sensorimotor network segregation was significantly reduced in older compared to younger adults, t(69)=2.55, p=.013 (See Figure 2C). To test whether sex influenced these results, we performed a follow-up ANCOVA using sex as a covariate in the model. After controlling for sex, we still found that sensorimotor segregation was significantly reduced in older compared to younger adults, (F(1, 67)=6.7, p=.012). Again, there were no significant sex by age group interactions.
Age differences in neural distinctiveness
The distinctiveness of activation patterns evoked by left vs. right hand movement was significantly lower in older compared to younger adults, t(62)=2.29, p=.025 (See Figure 2D). Further, this effect was still observed after controlling for sex, (F(1, 61)=5.11, p=.027), and there were no significant sex by age group interactions.
To test whether functional ROI size influenced these results, we performed a repeated measures ANOVA with a Greenhouse-Geisser correction using ROI size as the within-subjects factor (using ten functional ROI sizes of 50, 100, 200, 300, 400, 600, 1000, 2000, 5000, and 10,000 vertices) and age group as the between-subjects factor. The results revealed a significant within-subject effect of ROI size on neural distinctiveness, F(1.68, 107.71)=47.41, p<.001. Specifically, activation patterns for left vs. right hand movement were more distinctive at smaller ROI sizes and less distinctive at larger ROI sizes, F(1, 64) = 54.97, p<.001 (See Figure 3). More importantly, the age differences in neural distinctiveness that we observed did not vary with ROI size (i.e., there was not a significant ROI size x age group interaction (F(1.68, 107.71)=.70, p=.48). Neural distinctiveness was also significantly reduced in older compared to younger adults using the Power sensorimotor anatomical mask after processing the task-based data in MNI space, t(66)=2.1, p=.041.
Figure 3.
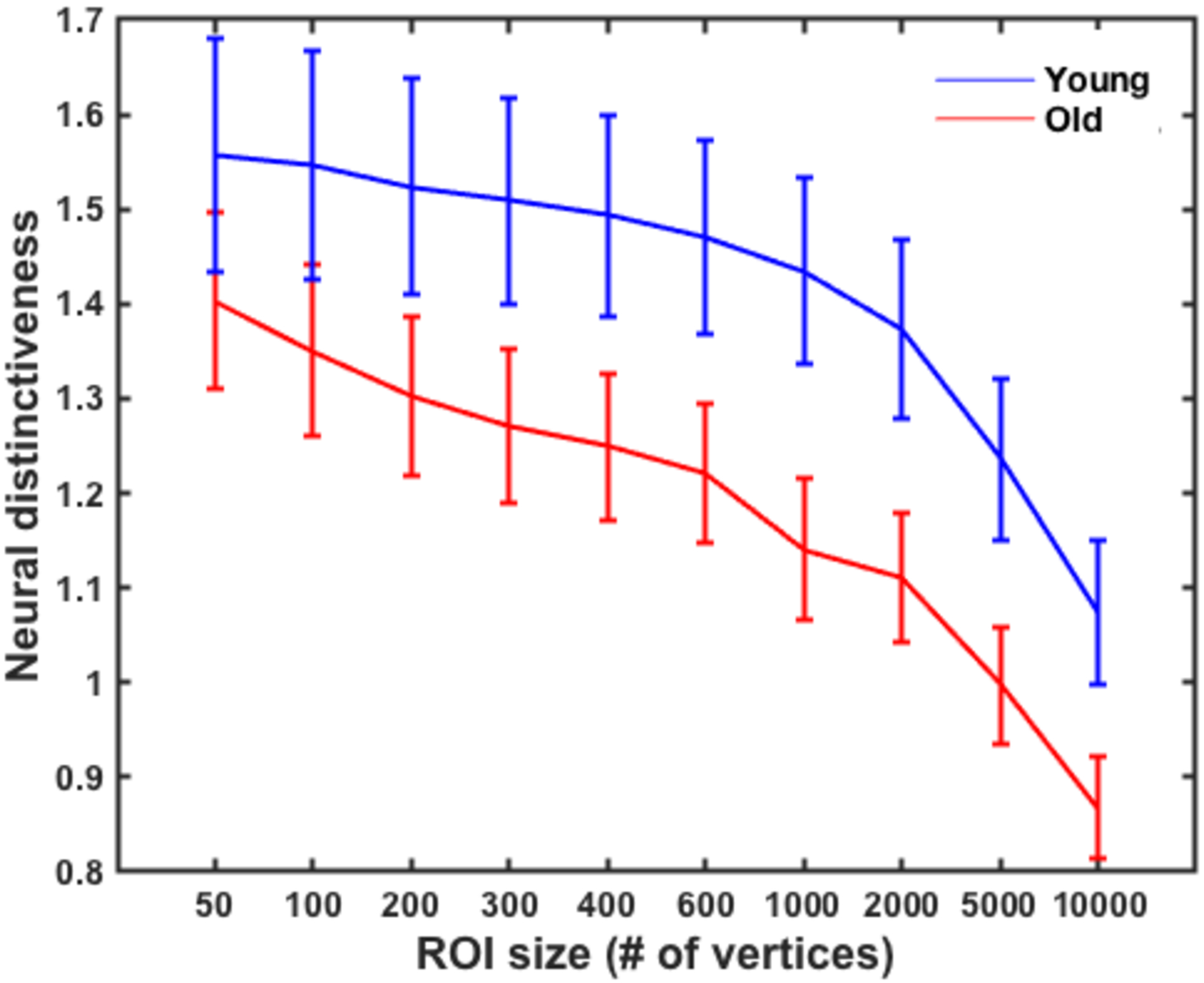
Neural distinctiveness as a function of ROI size in young (blue) and older (red) adults. Sensorimotor cortical activation patterns for right vs. left hand movement were more distinctive at smaller ROI sizes and less distinctiveness at larger ROI sizes across all participants. However, the effect of ROI size did not significantly influence the observed age differences in neural distinctiveness (F=.70, p=.48). Error bars denote the standard error of the mean.
In terms of univariate activation, we found that older adults activated a cluster including right precentral and superior frontal gyrus in addition to a smaller cluster including left precentral gyrus significantly more than young adults during right finger tapping (See Figure S1). We found no age differences in either left vs. rest or left vs. right finger tapping. For the significant clusters, we extracted the average beta value for each participant (YA group mean = −.34, SE = .06; OA group mean = .25, SE = .04) and included these as nuisance covariates in our subsequent statistical analyses (see below).
Relationship between network segregation and neural distinctiveness
We performed partial correlation analyses to assess the relationship between sensorimotor neural distinctiveness and sensorimotor network segregation. Controlling for age, GM volume, motion, and univariate activation, we observed a positive relationship between distinctiveness and segregation across all participants, r(57)=.36, p=.006 (see Figure 4A). Using the volume-based (rather than surface-based) distinctiveness measure in the partial correlation model (including the same nuisance covariates), we again observed a positive relationship between neural distinctiveness and network segregation, r(59)=.43, p=.001 (see Figure 4B).
Figure 4.
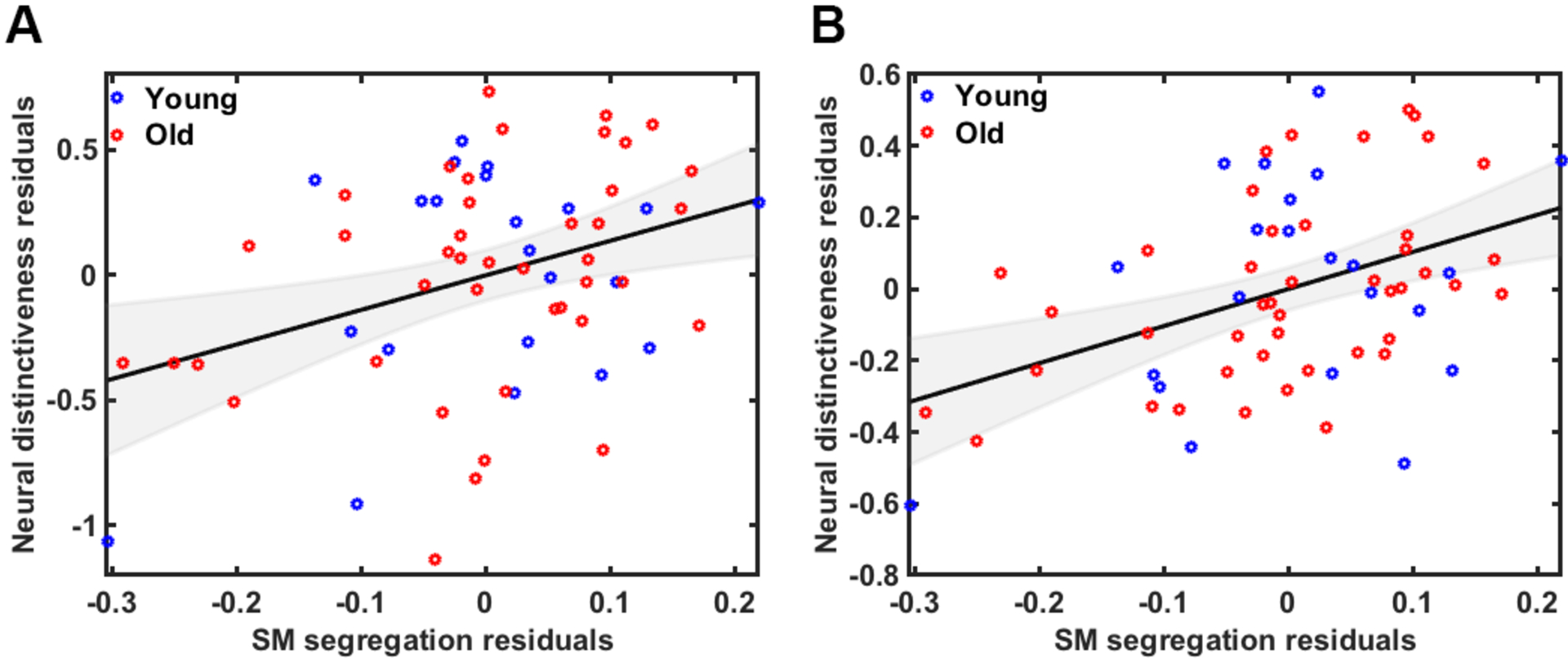
A) Relationship between sensorimotor network segregation and neural distinctiveness of sensorimotor representations using the functional ROI and B) using the Power sensorimotor network ROI across all participants after controlling for the effects of age, GM volume, motion and univariate activation.
To examine the specificity of the relationship between distinctiveness and segregation in the sensorimotor system, we calculated the partial correlation between sensorimotor neural distinctiveness and mean network segregation (averaged across all 10 networks). Controlling for age, GM volume, motion, and univariate activation, we observed a positive relationship, r(57)=.39, p=.003. We also examined the association between sensorimotor neural distinctiveness and network segregation outside of sensorimotor cortex (i.e., visual and auditory network segregation). We observed no significant relationships, either between sensorimotor neural distinctiveness and visual (r(59)=.13, p=.33) or auditory (r(59)=.17, p=.19) network segregation. Finally, we examined the association between sensorimotor network segregation and neural distinctiveness in ventral visual cortex (VVC) and auditory cortex (based on visual and auditory tasks, respectively). We did not observe a significant relationship between sensorimotor segregation and visual (r(61)=.25, p=.05) or auditory (r(60)=.14, p=.28) neural distinctiveness, although the association with visual distinctiveness approached significance.
Predicting behavior from models that include both network segregation and neural distinctiveness
We performed multiple regression analyses to predict sensorimotor performance based on age group, network segregation and neural distinctiveness (while controlling for GM volume, motion, and univariate activation). Please refer to Table S1 of supplemental material for correlation table that includes all factors used in model. We first created a model to predict gross sensorimotor performance. The fit of the overall model was significant (F(9,54)=3.7, p=.001) with an R2 of .38 and an adjusted R2 of .28. Segregation was a significant predictor (B=.36, t=2.4, p=.041) (Figure 5A) but distinctiveness was not (B=−.02, t=−.13, p=.90) (Figure 5B). There were no significant age group interactions, either between age group and segregation (B=−.48, t=−.7, p=.49) or between age group and distinctiveness (B=.49, t=−1.2, p=.22). We next created a model to predict fine sensorimotor performance from the same factors/covariates. Again, the fit of the overall model was significant (F(9,54)=4.57, p<.001) with an R2 of .43 and an adjusted R2 of .34. Segregation was a significant predictor (B=.36, t=2.6, p=.013) (Figure 5C) but distinctiveness was not (B=.13, t=.92, p=.36) (Figure 5D). There were no significant age group interactions, either between age group and segregation (B=−.5, t=−.75, p=.45) or between age group and distinctiveness (B=−.18, t=−.47, p=.64).
Figure 5.
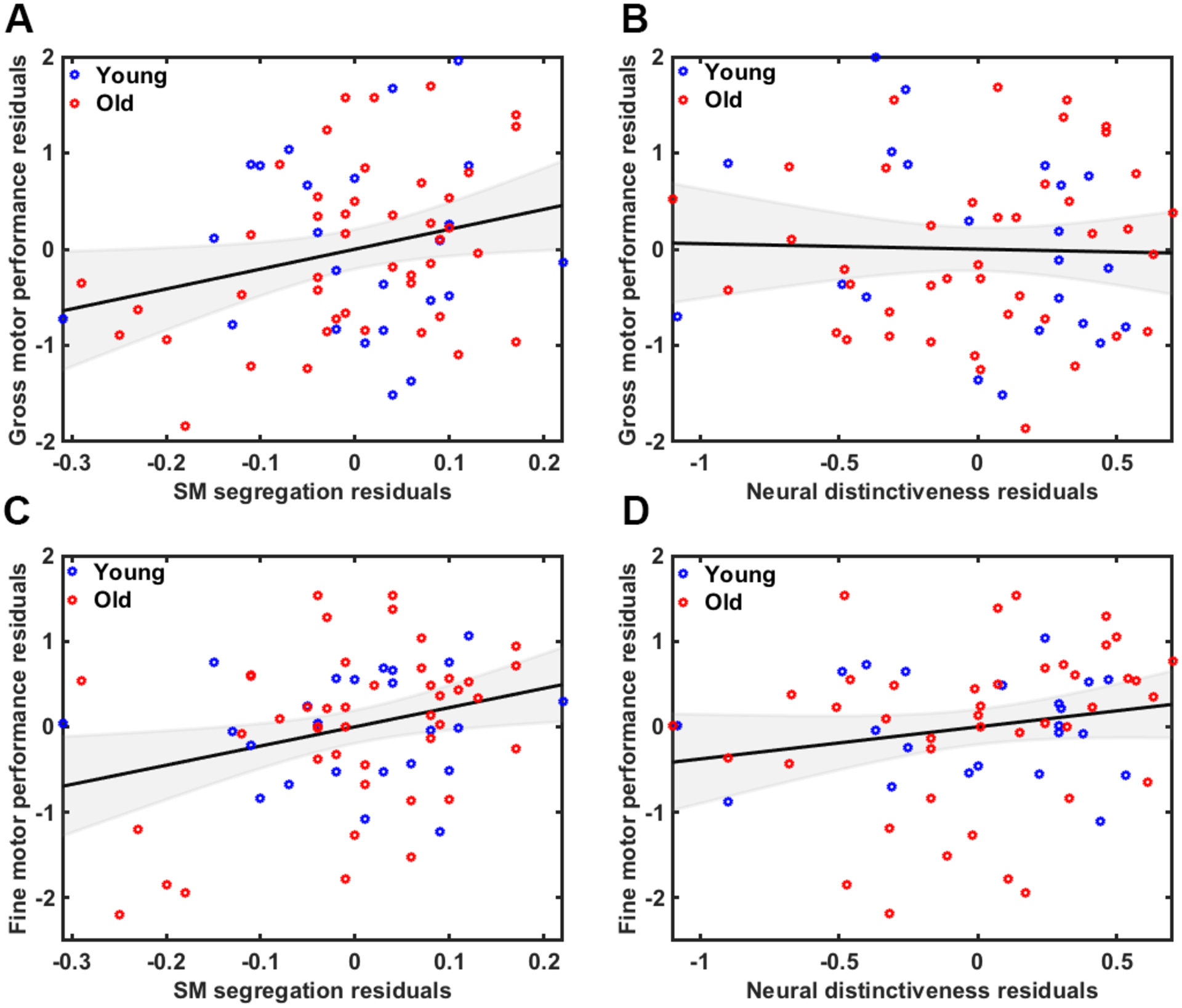
A) Relationship between gross motor performance and A) network segregation and B) neural distinctiveness. Relationship between fine motor performance and C) network segregation and D) neural distinctiveness. Plots are illustrated as partial correlations, controlling for the effects of age, GM volume, motion and univariate activation.
Discussion
Previous studies have found that measures of neural dedifferentiation are associated with worse behavior among older adults. However, neural dedifferentiation has been operationalized in two very different ways in the field: task-based fMRI studies have measured the distinctiveness of neural activation patterns evoked by different task conditions, while resting-state fMRI studies have measured the segregation or modularity of resting-state networks. Both measures decline with age and both have been associated with individual differences in behavior, but how (or whether) the two measures relate to each other is currently unknown. The present study examined the potential links between sensorimotor neural distinctiveness, sensorimotor network segregation, and sensorimotor behavior, all in the same participants. Consistent with previous findings, older adults exhibited reduced sensorimotor neural distinctiveness and reduced sensorimotor (resting state) network segregation relative to younger adults. A novel finding of the present study was that participants with the most distinct sensorimotor representations exhibited the most segregated sensorimotor networks. Further, sensorimotor network segregation was associated with individual differences in sensorimotor performance, particularly in older adults, whereas sensorimotor neural distinctiveness was not. These findings link, for the first time, sensorimotor network segregation to sensorimotor neural distinctiveness. They also suggest that sensorimotor network segregation may be a more sensitive predictor of age-related declines in sensorimotor behavior.
Age differences in network segregation and neural distinctiveness
Consistent with previous work (Cassady et al., 2019), we found that sensorimotor network segregation is significantly lower in older adults than in younger adults. Although we focused exclusively on the sensorimotor network in this study, previous studies have reported significant age differences in several sensorimotor and association (i.e., higher order cognitive) networks, suggesting that age-related reductions in network segregation occur at the whole-brain (i.e., multiple network) level.
We also demonstrated that older adults have significantly less distinctive neural representations in sensorimotor cortex than younger adults. This finding is consistent with previous studies that reported age differences in neural distinctiveness involving motor (Carp et al., 2011a), visual (Park et al., 2004, 2010; Carp et al., 2011b), auditory ((Lalwani et al., 2018)), and memory representations (Carp et al., 2010; Koen et al., 2018). Together, these findings suggest that age differences in neural distinctiveness are not limited to sensorimotor cortex, but rather are a general feature of the aging brain.
Network segregation is related to neural distinctiveness
Our study is the first to show that resting state sensorimotor network segregation varies with the distinctiveness of task-based activation patterns in sensorimotor cortex. This finding is consistent with previous data suggesting a relationship between the brain’s large-scale functional organization at rest and its functional recruitment during task performance (Langan et al., 2010; Chan et al., 2017)
In the context of aging, age differences in resting state brain organization may provide a network-based explanation for the commonly observed finding of age-related neural dedifferentiation of task-evoked activity. Or perhaps other neural factors influence the differentiation of both resting state networks and task-evoked activity. For instance, previous work by our group indicates that lower levels of the inhibitory neurotransmitter, gamma aminobutyric acid (GABA), are associated with reduced network segregation (Cassady et al., 2019) and reduced neural distinctiveness (Lalwani et al., 2019). Reduced white matter integrity (Burzynska et al., 2010) and amyloid deposition (Buckner et al., 2009; Mormino et al., 2011) also disrupt the organization of functional networks and brain activity. Future work could employ multimodal imaging to investigate potential interactions between brain structure, function, and chemistry.
We also observed a significant association between sensorimotor neural distinctiveness and mean network segregation (averaged across all 10 networks). It is therefore logical to question whether the relationship between neural distinctiveness and network segregation is regionally specific or not. Our results suggest that the relationship is regionally-specific, at least to some degree. For example, sensorimotor segregation was significantly correlated with sensorimotor distinctiveness, but not with neural distinctiveness outside of sensorimotor cortex (i.e., auditory and visual cortex, although the relationship with visual distinctiveness approached significance). Similarly, sensorimotor neural distinctiveness was not correlated with network segregation outside of sensorimotor cortex (i.e., auditory and visual cortex). Furthermore, given that sensorimotor segregation is highly correlated with mean segregation (r=.62, p<.001), it is not surprising that sensorimotor distinctiveness is associated with both sensorimotor and mean network segregation.
Network segregation (but not neural distinctiveness) is associated with sensorimotor performance
The present results demonstrate that sensorimotor network segregation (but not sensorimotor neural distinctiveness) is associated with sensorimotor performance, and that this relationship is particularly strong in older adults. Specifically, older individuals with less segregated networks exhibited worse sensorimotor performance (both gross and fine) than older adults with more segregated networks. Importantly, this relationship remained significant even after controlling for neural distinctiveness. In contrast, although there was a trend toward a positive relationship between neural distinctiveness and fine motor performance across all participants, this relationship was not observed within either age group separately. These findings suggest that network segregation may be a more sensitive predictor of age-related declines in sensorimotor performance than neural distinctiveness.
A natural question is why would segregation be a better predictor of sensorimotor behavior relative to distinctiveness? Although this study did not attempt to address that question specifically, we can rule out a few potential confounds. One potential confound is differences in data reliability; perhaps neural distinctiveness is a noisier measure than network segregation. To investigate that possibility, we analyzed resting state and task-based fMRI data from 47 participants who performed the fMRI tasks described here in two separate sessions, once on placebo and once after taking a benzodiazepine (Lorazepam) (see Gagnon et al., 2019, for details). And despite the pharmacological manipulation, the test-retest reliability of the neural distinctiveness measure was quite strong (intraclass correlation of 0.72) and actually higher than the reliability of the segregation measure (0.51). Another possibility is that the neural distinctiveness measure takes on a more restricted range of values and is therefore more difficult to relate to other measures (a restriction of range problem). However, variability in distinctiveness across participants was actually much higher than variability in segregation (across the entire sample and in both age groups separately). Thus, the finding that segregation is significantly related to behavior while distinctiveness is not cannot be attributed to differences in either reliability or in variability.
We also performed follow-up regional-mean activation analyses to rule out overall activation effects. In particular, if average activation is lower in the older group, then that could potentially reduce within-condition similarity because the signal is harder to distinguish from the noise. However, we found that for both the left vs. fixation and the right vs. fixation contrast, the older adult group actually exhibited slightly more activation than the young. If stronger responses lead to greater within-condition similarity and therefore greater distinctiveness, then distinctiveness should have increased with age in our sample. However, we observed the opposite. Thus, we do not think the observed results were driven by overall activation effects.
We hypothesize that one reason segregation may be a better predictor of sensorimotor performance than neural distinctiveness is that the segregation measure reflects the interaction of multiple large-scale distributed brain networks. In contrast, neural distinctiveness is based on task-based activation patterns in localized regions of interest. Given that most sensorimotor functions require the interaction of multiple brain areas, it is likely that regions outside of these localized ROIs are contributing to the associated declines in behavior. This may be one reason why segregation is a better predictor of sensorimotor performance compared to neural distinctiveness.
Another possibility is that resting state measures of fMRI provide a more stable, “trait-like” measure of brain function compared to task-based measures, which are less stable and more “state-like”. Because of the relative stability of resting state functional connectivity, many studies use it as a trait measure. In contrast, functional activity inherently provides a transient, state-dependent measure of brain characteristics. For instance, Cole and colleagues demonstrated that functional connectivity between brain regions at rest is more informative for predicting individual differences in fluid intelligence compared to task-evoked activity of functional regions in isolation (Cole et al., 2012). However, it could be the case that the task used to calculate neural distinctiveness in the present study was too specific or irrelevant to the sensorimotor (outside of the scanner) behavioral factors. It would be interesting for future studies to explore this question using similar in-scanner and out-of-scanner tasks, both to calculate neural distinctiveness and sensorimotor behavior. Overall, our findings suggest that trait-based measures such as resting state functional connectivity may be more sensitive in predicting individual differences in behavior compared to task-based state measures such as neural distinctiveness.
Limitations
One obvious limitation of this study is that our sample was cross sectional. We can therefore we can only make inferences about age differences rather than longitudinal changes that occur with age. Future longitudinal designs could examine age-related changes in neural and behavioral measures as well as the relationship between them over time.
Furthermore, the present study employed a simple unimanual thumb tapping task. We were therefore unable to examine the effects of aging on the neural representations of more realistic, complex movements or of different individual movements. Relatedly, previous research has found that, compared to younger adults, older adults are more likely to have “mirror movements”, i.e., unintended movements that occur in homologous muscles contralateral to the voluntarily active ones (Koerte et al., 2010). Thus, it could be that older participants have less differentiated right/left motor brain responses because their physical movements are less differentiated. However, the causality might actually go in the other direction: less differentiated left/right brain responses lead to less differentiated physical movements rather than the other way around. Regardless, we do not have a way to evaluate mirror movements in the current study. Future studies that use more complex sensorimotor tasks and that collect electromyography data to measure all bilateral movements rather than only recording button presses could add additional insight into the mechanisms of age-related neural dedifferentiation of sensorimotor representations.
Conclusions
The present study examined the relationship between sensorimotor network segregation, neural distinctiveness of sensorimotor representations, and sensorimotor behavior in young and older adults. Consistent with previous studies, we found that sensorimotor networks are less segregated and sensorimotor representations are less distinct in older relative to young adults. We also discovered that less segregated networks are associated with less distinct representations. Finally, we found that less segregated networks predict worse sensorimotor performance, particularly within older adults, whereas neural distinctiveness is not associated with performance. These findings link network segregation to neural distinctiveness and suggest that segregation is a more sensitive predictor of age-related declines in sensorimotor behavior.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health to TAP (R01AG050523) and KC (F31AG057205).
Footnotes
Factor 1 (including dominant and non-dominant dexterity) refers to fine motor performance whereas Factor 2 (including dominant and non-dominant grip strength and endurance) refers to gross motor performance.
Although mathematically, negative values are treated as smaller than zero, negative correlations may actually reflect the presence of inhibitory connections. Using absolute values is another alternative, but then it is impossible to distinguish node pairs whose time series look similar (and that are likely part of the same network) from node pairs whose time series look dissimilar (and that are likely part of different networks). For these reasons and others, existing graph theoretical measures were mostly developed for networks with only positive connections and many graph theory metrics largely ignore fMRI networks’ negative edges (Zhan et al., 2017).
References
- Behzadi Y, Restom K, Liau J, Liu TT (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD (2012) Evidence for motor cortex dedifferentiation in older adults. Neurobiol Aging 33:1890–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar Mri. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Brenkel M, Shulman K, Hazan E, Herrmann N, Owen AM (2017) Assessing Capacity in the Elderly: Comparing the MoCA with a Novel Computerized Battery of Executive Function. Dement Geriatr Cogn Disord Extra 7:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen F, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA (2009) Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. J Neurosci Off J Soc Neurosci 29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li S-C, Lindenberger U, Heekeren HR (2010) Age-related differences in white matter microstructure: region-specific patterns of diffusivity. NeuroImage 49:2104–2112. [DOI] [PubMed] [Google Scholar]
- Carp J, Park J, Hebrank A, Park DC, Polk TA (2011a) Age-Related Neural Dedifferentiation in the Motor System. Plos One 6 Available at: ://WOS:000299684700053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Park J, Polk TA, Park DC (2011b) Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. NeuroImage 56:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady K, Gagnon H, Lalwani P, Simmonite M, Foerster B, Park D, Peltier SJ, Petrou M, Taylor SF, Weissman DH, Seidler RD, Polk TA (2019) Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. NeuroImage 186:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain JD, Gagnon H, Lalwani P, Cassady KE, Simmonite M, Foerster BR, Petrou M, Seidler RD, Taylor SF, Weissman DH, Park DC, Polk TA (2019) GABA levels in ventral visual cortex decline with age and are associated with neural distinctiveness. bioRxiv:743674. [DOI] [PMC free article] [PubMed]
- Chan MY, Alhazmi FH, Park DC, Savalia NK, Wig GS (2017) Resting-State Network Topology Differentiates Task Signals across the Adult Life Span. J Neurosci 37:2734–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS (2014) Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U A 111:E4997–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole KJ, Rotella DL, Harper JG (1999) Mechanisms for age-related changes of fingertip forces during precision gripping and lifting in adults. J Neurosci 19:3238–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Yarkoni T, Repovš G, Anticevic A, Braver TS (2012) Global Connectivity of Prefrontal Cortex Predicts Cognitive Control and Intelligence. J Neurosci 32:8988–8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999) Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS (2017) Effects of aging on functional and structural brain connectivity. Neuroimage Available at: http://www.ncbi.nlm.nih.gov/pubmed/28159687. [DOI] [PubMed]
- Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Goh JOS (2011) Functional Dedifferentiation and Altered Connectivity in Older Adults: Neural Accounts of Cognitive Aging. Aging Dis 2:30–48. [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P (2001) Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293:2425–2430. [DOI] [PubMed] [Google Scholar]
- King BR, van Ruitenbeek P, Leunissen I, Cuypers K, Heise K-F, Santos Monteiro T, Hermans L, Levin O, Albouy G, Mantini D, Swinnen SP (n.d.) Age-Related Declines in Motor Performance are Associated With Decreased Segregation of Large-Scale Resting State Brain Networks. Cereb Cortex:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte I, Eftimov L, Laubender RP, Esslinger O, Schroeder AS, Ertl‐Wagner B, Wahllaender‐Danek U, Heinen F, Danek A (2010) Mirror movements in healthy humans across the lifespan: effects of development and ageing. Dev Med Child Neurol 52:1106–1112. [DOI] [PubMed] [Google Scholar]
- Lalwani P, Gagnon H, Cassady K, Simmonite M, Peltier S, Seidler RD, Taylor SF, Weissman DH, Polk TA (2018) Neural distinctiveness declines with age in auditory cortex and is associated with auditory GABA levels. bioRxiv:470781. [DOI] [PMC free article] [PubMed]
- Langan J, Peltier SJ, Bo J, Fling BW, Welsh RC, Seidler RD (2010) Functional implications of age differences in motor system connectivity. Front Syst Neurosci 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Lindenberger U (1999) Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. Cogn Neurosci Mem:103–146. [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, H. Onami S, Greicius MD, Rabinovici GD, Janabi M, Baker SL, Yen I V, Madison CM, Miller BL, Jagust WJ (2011) Relationships between Beta-Amyloid and Functional Connectivity in Different Components of the Default Mode Network in Aging. Cereb Cortex 21:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR (2004) Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci U S A 101:13091–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Hebrank A, Park DC, Polk TA (2010) Neural Specificity Predicts Fluid Processing Ability in Older Adults. J Neurosci 30:9253–9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE (2011) Functional network organization of the human brain. Neuron 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010) Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect 2:125–141. [DOI] [PubMed] [Google Scholar]
- Zhan L, Jenkins LM, Wolfson OE, GadElkarim JJ, Nocito K, Thompson PM, Ajilore OA, Chung MK, Leow AD (2017) The significance of negative correlations in brain connectivity. J Comp Neurol 525:3251–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


