Abstract
Background:
B7-H3 is an important immunomodulatory molecule, and clinical studies have confirmed that its expression level is closely correlated with prostate cancer prognosis. However, the mechanism of its biological action is unclear.
Methods:
An engineered cell line overexpressing B7-H3 was constructed. Cell apoptosis, growth and proliferation assays in vitro and an animal model in vivo were performed to analyze the role and possible mechanism of B7-H3 in promoting prostate cancer progression.
Results:
Compared with the control cell line (Mock-RM-1), the B7-H3-overexpressing prostate cancer cell line (B7-H3-RM-1) showed no significant growth differences in vitro, whereas the in vivo tumorigenesis rate of B7-H3-RM-1 was significantly higher than that of Mock-RM-1. These results suggest that B7-H3indirectly, rather than directly, promotes prostate cancer progression. Further analysis revealed that significantly higher levels of myeloid-derived suppressor cells (MDSCs) accumulated in vivo in B7-H3-RM-1 tumor-bearing mice than in Mock-RM-1 mice. In vitro and in vivo experiments showed that B7-H3-RM-1 cells significantly antagonized MDSC apoptosis. To further confirm the role of MDSCs in B7-H3-mediated prostate cancer progression, model mice were pretreated with cyclophosphamide before inoculation to clear immune cells and achieve myelo suppression. The results showed no significant differences in tumor growth between the B7-H3-RM-1 group and the Mock-RM-1 group.
Conclusions:
We found, for the first time, that B7-H3 can antagonize MDSC apoptosis, leading to MDSC accumulation in the tumor microenvironment and thereby promoting prostate cancer progression.
Keywords: B7-H3, prostate cancer, MDSC, apoptosis
Background
Prostate cancer is a malignancy, and seriously threatens to men’s health. Prostate cancer has the second highest mortality rate among men’s cancers.1 Immunological factors play key roles in prostate cancer development; therefore, understanding immune regulatory mechanisms is necessary for developing targeted biological treatments or prostate cancer.
B7-H3 belongs to the recently discovered B7 superfamily of costimulatory molecules.2 Human B7-H3 has 2 spliced forms: 2IgB7-H3a and 4IgB7-H3b. The role of B7-H3 in immune regulation is controversial. Initial functional studies have shown that human B7-H3 synergistically stimulates CD4+ and CD8+ T cell proliferation and specifically promotes interferon-γ (IFN-γ) secretion.2 Subsequent experiments have also confirmed that B7-H3 is an immunosuppressor that inhibits the immune response in vivo by down regulating the type I helper T cell-mediated immune response.3 Recently, our group confirmed that B7-H3 played an important role in the mononuclear macrophagocyte-mediated inflammatory response through the Toll-like receptor (TLR) signaling pathway.4 The clinical significance of B7-H3 expression in tumor tissue has also received much attention. Research groups in China and abroad have reported abnormal B7-H3 expression in cancer tissues, such as lung, gastric, renal, breast and prostate cancers and neuroblastomas, and have confirmed that its expression level is closely related to tumor metastasis and prognosis; however, the mechanism of action is unclear.5-7
Clinical statistical analysis has shown that B7-H3 expression in tumor tissues is closely related to tumor metastasis. Among these tissues, prostate cancer has received the most attention in terms of B7-H3 expression and its clinical significance.8-10 Roth et al.10 analyzed the immunohistochemistry of tissue specimens from338 patients who underwent radical prostatectomies and found that B7-H3 expression was detected in all prostate cancer tissue specimens. In addition, the intensity of B7-H3 expression was significantly correlated with prostate cancer recurrence, with higher intensity being correlated with higher recurrence rates after surgery. Zang et al.11 also found that higher B7-H3 expression levels in prostate cancer tissues were associated with higher tumor metastasis, postoperative recurrence and mortality rates; however, the role of B7-H3 in tumor progression remains unclear. Further studies have confirmed that B7-H3 negatively regulates T cell-mediated immune responses, andB7-H3 has been proposed to be the next most promising immune checkpoint molecule.7,12
Myeloid-derived suppressor cells (MDSCs) suppress immune cells, such asmononucleocytes/macrophages, granulocytes, immature dendritic cells, and other myeloid cells, and play important roles in tumor progression and metastasis.13 Mouse MDSCs are defined as Gr-1+ and CD11b+ double-positive cells. No uniform standard exists for defining human MDSCs. The widely accepted standard markers are HLA-DR-CD33+CD11b+ and CD14+HLA-DR-/low, with the former set being considered indicative of multinuclear-derived MDSCs and the latter set being indicative of mononuclear-like MDSCs.14-17 MDSCs can generate negative regulatory functions by inhibiting effect or T cell responses, inducing regulatory T (Treg) lymphocyte expansion, and forming a complex network with natural killer (NK) cells and Treg cells.13
MDSCs are present in tumor-bearing animal models and can form premetastatic niches and promote lymph node metastasis of tumors (breast cancer) through CCR5,18 demonstrating that the CCR5-CCL5 (-MIP-MIP-1β) chemokine axis is present in tumor-regional lymph nodes. However, how MDSCs survive and form abundant aggregates in the tumor microenvironment remains unclear. In this study, a prostate cancer tumor-bearing mouse model was constructed using the RM-1 cell line, which differentially expresses B7-H3, and the mechanism of action by which B7-H3 promotes prostate cancer progression was examined by analyzing the effects ofB7-H3on MDSC apoptosis in vitro and in vivo.
Materials and Methods
Ethics Approval
The use of the animal in current study was approved by the Ethics Board of Soochow University. The mouse were anesthetized according to the relevant ethical requirements.
Cell Lines and Experimental Animals
Mouse prostate cancer RM-1 cells were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. Four- to 6-week-old C57BL/6 mice weighing 18-22 g were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. and housed in clean-grade experimental animal rooms.
All post-operative supportive care were made to minimize suffering. Post-operative supportive care is mandated by the Public Health Service based on the Guide for the Care and Use of Laboratory Animals (National Research Council. 2011).
The healthy state of mice was judged according to the weight change of mice, normal appetite, asleep and calm.
Reagents and Equipment
The reagents used were as follows: 1) Gateway BP Clonase II Enzyme Mix, Invitrogen (Carlsbad, CA, USA), Cat. No. 11789-020; 2) Gateway LR Clonase II Plus Enzyme Mix, Invitrogen (Carlsbad, CA, USA), Cat. No. 12538-120; 3) QIA quick Gel Extraction Kit, QIAGEN (Germantown, MD, USA), Cat. No. 28704; and 4) cell culture medium 1640+ 10% fetal bovine serum (FBS, HyClone, Waltham, MA, USA). Cell culture equipment was purchased from Corning (NY, USA).The automated PCR instrument was purchased from Applied Biosystems (Waltham, MA, USA), and the FACS Calibur flow cytometer was purchased from Beckman (FC500, Brea, CA, USA).FlowJo software (Ashland, OR, USA) was used for the flow cytometry analysis. The Bio 1200-II-A2 biosafety cabinet was purchased from Shanghai Zhen Zi Chuang Air Purification Equipment Co., Ltd (Shanghai, China).Anti-CD11b-PC7 was purchased from BioLegend (San Diego, CA, USA); anti-B7-H3-PE was purchased from eBiosciences (San Diego, CA, USA); anti-B7-H3-PE, anti-Gr-1-FITC, andanti-GR-1-PC5 were purchased from Bio Legend(San Diego, CA, USA); and anti-B7-H3 with blocking function was purchased from R&D systems(Minneapolis, MN, USA).
Constructing and Identifying Engineered RM-1 Cells Overexpressing B7-H3
After RM-1 cells were transfected with the B7-H3 gene or an empty vector, RM-1/B7-H3-eGFP cells (B7-H3-RM-1) and RM-1/ eGFP cells (Mock-RM-1) of high purity were obtained by selection pressure and subcloning. The 2 engineered cell lines and RM-1 blank cells were examined by flow cytometry to measure the B7-H3 expression rate and determine the transfection efficiency.
Flow Cytometry and Analysis
Cells from tissues were treated and digested to prepare single-cell suspensions. Cell lines were directly subjected to flow cytometry after digestion. Polychromatic flow cytometry requires the prior establishment of compensation programs. A fluorescent antibody was added to the single-cell suspension, and the solution was incubated on ice for 30 minutes in the dark. After centrifugation and washing with 2 ml of phosphate-buffered saline (PBS), cells were resuspended in 0.5 ml of PBS and analyzed by flow cytometry. Data were analyzed entirely with FlowJo software.
Mouse Model Construction and Testing
Cyclophosphamide (CP) was intraperitoneally injected at 80 milligrams per kilogram of body weight once every 2 days for a total of 3 injections to construct the immunodeficient mouse model. The established stable RM-1B7-H3 cell line transfected with the B7-H3 gene and the negative control RM-1 cells were grown at 37°Cin 5% CO2 incubators. When cells reached 70% confluence, they were trypsinized, and suspensions were prepared. Cell concentrations were adjusted to approximately 5 × 107/ml with normal saline. Under aseptic conditions, 5×106cells (Mock-RM-1 and B7-H3-RM-1) were inoculated subcutaneously into the right groin of anormal or immunodeficient mouse at 100 µl per mouse. Mice inoculated with untransfected RM-1 cells were used as controls. The negative control group was the untransfected RM-1 cell group (Control); the experimental group was the B7-H3-transfected cell group.
Xenograft tumor occurrence and growth were observed daily, and the times at which the tumors appeared were recorded. Tumors were measured with a caliper every 3 days after the initial appearance to determine the longest diameter and width, and tumors over 1 mm were recorded with the formula size (mm3) = width ^ 2) x length/2.
Apoptosis Analysis
For the in vitro experiments, Gr-1+CD11b+ cells isolated from spleen (MDSC Sorting Kit, Miltenyi Biotec, Germany) were mixed with RM-1-B7-H3 and B7-H3-mock mouse prostate cancer RM-1 cells, and the cells were seeded in 24-well plates (1 × 105/ml/well)and incubated in humidified incubatorswith5% CO2 at 37°C. Cells were harvested, the annexin IV (ANXIV) and propidium iodide (PI) levels were examined by flow cytometry, and MDSC apoptosis was analyzed.
Apoptosis in tumor tissues was analyzed in vivo using spleen tissues. Cells were harvested and washed twice with PBS, and the collected cell yield was up to 5 × 105 cells/ml. A total of 0.5 ml of binding buffer was added to suspend the cells; PI was added and thoroughly mixed; and 0.005 ml of PI was then added and mixed for a 10-min reaction in the dark, followed by flow cytometry.
Cellomics Analysis of the Cell Growth Curve and Proliferation Rate
The transfected cells showed green fluorescence. The Cellomics instrument was able to detect and photograph fluorescent cells and then calculate the number of cells in the different groups via software analysis and processing. After 3-5 consecutive days of detection, the cell growth curve was plotted to show the cell growth status. After the digestion of each cell group in the logarithmic growth phase with trypsin, cell suspensions were prepared with complete medium, and the cells were counted with an improved Neubauer hemocytometer (cell density: 2,000 cells/well). Each experimental group contained 3 replicate wells with 100µl per well, and the number of cells added was consistent among all wells. After cells were seeded into the plates, the plates were incubated at 37°C in 5% CO2 incubators. Starting the second day after preparing the plates, 1 plate was tested every day with the Cellomics instrument for 5 consecutive days. Adjusting the input parameters allowed accurate calculation of the number of green fluorescent cells. The data were plotted to obtain a 5-day cell proliferation curve.
Statistical Analysis
GraphPad Prism5 was used to analyze the experimental data. The data were expressed as the mean ± standard deviation. A t-test or 1-way analysis of variance (ANOVA) was used to compare groups. P < 0.05 was considered statistically significant.
Results
Effects of B7-H3 on Prostate Cancer Cell Proliferation In Vitro
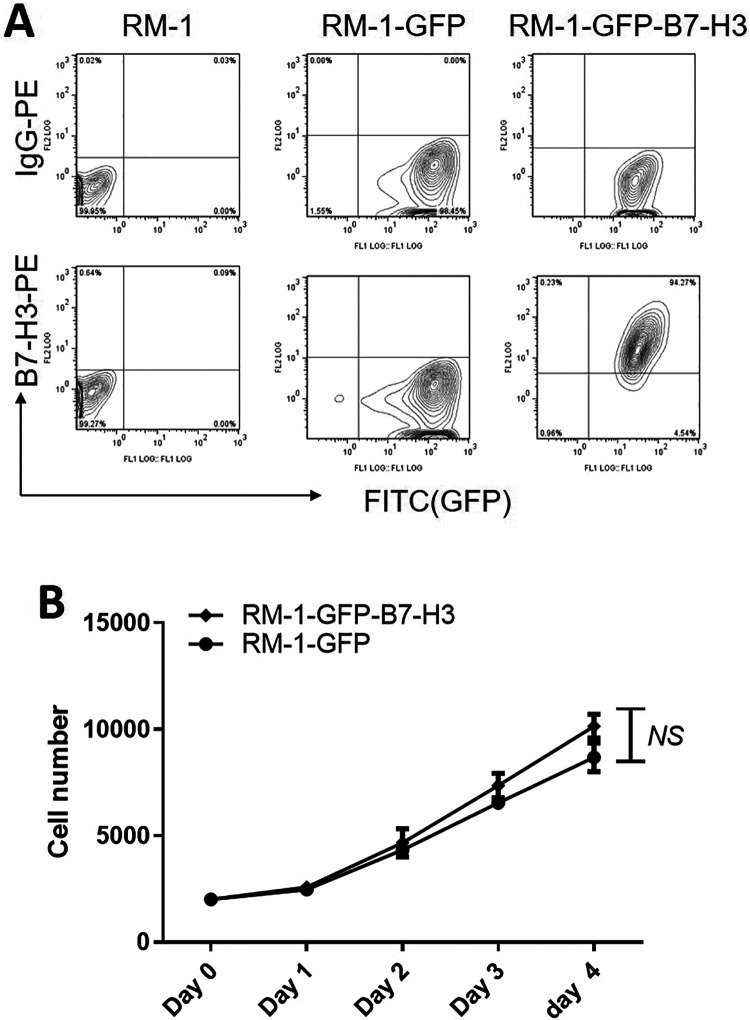
RM-1 mouse prostate cancer cells do not express B7-H3 or GFP. After successful transfection, we verified that the GFP group expressed only GFP but not B7-H3, while the GFP-B7-H3 group expressed both GFP and B7-H3 (Figure 1A). In vitro proliferation assays showed that when the proliferation rates of the B7-H3 overexpression group and the negative control group were compared, the mouse prostate cancer RM-1 cell proliferation rate was not significantly inhibited, suggesting that the B7-H3 gene is not associated with RM-1 cell proliferation (Figure 1B).
Figure 1.
Effect of B7-H3 on prostate cancer cell proliferation in vitro. A. Flow cytometry to analyze B7-H3 and GFP expression in cells after transfection. Left: Blank control group; Middle: GFP control group; Right: GFP-B7-H3 experimental group.B. Effect of B7-H3 on RM-1 cell proliferation. Tumor cells in the experimental and control groups were counted every 24 hours. The upper portion is the experimental group curve, and the lower portion is the control group curve.
Effect of B7-H3 on Prostate Cancer Xenograft Tumor Growth
Xenograft tumor growth in the RM-1 cell control group was slower, where as xenograft tumor growth in the RM-1-GFP-B7-H3 cell group was significantly accelerated. Tumors were removed after 22 days of in vivo implantation. The tumor volume was significantly larger in the experimental group than in the control group. Figure 2 shows that B7-H3 overexpression plays asignificant role in promoting prostate cancer RM-1 xenograft tumor growth in vivo.
Figure 2.
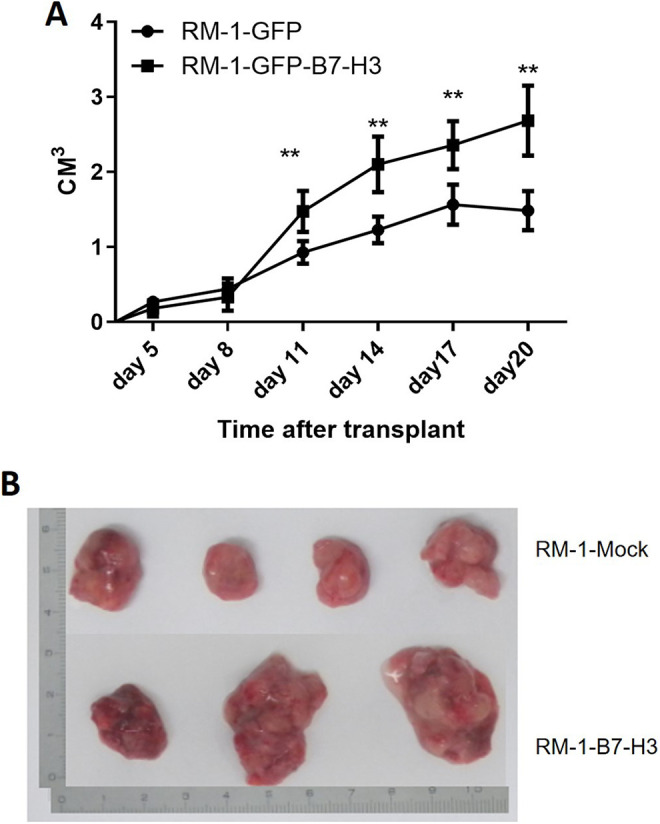
Effect of B7-H3 on prostate cancer cell tumorigenesis in vivo. A. Transplanted tumor growth curves. The top curve shows the changes in xenograft tumor volumes in the groins of the B7-H3 overexpression group, and the lower curve shows the changes in the tumor volumes in the B7-H3 underexpression group. B. Tumor morphology in the experimental and control group. The 4 tumor specimens shown at the top are from the control group, and the 3 specimens shown at the bottom are from the experimental group.
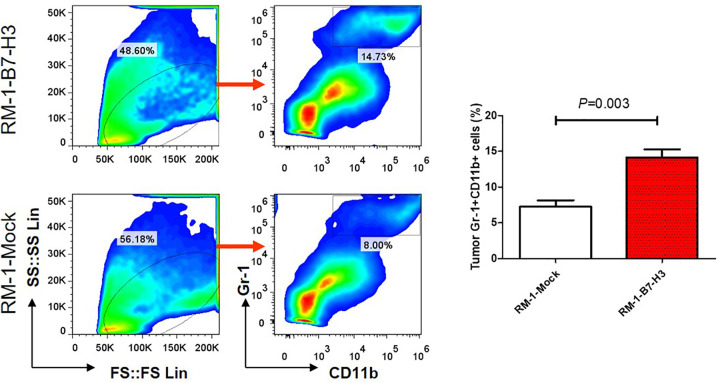
B7-H3 Promotes MDSC Accumulation in Prostate Cancer Tissue
MDSC accumulation in the tumor tissues of the B7-H3 overexpression group was significantly higher than in the tumor tissues of the B7-H3 under expression group (Figure 3). Similar results were also observed in the spleen (Figure 1), suggesting that B7-H3 may be involved in regulating MDSC accumulation in tissues.
Figure 3.
B7-H3 signaling regulates MDSC accumulation in prostate cancer tissues. After tumor growth for 20 days, MDSC accumulation in the tumor tissues of the B7-H3-RM-1 and Mock-RM-1 mice (n = 4) was analyzed.
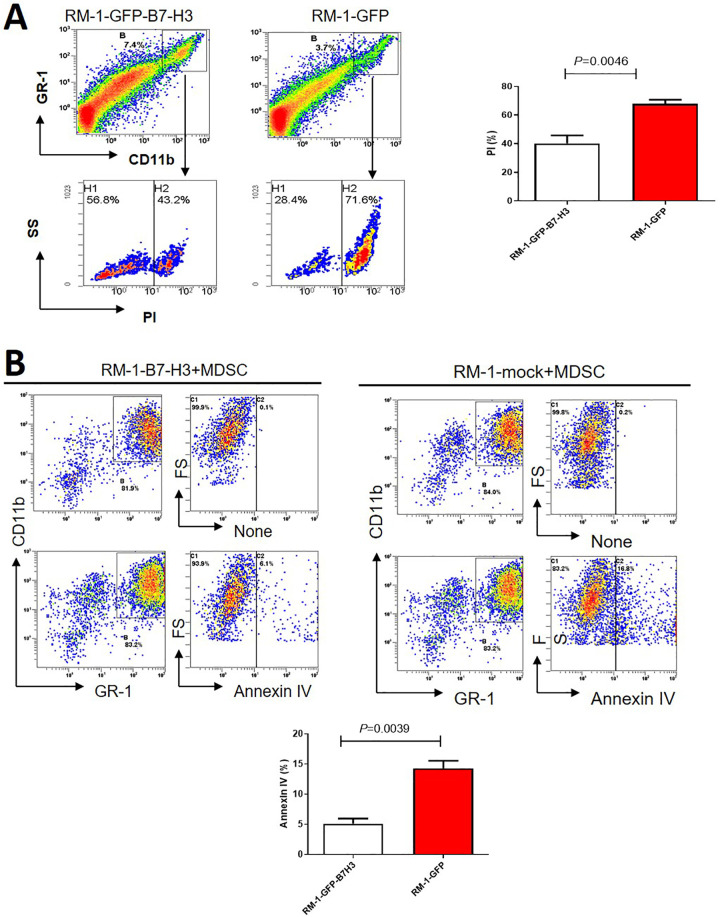
B7-H3 maybe Antagonizes MDSC Apoptosis
To investigate the possible mechanism by which B7-H3 promotes MDSC accumulation, we first analyzed MDSC apoptosis in tissues from the B7-H3-RM-1 and Mock-RM-1 groups. The results are shown in Figure 4A, revealing that the apoptosis rate of Gr-1+CD11b+MDSCs in the B7-H3-RM-1 group was lower than that in the Mock-RM-1 group, with statistical significance. Furthermore, we investigated the effect of B7-H3 on MDSC apoptosis in vitro and found that MDSC apoptosis was significantly inhibited in the B7-H3-RM-1 group compared with the Mock-RM-1 group (Figure 4B).
Figure 4.
B7-H3 significantly prevents MDSC apoptosis. A. In vitro experiments confirmed that B7-H3-RM-1 cells significantly inhibited MDSC apoptosis compared with Mock-RM-1 cells (n = 3). B. Tissue analysis from tumor-bearing mice shows the difference between B7-H3-RM-1 cells and Mock-RM-1 cells in antagonizing MDSC apoptosis in vivo (n = 4).
The Immunosuppressive Mouse Model
Cyclophosphamide is the most commonly used alkylating agent for treating malignant tumors. Cyclophosphamide treatment significantly inhibited mouse NK cell activity, lymphocyte transformation and neutrophil phagocytosis. The B7-H3 overexpression and under expression groups did not significantly differ in terms of tumor size, suggesting that upon inhibition of immune cell functions, the B7-H3 gene cannot stimulate mouse prostate cancer cell growth in vivo. Therefore, we hypothesize that B7-H3 promotes prostate cancer progression in a myeloid-dependent manner (Figure 5).
Figure 5.
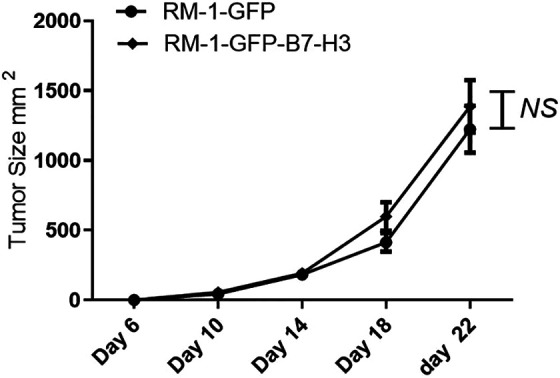
B7-H3 fails to promote prostate cancer progression in myelosuppressed mice (n = 3).
Discussion
B7-H3 expression has been observed in several human tumor tissue types, including prostate, renal clear cell, non-small cell lung, pancreatic, gastric, ovarian, colon, and urothelial cancers, as well as in tumor vascular endothelial cells.5,6 However, the exact role of B7-H3remains unclear, and functions in both the stimulation and inhibition of tumor immunity have been reported.19-22 However, clinical studies have shown that the expression level of B7-H3 is closely related to tumor prognosis,10,11,23 suggesting that B7-H3plays a key role in tumor progression.
This study investigated the biological behavior of mouse prostate cancer RM-1 cells overexpressing tumor-associated B7-H3. The in vitro growth rate of the RM-1 cells overexpressing B7-H3 did not change significantly, indicating that B7-H3 overexpression did not affect the proportion of proliferating RM-1 cells and that B7-H3 was uninvolved in regulating tumor cell apoptosis in the absence of external stimuli. However, the tumor growth indicator results showed that the growth of xenograft tumors in the B7-H3-RM-1 group was significantly higher than that in the control group. The above results suggest that the B7-H3 molecule itself cannot promote prostate cancer cell proliferation, but it can promote prostate cancer cell growth in vivo through other means. To further characterize this intermediate link, we selected MDSCs that are important for tumor progression as the entry point.
MDSCs are phenotypically heterogeneous cells that play important roles in tumor immune escape.24 Because their differentiation into normal dendritic cells or macrophages is blocked, myeloid cells secrete cytokines that promote tumor growth, participate in the formation of tumor blood vessels, and induce Treg production, thereby inhibiting the antitumor effects of T lymphocytes. MDSCs are abnormally amplified in tumor-bearing mouse models. MDSC amplification is found in the spleens and tumors of tumor-bearing mice. MDSCs are produced in a non-neoplastic state and differentiate under appropriate conditions into myeloid terminal cells that are morphologically and functionally normal but not immunosuppressive. In contrast, MDSCs produced under tumor conditions differentiate into morphologically and functionally mature terminal cells with immunosuppressive functions. MDSCs can promote negative regulatory functions by inducing the apoptosis of activated T-cells, releasing inhibitory cytokines, inducing Treg production and forming a complex network with NK cells and Tregs, thus inhibiting the development of tumor immunity and leading to tumor immune escape.
Many studies have been conducted on the role of MDSCs in tumor immune escape. MDSC is ubiquitous in the tumor microenvironment of solid tumors, and B7-H3 is over expressed in many other tumors. Hence it could suppose that this mechanism is common in biological systems. In this study, we examined the effect of B7-H3expression on MDSC regulation in the tumor tissues and spleen specimens of experimental mice and analyzed the biological characteristics of MDSC aggregation and apoptosis. This study showed that tumor tissues overexpressing B7-H3 accumulated significantly higher (P < 0.01) MDSC levels than tumor tissuesunderexpressingB7-H3. We compared the functional differences between B7-H3 overexpression and underexpression in inhibiting MDSC apoptosis. Compared with the control group, the B7-H3 overexpression group showed significant inhibition of MDSC apoptosis and significantly differed from the underexpression group. Therefore, we speculate that B7-H3 promotes mouse prostate cancer RM-1tumor development in vivo during this process, as promoting proliferation and inhibiting MDSC apoptosis constitute an important mechanism of B7-H3 action. To further confirm this result, we repeated this experiment in the cyclophosphamide-treated mouse immunosuppressed mouse model. No significant differences were found in the tumor volume between the B7-H3 overexpression group and underexpression groups. Cyclophosphamide can significantly decrease the total T cell, T helper cell and inhibitory T cell numbers, thus inhibiting immune function. The experiment further showed that the function of the B7-H3 gene in mouse prostate cancer cells is achieved by regulating immune cells.
Conclusion
In conclusion, we found, for the first time, that B7-H3 does not directly promote prostate cancer progression but instead requires the involvement of immune cells, specifically MDSCs. In addition, we found, for the first time, that B7-H3 can prevent MDSC apoptosis, but the molecular mechanism involved is unclear. Our previous work showed that B7-H3 binds to myeloid cells, indicating a potential B7-H3 receptor on these cells. In subsequent experiments, we will continue to explore the molecular signaling mechanism of B7-H3 in antagonizing MDSC apoptosis.
List of abbreviations
- (CP)
Cyclophosphamide
- (IFN-γ)
interferon-γ
- (MDSCs)
Myeloid-derived suppressor cells
- (NK)
natural killer
- (PBS)
phosphate-buffered saline
- (Treg)
regulatory T
- (TLR)
Toll-like receptor
Footnotes
Author Contributions: Author contributions: Y.Z., W.Z., J.H., Y.H. designed research; Y.Z., G.B., X.W. performed research and contributed to data analysis and interpretation; Y.Z., G.B. drafted the manuscript; J.H., Y.H., had the critical revision of the manuscript for scientific and factual content. Y.Z., G.Z. and W.Z. contributed equally to this work.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval: The use of the animal in current study was approved by the Ethics Board of Soochow University.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Nos. 81072085 and 81272839) and by the Jiangsu Provincial Key Medical Discipline ZDXKA2016012.
ORCID iD: Yuhua Huang  https://orcid.org/0000-0002-0341-0078
https://orcid.org/0000-0002-0341-0078
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Chapoval AI, Ni J, Lau JS, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2(3):269–74. [DOI] [PubMed] [Google Scholar]
- 3. Suh WK, Gajewska BU, Okada H, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906. [DOI] [PubMed] [Google Scholar]
- 4. Zhang G, Wang J, Kelly J, et al. B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol. 2010;185(6):3677–3684. [DOI] [PubMed] [Google Scholar]
- 5. Qin X, Zhang H, Ye D, Dai B, Zhu Y, Shi G. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. Onco Targets Ther. 2013;6(3):1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kraan J, van den Broek P, Verhoef C, et al. Endothelial CD276 (B7-H3) expression is increased in human malignancies and distinguishes between normal and tumour-derived circulating endothelial cells. Br J Cancer. 2014;111(1):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: friend or foe? Int J Cancer. 2014;134(12):2764–2771. [DOI] [PubMed] [Google Scholar]
- 8. Lehmann BD, Paine MS, Brooks AM, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68(19):7864–7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crispen PL, Sheinin Y, Roth TJ, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14(16):5150–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roth TJ, Sheinin Y, Lohse CM, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67(16):7893–7900. [DOI] [PubMed] [Google Scholar]
- 11. Zang X, Thompson RH, Al-Ahmadie HA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104(49):19458–19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7-H3 (CD276) for human cancer immunotherapy. Clin Cancer Res. 2016;22(14):3425–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thevenot PT, Sierra RA, Raber PL, et al. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41(3):389–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. [DOI] [PubMed] [Google Scholar]
- 15. Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-Sign. Cancer Res. 2010;70(11):4335–4345. [DOI] [PubMed] [Google Scholar]
- 16. Vuk-Pavlović S, Bulur PA, Lin Y, et al. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70(4):443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DRlow/- monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117(3):872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8(12):1369–1375. [DOI] [PubMed] [Google Scholar]
- 19. Hofmeyer KA, Ray A, Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci U S A. 2008;105(30):10277–10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci U S A. 2008;105(30):10495–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leitner J, Klauser C, Pickl WF, et al. B7-H3 is a potent inhibitor of human T-cell activation: no evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39(7):1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vigdorovich V, Ramagopal UA, Lázár-Molnár E, et al. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure. 2013;21(5):707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamato I, Sho M, Nomi T, et al. Clinical importance of B7-H3 expression in human pancreatic cancer. Br J Cancer. 2009;101(10):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]