Abstract
The microRNA-200 (miR-200) family has been reported to be vital for the inhibition of epithelial-to-mesenchymal transition (EMT) in tumor cells. The miR-200 family represents a complex multi-factorial regulatory network which has not been well described in breast cancer. This study aimed to clarify the underlying regulatory association between IL-8 and miR-200 family in the process of EMT in breast cancer cell. In estrogen-receptor (ER) positive breast cancer cell line MCF-7, IL-8 overexpression cells were performed by lentivirus transfection as endogenous regulation with additional exogenous IL-8 stimulation. Transient overexpressions of miR-200 family were performed after endogenous or exogenous IL-8 overexpression in MCF-7 cells. IL-8 knockdown cells were constructed via siRNA and shRNA transfection in triple negative breast cancer cell line MDA-MB-231. N-cadherin, vimentin and ZEB2 were down-regulated and E-cadherin was up-regulated in IL-8 knockdown group compared with control group. On the other hand, N-cadherin, vimentin and ZEB2 were up-regulated and E-cadherin was down-regulated in IL-8 overexpression group compared with control group. This indicated IL-8 promotes EMT in breast cancer cells. Transwell assay showed that IL-8 increased the migration and invasiveness of tumor cells. Furthermore, we performed transient overexpression of miR-200 family after endogenous or exogenous IL-8 overexpression in MCF-7 cells, which showed that the miR-200 family could inhibit EMT induced by IL-8. IL-8 promoted EMT via downregulation of miR-200 family expression in breast cancer cells and increases tumor cell migration and invasion.
Keywords: Breast cancer, Interleukin-8, miR-200, Epithelial-to-mesenchymal transition, Invasion, Migration
Introduction
Breast cancer is the most frequent malignancy of women worldwide. Due to remarkable advances in its diagnosis and therapeutic approaches, breast cancer has a good survival rate. However, tumor recurrence and distant metastasis remain the major clinical obstacles that often lead to patient death.
During the initial stage of metastasis, tumor cells down-regulate their epithelial specificities and acquire mesenchymal properties which lead to a loss of cell contracts as well as motile and invasive capabilities; this process is called epithelial-to-mesenchymal transition (EMT). Disseminated neoplastic cells spread through circulation and move to distant organs, where they then undergo the reverse process of mesenchymal-to-epithelial transformation (MET) to form epithelial phenotype and metastasize.1 During the process of EMT, the morphology and molecular mechanism of tumor cells undergo a series of changes. Epithelial biomarker E-cadherin and mesenchymal biomarkers N-cadherin, vimentin specifically respond to EMT changes in tumor cells.2
Interleukin-8 has a close relationship with tumorigenesis and plays important roles in tumor angiogenesis, invasiveness, metastasis and drug resistance.3 Zuccari et al. analyzed 72 breast cancer tissues and found IL-8 overexpression in tumor cells when compared to respective normal breast tissues, and elevated expression of IL-8 indicating a rather poor pathological grade, more advanced metastasis and a more common local recurrence.4 In colon cancer, it has also been reported that IL-8 is related to EMT and increases IL-8 levels accompanying EMT induced by upregulation of Snail.5 In our previous studies, IL-8 levels in human breast cancer cells were closely related to ER status. ER positive cells expressed low levels of IL-8 whereas ER negative cells expressed high levels of IL-8. IL-8 play a key factor in breast cancer invasion and angiogenesis.6,10
MiR-200 family is well known as a key factor to inhibit EMT by directly downregulating E-cadherin transcriptional repressors ZEB1 and ZEB2.7 Studies of hepatocellular carcinoma showed that the expression of miR-200a and miR-200b in both patient serum and cancer cells were decreased compared to normal controls; upregulation of miR-200a in liver cancer cell lines inhibited cell proliferation, migration and invasion.8
Our study aimed to clarify the relationship between IL-8 and miR-200 family and their regulatory mechanism on EMT in breast cancer.
Materials and Methods
Cell Culture
Human breast cancer cell lines MDA-MB-231 and MCF-7 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. Cells were regularly cultured in an incubator at 37°C with 5% CO2. Recombinant Human IL-8 (CXCL8) was purchased from Peprotech Company (Rocky Hill, NJ, US) and MCF-7 was treated with IL-8 of the 5, 10, 15ng/ml for 48 h respectively.
Transfection of Breast Cancer Cells
IL-8 siRNAs and negative control siRNA were purchased from Ribobio Company (Guangzhou, China). IL-8 shRNA and IL-8 cDNA were extracted by GeneCopoeia (Rockville, MD, US). MiR-200s mimics were purchased from Ribobio Company. IL-8 siRNAs were transiently transfected into MDA-MB-231 cells using Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) and miR-200 s mimics were transient transfected by Lipofectamine-iMAX (Invitrogen). IL-8 shRNA and IL-8 cDNA were stably transfected into MDA-MB-231 and MCF-7 cells with lentivirus vectors, puromycin-resistance was representative of transfection cells and was selected by 1-6ug/ml puromycin. All the procedures were manipulated under the manufacturer’s protocol.
Isolation of RNA and Quantitative Real-Time PCR (RT-qPCR)
Total RNA was extracted from respective cell lines by Trizol (Invitrogen), and reverse transcribed by TaKaRa PrimeScript RT reagent Kit according to the manufacturer’s instructions. Primers were synthesized by TaKaRa Company and TaKaRa SYBR PCR Kit was used to perform the reaction with a LightCycler 480 (Roche Applied Science) for Quantitative real-time PCR. The glyceralde-hyde 3-phosphate dehydrogenase (GAPDH) and U6 were used as reference genes. Primers used for RT-qPCR were as follows: E-cadherin, 5’-GCCCCATCAGGCCTCCGTTT-3’ (sense) and, 5’-ACCTTGCCTTCTTTGTCTTTGTTGGA-3’ (anti-sense); N-cadherin, 5’-TGGACCATCACTCGGCTTA-3’ (sense) and 5’-ACACTGGCAAACCTTCACG-3’ (anti-sense); vimentin, 5’-CCTGAACCTGAGGGAAACTAA-3 (sense) and 5’-GCAGAAAGGCACTTGAAAGC-3’ (anti-sense); ZEB2, 5’-TGAGGATGACGGTATTGC-3’ (sense) and 5’-ATCTCGTTGTTGTGCCAG-3’ (anti-sense).
Western Blotting and Immunofluorescence
Total cell protein was separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) then transferred to 0.22 µm PVDF membranes (Roche). The membranes were incubated with 5% non-fat milk for 2 h at room temperature to block nonspecific binding. The respective specific primary antibody was then incubated on the membranes at 4°C overnight. The following primary antibodies were used: GAPDH (1:2000, CST, Beverly, MA, USA), E-cadherin (1:1000, CST), N-cadherin (1:1000, CST), vimentin (1:1000, CST), ZEB2 (1:500, Millipore). For immunofluorescence, cells were plated on 24-well plate and stained using E-cadherin (1:500, CST), and N-cadherin (1:500, CST). After washing 3 times with PBST, horseradish peroxidase (HRP)-conjugated secondary antibodies were incubated on the membranes at 25°C for 1 h. GAPDH was used as the internal control. All western blot experiments were replicated 3 times. The membranes were then stained by enhanced chemiluminescence solution (Millipore). Nuclei were visualized by co-staining with DAPI and data was imaged with an Olympus BX63.
ELISA Assay
The protein level of IL-8 was quantified by ELISA assay using the DuoSet Immunoassay kit (R&D Systems, Minneapolis, MN, USA). IL-8 expression was manipulated with stable transfection of MDA-MB-231 and MCF-7 cells. Cells were cultured in six-well plates and media supernatant was collected after 48 h and stored at −80°C. All the processes were completed using the manufacturer’s protocol.
Migration and Invasion Assays
For migration assay, transwell chambers were grown with cells on the upper chamber in 24-well plate, medium in the upper chamber was DMEM without FBS while in the bottom chamber was DMEM containing 10% FBS. For invasion assay, Transwell chambers were coated with Matrigel before plating. After 24 h, cells in the upper chamber were removed and cells on the bottom were stained with crystal violet solution. Data was visualized and imaged with Olympus BX63 microscope. Stained cells on the bottom of the chamber were counted and analyzed statistically to demonstrate migration and invasion ability.
Results
Manipulation of IL-8 in Breast Cancer Cells
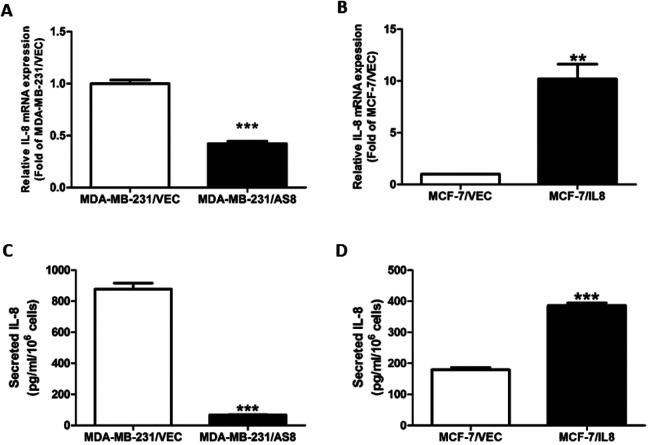
shRNA/IL-8 was transfected into MDA-MB-231 cells to construct the knockdown group, MDA-MB-231/AS8, and control group, MDA-MB-231/VEC. A lentivirus of IL-8 was transfected into MCF-7 to construct overexpression group MCF-7/IL-8 and control group MCF-7/VEC. RT-qPCR was performed to analysis IL-8 mRNA expression. IL-8 level decreased 53.3% in the knockdown group and IL-8 level increased 10.26-fold in the overexpression group compared with respective control group (Figure 1A, B).
Figure 1.
Level of IL-8 expression in IL-8 manipulated cells. (A, B) RT-qPCR analysis of IL-8 mRNA expression following transfection with IL-8 knockdown in MDA-MB-231 cells and IL-8 overexpression in MCF-7 cells compared with control cells. (C, D) Results from ELISA of IL-8 protein secretion in IL-8 knockdown and IL-8 overexpression groups. (*** P < 0.001).
The ELISA revealed that IL-8 protein level dropped to 11.4% in MDA-MB-231/AS8 group and rose to 1.92-fold in MCF-7/IL-8 group (Figure-1C, D).
IL-8 Promotes EMT in Breast Cancer Cells
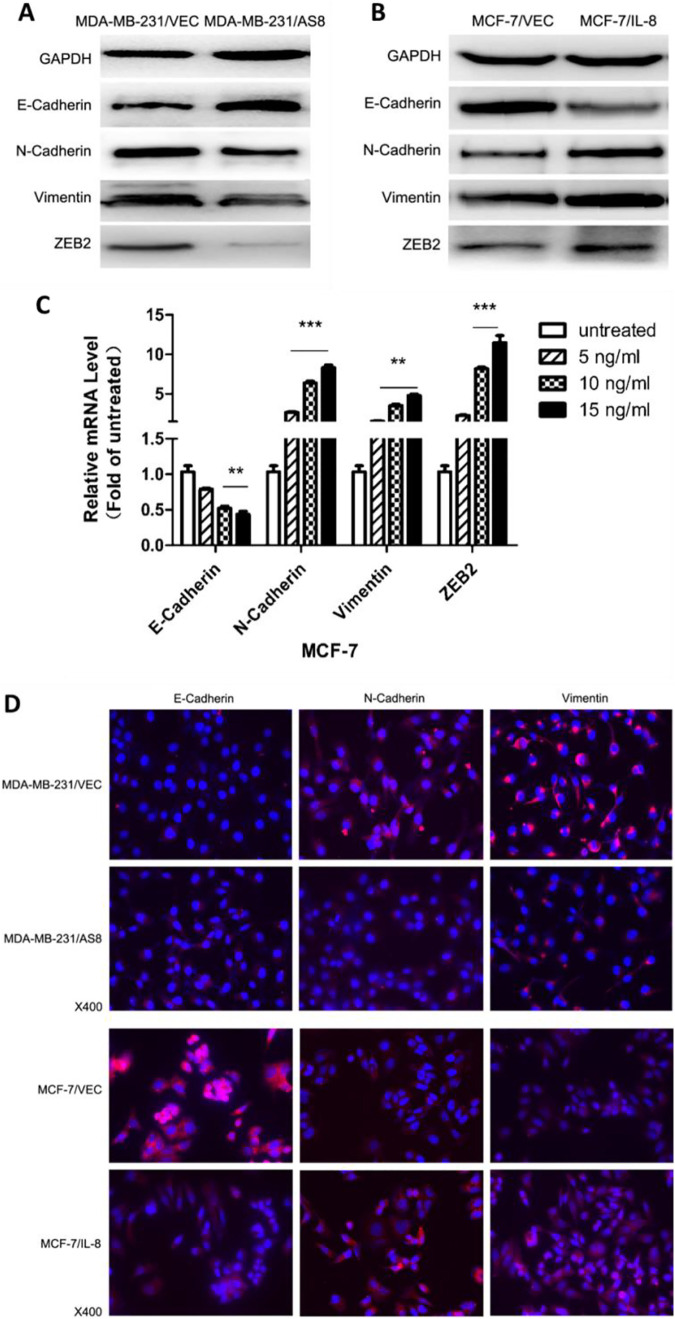
We evaluated the expression of EMT biomarkers in transfected breast cancer cell lines to validate the role of IL-8 in EMT. Knockdown of IL-8 in MDA-MB-231 cells showed a significant decrease in N-cadherin, vimentin and ZEB2 expression, as well as a significant increase in E-cadherin expression. Meanwhile, overexpression of IL-8 in MCF-7 cells showed the opposite result: an increase in N-cadherin, vimentin and ZEB2 and a decrease in E-cadherin (Figure-2A, B, D). Exogenous stimulation of IL-8 was also performed in MCF-7 with treatment of IL-8 concentrations of 5, 10, 15ng/ml IL-8 compared with untreated cells. Results showed that IL-8 stimulation promoted EMT in MCF-7 in accordance with the overexpression group (Figure 2C). Taken together, these results demonstrate that endogenous IL-8 and exogenous IL-8 stimulation promote EMT in breast cancer cells.
Figure 2.
Effect of IL-8 on EMT in breast cancer cells. (A, B) Western blot analysis of expression of EMT biomarkers E-cadherin, N-cadherin, vimentin, ZEB2 in IL-8 knockdown and IL-8 overexpression groups compared with respective controls. GAPDH was used as the internal control. (C) MCF-7 cells were treated with human recombinant IL-8 at a concentration of 5, 10, 15 ng/ml. After 48 h treatment, RT-qPCR was performed to evaluate the expression of EMT biomarkers in treated cells compared with untreated cells. (D) Immunofluorescence microscopy analysis of the expression and localization of EMT biomarkers in IL-8 knockdown and IL-8 overexpression groups compared with respective controls. (** P < 0.01, *** P < 0.001).
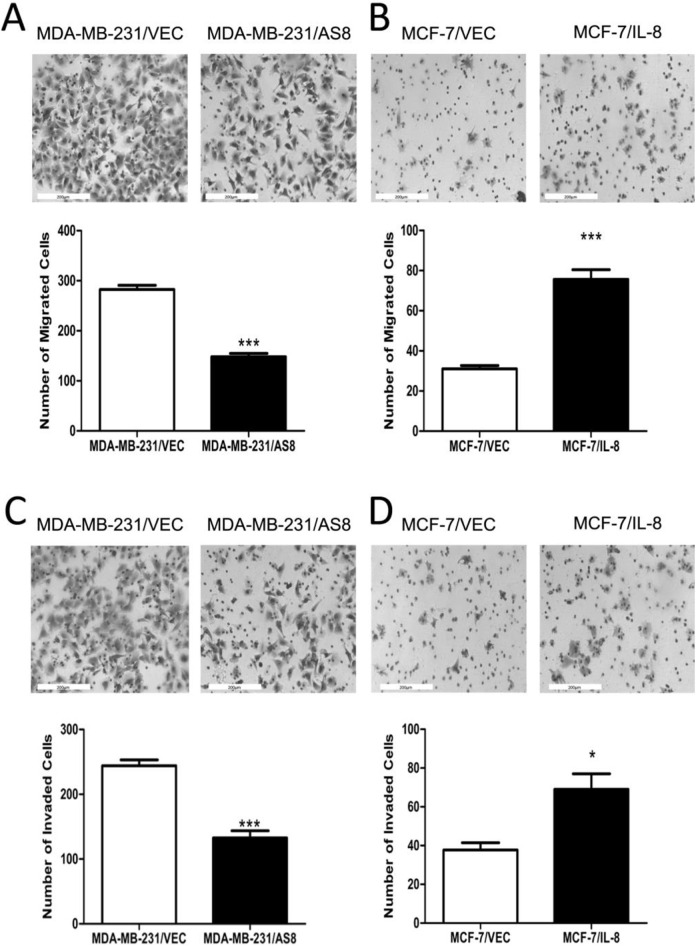
IL-8 Increases Breast Cancer Cells Migration and Invasion in Vitro
Significant changes to EMT biomarkers in IL-8 manipulated breast cancer cells prompted us to investigate the possible biological impact of IL-8 on tumorigenesis. We performed Transwell experiments to directly test whether IL-8 could promote invasive behavior by promoting EMT-like morphological changes in cancer cells. We observed that knockdown of IL-8 in MDA-MB-231 cells significantly decreased cell migration and invasiveness compared with the control group (Figure 3A, C). Conversely, overexpression of IL-8 in MCF-7 cells significantly increased cell migration and invasion compared with control group (Figure 3B, D). These data indicated that IL-8 has oncogenic properties that can lead to a more migratory and invasive phenotype in breast cancer cells.
Figure 3.
Effect of IL-8 on migration and invasion in vitro. (A, B) Migration and (C, D) invasiveness were demonstrated by Transwell assay analysis in IL-8 knockdown and IL-8 overexpression groups compared with respective controls. After 24 h, cells were stained and counted.(*P < 0.05, ***P < 0.001).
MiR-200 Family Expression in Breast Cancer Cells
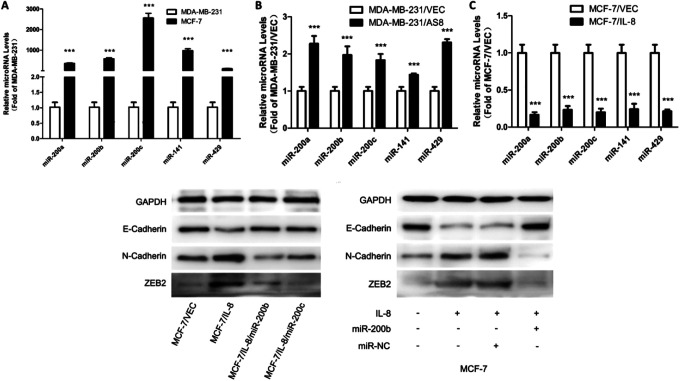
To investigate the role of the miR-200 family in breast cancer, we firstly evaluated miR-200 family expression in MDA-MB-231 and MCF-7 cell lines. MiR-200 family had low expression in MDA-MB-231 cells and a high expression in MCF-7 cells (Figure-4A). This indicated a negative correlation between miR-200 family expression and invasiveness in breast cancer cells.
Figure 4.
Association between IL-8 and miR-200 family, and effect of miR-200 family on IL-8-induced EMT. (A) RT-qPCR was performed to detect miR-200 family expression in MCF-7 cells compared with MDA-MB-231 cells. (B, C) MiR-200 family expression in IL-8 knockdown and IL-8 overexpression groups compared with respective controls by RT-qPCR analysis. (D) MCF-7 cells were transfected to overexpress IL-8, then miR-200b and miR-200c mimics were transfected compared with miR-NC. (E) MCF-7 cells were treated with human IL-8 at a concentration of 15 ng/ml for 48 h. Then miR-200b mimic was transfected compared with miR-NC. After 72 h, western blot was performed to demonstrate the expression level of EMT biomarkers. (***P < 0.001).
IL-8 Downregulates miR-200 Family
MiR-200 family had a significant upregulation in IL-8 knockdown cells (MDA-MB-231/AS8) while with a significant downregulation in IL-8 overexpression cells (MCF-7/IL-8), as compared to respective control cells (Figure-4B, C). These results indicated that there was a negative relationship between IL-8 and miR-200 family.
MiR-200 Family Inhibits IL-8-Induced EMT in Breast Cancer Cells
It was important to determin whether IL-8 promotes EMT in breast cancer cells by regulating miR-200 family. We therefore constructed IL-8 overexpression in MCF-7 cells using either a endogenous approach of IL-8 transfection or exogenous approach of 15ng/ml IL-8 stimulation in order to induce EMT process in breast cancer cells. At the same time miR-200b and miR-200c were overexpressed with transient transfection to demonstrate the rescue experiment. We chose miR-200b and miR-200c as representatives for miR-200a/200b/429 cluster and miR-200c/141 cluster.9 Our findings showed that upregulation of IL-8 raised N-cadherin and ZEB2 level and reduced E-cadherin level, leading to EMT. On the other hand, upregulation of miR-200b and miR-200c presented a reverse phenomenon which indicates that upregulation of miR-200 family inhibits IL-8 induced EMT.
Discussion
In our previous study, we found that IL-8 significantly highly expressed in ER negative breast cancer cells and promoted invasion and angiogenesis.6 IL-8 upregulated integrin beta3 via PI3K/Akt/NF-kB pathway to promote breast cancer cell invasion.10 In order to prove that IL-8 could promote EMT as an oncogene, we performed an IL-8 knockdown in triple negative breast cancer cells, MDA-MB-231, to impair their invasiveness. Contrastingly, we overexpressed IL-8 in ER positive cell MCF-7 to enhance its invasiveness.
The multistage process of EMT comprises the gradual remodeling of epithelial cell architecture and functional capabilities. Decreased cellular expression of E-cadherin leads to the loss of epithelial barrier function. On the other hand, N-cadherin expression increases lead to intermediate cell transformation to mesenchymal phenotype.11 In this study, E-cadherin and N-cadherin were obvious biomarkers of functional cellular changes. Remarkably, the IL-8 knockdown group, MDA-MB-231/AS8, showed a decrease in N-cadherin levels and an increase in E-cadherin levels compared with the control group, MDA-MB-231/VEC. This finding illustrated the transition of cancer cells to MET-like morphology. On the other hand, IL-8 overexpression group (MCF-7/IL-8) exhibited a reverse phenomenon where cancer cells transformed into an EMT-like morphology. These data proved that in breast cancer cells, downregulation of IL-8 inhibited EMT and upregulated IL-8 to promote EMT. The aberrant activation of EMT promoted tumor cell invasion and dissemination, while its reverse process, MET, is believed to support metastatic growth once tumor cells have arrived in distant sites.12 Furthermore, Transwell assays were performed to investigate motility and invasiveness of breast cancer cells and showed that IL-8 increases cell migration and invasion.
It was reported that miR-200 family can directly bind to the 3’UTRs of ZEB1 and ZEB2 causing ZEB1 and ZEB2 downregulation.12 ZEB can bind to the promoter E-box of E-cadherin in order to inhibit E-cadherin expression and upregulate vimentin, finally inducing EMT.13 In this study we also observed ZEB2 changes in manipulation of IL-8, so we supposed there was a relationship and regulatory mechanism between IL-8 and miR-200 family. We detected miR-200 family expression in IL-8 manipulated cells which identified a negative correlation between them and proved IL-8 could downregulate miR-200 family. In order to theoretically link IL-8 and miR-200 family together in the process of EMT, we overexpressed miR-200 as rescue experiment after overexpression of IL-8 in MCF-7 cells to induce EMT. We found upregulation of miR-200 family could inhibit EMT induced by endogenous and exogenous IL-8 upregulation.
Next we searched for an in-depth mechanism of the IL-8/miR-200 pathway. The miR-200 family has been shown to downregulate the expression of IL-8 and its receptor CXCL1 by directly binding to their 3’UTRs, therefore inhibiting tumor cell angiogenesis.14 To invalidate IL-8 and CXCL1 as direct miR-200 targets, these researchers performed luciferase assay and binding site mutation. Based on their results, we suppose that IL-8 and miR-200 family have bidirectional regulation. Another study of epithelial cancer cells identified a double-negative feedback loop between ZEB1/ZEB2 and the miR-200 family. This indicates ectopic miR-200a and miR-200b can downregulate ZEB1/ZEB2 expression while ectopic ZEB1/ZEB2 can repress miR-200 promotion. Cancer cells were shown to be triggered to undergo EMT by ectopic ZEB1/ZEB2 expression and inhibition of the miR-200 family. These cells could revert to MET phenotype by ectopic miR-200 expression.15 While the double-negative feedback loop allows the system to remain reversible, it also allows the maintenance of bistable states. Combining these theories, we hypothesize that a double-negative feedback loop exists between IL-8 and the miR-200 family.
In addition to regulating EMT process, miR-200 family is reported to function as a central regulator of many cancer behaviors.16 Several studies have shown that low miR-200 levels are linked to increased chemotherapy resistance as well as anti-epidermal growth factor receptor therapy resistance and increased expression of ZEB1/2.17-19 These studies suggest that miR-200 family has a significant effect on cancer progression and may have therapeutic potential.
In summary, we demonstrated the regulatory mechanism of IL-8 promotes the EMT in breast cancer cells as well promotes cell migration and invasion via downregulation of the miR-200 family. These data uncovered the significance of the IL-8/miR-200 pathway in governing the plasticity of EMT and provided new idea on regulation of EMT in breast cancer cells. Modulation of this new pathway may be one method to inhibit breast cancer invasion and metastasis via restoration and upregulation of miR-200 expression.
Acknowledgments
Thanks to all the authors.
Abbreviations
- DMEM
Dulbecco’s modified Eagle’s medium
- GAPDH
glyceralde-hyde 3-phosphate dehydrogenase
- EMT
epithelial-to-mesenchymal transition
- MET
mesenchymal-to-epithelial transformation
- RT-qPCR
quantitative real-time PCR
Footnotes
Author Contributions: Jin Zhang and Nan Shao contribute equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China [grant number 81702848].
ORCID iD: Ying Lin  https://orcid.org/0000-0002-5923-3306
https://orcid.org/0000-0002-5923-3306
References
- 1. Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27(20):2192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–6741. [DOI] [PubMed] [Google Scholar]
- 4. Zuccari DA, Leonel C, Castro R, et al. An immunohistochemical study of interleukin-8 (IL-8) in breast cancer. Acta Histochem. 2012;114(6):571–576. [DOI] [PubMed] [Google Scholar]
- 5. Bates RC, DeLeo MR, Mercurio AM. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res. 2004;299(2):315–324. [DOI] [PubMed] [Google Scholar]
- 6. Lin Y, Huang R, Chen L, et al. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. 2004;109(4):507–515. [DOI] [PubMed] [Google Scholar]
- 7. Shao N, Lu Z, Zhang Y, et al. Interleukin-8 upregulates integrin beta3 expression and promotes estrogen receptor-negative breast cancer cell invasion by activating the PI3K/Akt/NF-kappaB pathway. Cancer Lett. 2015;364(2):165–172. [DOI] [PubMed] [Google Scholar]
- 8. Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22(7):894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dhayat SA, Mardin WA, Kohler G, et al. The microRNA-200 family—a potential diagnostic marker in hepatocellular carcinoma? J Surg Oncol. 2014;110(4):430–438. [DOI] [PubMed] [Google Scholar]
- 10. Korpal M, Kang Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. Rna Biol. 2008;5(3):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Xu H, Fu W, et al. 20(S)-Protopanaxadiol inhibits angiotensin II-induced epithelial- mesenchymal transition by downregulating SIRT1. Front Pharmacol. 2019;10:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342(6159):1234850. [DOI] [PubMed] [Google Scholar]
- 13. Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66(5):773–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pecot CV, Rupaimoole R, Yang D, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun. 2013;4:2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bracken CP, Gregory PA, Kolesnikoff N, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68(19):7846–7854. [DOI] [PubMed] [Google Scholar]
- 16. Iliopoulos D, Lindahl-Allen M, Polytarchou C, et al. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39(5):761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cochrane DR, Howe EN, Spoelstra NS, Richer JK. Loss of miR-200c: a marker of aggressiveness and chemoresistance in female reproductive cancers. J Oncol. 2010;2010:821717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adam L, Zhong M, Choi W, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15(16):5060–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ceppi P, Mudduluru G, Kumarswamy R, et al. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol Cancer Res. 2010;8(9):1207–1216. [DOI] [PubMed] [Google Scholar]