Abstract
Background
A linear increase in the number of valvular heart disease is expected due to the aging population, yet most patients with severe valvular heart disease remain undiagnosed.
Hypothesis
POCUS can serve as a screening tool for valvular heart disease.
Methods
We reviewed the literature to assess the strengths and limitations of POCUS in screening and diagnosing valvular heart disease.
Results
POCUS is an accurate, affordable, accessible, and comprehensive tool. It has a fast learning curve and can prevent unnecessary and more expensive imaging. Challenges include training availability, lack of simplified screening protocols, and reimbursement. Large scale valvular screening data utilizing POCUS is not available.
Conclusion
POCUS can serve as a screening tool and guide the management of patients with valvular heart disease. More data is needed about its efficacy and cost‐effectiveness in the screening of patients with valvular heart disease.
Keywords: POCUS, point of care ultrasound, screening, valvular heart disease
1. INTRODUCTION
As the population continues to age, the adverse effects associated with valvular heart disease are increasing as well. 1 With the introduction of the transcatheter aortic valve replacement (TAVR), the number of patients who will be eligible for TAVR is estimated to increase 4‐fold over the next 5 years. 2 Similarly, the number of patients who may benefit from transcatheter mitral valvular interventions such as Mitraclip is expected to increase. 3 Unfortunately, many patients with severe valvular heart disease remain undiagnosed. 4 , 5 One possible limiting factor is relying only on physical exam as an initial screening test for detecting valvular abnormalities. One study that surveyed a group of primary care physicians and cardiologist showed that the confidence of the physicians to detect mitral regurgitation with a stethoscope was less than 50%. 5 , 6 Thus, to meet such demand, a reliable and accessible technology is essential to enhance the physical exam quality and sensitivity in screening for valvular heart disease. The prevalence of moderate or severe valvular heart disease in a large‐scale community screening program of patients over 65 years in the United Kingdom exceeded 11%, with a projected doubling before 2050. 4 An early detection of valvular heart disease, through echocardiograms, can have a lasting impact on morbidity, mortality, and cost of care. 7
With the advancement in technology, portable ultrasound machines became readily available. These include both stationary high‐end ultrasound system, and small handheld ultrasound devices (HUD). Point of care ultrasound (POCUS) refers to a goal‐oriented, limited ultrasound examination of a particular body structure with a predefined limited protocol. 8 Performing POCUS using HUD is an accurate, affordable, accessible tool that can aid physical exam and streamline unnecessary clinical testing. 6 , 9 In addition, visual representation allows physicians to glimpse inside the patient to better examine and diagnose a condition. 6 , 9 POCUS has a high correlation with standard echocardiogram in evaluating left ventricular function and valvular abnormalities, making it a potential useful tool for screening for valvular abnormalities. We aim in this article to review the role of POCUS in cardiac evaluation and its potential rule in screening for valvular heart disease.
2. CURRENT SCREENING METHODS FOR VALVULAR HEART DISEASE ‐ A ROOM FOR IMPROVEMENT
The current screening practice of valvular heart disease is mainly dependent on cardiac auscultation. Only those with abnormal findings, are referred for a standard echocardiogram. There are no clear recommendations about who and how to screen by neither the American college of cardiology, nor American heart association. 7 The stethoscope, a 200 year old device discovered by Dr Laennec, remains the solo screening tool for valvular abnormalities. 10 , 11 Yet the amount of information that can be gained through auscultation is not comparable to that of the ultrasound. 12 Expert cardiologists still experience the limitation of auscultation when it comes to confidently diagnosing valvular abnormalities. 11 , 13 , 14 , 15 POCUS has been shown to perform better than traditional auscultation methods in evaluating valvular heart disease, even when carried‐out by non‐cardiologists. 13 , 16 , 17 , 18 , 19 Initial diagnosis gathered through traditional methods like auscultation, can be easily verified using POCUS, making ultrasound imaging a better option. 20 Therefore, performing POCUS examination improves the accuracy of diagnosis of valvular heart disease from 50% to 80% in as little as 15 minutes after a patient exam has started. 21 After the introduction of HUD, physicians have been able to increase the range of acute and chronic conditions that can be diagnosed using POCUS. 22 One small study has compared the performance of board certified cardiologists utilizing standard physical exams, with medical students trained for 18 hours to perform POCUS using HUD. The use of POCUS outperformed the experienced cardiologists in detecting abnormal cardiac pathologies (75% accuracy), and valvular pathology (93% vs 49%). 23 Another study has compared the results of physical examination performed by board certified cardiologist with the results of POCUS in a sample of 36 patients with cardiovascular disease. Cardiac examination alone failed to detect 59% of the overall cardiovascular findings and missed about 43% of the major findings. POCUS reduced this to 21% without significant inter‐physician variation. 13 Thus, POCUS can reduce the time it takes to reach a conclusion and the price of the device can reduce the cost of echocardiography as well. 6 , 24 , 25 , 26 , 27
3. HUD VS STANDARD ECHOCARDIOGRAPHY
Though portable echocardiogram devices have the potential to enhance auscultation, they are by no means a substitute for standard echocardiography. Therefore, its optimal role in healthcare has yet to be officially defined. Currently, standard echocardiography machines tend to be too big and expensive for primary care medical clinics, potentially hindering immediate ultrasound access. 9 , 13 Performing POCUS using HUD has been shown by one study to decrease the number of rarely appropriate standard echocardiography by 59%. 6 , 9 The study by Vourvouri et al, has shown that screening with point of care ultrasound avoids the use of standard echocardiography in approximately 80% of unselected patients and leads to a 33% cost reduction. 28
HUDs are equipped with color Doppler, which can provide a qualitative evaluation of valvular heart disease. However, the lack of spectral Doppler limits their ability for quantitative assessment. 6 , 24 , 25 , 26 , 29 , 30 HUDs have good sensitivity and specificity in evaluating regional wall motion abnormality and left ventricular global function. However, they have only modest accuracy in evaluating valvular heart abnormalities such as severe aortic stenosis, and mitral/tricuspid valve regurgitation. 6 , 8 , 24 , 25 , 26 , 31 , 32 POCUS using HUD has been shown to have a very good sensitivity and specificity for diagnosis of rheumatic heart disease. 31 , 33 Several devices are now equipped with online internet connection and Cloud image storage. Newer devices are also equipped with artificial intelligence which can guide the provider how to improve image acquisition. 34
4. HUD AND POCUS IN CLINICAL PRACTICE
With the expansion of availability of HUD and portable ultrasound stations, there have been several developments on how they can be utilized. 35 POCUS was first utilized in trauma patients in the 1970s in Europe, and later on was adopted in the US in the 1990s, mainly in the emergency department where the health care provider can rapidly identify life threatening conditions, such as pericardial effusion, pneumothorax, and bowel injury. 33 , 36 , 37 Several protocols were adopted by different centers and utilized by providers with variable degree of training. 20 , 26 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 Currently, POCUS is mainly used in the emergency department and intensive care units. Its role in non‐emergent settings is still emerging. Among non‐emergent conditions, POCUS can improve physical exam, 46 guide diuresis, 47 predict risk of rehospitalization, 48 and safely discharge cardiac patients from the clinic. 36
5. POCUS TRAINING AND CHALLENGES
Although the HUD has been available since the 1970s, there is still hesitancy by many physicians about incorporating POCUS in their clinical practice, mainly due to a lack of formal rules or guidance on when and how a proper exam should be conducted. 21 , 26 , 35 , 49 , 50 Given its importance, increasing availability and high accuracy, POCUS could became an essential part of the physical exam and can provide comparable information to auscultation and palpation. 26 , 50 Limitations in training availability has slowed its implementation. Though progress has been slow, there has been an increase in POCUS training in undergraduate and graduate level and continuing in professional programs. 51 , 52 Recently, Harvard medical school incorporated POCUS training in first‐ and second‐year medical students' curriculum as part of the physical exam training. 53 , 54 Similarly, residency programs have included POCUS training in their curriculum. 54 , 55 Though some guidelines currently exist, there is a need for new and updated data on the current methods of practicing. 5
The American Society of Echocardiography has predicted an exponentially increasing number of POCUS users and has emphasized the importance of high quality training, interpretation, and proper usage. 29 Several efforts are being made to encourage physicians to use ultrasound in their practice with hopes of reducing the healthcare cost by not requesting more expensive imaging. 22 It became crucial to better understand the possibilities and necessities for POCUS, and to have guidelines and protocols for its use. For example, the focused assessment with sonography in trauma (FAST) protocol has been widely adopted due to its ease and accuracy, and importantly speeding up and advancing patient care. 39 , 42 , 56 , 57 Currently, the only field that has a dedicated and clear echocardiography training is cardiology. Cardiology fellows need to perform and read certain numbers of echocardiograms, as assigned by the American college of cardiology, to achieve different levels of competencies. 58 The American Society of echocardiography and American college of emergency physician have issued consensus statement about the use of focused cardiac ultrasound in emergency settings. 57 The American college of chest physician (ACCP) as well as several critical care societies have issued several requirements on competencies in critical care ultrasonography and echocardiography. 59 , 60 , 61 , 62 Unfortunately, POCUS training is still not a requirement nor formally incorporated during internal medicine training. 63 A 2014 survey of family medicine program directors found that only 2% of residency programs had a formal POCUS curriculum. 64 There is still no consensus on the training requirements to achieve adequate competency level. However, it's generally agreed that training must include basic ultrasound physics knowledge, supervised image acquisition and interpretation. 46 Some studies showed residents could perform accurate echocardiograms after a training for several hours to only a few days, 54 , 65 , 66 or as little as 25 scanning exams. 60
6. POCUS IN PRIMARY CARE SETTING
In the outpatient setting, the utilization of HUD is also gradually increasing. 64 This technological innovation has brought the clinician closer to patients when it comes to diagnosis, increasing the relationship and overall patient satisfaction. 67 , 68 POCUS allows patients and physicians to view images together where changes can be easily seen and tracked, and images can serve as a guide to explain more physiological concepts. 69 (Figure 1) This can also improve the quality of care and patient safety. 69 With the advancement of transcatheter treatment, screening for valvular heart disease in the outpatient setting might help in early detection and treatment, which may prevent downstream complications of valvular heart disease. 4 , 7 , 68 , 70 In a recent randomized clinical trial that assessed patient with known structural heart disease, the addition of POCUS to clinic evaluation resulted in earlier referral for valvular intervention, and decreased the risk of hospitalization and mortality. 27 Given its ease and low cost, POCUS using HUD will be the tool of choice to screen high risk patients. 4 Patients with any valvular abnormalities, can be confirmed by standard echocardiography. Those with valvular abnormalities, can also be followed using POCUS without the need for a more expensive echocardiogram. 27 There is an increasing evidence, mainly from low to middle income countries, that HUD can be used to screen for structural heart disease and can improve patients outcomes. 27 , 31 , 33 , 71 , 72 , 73 , 74 , 75
FIGURE 1.
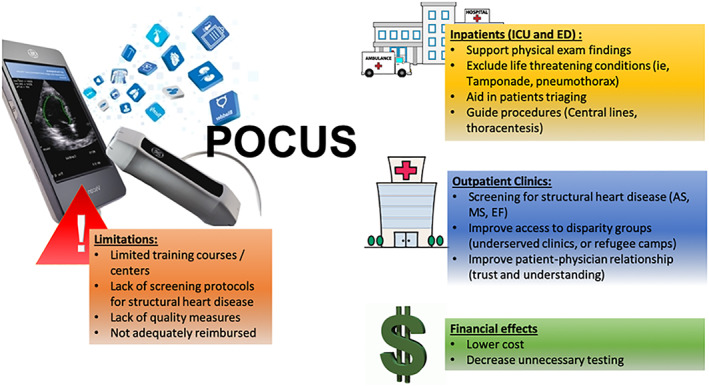
Clinical application and limitations of point of care ultrasound
In comparison to the in hospital setting where POCUS can be easily performed in the emergency department and critical care units, POCUS availability and utilization in the primary care setting is still limited and faces multiple challenges including device availability, lack of specific screening protocols, training programs and reimbursement. 64 , 66 , 67 With the rapidly growing technology, the cost of HUD is expected to drop significantly, and when compared to the formal echocardiography, they are at least 10 times cheaper. 66 A simplified protocol for screening for valvular heart disease and cardiac abnormalities is needed before POCUS can be used as a practical tool to screen for valvular heart disease. Although it has not been widely adopted, a brief focused POCUS training focused on valvular heart disease and cardiac function is promising. 55 One study has implemented a POCUS training program consist of a 50‐question test, 4‐lectures on basic echocardiography & imaging interpretation, a supervised interpretation of 50 echocardiograms, and performance of 30 exams using HUD. 55 They have trained 12 residents, and compared their performance in 30 cases to experienced cardiologist. The performance of the trained residents was comparable to experienced cardiologist in detecting normal findings (95% correct interpretation), and to a lesser degree of abnormal findings (75% correct interpretation). The performance of the residents was also very good in detecting valve abnormalities (85% correct interpretation). 55 It remains that, the biggest limitation to this new technology is the fact that there are not enough trainers of POCUS. 55 Learning programs, like the GE Digital Expert that provides virtual and flexible face to face training could also be tailored to teach physicians POCUS and help face some of these challenges mentioned in this review. 59
7. USING POCUS IN SCREENING FOR VALVULAR HEART DISEASE
The data about using POCUS in screening for valvular heart disease is scarce. Most of the published studies have enrolled small samples of subjects with and without valvular heart disease and focused on sensitivity and accuracy of detecting valvular heart abnormalities. The use POCUS on a large scale to screen individuals without known valvular heart disease yet faces multiple challenges. The prevalence of valvular heart disease increases with age, and this will have implications on the sensitivity and specificity of any screening tests. The prevalence of rheumatic heart disease is higher in younger age and estimated at 12.9 per 1000 people in developing countries, 74 while its exceedingly rare in the United States (~0.04 cases per 1000 children). 74 , 75 In a landmark U.S. study by Nkomo et al, that included 11 911 subjects who prospectively underwent echocardiographic examination in three large national population‐based epidemiological studies: the CARDIA (Coronary Artery Risk Development in Young Adults), 76 ARIC (Atherosclerosis Risk In Communities), 77 and CHS (Cardiovascular Health Study), 78 the age‐adjusted prevalence of moderate or severe valvular heart disease was 2.5%, and was significantly influenced by age: <2.0% prevalence in those <65 years of age and 13.2% in those ≥75 years of age. Increasing age (per 10 years) was significantly associated with mitral regurgitation (odds ratio of 1.84; 95% CI: 1.70 to 1.99; P < .0001), mitral stenosis (odds ratio of 1.65; 95% CI: 1.12 to 2.43; P = .01), aortic regurgitation (odds ratio of 1.49; 95% CI: 1.30 to 1.70; P < .0001), and aortic valve stenosis (odds ratio of 2.51; 95% CI: 2.02 to 3.12; P < .0001). In subjects ≥75 years of age, the most frequent valvular heart disease was mitral regurgitation (9.3%), followed by aortic stenosis (2.8%), aortic regurgitation (2.0%) and mitral stenosis (0.2%). 79 The prevalence of moderate to severe aortic stenosis was also reported to range between 2.9% ‐ 4% in other smaller studies. 80 , 81 In a systematic review and meta‐analysis including 9723 patients >75 years of age reported that the prevalence of AS was 12.4%, while severe AS was 3.4%. 82 Another study has reported the prevalence of bicuspid aortic valve to be 22% among octogenarians patients undergoing aortic valve surgery. 83 In the Framingham heart study that screened 1696 men and 1893 women (aged 54 +/− 10 years) for valvular regurgitation during routine examination, the prevalence of at least mild mitral regurgitation and tricuspid regurgitation was 19.0% and 14.8% in men, and 19.1% and 18.4% in women, respectively. 84 In the OxVALVE population cohort study that screened individuals aged≥65 years from a primary care population without known valvular heart disease, the prevalence of any valvular heart disease was 51% of participants. 4 The most common abnormalities were aortic sclerosis (34%), mitral regurgitation (22%), and aortic regurgitation (15%). The prevalence increased linearly with age, from 42.4% (379/894) in those aged 65 to 69 years to 76.3% (103/135) in those aged 85 to 95 years. The proportion with moderate or severe valvular heart disease was 3.3% (54/1621) among those aged 65 to 74 years, rising to 11.9% (105/879) in those aged ≥75 years. Based on these results, the yield of screening of valvular heart disease might be more effective in the elderly, particularly those older than 75 years.
The other challenge in screening for valvular heart disease is how asymptomatic patients should be treated. The current American college of cardiology / American heart association guidelines largely recommend treatment only in those with severe valvular heart disease who are symptomatic, or with evidence of left ventricular or right ventricular dysfunction. 7 , 68 , 70 Those without are usually recommended to get periodic echocardiograms. It's possible that early diagnosis of valvular dysfunction may lead to additional imaging and additional cost. Ongoing trials such as early TAVR in asymptomatic aortic stenosis patients might help in addressing this question. Indeed, prospective data about large scale screening and its effect on outcomes and health care cost is needed.
The other potential group that may benefit from screening using POCUS are the young athletes before participating in strenuous competitive exercises. Sudden cardiac death remains a devastating event among athletes, and the European society of cardiology recommends history, physical exam, and electrocardiogram screening for these individuals. 85 Several studies have utilized POCUS in screening athletes for different causes of sudden cardiac death, such as hypertrophic cardiomyopathy, and anomalous coronary artery. Using a simplified protocol to assess septal wall thickness, left ventricular function, and aortic root dilation, POCUS has been shown to be an easy and effective method for screening for hypertrophic cardiomyopathy. 86 A large metanalysis that included five studies, representing 2646 athletes, demonstrated that electrocardiogram‐inclusive preparticipation screening strategies for potential causes of sudden cardiac death, resulted in positive results in 19.9% of the cohort. With the addition of POCUS, positive results were reduced to 4.9%, and 1 additional condition potentially associated with sudden cardiac death was identified. 87 The cost of POCUS was reported to range between $20 to $28 per athlete / student screened. 86 , 88 If the cost to perform POCUS is modest, and it results in a reduction in false‐positive results and subsequent secondary investigations and cardiologic consultations, then POCUS may represent a cost‐saving screening modality. However, there are insufficient data to draw conclusions regarding cost‐effectiveness from these studies. 87
In developing countries, POCUS has been utilized by several large‐scale studies to screen for rheumatic heart disease. 27 , 31 , 33 , 71 , 72 , 73 , 89 , 90 The world health organization has developed a standard echocardiography protocol for screening, and the widely available handheld devices allowed for active screening and identification of undiagnosed and subclinical carditis in endemic areas. Most of the studies have screened school students. 31 , 91 In comparison to a standard echocardiogram, POCUS has been shown to be equally effective in the diagnosis of definite rheumatic heart disease with comparable sensitivity and specificity. 31 More recent studies focused on training non‐expert healthcare workers (eg, nurses, medical students, clinical officers), 72 , 92 in using POCUS to screen for rheumatic heart disease have shown a promise. This might be a viable strategy to implement a screening program in areas where there is a deficiency of highly trained sonographers or cardiologists. 93
8. POCUS CHALLENGES AND LIMITATIONS
With the decreased resources available and increased need for healthcare, new medical innovations are always being evaluated for cost, effectiveness, and reliability. 50 Individual reimbursement still remains an important barrier for its more ubiquitous use. 29 This is especially true in clinics where physicians get compensated through the relative value unit (RVU). 29 In a RVU‐based compensation system, POCUS exam may cause financial loss to the practice, due to increased time of the clinic visit, not appropriate compensation, and less income from ordering additional imaging tests. 6 POCUS was shown to be very successful in reimbursement systems based on time (not RVUs), where the total cost of care matters more than individual income. 66 To improve efficiency, some groups have added a “POCUS clinic” to their primary care clinic, where patients felt to benefit from imaging can get scanned without interrupting the clinic workflow. 66 A future shift in reimbursement based on quality of care rather than quantity, may help address this issue. A proper documentation of the indication and findings, as well as image storage are essential for reimbursement. Additional system barriers include availability of training programs, unclear credentialing requirements, efficiency, electronic storage for image archiving, and policies and procedures for quality assurance. 46
It's important to note that most of the published studies about POCUS utilization are designed to show feasibility and accuracy of POCUS with little or no evidence how it can impact patients' outcomes and healthcare costs. It's possible that POCUS may lead to a higher number of unnecessary testing due to false positive findings. Further studies are needed. 8
9. A PROPOSED TRAINING PROGRAM
As stated earlier in the manuscript, there is no consensus about training protocols and the minimum amount of training needed for cardiac POCUS. It's generally agreed that training must include basic ultrasound physics knowledge, supervised image acquisition, and interpretation. 46 , 94 Several protocols were proposed by small studies to train non‐cardiologist or non‐sonographers. The VISION‐in‐Tele‐Echo protocol 95 was validated in two separate cohorts and could serve as a training protocol for those interested in developing a screening program for structural heart disease. 27 , 95 The program was first developed to train 17 non‐cardiologist physicians to performed cardiac POCUS. The protocol consists of 6 hours of focused training in echocardiography in a tertiary care center. The training was performed in‐site for nine physicians, and remotely in the remaining ones. The training was performed by expert sonographers who were American society of echocardiography members. The training began with a 1‐hour lecture that introduced the fundamentals of basic echocardiographic examination and oriented the participants to the specifically designed scanning protocol. The scanning protocol consisted of 11 standard views, including color‐flow Doppler images of all valves. The standard views included: 2D and color images of parasternal long, parasternal short, apical four‐chamber, and apical five‐chamber views. This was followed by hands‐on training using the pocket‐size and HUD units. The trained physicians subsequently scanned elderly patients undergoing cataract surgery. The quality of images was graded, and agreement between local physicians' interpretations and Web‐based interpretations by worldwide experts was compared. A total of 968 studies were performed, 660 were used for validating physicians' competence. The trained physicians could recognize the major echocardiographic abnormalities with 58.7% sensitivity and 97.0% specificity (overall k = 0.62, P < .001). Diagnostic accuracy was the best for valve lesions (sensitivity, 80.9%; specificity, 99.8%; k = 0.88; P < .001) and relatively modest for left ventricular systolic dysfunction (sensitivity, 58.0%; specificity, 98.3%; k = 0.62; P < .001). 95 This protocol was then utilized in a randomized control trial lead by the American society of echocardiography foundation (ASEF‐VALUES; American Society of Echocardiography Foundation‐Valvular Assessment Leading to Unexplored Echocardiographic Stratagems). POCUS in this study was performed by non‐cardiologist physicians. Among patients with structural heart disease, and in comparison, to standard clinical practice, the utilization of POCUS has led to early referral for valvular interventions and a lower probability of hospitalization or death. 27 Readers in both of these cohorts were requested to give only visual, and qualitative assessments (mild, moderate, or severe) on specific pathologic issues: left ventricular dilation, wall hypertrophy (concentric or asymmetric), reduction of function (visual ejection fraction), segmental wall motion abnormality (yes or no), right ventricular dilation, left atrial dilatation, aortic root dilatation, valve calcification, pericardial effusion, pleural effusion, and dilation with reduced inspiratory reactivity of inferior vena cava.
10. CONCLUSION
Performing POCUS is an accurate, affordable, accessible, and comprehensive tool. It has a fast learning curve, and can prevent unnecessary and more expensive imaging. 6 , 9 POCUS can serve as a screening tool and guide management of patients with valvular heart disease. 54 Thus, it is important to acknowledge the limited training availability, lack of simplified screening protocols, and importantly reimbursement. As the utilization of POCUS increases in the outpatient clinic, more research is needed about its impact on screening, management, outcomes and cost of care.
Hammadah M, Ponce C, Sorajja P, Cavalcante JL, Garcia S, Gössl M. Point‐of‐care ultrasound: Closing guideline gaps in screening for valvular heart disease. Clin Cardiol. 2020;43:1368–1375. 10.1002/clc.23499
Abbreviations: FAST, focused assessment with sonography in trauma; HUD, handheld ultrasound devices; POCUS, point of care ultrasound; RVU, relative value unit: TAVR, transcatheter aortic valve replacement
DATA AVAILABILITY STATEMENT
No new data generated
REFERENCES
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005‐1011. [DOI] [PubMed] [Google Scholar]
- 2. Carroll JD. TAVR prognosis, aging, and the second TAVR tsunami insights from France. J Am Coll Cardiol. 2016;68:1648‐1650. [DOI] [PubMed] [Google Scholar]
- 3. Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral‐valve repair in patients with heart failure. New England J Med. 2018;379:2307‐2318. [DOI] [PubMed] [Google Scholar]
- 4. d'Arcy JL, Coffey S, Loudon MA, et al. Large‐scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE population cohort study. Eur Heart J. 2016;37:3515‐3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang A, Grayburn P, Foster JA, et al. Practice gaps in the care of mitral valve regurgitation: insights from the American College of Cardiology mitral regurgitation gap analysis and advisory panel. Am Heart J. 2016;172:70‐79. [DOI] [PubMed] [Google Scholar]
- 6. Pathan F, Fonseca R, Marwick TH. Usefulness of hand‐held ultrasonography as a gatekeeper to standard echocardiography for "rarely appropriate" echocardiography requests. Am J Cardiol. 2016;118:1588‐1592. [DOI] [PubMed] [Google Scholar]
- 7. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the Management of Patients with Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:2440‐2492. [DOI] [PubMed] [Google Scholar]
- 8. Cardim N, Dalen H, Voigt JU, et al. The use of handheld ultrasound devices: a position statement of the European Association of Cardiovascular Imaging (2018 update). Eur Heart J Cardiovasc Imaging. 2019;20:245‐252. [DOI] [PubMed] [Google Scholar]
- 9. Tse KH, Luk WH, Lam MC. Pocket‐sized versus standard ultrasound machines in abdominal imaging. Singapore Med J. 2014;55:325‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. David L, Dumitrascu DL. The bicentennial of the stethoscope: a reappraisal. Clujul Med. 2017;90:361‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel A, Tomar NS, Bharani A. Utility of physical examination and comparison to echocardiography for cardiac diagnosis. Indian Heart J. 2017;69:141‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas F, Flint N, Setareh‐Shenas S, Rader F, Kobal SL, Siegel RJ. Accuracy and efficacy of hand‐held echocardiography in diagnosing valve disease: a systematic review. Am J Med. 2018;131:1155‐1160. [DOI] [PubMed] [Google Scholar]
- 13. Spencer KT, Anderson AS, Bhargava A, et al. Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient. J Am Coll Cardiol. 2001;37:2013‐2018. [DOI] [PubMed] [Google Scholar]
- 14. Sztajzel JM, Picard‐Kossovsky M, Lerch R, Vuille C, Sarasin FP. Accuracy of cardiac auscultation in the era of Doppler‐echocardiography: a comparison between cardiologists and internists. Int J Cardiol. 2010;138:308‐310. [DOI] [PubMed] [Google Scholar]
- 15. Mehta M, Jacobson T, Peters D, et al. Handheld ultrasound versus physical examination in patients referred for transthoracic echocardiography for a suspected cardiac condition. JACC Cardiovasc Imaging. 2014;7:983‐990. [DOI] [PubMed] [Google Scholar]
- 16. Khan HA, Wineinger NE, Uddin PQ, Mehta HS, Rubenson DS, Topol EJ. Can hospital rounds with pocket ultrasound by cardiologists reduce standard echocardiography? Am J Med. 2014;127:669.e1‐669.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mjølstad OC, Andersen GN, Dalen H, et al. Feasibility and reliability of point‐of‐care pocket‐size echocardiography performed by medical residents. Eur Heart J Cardiovasc Imaging. 2013;14:1195‐1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prinz C, Voigt JU. Diagnostic accuracy of a hand‐held ultrasound scanner in routine patients referred for echocardiography. J Am Soc Echocardiogr. 2011;24:111‐116. [DOI] [PubMed] [Google Scholar]
- 19. Kobal SL, Tolstrup K, Luo H, et al. Usefulness of a hand‐carried cardiac ultrasound device to detect clinically significant valvular regurgitation in hospitalized patients. Am J Cardiol. 2004;93:1069‐1072. [DOI] [PubMed] [Google Scholar]
- 20. Shen‐Wagner J, Wagner M, Hughes A. A Patient's perspective: pairing the stethoscope with POCUS to evaluate acute dyspnea in the clinic. South Med J. 2018;111:401‐403. [DOI] [PubMed] [Google Scholar]
- 21. Jones AE, Tayal VS, Sullivan DM, Kline JA. Randomized, controlled trial of immediate versus delayed goal‐directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004;32:1703‐1708. [DOI] [PubMed] [Google Scholar]
- 22. Holt GR. Introduction to special issue on point‐of‐care ultrasound. South Med J. 2018;111:371. [DOI] [PubMed] [Google Scholar]
- 23. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination. American J Cardiol. 2005;96:1002‐1006. [DOI] [PubMed] [Google Scholar]
- 24. Kitada R, Fukuda S, Watanabe H, et al. Diagnostic accuracy and cost‐effectiveness of a pocket‐sized transthoracic echocardiographic imaging device. Clin Cardiol. 2013;36:603‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vourvouri EC, Poldermans D, Deckers JW, Parharidis GE, Roelandt JR. Evaluation of a hand carried cardiac ultrasound device in an outpatient cardiology clinic. Heart. 2005;91:171‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narula J, Chandrashekhar Y, Braunwald E. Time to add a fifth pillar to bedside physical examination: inspection, palpation, percussion, auscultation, and Insonation. JAMA Cardiol. 2018;3:346‐350. [DOI] [PubMed] [Google Scholar]
- 27. Bhavnani SP, Sola S, Adams D, Venkateshvaran A, Dash PK, Sengupta PP. A randomized trial of pocket‐echocardiography integrated Mobile health device assessments in modern structural heart disease clinics. JACC Cardiovasc Imaging. 2018;11:546‐557. [DOI] [PubMed] [Google Scholar]
- 28. Vourvouri EC, Koroleva LY, Ten Cate FJ, et al. Clinical utility and cost effectiveness of a personal ultrasound imager for cardiac evaluation during consultation rounds in patients with suspected cardiac disease. Heart. 2003;89:727‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rigolin VH. What's in store for cardiovascular ultrasound in the future. J Am Soc Echocardiogr. 2018;31:A17. [DOI] [PubMed] [Google Scholar]
- 30. Salustri A, Trambaiolo P. The "ultrasonic stethoscope": is it of clinical value? Heart British Cardiac Soc. 2003;89:704‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beaton A, Lu JC, Aliku T, et al. The utility of handheld echocardiography for early rheumatic heart disease diagnosis: a field study. Eur Heart J Cardiovasc Imaging. 2015;16:475‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giusca S, Jurcut R, Ticulescu R, et al. Accuracy of handheld echocardiography for bedside diagnostic evaluation in a tertiary cardiology center: comparison with standard echocardiography. Echocardiography. 2011;28:136‐141. [DOI] [PubMed] [Google Scholar]
- 33. Beaton A, Aliku T, Okello E, et al. The utility of handheld echocardiography for early diagnosis of rheumatic heart disease. J Am Soc Echocardiogr. 2014;27:42‐49. [DOI] [PubMed] [Google Scholar]
- 34. US Food and Drug Administration . FDA authorizes marketing of first cardiac ultrasound software that uses artificial intelligence to guide user 2020.
- 35. Scalea TM, Rodriguez A, Chiu WC, et al. Focused assessment with Sonography for trauma (FAST): results from an international consensus conference. J Trauma. 1999;46:466‐472. [DOI] [PubMed] [Google Scholar]
- 36. Cardim N, Fernandez Golfin C, Ferreira D, et al. Usefulness of a new miniaturized echocardiographic system in outpatient cardiology consultations as an extension of physical examination. J Am Soc Echocardiogr. 2011;24:117‐124. [DOI] [PubMed] [Google Scholar]
- 37. Gorcsan J 3rd, Pandey P, Sade LE. Influence of hand‐carried ultrasound on bedside patient treatment decisions for consultative cardiology. J Am Soc Echocardiogr. 2004;17:50‐55. [DOI] [PubMed] [Google Scholar]
- 38. Richards JR, McGahan JP. Focused assessment with Sonography in trauma (FAST) in 2017: what radiologists can learn. Radiology. 2017;283:30‐48. [DOI] [PubMed] [Google Scholar]
- 39. Boulanger BR, Kearney PA, Brenneman FD, Tsuei B, Ochoa J. Utilization of FAST (focused assessment with Sonography for trauma) in 1999: results of a survey of north American trauma centers. Am Surg. 2000;66:1049‐1055. [PubMed] [Google Scholar]
- 40. Tayal VS, Beatty MA, Marx JA, Tomaszewski CA, Thomason MH. FAST (focused assessment with sonography in trauma) accurate for cardiac and intraperitoneal injury in penetrating anterior chest trauma. J Ultrasound Med. 2004;23:467‐472. [DOI] [PubMed] [Google Scholar]
- 41. Rozycki GS, Feliciano DV, Ochsner MG, et al. The role of ultrasound in patients with possible penetrating cardiac wounds: a prospective multicenter study. J Trauma. 1999;46:543‐552. [DOI] [PubMed] [Google Scholar]
- 42. Via G, Hussain A, Wells M, et al. International evidence‐based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27:683.e1‐683.e33. [DOI] [PubMed] [Google Scholar]
- 43. Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: rapid ultrasound in SHock in the evaluation of the critically lll. Emerg Med Clin North Am. 2010;28:29‐56. vii. [DOI] [PubMed] [Google Scholar]
- 45. Kimura BJ, Shaw DJ, Agan DL, Amundson SA, Ping AC, DeMaria AN. Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic. Am J Cardiol. 2007;100:321‐325. [DOI] [PubMed] [Google Scholar]
- 46. Bhagra A, Tierney DM, Sekiguchi H, Soni NJ. Point‐of‐care ultrasonography for primary care physicians and general internists. Mayo Clin Proc. 2016;91:1811‐1827. [DOI] [PubMed] [Google Scholar]
- 47. Krishnan DK, Pawlaczyk B, McCullough PA, Enright S, Kunadi A, Vanhecke TE. Point‐of‐care, ultraportable echocardiography predicts diuretic response in patients admitted with acute decompensated heart failure. Clin Med Insights Cardiol. 2016;10:201‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595‐601. [DOI] [PubMed] [Google Scholar]
- 49. Kimura BJ, Yogo N, O'Connell CW, Phan JN, Showalter BK, Wolfson T. Cardiopulmonary limited ultrasound examination for "quick‐look" bedside application. Am J Cardiol. 2011;108:586‐590. [DOI] [PubMed] [Google Scholar]
- 50. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med. 2012;38:577‐591. [DOI] [PubMed] [Google Scholar]
- 51. Schnobrich DJ, Gladding S, Olson AP, Duran‐Nelson A. Point‐of‐care ultrasound in internal medicine: a National Survey of educational leadership. J Grad Med Educ. 2013;5:498‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mathews BK, Reierson K, Vuong K, et al. The design and evaluation of the comprehensive hospitalist assessment and mentorship with portfolios (CHAMP) ultrasound program. J Hosp Med. 2018;13:544‐550. [DOI] [PubMed] [Google Scholar]
- 53. Rempell JS, Saldana F, DiSalvo D, et al. Pilot point‐of‐care ultrasound curriculum at Harvard medical school: early experience. West J Emerg Med. 2016;17:734‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Skalski JH, Elrashidi M, Reed DA, McDonald FS, Bhagra A. Using standardized patients to teach point‐of‐care ultrasound‐guided physical examination skills to internal medicine residents. J Grad Med Educ. 2015;7:95‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Siqueira VN, Mancuso FJ, Campos O, De Paola AA, Carvalho AC, Moises VA. Training program for cardiology residents to perform focused cardiac ultrasound examination with portable device. Echocardiography. 2015;32:1455‐1462. [DOI] [PubMed] [Google Scholar]
- 56. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567‐581. [DOI] [PubMed] [Google Scholar]
- 57. Labovitz AJ, Noble VE, Bierig M, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J American Soc Echocardiography. 2010;23:1225‐1230. [DOI] [PubMed] [Google Scholar]
- 58. Wiegers SE, Ryan T, Arrighi JA, et al. ACC/AHA/ASE advanced training statement on echocardiography (revision of the 2003 ACC/AHA clinical competence statement on echocardiography). A Report ACC Competency Management Committee. 2019;74:377‐402. [DOI] [PubMed] [Google Scholar]
- 59. Ma IWY, Cogliati C, Bosch FH, et al. Point‐of‐care ultrasound for internal medicine: an international perspective. South Med J. 2018;111:439‐443. [DOI] [PubMed] [Google Scholar]
- 60. Dversdal RK, Piro KM, LoPresti CM, Northcutt NM, Schnobrich DJ. Point‐of‐care ultrasound in the inpatient setting: a tale of four patients. South Med J. 2018;111:382‐388. [DOI] [PubMed] [Google Scholar]
- 61. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Societe de reanimation de langue Francaise statement on competence in critical care ultrasonography. Chest. 2009;135:1050‐1060. [DOI] [PubMed] [Google Scholar]
- 62. International expert statement on training standards for critical care ultrasonography . Springer. Intensive Care Med. 2011;37:1077‐1083. [DOI] [PubMed] [Google Scholar]
- 63. Education ACfGM . ACGME Program Requirements for Graduate Medical Education in Internal Medicine. 2019.
- 64. Hall JW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: a CERA study. Fam Med. 2015;47:706‐711. [PubMed] [Google Scholar]
- 65. Kelm DJ, Ratelle JT, Azeem N, et al. Longitudinal ultrasound curriculum improves long‐term retention among internal medicine residents. J Grad Med Educ. 2015;7:454‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosborough TK, Tierney DM. A late‐career internist and point‐of‐care‐ultrasound in a primary care clinic. South Med J. 2018;111:418‐420. [DOI] [PubMed] [Google Scholar]
- 67. Wagner M, Shen‐Wagner J, Zhang KX, Flynn T, Bergman K. Point‐of‐care ultrasound applications in the outpatient clinic. South Med J. 2018;111:404‐410. [DOI] [PubMed] [Google Scholar]
- 68. Baumgartner H, Falk V, Bax JJ, et al. ESC/EACTS Guidelines for the management of valvular heart disease. European Heart J. 2017;38:2739‐2791. [DOI] [PubMed] [Google Scholar]
- 69. Mathews BK, Miller PE, Olson APJ. Point‐of‐care ultrasound improves shared diagnostic understanding between patients and providers. South Med J. 2018;111:395‐400. [DOI] [PubMed] [Google Scholar]
- 70. Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. Report American College of Cardiol/American Heart Assoc Task Force Clinic Pract Guidelines. 2017;70:252‐289. [DOI] [PubMed] [Google Scholar]
- 71. Godown J, Lu JC, Beaton A, et al. Handheld echocardiography versus auscultation for detection of rheumatic heart disease. Pediatrics. 2015;135:e939‐e944. [DOI] [PubMed] [Google Scholar]
- 72. Ploutz M, Lu JC, Scheel J, et al. Handheld echocardiographic screening for rheumatic heart disease by non‐experts. Heart. 2016;102:35‐39. [DOI] [PubMed] [Google Scholar]
- 73. Singh S, Bansal M, Maheshwari P, et al. American Society of Echocardiography: remote echocardiography with web‐based assessments for referrals at a distance (ASE‐REWARD) study. J Am Soc Echocardiogr. 2013;26:221‐233. [DOI] [PubMed] [Google Scholar]
- 74. Rothenbühler M, O'Sullivan CJ, Stortecky S, et al. Active surveillance for rheumatic heart disease in endemic regions: a systematic review and meta‐analysis of prevalence among children and adolescents. The Lancet Global Health. 2014;2:e717‐e726. [DOI] [PubMed] [Google Scholar]
- 75. Beaudoin A, Edison L, Introcaso CE, et al. Acute rheumatic fever and rheumatic heart disease among children–American Samoa, 2011‐2012. MMWR Morb Mortal Wkly Rep. 2015;64:555‐558. [PMC free article] [PubMed] [Google Scholar]
- 76. Hughes GH, Cutter G, Donahue R, et al. Recruitment in the coronary artery disease risk development in young adults (Cardia) study. Control Clin Trials. 1987;8:68s‐73s. [DOI] [PubMed] [Google Scholar]
- 77. The ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687‐702. [PubMed] [Google Scholar]
- 78. Jones EC, Devereux RB, Roman MJ, et al. Prevalence and correlates of mitral regurgitation in a population‐based sample (the strong heart study). Am J Cardiol. 2001;87:298‐304. [DOI] [PubMed] [Google Scholar]
- 79. Kodali SK, Velagapudi P, Hahn RT, Abbott D, Leon MB. Valvular heart disease in patients ≥80 years of age. J Am Coll Cardiol. 2018;71:2058‐2072. [DOI] [PubMed] [Google Scholar]
- 80. Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease fn1fn1This study was supported in part by contracts NO1‐HC85079 through HC‐850086 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland. J American College Cardiol. 1997;29:630‐634. [DOI] [PubMed] [Google Scholar]
- 81. Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart. 2013;99:396‐400. [DOI] [PubMed] [Google Scholar]
- 82. Osnabrugge RLJ, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for Transcatheter aortic valve replacement: a meta‐analysis and modeling study. J American College Cardiol. 2013;62:1002‐1012. [DOI] [PubMed] [Google Scholar]
- 83. Roberts WC, Janning KG, Ko JM, Filardo G, Matter GJ. Frequency of congenitally bicuspid aortic valves in patients ≥80 years of age undergoing aortic valve replacement for aortic stenosis (with or without aortic regurgitation) and implications for transcatheter aortic valve implantation. Am J Cardiol. 2012;109:1632‐1636. [DOI] [PubMed] [Google Scholar]
- 84. Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham heart study). Am J Cardiol. 1999;83:897‐902. [DOI] [PubMed] [Google Scholar]
- 85. Pelliccia A, Sharma S, Gati S, et al. ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2020;2020. [DOI] [PubMed] [Google Scholar]
- 86. Fox JC, Lahham S, Maldonado G, et al. Hypertrophic cardiomyopathy in youth athletes: successful screening with point‐of‐care ultrasound by medical students. J Ultrasound Med. 2017;36:1109‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cassels M, Moulson N, Regan J, et al. Point‐of‐care ultrasound as a component of Preparticipation screening of athletes: a systematic review. J Ultrasound Med. 2019;38:3123‐3130. [DOI] [PubMed] [Google Scholar]
- 88. Mitchell ARJ, Hurry R, Le Page P, MacLachlan H. Pre‐participation cardiovascular screening: is community screening using hand‐held cardiac ultrasound feasible? Echo Res Pract. 2015;2:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Becker DM, Tafoya CA, Becker SL, Kruger GH, Tafoya MJ, Becker TK. The use of portable ultrasound devices in low‐ and middle‐income countries: a systematic review of the literature. Trop Med Int Health. 2016;21:294‐311. [DOI] [PubMed] [Google Scholar]
- 90. Beaton A, Okello E, Lwabi P, Mondo C, McCarter R, Sable C. Echocardiography screening for rheumatic heart disease in Ugandan schoolchildren. Circulation. 2012;125:3127‐3132. [DOI] [PubMed] [Google Scholar]
- 91. Lu JC, Sable C, Ensing GJ, et al. Simplified rheumatic heart disease screening criteria for handheld echocardiography. J American Soc Echocardiography. 2015;28:463‐469. [DOI] [PubMed] [Google Scholar]
- 92. Engelman D, Kado JH, Reményi B, et al. Screening for rheumatic heart disease: quality and agreement of focused cardiac ultrasound by briefly trained health workers. BMC Cardiovasc Disord. 2016;16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Woldu B, Bloomfield GS. Rheumatic heart disease in the twenty‐first century. Curr Cardiol Rep. 2016;18:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Anstey JE, Jensen TP, Afshar N. Point‐of‐care ultrasound needs assessment, curriculum design, and curriculum assessment in a large academic internal medicine residency program. South Med J. 2018;111:444‐448. [DOI] [PubMed] [Google Scholar]
- 95. Bansal M, Singh S, Maheshwari P, et al. Value of interactive scanning for improving the outcome of new‐learners in transcontinental tele‐echocardiography (VISION‐in‐Tele‐Echo) study. J Am Soc Echocardiogr. 2015;28:75‐87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data generated


