Abstract
Objective:
To characterize the impact on kidney injury of recurrent urinary tract infections (RUTI) in the frail elderly.
Methods:
Prospective observational study in 200 frail elderly subjects for 1 year. Groups: GA (n = 100): subjects without RUTI, GB (n = 100): subjects with RUTI. Variables: age, concomitant diseases, glomerular filtration rate (GFR), urine neutrophil gelatinase-associated lipocalin (NGAL) at the beginning (NGAL-1) and end (NGAL-2) of the study, urine N-acetyl glucosaminidase (NAG) at the beginning (NAG-1) and the end (NAG-2) of the study, urine transforming growth factor-beta 1 (TGFβ-1). Descriptive statistics, Mann–Whitney test, Chi-squared test, Fisher’s exact test, and multivariate analysis were used.
Results:
Mean age was 84.33 (65–99) years old, with no difference between GA and GB. Mean NGAL-1 was 1.29 ng/ml (0.04–8). There was lower in GA than in GB. Mean NGAL-2 was 1.41 ng/ml (0.02–9.22). NGAL-2 was lower in GA than in GB. Mean NAG-1 was 0.38 UU.II/ml (0.01–2.63. NAG-1 in GA was lower than in GB. Mean NAG-2 was 0.44 UU.II/ml (0–3.41). NAG-2 was lower in GA compared with GB. Mean TGFβ-1 was 23.43 pg/ml (0.02–103.76). TGFβ-1 was lower in GA than GB. There were no differences in the presence of secondary diagnoses between GA and GB. NAG-2 and NGAL-1 were the most determining factors of renal function; in GA it was NGAL-2, followed by NAG-1; in GB it was NGAL-1, followed by NAG-2.
Conclusion:
Frail elderly with RUTI have higher urinary levels of renal injury markers, specifically NGAL, NAG, and TGFβ-1, chronically in periods between urinary tract infection (UTI). Urinary markers of renal injury, specifically NGAL, NAG, and TGFβ-1, identify early deterioration of renal function, compared with serum creatinine, or albuminuria, in frail elderly with recurrent urinary infections.
Keywords: elderly, glomerular filtration, NAG, NGAL, RUTI, TFG-beta 1
Introduction
It is crucial to determine the risk of recurrent urinary tract infections (RUTI) in the frail elderly as a functional loss risk and a dependency state.
The frail elderly are defined as those who have a decrease in physiological reserves and a greater risk of decline, placing them in a situation of vulnerability to external disturbances and at a greater probability of presenting adverse health episodes (e.g., hospitalization, institutionalization, death, falls) and function loss, disability, or dependency.
Fragility is a difficult concept to define. It is not a rigidly defined clinical state. It can be defined as an intermediate state between the following two situations: (a) being in good health, i.e., the human body is physiologically and functionally well; and (b) loss of health and with pathology. The next step to frailty is being disabled and dependent.1–3
In the frail elderly, urine infections are the most frequent pathology, ahead of respiratory infections. Wojszel has shown that bacterial urinary tract infections (UTIs) affect about one in five hospitalized geriatric patients, and that the clinical picture of these infections is often atypical and needs diagnostic vigilance.4
Fragility causes a significant expense to society, quantified in the large number of health resources the frail elderly consume: 15% of all antibiotics in both primary care and specialized care.4 The prevalence of fragility increases with age, since aging produces an alteration of the defensive mechanisms against infection. Besides, aging people have high comorbidity, instrumentation, and hospitalization is frequent, thus there is increasing nosocomiality. Clinical manifestations have more severe presentation and worse prognosis. Management is complicated since aging carries a decrease in antimicrobial clearance, which produces an increase in side effects. Furthermore, the increasing risk of bacterial resistance to antibiotics is noteworthy.5
Risk factors for RUTI in women and men over 65 years are age, place of residence (institutionalized or not), health status, previous urinary tract instrumentation, and previous antibiotic treatments.6
The following are also postulated as RUTI predisposing factors in the elderly: age-related decrease in the immune response; alteration in natural defenses: decrease in skin thickness, gastric achlorhydria, mucociliary clearance decrease, vaginal and urethral mucosa atrophy, prostatic hypertrophy, sphincter dysfunction; comorbidities such as diabetes or advanced dementia (aspiration risk); instrumentation and nosocomiality; drugs such as antibiotics or steroids that favor infection. Specifically, antibiotics alter the natural balance of the intestinal microbiome, causing genitourinary infections.7
Urinary markers of kidney injury
Markers of kidney damage that have been demonstrated in other studies have been chosen.8–10 Our research’s great innovation is that the markers of kidney damage were not determined when the patients had an episode of UTI, but rather in the intervening periods between one UTI and another UTI.
Serum creatinine and glomerular filtration rate
Serum creatinine (sCr) and glomerular filtration rate (GFR) are the best measures with which to study kidney function in adult patients without kidney disease or with chronic kidney failure.
In patients with acute kidney injury (AKI), sCr limitations are several: sCr is significant as a functional marker when more than 50% of the GFR has been lost, and is useful only after it has reached a stationary state.11
Neutrophil gelatinase-associated lipocalin
Neutrophil gelatinase-associated lipocalin (NGAL) is a small 178-amino-acid protein that belongs to the lipocalin superfamily. NGAL was initially discovered as a neutrophil gelatinase-bound protein.
NGAL is expressed at low levels in different tissues, such as kidney, trachea, lungs, stomach, and colon, and its expression increases noticeably in inflammation. Therefore, it constitutes a biomarker of systemic leukocyte activation, considered an acute phase reactant.12
N-acetyl glucosaminidase
N-acetyl glucosaminidase (NAG) is a large protein (about 140 kDa) originating from the lysosomes of cells in the proximal tubule. Urinary NAG levels are a sensitive marker of tubular injury. These levels increase in urine 12 h after renal tubule injury onset. Elevated urinary NAG values have been described in patients with diabetes, rheumatoid arthritis, or hyperthyroidism without acute kidney injury.13
Transforming growth factor-beta-1
Transforming growth factor-beta-1 (TGFβ-1) belongs to a multifunctional cytokine family involved in the development and tissue repair regulation.
The most crucial property of TGF-β1 is the extracellular matrix protein synthesis regulation, and it is fundamentally responsible for renal tubule-interstitial and glomerular fibrosis.14 High levels of TGFβ-1 in urine have been observed during active renal fibrosis,15 and in patients with diabetes is a leading cause of kidney disease.16
Albuminuria
Albuminuria is an excess of albumin in the urine and is a sign of kidney disease. Together with GFR estimation, diagnosis of chronic kidney disease (CKD) is based on albuminuria. The presence of albuminuria identifies a group of patients at higher risk of kidney disease progression and more significant cardiovascular morbidity.17,18
This objective of this study was to characterize the impact on chronic kidney injury attributable to RUTI in the frail elderly. Urinary renal injury markers were investigated in a sample of the elderly population classified as “frail elderly” using these new generation urinary markers. Those markers have been demonstrated previously experimentally but have not been used in clinical practice until now. In frail elderly, two groups are compared: those with RUTI and those without RUTI. Multivariate analysis was carried out to avoid biases that could occur because concomitant diseases in some elderly people could cause kidney failure.
Methods
A prospective observational study of 200 elderly people classified as “frail elderly” consulted in primary care. The frail elderly diagnostic criteria were: frail elderly defined as individuals over 65 years old, dependent on others for daily living activities, and often in institutional care.
All individuals participating in the study met the frail elderly criteria according to the definition in routine clinical practice.19 All 200 subjects were institutionalized. Individuals were included in one group or the other depending on whether or not they had RUTI. The multivariate analysis distinguished whether concomitant diseases that can affect kidney function had a different distribution between the groups.
The identification and number of UTIs registered in the previous year was vital to create the study groups, with criteria described in this section. During the follow-up year, no patient changed groups, as those without UTIs continued without them, and those with UTIs continued with the same incidence level of UTIs. When recruiting patients for each group, the investigators did not know whether any patient would change status to RUTIs over time. The fact was that no patient changed group.
However, as research on kidney function in elderly people requires using a larger sample size to allow multivariate analysis to discriminate concomitant diseases that may affect kidney function, it was decided to use n = 100 patients in each group.
In the GA group were 55 patients with asymptomatic bacteriuria in the year before starting the study; among these 55 were 40 elderly who did have any UTI in that previous year. During the year of study follow up, in the GA group, 50 elderly patients had asymptomatic bacteriuria, among these were also 30 who presented with a UTI. In GB, all the elderly had significant bacteriuria: either in the UTI phase or in the asymptomatic bacteriuria phase. No elderly patient in GB was without bacteriuria in the 2 years investigated. Urine samples were collected at baseline, and at months 1, 3, 6, and 12. Urine culture was also performed before the start of antibiotic treatment in each episode of UTI. In this way, it was possible to find differences between the absence of significant bacteriuria, asymptomatic bacteriuria, and UTI. Urinary marker analysis was done at the beginning of the study for NGAL and NAG, and the end of the study for all variables, again including NGAL and NAG.
Study groups
Follow up was carried out for 1 year, measuring the variables that quantify kidney injury. Two study groups were established:
Group GA (n = 100): frail elderly subjects without RUTI.
Group GB (n = 100): frail elderly subjects with RUTI.
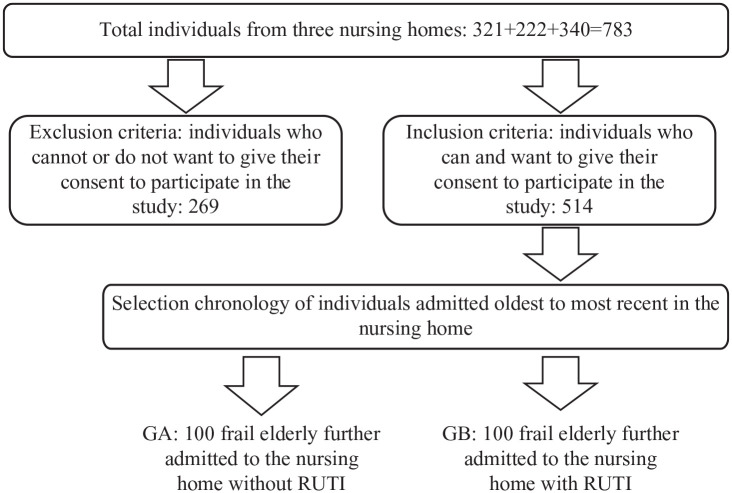
A flow chart diagram show the strategy of inclusion of individuals in the study.
Figure 4.
GA, group A; GB, group B.
Regarding sample size, there is no history regarding the investigation of kidney injury urine markers compared with controls, so taking a pilot study of our research group as a reference that compared GFR in long-term follow up of patients with RUTI with patients without RUTI using Epidat 3.1 software to compare two groups of RUTI prophylaxis,20,21 we made the decision to propose two groups of 100 patients each.
Study variables
Age, gender, secondary diagnoses, concomitant treatments, and GFR were registered at the beginning of the study and at 12 months. In addition to health controls’ routine management in these frail elderly people, the following specific variables for this study were determined in both patients and controls: proteinuria, albuminuria, urine NGAL, urine NAG, urinary creatinine, and urine TGF-beta 1.
The definition for UTI in the study was: significant bacteriuria plus symptoms. Asymptomatic bacteriuria was not included. Positive urine culture was considered bacterial counts of 100,000 colony-forming units per milliliter or higher in midstream urine cultures. The definition for RUTI was suffering from three, or more than three, UTIs in the previous year.22
We considered UTI symptoms: dysuria, urinary frequency, urinary urgency, bladder pain, suprapubic pain, fever, or low-grade fever. One or more symptoms plus a positive urine culture were considered to diagnose an episode of UTI. We diagnosed non-febrile UTIs as cystitis and febrile UTIs as uncomplicated pyelonephritis in women. Febrile UTIs were found in males when they presented pyelonephritis or acute bacterial prostatitis. There were no cases of septic shock or orchiepididymitis. The same multidisciplinary team treated all patients with the same protocol. A urine sample was collected for urine culture in all UTI episodes as soon as symptoms started and before starting empirical antibiotic treatment. Treatment was based on the historical record of sensitivity and resistance of that patient’s previous urine cultures. The most frequently used antibiotic in non-febrile UTIs was phosphomycin at a 500 mg dose by mouth every 8 h for 8 days. In febrile UTIs, a combined treatment of aminoglycoside (tobramycin) was administered intramuscularly at a dose of 100 mg every 24 h for 6 days concomitantly with amoxicillin/clavulanate 875/125 mg by mouth every 8 h for 14 days in the case of uncomplicated pyelonephritis or 21 days if it was bacterial prostatitis. The tobramycin dose was adjusted for renal function, without finding data on the deterioration of renal function associated with its limited use, and it was adjusted for renal function, the minimum time possible, and according to the sensitivity results of the antibiogram in the urine culture. The physician changed the initial empirical treatment according to the result of the urine culture. Patients received treatment only if they met the criteria for UTI. That is, significant asymptomatic bacteriuria were not treated.
Protocol
Sample collection
A urine sample was obtained from each patient at the time of enrollment, and at 1, 3, and 6 months thereafter. Urine sample collection was carried out in standard containers for urine collection. Urine samples were centrifuged immediately at 10,000 g to remove any formed debris, and the supernatant was frozen at –80 C until use. Standard blood tests were also performed in these patients’ follow up in primary care centers that included parameters related to kidney function, such as plasma creatinine concentration.
Proteinuria
Daily urinary protein excretion was determined by colorimetry using the Bradford method (1976). It consists of the formation of a blue-colored adsorption compound between the basic amino acid residues of proteins and the Coomassie Blue G-250 dye, which absorption maximum is at 595 nm.23
Albuminuria was determined by ELISA (Bethyl Laboratories, Montgomery, TX, USA. Ref: E-110-125-17). Absorbance was measured by spectrophotometry at a wavelength of 450 nm. NGAL was determined by a Human Lipocalin-2/NGAL Quantikine ELISA Kit/DLCN20 (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s specifications. Urine NGAL was measured in ng/ml, at the start and at the end of the study: these values were called initial NGAL or NGAL-1, and final NGAL or NGAL-2, respectively.
Urine NAG was determined by NAG: kit colorimétrico (Dyazime Laboratories, Poway, CA, USA, EEUU. Ref: DZ-062AKA), following the manufacturer’s specifications. Urine NAG was measured in UU.II./ml at the start and at the end of the study: these values were called initial NAG or NAG-1, and final NAG or NAG-2, respectively.
TGF-β1) was determined by Human TGF-β1 Quantikine ELISA Kit/DB100B (R&D Systems), following the manufacturer’s specifications. Urine TGF-β1 was measured in pg/ml at the end of the study.
At the time of determining the marker N-GAL-2, NAG-2, and TGF-β1, at the end of the study, the individual did not have to meet the criteria for active UTI.
Urine creatinine (uCr) was determined by a colorimetric kit (BioAssay Systems, Hayward, CA, USA, Ref: DICT-500), according to the manufacturer’s instructions. This analysis technique was based on the Jaffe method, which uses picric acid as a generating agent for a colored complex and creatinine present in plasma; its intensity can be measured by spectrophotometry at a wavelength between 490 nm and 530 nm. Measured absorbance is directly proportional to creatinine concentration in the sample.
GFR was determined using HUGE, MDRD, and Cockcroft-Gault formulas, based on plasma creatinine and anthropometric data.
Data analysis
The study data was organized in an Excel document. Results were analyzed with descriptive statistics, nonparametric statistical methods (Mann–Whitney test), Chi-squared test, Fisher’s exact test, and multivariate analysis two-step cluster. Statistical significance was accepted for p < 0.05.
Ethical Approval
Patients received an information sheet and signed the informed consent in order to participate in the study (Royal Decree 651/93). Good Clinical Practice Guidelines standards were respected.23,24
Ethical Research Committee on Medicines of Avila, Spain approved the project with code GRS1598/A/17 for Research with Medicines of Avila, Spain. All 200 subjects were institutionalized. Informed consents were obtained from the elders themselves, if possible, or from their legal representative when not possible due to any physical or mental impairment.
Results
All the variables presented a normal distribution. Mean age was 84.33 years old, standard deviation (SD) 8.11, median 86, range 65–99. There was no difference between GA and GB p = 0.336. There were 125 (62.5%) women and 75 (37.5%) men, there was no difference between men and women (p = 0.8838). Mean urine Cr was 81.05 mg/dl, SD 44.66, median 69, range 5–225.18, there were no differences in urine Cr (p = 0.4980). Mean NGAL-1 was 1.29 ng/ml, SD 1.40, median 0.88, range 0.04–8; NGAL-1 was lower in GA (p = 0.0010) (Table 1).
Table 1.
Distribution of the statistical results of the variables in the non-RUTI group and RUTI group.
| Variables | Non-RUTI group |
RUTI group |
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | p value | |
| Age (years) | 83.69 | 8.59 | 65–98 | 84.97 | 7.60 | 65–99 | 0.336 |
| Urine Cr (mg/dl) | 77.58 | 4.04 | 5–202.10 | 84.51 | 4.80 | 13–225.18 | 0.4980 |
| NGAL-1 (ng/ml) | 0.96 | 1.10 | 0–6.2 | 1.61 | 1.58 | 0.1–8 | 0.0010 |
| NGAL-2 (ng/ml) | 1.07 | 1.14 | 0–5.8 | 1.76 | 1.75 | 0.1–9.2 | 0.004 |
| NAG-1 (UU.II/ml) | 0.27 | 0.36 | 0.01–2.02 | 0.49 | 0.53 | 0.02–2.63 | 0.006 |
| NAG-2 (UU.II/ml) | 0.32 | 0.39 | 0–1.98 | 0.57 | 0.62 | 0.02–3.41 | 0.0009 |
| sCr (mg/dl) | 1.08 | 0.95 | 0.33–7.38 | 1.11 | 0.76 | 0.44–7.38 | 0.7753 |
| GFR (ml/min/m2) | 65.73 | 20.80 | 9.0–90.0 | 62.20 | 22.74 | 9.0–90.0 | 0.3530 |
| Pr Urine (mg/dl) | 12.34 | 11.85 | 0–75 | 21.00 | 36.88 | 0–247 | 0.0120 |
| Album Urine (mg/dl) | 8.42 | 16.85 | 0–75 | 11.92 | 26.19 | 0–150 | 0.8590 |
| TGFβ-1 (pg/ml) | 10.63 | 22.26 | 0.02–78.26 | 36.22 | 37.97 | 0.02–103.76 | 0.008 |
| Protein/creatinine ratio | 27.73 | 90.59 | 0.49–900 | 33.12 | 73.97 | 0.99–633.33 | 0.0162 |
| Albumin/creatinine ratio | 38.20 | 61.81 | 2.06–500 | 48.58 | 55.73 | 1.50–384.62 | 0.0071 |
| Degree of disability (Barthel Index)* | 50.00 | 0.10 | 41–60 | 25 | 0.15 | 21–40 | 0.0020 |
| Other variables | Non-RUTI group | RUTI group | |||||
| n | % | n | % | p value | |||
| Sex female/male | 63:37 | 63.00/37.00 | 62:38 | 62.00/38.00 | 0.8838 | ||
| Chronic diseases | |||||||
| Arterial hypertension | 42 | 42.86 | 56 | 57.14 | 0.0630 | ||
| Diabetes mellitus | 16 | 43.24 | 21 | 56.76 | 0.3525 | ||
| Dementia | 45 | 56.25 | 35 | 43.75 | 0.1545 | ||
| Dyslipidemia | 17 | 34.69 | 32 | 65.31 | 0.0044 | ||
| Medications | |||||||
| Metformin | 4 | 26.67 | 11 | 73.33 | 0.0268 | ||
| First step: Mild pain | 24 | 52.17 | 22 | 47.83 | 0.8350 | ||
| Diuretics | 24 | 45.28 | 29 | 54.72 | 0.4373 | ||
| ACE | 7 | 35.00 | 13 | 65.00 | 0.1128 | ||
| PPIs | 30 | 46.15 | 35 | 53.85 | 0.4831 | ||
| BZO | 37 | 47.44 | 41 | 52.56 | 0.6311 | ||
| Neuroleptics | 30 | 56.60 | 23 | 43.40 | 0.2437 | ||
Barthel Index: Mild dependence (61–99). Moderate dependence (41–60). Severe dependency (21–40). Total dependence (0–20).
ACE, angiotensin converting enzyme inhibitors; BZO, benzodiazepines; GFR, glomerular filtration rate; NAG, N-acetyl glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; PPIs, proton pump inhibitors; Pr Urine, proteins in urine; RUTI, recurrent urinary tract infection; sCr, serum creatinine; SD, standard deviation; TGFβ-1, transforming growth factor beta 1.
NGAL-2 mean was 1.41 ng/ml, SD 1.51, median 0.96, range 0.02–9.22. NGAL-2 was lower in GA than in GB (p = 0.004). Mean NAG-1 was 0.38 UU.II/ml, SD 0.46, median 0.22, range 0.01–2.63; NAG-1 in GA was lower than in GB (p = 0.006). Mean NAG-2 was 0.44 UU.II/ml, SD 0.53, median 0.29, range 0–3.41; NAG-2was lower in GA (p = 0.0009). Mean serum Cr was 1.10 mg/dl, SD 0.86, median 0.89, range 0.33–7.38; serum Cr was lower in GA (p = 0.7753) (Table 1).
Mean GFR was 63.96 ml/min/1.7603m2, SD 21.81, median 70, range 9–90. There were no differences in GFR between groups (p = 0.3530). Mean proteins in urine (PrUrine) was 16.67 mg/dl, SD 7.67, median 10, range 0–247; PrUrine was lower in GA (p = 0.0120). Mean albumin in urine (album urine) was 10.17 mg/dl, SD 22.04, median 0.10, range 0–150; there were no differences in urinary albumin between groups (p = 0.8590). Mean TGFβ-1 was 23.43 pg/ml, SD 33.58, median 4.81, range 0.02–103.76; TGFβ-1 was lower in GA (p = 0.008) (Table 1).
Table 1 shows the percentage of patients who presented with concomitant disease within each group. There were no differences between GA and GB (test Pearson’s Chi-Square: 13.8539; p = 0.3842). This aspect is essential: given that they are frail elderly subjects, comorbidities could suppose a significant bias that could affect renal function; it is vital that the two groups are comparable in terms of comorbidities or secondary diagnoses.
There were no differences between GA and GB regarding the percentage of patients undergoing concomitant treatment in each group (test Pearson’s Chi-Square: 13.8539; p = 0.3842). This aspect correlates with comorbidities (Table 1). However, since the same diseases can be treated with different types of drugs, which may have different nephrotoxic potentials, this aspect has also been analyzed. Mean protein/creatinine ratio was 30.42 mg/g, SD 82.53, median 14.28, range 0.49–900, and was lower in GA (p = 0.0162). Mean albumin/creatinine ratio was 43.39 mg/g, SD 58.93, median 27.91, range 1.50–500, and was lower in GA (p = 0.0071) (Table 1).
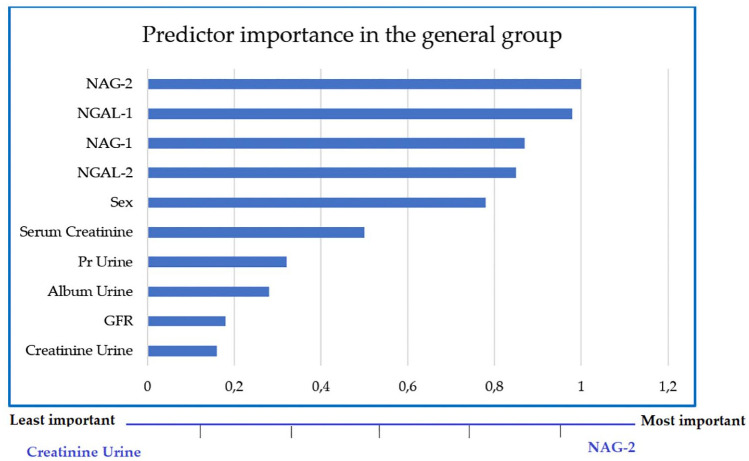
Multivariate analysis was performed using a two-step cluster; the 200 subjects (general group) represent four types of differentiation, the smallest cluster corresponds to 5.5% and the largest cluster is 49.7% of the group. NAG-2 and NGAL-1 were found to be the most important and determining factors and urine Cr was the least important factor among all the investigated variables (Figure 1).
Figure 1.
Most important variables in the clusters of the general group.
GFR, glomerular filtration rate; NAG, N-acetyl glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; Pr Urine, proteins in urine.
GA cluster sizes were 9.1% for the smallest group and 57.6% for the largest. It was found that the most crucial factor was NGAL-2, followed by NAG-1, and the least important was sCr (Figure 2).
Figure 2.
Most important variables in the clusters of GA.
GA, group A; GFR, glomerular filtration rate; NAG, N-acetyl glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin.
In GB, the two-step cluster revealed a more radical distribution than GA, the smallest group was 18% and the largest 82%, and in this case the factor with the most importance was NGAL-1, followed by NAG-2. GFR was the least essential variable in GB (Figure 3).
Figure 3.
Most important variables in the clusters of GB.
GB, group B; GFR, glomerular filtration rate; NAG, N-acetyl glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; Pr Urine, proteins in urine.
Gender was the most important factor, with being female explaining between 63.60% and 83.30% of variance; this is a determinant for evaluating kidney damage markers. With its level being the highest in the individual indicators, sex was most frequent and the most important. Therefore, it has been distinguished that the determining variables in groups A and B differ, with the markers NGAL-2 and NAG-1 being critical determinants for patients with non-recurrent UTIs, and NGAL-1 and NAG-2 for patients with RUTIs.
In GA, 60 elderly patients presented no UTI in the year prior to the start of the study, 30 elderly patients presented one UTI in the year before the start of the study, 10 elderly patients presented two UTIs in the year before the start of the study with a separation of more than 6 months between the two ITUs. Therefore, when a subject had two UTIs, they did not meet RUTI criteria.
In GB, all subjects presented more than three UTIs in the year prior to the start of the study: mean 10.63, SD 3.21, median 8, range 6–16. Of all the UTIs presented in GB in the year before the study began, 30% were feverish: 28% uncomplicated pyelonephritis and 2% prostatitis. During the year of follow up in the study, in GA 70 subjects had no UTIs, 30 subjects had one UTI, nobody had two UTIs. Nobody in GA had febrile UTI, whether pyelonephritis or prostatitis.
During the study follow-up year, in GB all the elderly presented more than three UTIs: mean 9.22, SD 4.52, median 8, range 5–17. Of all the UTIs presented in GB in the following year of the study, 28% were feverish: 26% uncomplicated pyelonephritis and 2% prostatitis. Asymptomatic bacteriuria is not considered a UTI.
Discussion
UTIs are the most frequent bacterial infections in the elderly population. UTI prevalence increases with age as aging produces alterations in defensive mechanisms against infection. Adding to this, this population group has high comorbidity, instrumentation, and frequent hospitalization, which increases nosocomiality. Complicated UTI can arise in a heterogeneous group of patients. However, neither age nor patient gender seems to have relevance in renal function deterioration of the elderly.24,25
According to the Guide of the European Association of Urology,22 patients included in the high-risk group for complicated UTI require rapid evaluation, designed specifically to reduce both short-term and long-term morbidity and mortality. A urine culture is required (unlike uncomplicated UTIs). An imaging study is essential to rule out other complications, mainly in patients with congenital kidney malformations, immunosuppressed patients, or the elderly.25,26
Despite the high prevalence of RUTI, and the damage in the health status attributed to UTI in the elderly, no specific scientific articles reflect chronic kidney damage or the affectation of permanent renal function as a sequel due to the RUTI. It has been demonstrated that kidney damage can be evidenced by the elevation of urinary markers of kidney damage (NGAL, GAL, and TGFβ-1 among others).11 For this reason, we have developed this research to clarify if RUTI can be associated with chronic kidney damage. The main finding of this research is that we have found that UTIs can cause renal damage in frail elderly.
Regarding the renal function markers studied, we found no differences in Urine Cr in between GA and GB (p = 0.4980). Likewise, there were no differences in serum creatinine between GA and GB (p = 0.77539). GFR was lower in GA (p = 0.7753). Pr Urine was lower in GA (p = 0.0120). There were no differences in urinary albumin between GA and GB (p = 0.8590).
Secondary diagnoses and concomitant medications must be considered carefully in the frail elderly setting, as these patients often have comorbidities and treatments that could affect kidney function. There were no differences on secondary diagnoses and concomitant treatments between GA and GB.
Many studies have reported that NAG and NGAL biomarkers are important in early detection and prediction of kidney damage in patients with diabetes, and are considered biomarkers for acute kidney injury.23,24
We intend to demonstrate that UTIs are, a priori, not complicated. However, RUTIs are a threat to kidney function, entailing a deterioration of health status that must be taken into account in frail elderly people. This research is the first step for a subsequent action plan to protect these frail elderly people against RUTIs, and consequently to elevate their health status.
In our study, we found outstanding kidney damage diagnostic accuracy for patients with RUTI. Both urinary NGAL and urinary NAG tested in this study demonstrated significantly higher mean values among RUTI patients, supporting the fact that these two biomarkers could help detect renal damage caused by RUTI.
Although patients with RUTI are thought to have less kidney function and more kidney damage, there is no published data, and it has not been demonstrated in individuals who only have RUTI as a threat to their kidney function. Our study demonstrates the burden of impaired kidney function caused by RUTI.
NAG is excreted from the renal tubular tissue and is relatively stable in the urine, it has minimal diurnal variations and a high molecular weight that prevents its filtration by the glomerulus.26,27 Multivariate analysis shows that, in frail elderly subjects, the variables with the most significant weight concerning renal damage are NAG and NGAL, before gender, sCr or the GFR.
NGAL-1 and NGAL-2 were lower in GA than in GB. NGAL is increased in chronic renal injury.27,28,29 If NGAL is elevated, there is more tubular production, as several authors have demonstrated, or because there is less tubular reabsorption, as has just been shown by Sancho-Martínez et al.29,30
Two urinary markers of kidney damage – NGAL and NAG – were lower in GA than GB both at the beginning and at the end of the study. These data are new: until now, it has not been described that the elderly with RUTI had these higher urine markers. Furthermore, the data is consistent with lower sCr in GA versus GB. Also TGFβ-1 is lower at the end of the study in GA versus GB. However, there are no differences between eGFR between GA and GB at the study endpoint. We hypothesized that this mismatch is due to greater fineness, precision, or that urinary markers can indicate kidney damage earlier than eGFR data. Multivariate analysis supports the hypothesis that NGAL and NAG are the most important markers related to kidney damage in these patients.
Does this observation matter at the level of clinical management of frail elderly with RUTI? The authors of this article are firmly convinced that yes: to establish preventive measures and improve management, the damage attributed to the condition must first be demonstrated. We understand that it is crucial to keep the frail elderly as protected as possible from kidney damage. This article demonstrates two issues: 1. RUTI can cause kidney damage; 2. NGAL and NAG markers can indicating this damage earlier than eFGR.
Neither the protein/creatinine ratio nor the albumin/creatinine ratio in the multivariate analysis was performed with renal damage or renal function markers.
Therefore, the contribution of our research is to demonstrate that RUTI are a significant threat that can cause renal damage, which leads to functional sequelae in the frail elderly.
Conclusion
The frail elderly with criteria for RUTI, without differences with a comparable group in age or gender, presented increased urinary markers of renal damage, specifically NGAL, NAG, and TGFβ-1, compared with the elderly without UTIs.
People with RUTI had higher levels of markers of kidney damage than those without RUTI. The fact that this difference is observed, in the absence of GFR and creatinine differences, may indicate that these markers are more sensitive markers of kidney damage than traditional markers in elderly disabled people.
This finding is of great importance since residual permanent kidney damage is not attributed currently to UTI, either in the young population or in the elderly. To develop drastic strategies in the management of RUTI in the frail elderly, the first step is to determine the problem’s clinical significance.
Footnotes
Author contributions: Conceptualization, M.F.L.G and M.B.G.C.; Methodology, MFLG.; Software, M.M.S; Validation, MFLG; Formal Analysis, M.M.S; Investigation, I.G.C, THS, CMA; Resources, PUBLIC HEALTH SYSTEM OF CASTILLA Y LEON; Data Curation, M.M.S; Writing – Original Draft Preparation, JFF; B.P.F; Writing – Review & Editing, J.F.F and M.C.F.F.; Visualization, J.F.F; Supervision, M.F.L.G; Project Administration, S.V.M.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study has been funded with a research grant from the Regional Management of Public Health System of Castilla y León (SACYL) with the code GRS1598/A/17.
ORCID iDs: Magaly Márquez-Sánchez  https://orcid.org/0000-0003-1046-0118
https://orcid.org/0000-0003-1046-0118
Javier Flores-Fraile  https://orcid.org/0000-0003-1338-0551
https://orcid.org/0000-0003-1338-0551
Contributor Information
María-Fernanda Lorenzo-Gómez, Department of Surgery, University of Salamanca, Salamanca, Spain Multidisciplinary Renal Research Group) of the Institute for Biomedical Research of Salamanca (IBSAL), Spain Urology Service of the University Hospital of Salamanca, Salamanca, Spain.
María-Carmen Flores-Fraile, Department of Surgery, University of Salamanca, Salamanca, Spain.
Magaly Márquez-Sánchez, Multidisciplinary Renal Research Group) of the Institute for Biomedical Research of Salamanca (IBSAL), Spain.
Javier Flores-Fraile, Department of Surgery, University of Salamanca, Alfonso X el sabio Campus Miguel de Unamuno, Salamanca, 37008, Spain.
Ignacio González-Casado, Multidisciplinary Renal Research Group) of the Institute for Biomedical Research of Salamanca (IBSAL), Spain.
Bárbara Padilla-Fernández, Department of Surgery, Section of Urology, University La Laguna, Tenerife, Spain.
Sebastián Valverde-Martínez, Department of Surgery, University of Salamanca, Salamanca, Spain Multidisciplinary Renal Research Group) of the Institute for Biomedical Research of Salamanca (IBSAL), Spain Department of Urology of University Hospital of Avila, Spain.
Teresa Hernández Sánchez, Urology Service of the University Hospital of Salamanca, Salamanca, Spain.
Carlos Muller-Arteaga, Department of Urology of University Hospital of Ourense, Spain.
María-Begoña García-Cenador, Department of Surgery, University of Salamanca, Salamanca, Spain.
References
- 1. Baztan-Cortés J-J. Función y fragilidad: ¿ qué tenemos que medir? Revista Española de Geriatría y Gerontología 2006; 41: 36–42. [Google Scholar]
- 2. Abizanda Soler P, Gómez-Pavón J, Martín Lesende I, et al. Detección y prevención de la fragilidad: una nueva perspectiva de prevención de la dependencia en las personas mayores. Medicina Clínica 2010; 135: 713–719. [DOI] [PubMed] [Google Scholar]
- 3. Whitson HE, Purser JL, Cohen HJ. Frailty thy name is . . . Phrailty? J Gerontol A Biol Sci Med Sci 2007; 62: 728–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wojszel ZB, Toczyńska-Silkiewicz M. Urinary tract infections in a geriatric sub-acute ward-health correlates and atypical presentations. Eur Geriatr Med 2018; 9: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giarratano A, Green SE, Nicolau DP. Review of antimicrobial use and considerations in the elderly population. Clin Interv Aging 2018; 13: 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gutierrez-Perez M-I, Lorenzo-Gomez M-F. Infecciones urinarias. In: Gutierrez-Perez M, Amón-Sesmero J. (eds) Manejo De La Patología Urológica En Atención Primaria. 1. Valladolid: MI Gutiérrez-Pérez; 2013, pp.47–105. [Google Scholar]
- 7. Anton-Jimenez M, Esteban-Saiz R, Ortes-Gomez R. Infección urinaria. In: Sedgy Gerontología. (ed.) Tratado de Geriatría para residentes. Madrid, España: International Marketing & Communication, S.A; (IM&C), 2006, pp.429–433. [Google Scholar]
- 8. Blázquez-Medela AM, García-Sánchez O, Blanco-Gozalo V, et al. Hypertension and hyperglycemia synergize to cause incipient renal tubular alterations resulting in increased NGAL urinary excretion in rats. PLoS One 2014; 9: e105988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Macías-Núñez JF, López-Novoa JM, Martínez-Maldonado M. Acute renal failure in the aged. Semin Nephrol 1996; 16: 330–338. [PubMed] [Google Scholar]
- 10. Quirós Y, Blanco-Gozalo V, Sanchez-Gallego JI, et al. Cardiotrophin-1 therapy prevents gentamicin-induced nephrotoxicity in rats. Pharmacol Res 2016; 107: 137–146. [DOI] [PubMed] [Google Scholar]
- 11. López-Novoa JM, Rodríguez-Peña AB, Ortiz A, et al. Etiopathology of chronic tubular, glomerular and renovascular nephropathies: clinical implications. J Transl Med 2011; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Treeprasertsuk S, Wongkarnjana A, Jaruvongvanich V, et al. Urine neutrophil gelatinase-associated lipocalin: a diagnostic and prognostic marker for acute kidney injury (AKI) in hospitalized cirrhotic patients with AKI-prone conditions. BMC Gastroenterol 2015; 15: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrology Dialysis Transplantation 2014; 29: 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994; 331: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 15. Raptis V, Bakogiannis C, Loutradis C, et al. Serum fas ligand, serum myostatin and urine TGF-beta1 are elevated in autosomal dominant polycystic kidney disease patients with impaired and preserved renal function. Kidney Blood Press Res 2018; 43: 744–754. [DOI] [PubMed] [Google Scholar]
- 16. Tsapenko MV, Nwoko RE, Borland TM, et al. Measurement of urinary TGF-beta1 in patients with diabetes mellitus and normal controls. Clin Biochem 2013; 46: 1430–1435. [DOI] [PubMed] [Google Scholar]
- 17. Iseki K, Ikemiya Y, Iseki C, et al. Proteinuria and the risk of developing end-stage renal disease. Kidney Int 2003; 63: 1468–1474. [DOI] [PubMed] [Google Scholar]
- 18. Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106: 1777–1782. [DOI] [PubMed] [Google Scholar]
- 19. Martín Lesende I, Gorroñogoitia Iturbe A, Gómez Pavón J, et al. El anciano frágil. Detección y tratamiento en AP. Atención Primaria 2010; 42: 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muller-Arteaga C. Prevención de la afectación de la función renal en las infecciones del tracto urinario con profilaxis antibiótica frente a vacuna bacteriana. [Investigación clínica original. Trabajo experimental.]. Salamanca, España: Universidad de Salamanca, 2016. [Google Scholar]
- 21. Ahnn S, Anderson SJ. Sample size determination for comparing more than two survival distributions. Stat Med 1995; 14: 2273–2282. [DOI] [PubMed] [Google Scholar]
- 22. Bonkat G, Bartoletti RR, Bruyère F, et al. EAU Guidelines on urological infections. In: European Association of Urology (ed.) EAU guidelines. Arnhem, The Netherlands: European Association of Urology, 2019. [Google Scholar]
- 23. Bradford M-M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976; 72: 248–54. [DOI] [PubMed] [Google Scholar]
- 24. Lorenzo-Gómez M-F. Sujetos participantes en la investigación. Garantías. In: Comisión-de-Investigación-del-Complejo-Asistencial-Universitario-de-Salamanca© (ed.) Guía de buenas prácticas en investigación. Salamanca, España: Comisión de Investigación del Complejo Asistencial Universitario de Salamanca©; 2015, pp.41–53. [Google Scholar]
- 25. Lorenzo-Gómez M-F. Urinary tract infection in the frail elderly. Salamanca, España: Universidad-de-Salamanca&Asociacion-Española-de-Urologia, 2017. [Google Scholar]
- 26. Neal DE., Jr Complicated urinary tract infections. Urol Clin N Am 2008; 35: 13–22. [DOI] [PubMed] [Google Scholar]
- 27. Sharifi AM, Zare B, Keshavarz M, et al. Urinary N-acetyl-β-D-glucosaminidase (NAG) activity in the early detection of diabetic nephropathy. Int J Diabetes Dev Ctries 2015; 35: 369–374. [Google Scholar]
- 28. Skálová S. The diagnostic role of urinary N-acetyl-beta-D-glucosaminidase (NAG) activity in the detection of renal tubular impairment. Acta Medica (Hradec Kralove) 2005; 48: 75–80. [PubMed] [Google Scholar]
- 29. Lábr K, Špinar J, Paˇrenica J, et al. Renal functions and prognosis stratification in chronic heart failure patients and the importance of neutrophil gelatinase-associated lipocalin. Kidney Blood Press Res 2018; 43: 1865–1877. [DOI] [PubMed] [Google Scholar]
- 30. Sancho-Martínez SM, Blanco-Gozalo V, Quiros Y, et al. Impaired tubular reabsorption is the main mechanism explaining increases in urinary NGAL excretion following acute kidney injury in rats. Toxicol Sci 2020; 175: 75–86. [DOI] [PubMed] [Google Scholar]