Abstract
Background:
Neoadjuvant anthracycline-taxane-based chemotherapy (ChT) is a standard of care treatment option for stage II–III breast cancer (BC) patients. However, the optimal duration of neoadjuvant ChT has been poorly investigated so far.
Material and methods:
We retrospectively retrieved clinical data of patients with stage II–III human epidermal growth factor receptor 2-negative (HER2–) BC who were treated between October 2007 and January 2018 with neoadjuvant AT (doxorubicin-paclitaxel) for three cycles followed by CMF (cyclophosphamide-methotrexate-5-fluorouracil) for three cycles (cohort A) or with four AT cycles followed by four CMF cycles (cohort B). The aim of our study was to investigate the impact of neoadjuvant ChT duration (cohort A versus cohort B) on pathological complete response (pCR) rates, disease-free survival (DFS) and overall survival (OS).
Results:
Of 209 HER2– BC patients included, 62 had triple-negative breast cancer (TNBC) and 147 had hormone receptor-positive (HR+) BC. Median age was 48 years (range 30–74 years). A total of 111 patients belonged to cohort A and 98 patients belonged to cohort B. pCR was detected in 29 (13.9%) patients, 25 (40.3%) of whom had TNBC and four (2.7%) had HR+ HER2– BC. Patients achieving pCR had significantly longer DFS and OS, with statistical significance reached only in patients with TNBC. We found no differences between cohort A and cohort B in terms of pCR rates (15.3% versus 12.2%; p = 0.55), DFS (p = 0.49) or OS (p = 0.94). The incidence of grade 3/4 adverse events was similar in cohort A versus cohort B as well (22.5% versus 19.4%; p = 0.54).
Conclusion:
Shorter duration of neoadjuvant anthracycline-taxane ChT was not associated with worse clinical outcomes in patients with stage II–III BC. Prospective studies are needed to evaluate whether the duration of neoadjuvant anthracycline-taxane-based ChT can be reduced in specific patient subgroups without negatively affecting clinical outcomes.
Keywords: chemotherapy duration, disease-free survival, HER2-negative breast cancer, neoadjuvant chemotherapy, overall survival, pathological complete response, stage II–III breast cancer
Introduction
Anthracyclines and taxanes are the backbone of (neo)adjuvant chemotherapy (ChT) regimens in patients with early-stage breast cancer (BC).1–3 Although results of large randomized clinical trials demonstrated that neoadjuvant and adjuvant ChT are associated with similar long-term outcomes,4–6 preoperative ChT has the advantage to induce tumor downstaging and downsizing before surgery, thus increasing the rate of breast-sparing surgery.7,8 In addition, the antitumor activity of neoadjuvant ChT can provide valuable prognostic information, because patients achieving pathological complete response (pCR) have significantly longer disease-free survival (DFS) and overall survival (OS) when compared to patients with residual disease after primary systemic treatment.9–11 Notably, although the association between pCR and improved long-term outcomes is stronger in patients with triple-negative breast cancer (TNBC) and HER2-positive (HER2+) BC when compared to patients with hormone receptor-positive (HR+) BC, recent pooled analyses from large patient series showed that pCR is predictive of long-term clinical outcomes in all BC subgroups.12,13
Finally, failure to achieve pCR during neoadjuvant therapy can indicate the necessity to administer additional adjuvant therapies to reduce the risk of disease relapse and patient death. For instance, in the CREATE-X trial, six to eight triweekly cycles of adjuvant capecitabine prolonged DFS and OS in Asian HER2– BC patients with residual disease after preoperative ChT,14 while the KATHERINE study demonstrated that adjuvant Trastuzumab emtansine (T-DM1) reduces disease relapse rates in HER2+ BC patients failing to achieve pCR after neoadiuvant ChT-trastuzumab biochemotherapy.15 Based on these studies, several cancer centers have adopted adjuvant capecitabine or T-DM1 after neoadjuvant systemic therapies and surgery in patients with HER2– BC and HER2+ BC, respectively. Ongoing experimental trials are testing the efficacy of new adjuvant treatments, such as platinum compounds, PARP inhibitors or immunotherapy, after surgery in BC patients failing to achieve pCR during standard preoperative ChT (NCT02445391; NCT02032823; NCT02926196).16,17
Over the past few decades, there has been a remarkable evolution in (neo)adjuvant ChT regimens, from the ‘old’ CMF regimen (consisting of a combination of cyclophosphamide, methotrexate and fluorouracil)18 until more recent anthracycline and taxane-containing regimens.19–21 Currently, the sequential administration of cytotoxic ChT combinations with different mechanisms of action represents the standard of care of (neo)adjuvant ChT in BC patients. The first sequential regimen to demonstrate clinical efficacy consisted of doxorubicin (adriamicin)/epirubicin monotherapy followed by CMF (A→CMF), which showed superior clinical results when compared with CMF alone.22 Then, the phase III ECTO (European Cooperative Trial in Operable Breast Cancer) trial showed higher efficacy of doxorubicin-paclitaxel (AT) combination followed by CMF (AT→CMF) when compared with A→CMF in both the neoadjuvant and adjuvant settings, with superior rates of conservative surgery in patients receiving preoperative ChT.23 After the publication of the ECTO study, other trials have established sequential anthracycline-taxane regimens, and in particular four triweekly cycles of doxorubicin-cyclophosphamide followed by weekly paclitaxel or triweekly docetaxel, as effective and well tolerated (neo)adjuvant treatments for BC patients.4,13,24–26
One much less explored clinical issue is the optimal duration of neoadjuvant anthracycline-taxane ChT in patients with limited-stage BC. On the one hand, some phase III trials did not show significant differences between shorter and longer adjuvant ChT duration in limited-stage BC patients.27–29 On the other hand, none of these studies was conducted in the neoadjuvant setting, and most of them did not use anthracycline-taxane concomitant or sequential treatments, thus reducing their informative potential in the current clinical scenario. Finally, in those studies that compared neoadjuvant ChT regimens of different duration, different cytotoxic compounds and treatment schemes were used, thus limiting the possibility to draw definitive conclusions about the clinical impact of modifying treatment duration. For these reasons, it remains unclear which is the duration of preoperative ChT that is associated with the maximum therapeutic effect in terms of short-term (pCR rates) and long-term (e.g. DFS, OS) clinical outcomes.
Here, we conducted a retrospective study to assess the impact of shorter versus longer duration of sequential AT→CMF on pCR rates, DFS and OS in stage II–III HER2– BC patients.
Materials and methods
Study setting and inclusion criteria
This was a retrospective, independent, monocentric study in stage II–III HER2– BC patients treated between October 2007 and January 2018 at Fondazione IRCCS Istituto Nazionale dei Tumori (Milan, Italy). Eligibility criteria were: (a) women with pathologically/cytologically confirmed diagnosis of clinical stage II–III HER2– BC; (b) age ⩾18 years; (c) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2; (d) neoadjuvant ChT with AT (doxorubicin 60 mg/m2 i.v. plus paclitaxel 200 mg/m2 i.v. every 3 weeks) for three cycles followed by CMF (cyclophosphamide 600 mg/m2 i.v. plus methotrexate 40 mg/m2 i.v. and 5-FU 600 mg/m2 i.v. on days 1 and 8 every 4 weeks) for three cycles (cohort A), or AT for four cycles followed by CMF for four cycles (cohort B); (e) available data regarding clinical outcomes, including the type of pathological response at surgery (pCR versus no pCR), patient DFS and OS; (f) availability of medical records containing adequate information about treatment-related adverse events (AEs).
Patients in the shorter ChT duration cohort had been enrolled in an institutional, single-arm prospective study, namely the ASTER trial, which evaluated the safety and long-term clinical outcomes of AT×3→CMF×3 (neo)adjuvant ChT in limited-stage BC patients.30
Objectives of the study
The objective of this study was to assess the impact of the duration of neoadjuvant ChT (cohort A: shorter ChT course versus cohort B: longer ChT course) on clinical outcomes and safety profile in the whole patient population of HER2– BC patients, as well as in subgroups of patients with TNBC or HR+ HER2– BC. The primary study endpoint was pCR, as defined by the absence of invasive neoplastic cells at microscopic examination in surgically removed breast tissue and lymph node(s). DFS and OS were secondary activity/efficacy endpoints. DFS was defined as the time between surgery and any event of locoregional recurrence, distant recurrence or patient death from any cause (both BC-related and non- BC-related), whichever occurred first. OS was defined as the time between diagnosis and death from any cause. Patient data were collected according to the ethical principles for medical research involving human subjects adopted in the Declaration of Helsinki. The ethics committee of Fondazione IRCCS Istituto Nazionale dei Tumori approved the study design (INT 92/20). Patients alive at the time of data collection and/or analysis signed an informed consent for the use of their personal data for research purposes.
Assessment of efficacy and safety
To assess treatment safety, we recorded all AEs from blood evaluations and medical records. AEs were classified according to the common terminology criteria for adverse events (CTCAE), version 5.0 of November 2017, National Institutes of Health, National Cancer Institute. Hematological toxicities were collected from computerized blood sample data. Non-hematological toxicities were retrieved from medical records, where they had been regularly annotated during patient visits.
Statistical analyses
The χ2 test was used to study the distribution of clinically meaningful dichotomous variables (patientor tumor-related) in cohort A versus cohort B, whereas the Wilcoxon–Mann–Whitney test was used to compare the distribution of continuous variables in the two patient cohorts. DFS and OS were represented according to the Kaplan–Meier method, and survival distributions were compared with the log-rank test. Patients who had not undergone disease recurrence or death at the time of data cut-off and analysis were censored at their last disease evaluation. The impact of treatment duration on continuous (DFS, OS) or categorical outcomes (pCR) was also tested at multivariable analysis by using Cox proportional hazard models or logistic regression models, respectively.
A threshold of significance of 0.05 was set for all statistical evaluations. All statistical analyses were performed using the software R [version 3.5.2 (2018-12-20)].
Results
Patient population
Patient and tumor characteristics are described in Table 1. Median patient age was 48 years (range 30–74 years). Out of 209 HER2– BC patients included in this study, 62 (29.7%) had TNBC and 147 (70.3%) had HR+ HER2– BC. Clinically positive nodes at diagnosis (cN1–cN3) were detected in 135 (64.6%) patients. Regarding patient distribution in the two treatment cohorts, 111 (53%) belonged to cohort A, whereas 98 (47%) patients belonged to cohort B. Patients in cohort B were more likely to have larger primary tumors at diagnosis (cT3–cT4) when compared with patients in cohort A (54.1% and 9.0%, respectively; χ2 test p-value < 0.00002), with a statistically significant difference in the percentage of cT4 tumors (cohort A: 3.6%, cohort B: 40.8%; χ2 test p-value < 0.0001) but not of cT3 tumors (χ2 test p-value = 0.07). Regarding other clinical and tumor-related variables, cohorts A and B were well balanced in terms of patient age, tumor biology (HR+ BC versus TNBC), involvement of axillary nodes (cN0 versus cN1–N3), tumor grading (G1–G2 versus G3), percentage of Ki-67 positive tumor cells (<20 versus patient body mass index (BMI) ⩾20%) and patient (<25 versus ⩾25 kg/m2).
Table 1.
Patient and tumor characteristics.
| Overall n° 209 | AT×3→CMF×3 n° 111 | AT×4→CMF×4 n° 98 | AT×3→CMF×3 TN n° 28 | AT×4→CMF×4 TN n° 34 | AT×3→CMF×3 HR+ n° 83 | AT×4→CMF×4 HR+ n° 64 | |
|---|---|---|---|---|---|---|---|
| Age, | 48 | 47 | 51 | 45 | 54 | 48 | 50 |
| median (range) | (30–74) | (30–74) | (31–73) | (32–69) | (31–73) | (30–74) | (32–72) |
| p-value = 0.06 | p-value = 0.075 | p-value = 0.29 | |||||
| BMI | |||||||
| <25 | 114 (54.5) | 56 (50.5) | 58 (59.2) | 16 (57.1) | 20 (58.8) | 39 (47.0) | 38 (59.4) |
| ⩾25 | 79 (37.8) | 42 (37.8) | 37 (37.7) | 7 (25.0) | 13 (38.2) | 36 (43.4) | 24 (37.5) |
| NA | 16 (7.7) | 13 (11.7) | 3 (3.1) | 5 (17.9) | 1 (3.0) | 8 (9.6) | 2 (3.1) |
| p-value = 0.05 | p-value = 0.11 | p-value = 0.16 | |||||
| Biology | |||||||
| HR+ | 147 (70.3) | 83 (74.8) | 64 (65.3) | ||||
| TN | 62 (29.7) | 28 (25.2) | 34 (34.7) | ||||
| p-value = 0.18 | |||||||
| Primary tumor | |||||||
| cT1–cT2 | 145 (69.4) | 100 (90.1) | 45 (45.9) | 24 (85.7) | 20 (58.8) | 76 (91.6) | 25 (39.1) |
| cT3–cT4 | 63 (30.1) | 10 (9.0) | 53 (54.1) | 3 (10.7) | 14 (41.2) | 7 (8.4) | 39 (60.9) |
| NA | 1 (0.5) | 1 (0.9) | 0 (0) | 1 (3.6) | 0 (0) | 0 (0) | 0 (0) |
| p-value < 0.00002 | p-value = 0.019 | p-value < 0.00006 | |||||
| Axillar nodes | |||||||
| cN0 | 74 (35.4) | 44 (39.6) | 30 (30.6) | 11 (39.3) | 12 (35.3) | 33 (39.8) | 18 (28.1) |
| cN1–3 | 135 (64.6) | 67 (60.4) | 68 (69.4) | 17 (60.7) | 22 (64.7) | 50 (60.2) | 46 (71.9) |
| p-value = 0.22 | p-value = 0.95 | p-value = 0.12 | |||||
| Grading* | |||||||
| G1–G2 | 106 (50.7) | 60 (54.1) | 46 (46.9) | 4 (14.3) | 6 (17.6) | 56 (67.5) | 40 (62.5) |
| G3 | 73 (34.9) | 34 (30.6) | 39 (39.8) | 20 (71.4) | 26 (76.5) | 14 (16.9) | 13 (20.3) |
| NA | 30 (14.4) | 17 (15.3) | 13 (13.3) | 4 (14.3) | 2 (5.9) | 13 (15.6) | 11 (17.2) |
| p-value = 0.38 | p-value = 0.53 | p-value = 0.81 | |||||
| Ki67% | |||||||
| <20 | 41 (19.6) | 20 (18.0) | 21 (21.4) | 1 (3.6) | 3 (8.8) | 19 (22.9) | 18 (28,1) |
| ⩾20 | 147 (70.3) | 75 (67.6) | 72 (73.5) | 25 (89.3) | 29 (85.3) | 50 (60.2) | 43 (67.2) |
| NA | 21 (10.1) | 16 (14.4) | 5 (5.1) | 2 (7.1) | 2 (5.9) | 14 (16.9) | 3 (4.7) |
| p-value = 0.08 | p-value = 0.70 | p-value = 0.07 | |||||
Data are presented as n (%) except where otherwise noted. The p-value of the Wilcoxon–Mann–Whitney test (age) or χ2 test (other variables) is indicated in bold numbers when statistically significant. In case of not available (NA) information, the p-value refers to the χ2 test performed after excluding NA data.
According to Elston–Ellis breast cancer grading system.
AT, doxorubicin-paclitaxel; BMI, body mass index; CMF, cyclophosphamide-methotrexate-5-fluorouracil; HR+, hormone receptor-positive; TN, triple-negative.
Treatment activity and efficacy
At the time of data analysis, median follow-up was 76.96 months (interquartile range [IQR] 46.98–96.16) and median DFS and OS had not been reached. Overall, 60 events (28.7% of patients) of local and/or distant disease recurrence had occurred; of them, 34 events (30.6%) were observed in cohort A and 26 (26.5%) in cohort B (p = 0.587). A total number of 29 patients (13.9%) achieved pCR, with significantly lower pCR rates among HR+ HER2– BC patients (four out of 147; 2.7%) than among TNBC patients (25 out of 62; 40%) (p = 0.009). In the whole patient population, pCR was associated with a statistically significantly longer DFS (p = 0.047) and with a trend towards longer OS (p = 0.073) (Supplemental Figure 1A–1B). When considering tumor biology subgroups, pCR was associated with significantly longer DFS and OS in TNBC patients (DFS: p = 0.0014; OS: p = 0.0025), but not in HR+ HER2– BC patients (DFS: p = 0.33; OS: p = 0.54) (Supplemental Figure 1C–1D).
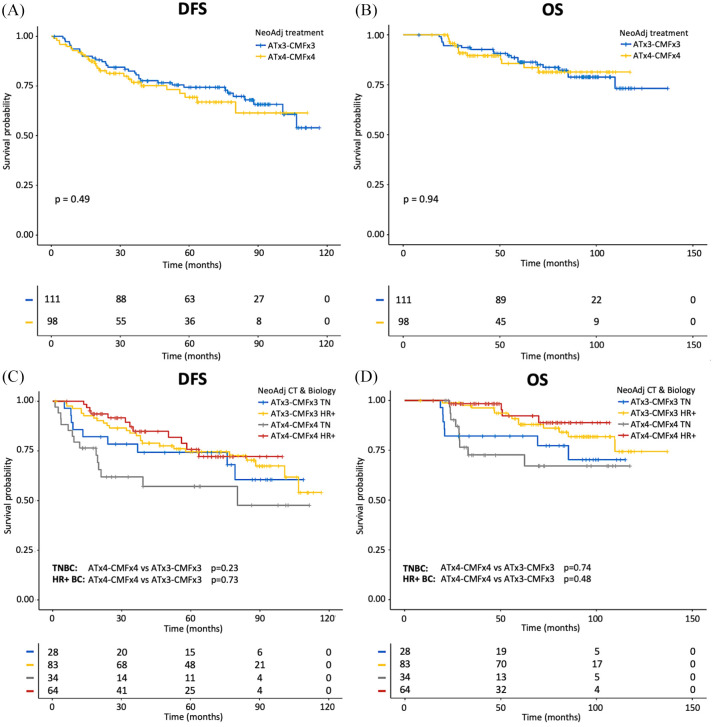
We found no statistically significant differences between patients in cohort A and cohort B in terms of pCR rates (15.3% versus 12.2%; p = 0.55), DFS (p = 0.49) (Figure 1A) or OS (p = 0.94) (Figure 1B). Similarly, in the subgroups of patients with TNBC and HR+ HER2– BC, cohorts A and B patients had non-statistically significantly different DFS (TNBC: p = 0.23; HR+ BC: p = 0.73, respectively) (Figure 1C) or OS (TNBC: p = 0.74; HR+ BC: p = 0.48) (Figure 1D).
Figure 1.
Kaplan–Meier curves for DFS and OS according to duration of neoadjuvant ChT in the whole population of enrolled patients.
A, C: Kaplan–Meier curves of DFS according to duration of treatment (A); Kaplan–Meier curves of DFS according to duration of treatment and tumor biology (TN and HR+ BC) (C). B, D: Kaplan–Meier curves of OS according to duration of treatment (B); Kaplan–Meier curves of OS according to duration of treatment and tumor biology (TN and HR+ BC) (D). The + symbol indicates patients censored at the time of data cut-off and analysis.
BC, breast cancer; ChT, anthracycline-taxane-based chemotherapy; DFS, disease-free survival; HR+, hormone receptor-positive; OS, overall survival; TN, triple-negative.
At multivariable analysis adjusting for other patient or tumor-related characteristics, ChT duration was not associated with clinical outcomes. TNBC biology was the only factor associated with significantly worse DFS (p = 0.028), worse OS (p = 0.003), and with a trend towards higher pCR rates (p = 0.0595) at multivariable analysis (Table 2).
Table 2.
Multivariable analysis of (a) DFS, (b) OS and (c) pCR (binary logistic) according to patients and treatment characteristics.
| (a) DFS | ||||
|---|---|---|---|---|
| Variables | HR | 95% CI | p-value | |
| Tumor biology | TNBC versus HR+ BC | 1.81 | 1.07–3.09 | 0.028 |
| Primary tumor | cT3–cT4 versus cT1–cT2 | 0.98 | 0.5–1.91 | 0.959 |
| Axillar nodes | cN1–3 versus cN0 | 1.13 | 0.66–1.93 | 0.656 |
| ChT regimen | AT×3 CMF×3 versus AT×4 CMF×4 | 0.85 | 0.50–1.63 | 0.717 |
| (b) OS | ||||
| Variables | HR | 95% CI | p-value | |
| Tumor biology | TNBC versus HR+ BC | 16.86 | 3.62–78.4 | 0.003 |
| Primary tumor | cT3–cT4 versus cT1–cT2 | 0.46 | 0.13–1.70 | 0.245 |
| Axillar nodes | cN1–3 versus cN0 | 0.72 | 0.36–1.44 | 0.075 |
| No. of treatment cycles | AT×3 CMF×3 versus AT×4 CMF×4 | 4.31 | 0.86–21.7 | 0.075 |
| (c) pCR | ||||
| Variables | OR | 95% CI | p-value | |
| Tumor biology | TNBC versus HR+ BC | 2.61 | 1.32–5.17 | 0.0595 |
| Primary tumor | cT3–cT4 versus cT1–cT2 | 0.98 | 0.45–2.13 | 0.966 |
| Axillar nodes | cN1–3 versus cN0 | 0.72 | 0.36–1.44 | 0.355 |
| No. of treatment cycles | AT×3 CMF×3 versus AT×4 CMF×4 | 0.98 | 0.48–1.98 | 0.941 |
The p-value of the multivariable analysis for each characteristic is indicated in the right column of the table. The p-value of the test is indicated in bold numbers when statistically significant.
AT, doxorubicin-paclitaxel; CI, confidence interval; CMF, cyclophosphamide-methotrexate-5-fluorouracil; DFS, disease free survival; HR, hazard ratio; HR+ BC: hormone receptor-positive breast cancer; OS, overall survival; pCR, pathologic complete response; TNBC, triple-negative breast cancer.
Sensitivity analysis
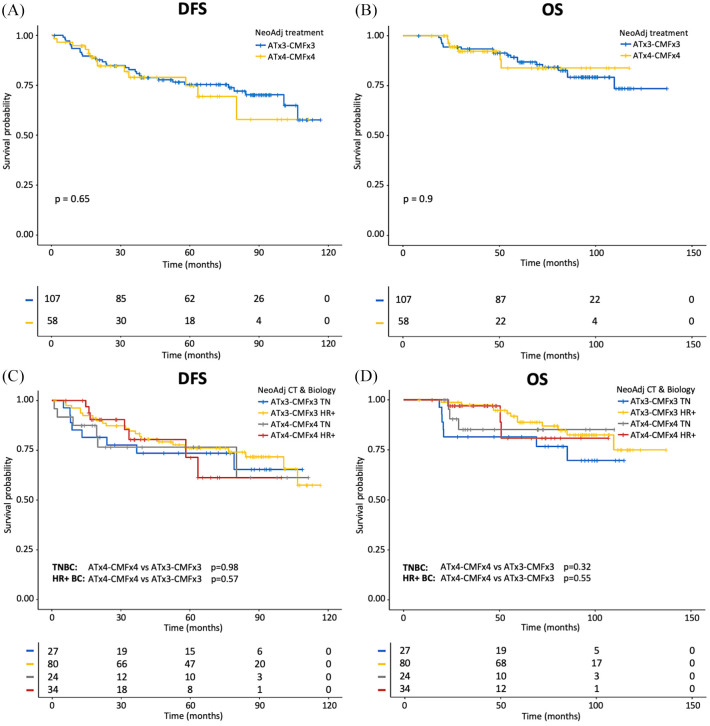
As cohort B patients were more likely to have cT4 stage tumors, we hypothesized that the observed lack of differences in terms of pCR rates, DFS and OS between patients treated with shorter and longer ChT duration could result, at least in part, from an imbalance of crucial prognostic factors in the two treatment cohorts. For this reason, we performed a sensitivity analysis, in which we excluded cT4 patients from our evaluations. Of note, even after removing patients with cT4 stage disease, we found no significant differences between cohorts A and B in terms of pCR rates (15.5% versus 14.2%; p = 0.80), DFS (p = 0.65; Figure 2A) and OS (p = 0.9; Figure 2B). We found similar results when TNBC and HR+ HER2– BC patient subgroups were evaluated separately (Figure 2C–D).
Figure 2.
Kaplan–Meier curves for DFS and OS according to duration of neoadjuvant ChT after excluding cT4 stage patients.
A, C: Kaplan–Meier curves of DFS according to duration of treatment (A); Kaplan–Meier curves of DFS according to duration of treatment and tumor biology (TN and HR+ BC) (C). B, D: Kaplan–Meier curves of OS according to duration of treatment (B); Kaplan–Meier curves of OS according to duration of treatment and tumor biology (TN and HR+ BC) (D). The + symbol indicates patients censored at the time of data cut-off and analysis.
BC, breast cancer; ChT, anthracycline-taxane-based chemotherapy; DFS, disease-free survival; HR+, hormone receptor-positive; OS, overall survival; TN, triple-negative.
Treatment safety and tolerability
Treatment-related AEs are summarized in Table 3. When considering the whole patient population, any grade neutropenia was detected in 101 (48.3%) patients, of whom 90 (43.1%) reported G1–G2 neutropenia and 30 (14.4%) patients reported severe (G3–G4) neutropenia. Fatigue was reported by 42 patients (20.9%), and was G1–G2 in all patients. G1–G2 peripheral neuropathy was reported by 41 (19.6%) patients, while G3–G4 neuropathy only occurred in three (1.4%) patients. Any grade nausea was reported by 65 (31.1%) patients, while only two (1%) patients developed G3/G4 nausea. Aspartate aminotransferase (AST) and analine amonotransferase (ALT) increase occurred quite commonly, with 95 (45.5%) patients experiencing AST increase and 109 (52.2%) patients experiencing ALT increase, respectively. Nonetheless, G3/G4 increase of ALT occurred only in six (2.9%) patients, while no G3/G4 increase of AST was reported.
Table 3.
Toxicities of different treatment arms.
| AEs |
Any grade n° (%)
|
p-value |
Grade ⩾3 n° (%)
|
p-value | ||
|---|---|---|---|---|---|---|
| AT×3→CMF×3 n° 111 | AT×4→CMF×4 n° 98 | AT×3→CMF×3 n° 111 | AT×4→CMF×4 n° 98 | |||
| Anemia | ||||||
| No | 47 (42.3) | 30 (30.6) | 0.11 | 110 (99.1) | 96 (98.0) | 0.91 |
| Yes | 64 (57.7) | 68 (69.4) | 1 (0.9) | 2 (2.0) | ||
| Neutropenia | ||||||
| No | 56 (50.4) | 52 (53.1) | 0.81 | 90 (81.1) | 89 (90.8) | 0.07 |
| Yes | 55 (49.6) | 46 (46.9) | 21 (18.9) | 9 (9.2) | ||
| Thrombocytopenia | ||||||
| No | 103 (92.8) | 88 (89.8) | 0.60 | 110 (99.1) | – | – |
| Yes | 8 (7.2) | 10 (10.2) | 1 (0.9) | |||
| Peripheral neuropathy | ||||||
| No | 91 (82.0) | 77 (78.6) | 0.66 | 110 (99.1) | 96 (98.0) | 0.91 |
| Yes | 20 (18.0) | 21 (21.4) | 1 (0.9) | 2 (2.0) | ||
| Diarrhea | – | – | – | |||
| No | 107 (96.4) | 92 (93.9) | 0.60 | |||
| Yes | 4 (3.6) | 6 (6.1) | ||||
| Constipation | – | – | – | |||
| No | 109 (98.2) | 93 (94.9) | 0.35 | |||
| Yes | 2 (1.8) | 5 (5.1) | ||||
| Nausea | ||||||
| No | 82 (73.9) | 61 (62.2) | 0.10 | 110 (99.1) | 97 (99.0) | 1.00 |
| Yes | 29 (26.1) | 37 (37.8) | 1 (0.9) | 1 (1.0) | ||
| Vomiting | – | – | ||||
| No | 100 (90.1) | 84 (85.8) | 0.45 | 110 (99.1) | ||
| Yes | 11 (9.9) | 14 (14.2) | 1 (0.9) | |||
| Mucositis | ||||||
| No | 93 (83.8) | 73 (74.5) | 0.14 | 108 (97.3) | 95 (96.9) | 1.00 |
| Yes | 18 (16.2) | 25 (25.5) | 3 (2.7) | 3 (3.1) | ||
| Fatigue | – | – | – | |||
| No | 94 (84.7) | 73 (74.5) | 0.10 | |||
| Yes | 17 (15.3) | 25 (25.5) | ||||
| AST increase | – | – | – | |||
| No | 68 (61.3) | 46 (46.9) | 0.053 | |||
| Yes | 43 (38.7) | 52 (53.1) | ||||
| ALT increase | ||||||
| No | 57 (51.4) | 43 (43.9) | 0.35 | 106 (95.5) | 97 (99.0) | 0.28 |
| Yes | 54 (48.6) | 55 (56.1) | 5 (4.5) | 1 (1.0) | ||
| Transaminitis | ||||||
| No | 56 (50.4) | 39 (39.8) | 0.16 | 106 (95.5) | 97 (99.0) | 0.28 |
| Yes | 55 (49.6) | 59 (60.2) | 5 (4.5) | 1 (1.0) | ||
Data are presented as n (%) except where otherwise noted. The p-value of the χ2 test assessing the association between each AE and the type of treatment received is indicated in the right column of the table.
AE, adverse event; AT, doxorubicin-paclitaxel; CMF, cyclophosphamide-methotrexate-5-fluorouracil.
We found a trend towards a higher incidence of any grade increase of AST levels in cohort B versus cohort A (53.1% versus 38.7%) (p = 0.053). The incidence of all other AEs was similar in the two treatment cohorts. At least one delay (equal or more than 7 days) in treatment administration for drug-related AEs was deemed necessary in 22 out of 111 (19.8%) patients in cohort A and in 13 out of 98 (13.3%) patients in cohort B (p = 0.26). Overall, 19 patients required a dose reduction to manage ChT-related AEs, nine of whom were in cohort A and 10 in cohort B, respectively (p = 0.82).
Discussion
In this study we performed a retrospective analysis to investigate the impact of shorter (approximately 5 months) versus longer (approximately 7 months) duration of the same neoadjuvant anthracycline-taxane-containing ChT regimen on clinical outcome of stage II–III HER2– BC patients.
We found that shorter and longer ChT duration are associated with similar antitumor activity (pCR rates) and long-term efficacy (DFS; OS) in the whole patient population, as well as in TNBC or HR+ BC subgroups. In our patient population, pCR rates were significantly higher in TNBC patients than in HR+ HER2– BC patients. In addition, achieving pCR was associated with better DFS and with a trend towards better OS in the whole patient population, thus confirming the prognostic role of pCR in the neoadjuvant setting regardless of treatment duration. Together, these data indicate that the findings of our study are reliable, and in line with the literature.
In this study, the two treatment cohorts were quite well balanced in terms of tumor and patient characteristics, with the only exception of a significantly higher proportion of patients with cT4 tumors in cohort B, which depends on the fact that patients with cT4 tumors were excluded from the ASTER trial.30 As an imbalanced distribution of cT4 tumors in the two treatment cohorts might have significantly contributed to the observed lack of differences, in terms of antitumor activity/efficacy, between shorter and longer ChT duration, we performed a sensitivity analysis in which we excluded patients with cT4 tumors. Notably, this analysis confirmed the main study results, thus supporting the clinical solidity of our findings.
To the best of our knowledge, this was the first study to show similar antitumor activity and efficacy of shorter versus longer ChT duration in the neoadjuvant setting in patients with stage II–III HER2– BC. Although these results need prospective validation, they suggest that reducing the duration of standard preoperative ChT might be associated with non-inferior treatment efficacy, provided that the most active cytotoxic agents, namely anthracyclines, taxanes and cyclophosphamide, are sequentially or concomitantly administered for a minimum number of cycles. In particular, we hypothesize that a lower number of total anthracycline-taxane ChT cycles may be sufficient to treat tumors that are more sensitive to cytotoxic agents efficaciously; at the same time, prolonging ChT duration may provide no additional benefit to patients with chemo-resistant neoplasms. In this latter patient population, adjuvant systemic treatments with different mechanisms of action could be more effective to kill residual chemo-resistant cells and to improve long-term outcomes. Although provocative, these hypotheses need to be tested in large prospective trials randomly assigning patients to the same ChT schedule, but for a different number of total ChT cycles.
Recent clinical trials have shown that concomitant or sequential administration of the most effective cytotoxic compounds (namely anthracyclines, taxanes, cyclophosphamide and, in some contexts, platinum compounds) in the neoadjuvant treatment setting yields the best clinical results in terms of pCR and reduction of the risk of disease relapses.25,31–35 Some of these studies compared neoadjuvant anthracycline-taxane regimens of different duration, and showed that longer ChT duration is associated with a higher percentage of pCR rates. However ChT schedules that were compared in these studies also contained different cytotoxic agents and different combination regimens, thus making it unclear if treatment duration is actually responsible for the observed differences in terms of clinical outcomes.
The issue of escalating or de-escalating adjuvant ChT in localized BC treatment is one of the most debated topics in the breast clinical oncology community. In patients with limited-stage HER2+ BC, anthracycline-free adjuvant regimens consisting of 12 weekly paclitaxel administrations plus 1 year of trastuzumab demonstrated excellent long-term outcomes in patients with low-risk disease (pT1-pT2pN0 tumors with lower than 3 cm maximum diameter),36 while the addition of pertuzumab to adjuvant anthracycline-paclitaxel-trastuzumab therapy,37 or adjuvant T-DM1 in patients failing to achieve pCR after preoperative treatment,15 resulted in improved clinical outcomes in patients with high-risk disease. Similarly, TNBC patients failing to achieve pCR after standard neoadjuvant anthracycline-taxane ChT might benefit from additional adjuvant cytotoxic treatments, such as capecitabine.14
One crucial goal of de-escalating neoadjuvant ChT consists in reducing ChT-related adverse events without negatively affecting clinical outcomes. In this perspective, identifying and validating clinical and tumor-related parameters associated with a lack of detrimental effects with shorter ChT course (e.g. clinical tumor stage; lymph node status; proliferation index; tumor genomic or gene expression profiles) will be of paramount importance properly to select patients who are candidates for (neo)adjuvant ChT de-escalation. In the neoadjuvant setting, monitoring tumor response to treatment through multiparametric radiological techniques (magnetic resonance imaging; radio-labeled glucose and glutamine positron emission tomography scans) could add valuable information to predict pCR before the completion of a full-course ChT programme.
Our study has some limitations. Firstly, it was a retrospective study, and its results need to be validated in the context of prospective clinical trials. Secondly, we enrolled a relatively low number of patients, which might have reduced the power to detect small differences, in terms of antitumor activity and efficacy, between the two treatment cohorts. Thirdly, there was a significant imbalance in the percentage of cT4 tumors between patients receiving shorter versus longer chemotherapy. Nevertheless, the fact that a sensitivity analysis confirmed the main study findings after excluding patients with cT4 tumors indicates that the study results are solid.
Finally, the chemotherapy regimen used in this study, which consisted of triweekly AT cycles followed by every 4-weeks CMF cycles, is rarely used in clinical practice now. This is especially true in the case of the CMF regimen which, although still included in international guidelines for (neo)adjuvant BC treatment (https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf), is not commonly prescribed now. Although the AT→CMF combination is different from the most commonly used biweekly (dose-dense) or triweekly AC (doxorubicin-cyclophosphamide) followed by 12 weekly paclitaxel (wP) or four triweekly docetaxel (T) cycles, it actually contains the most active cytotoxic agents against BC, including doxorubicin, paclitaxel and cyclophosphamide, and its efficacy has been previously demonstrated in a phase III trial.23 Therefore, we consider it reasonable that the results of our study could be extended to the most commonly used AC→wP or AC→T regimens. In future clinical trials, it would be interesting to compare the antitumor activity/efficacy of the following anthracycline-taxane-containing chemotherapy regimens of different duration: (a) AC×4 followed by weekly paclitaxel ×12 versus AC×3 followed by weekly paclitaxel ×9; (b) AC×4 followed by docetaxel ×4 versus fluorouracil-epirubicin-cyclophosphamide (FEC)×3 followed by docetaxel ×3; (c) AT×4 versus AT×6; (d) AC×4 followed by weekly paclitaxel ×12 versus docetaxel-cyclophosphamide (TC)×4.
Conclusion
This is one of the first studies to indicate that the duration of standard anthracycline-taxane neoadjuvant ChT could be reduced without negatively affecting clinical outcomes. However, due to the retrospective nature of the study and the type of chemotherapy used, which does not represent the standard of care in many countries, prospective studies are needed to confirm these data in specific BC populations (i.e. TNBC or HR+ BC) and by using more recent neoadjuvant ChT regimens, such as the triweekly AC followed by weekly paclitaxel or triweekly docetaxel, dose-dense AC followed by weekly paclitaxel, AC followed by carboplatin plus paclitaxel, or paclitaxel plus carboplatin and pembrolizumab followed by AC/EC in TNBC patients.
Supplemental Material
Supplemental material, sj-tiff-1-tam-10.1177_1758835920970081 for Antitumor activity and efficacy of shorter versus longer duration of anthracycline-taxane neoadjuvant chemotherapy in stage II–III HER2-negative breast cancer: a 10-year, retrospective analysis by Riccardo Lobefaro, Emma Zattarin, Federico Nichetti, Michele Prisciandaro, Francesca Ligorio, Marta Brambilla, Pierangela Sepe, Francesca Corti, Giorgia Peverelli, Arianna Ottini, Teresa Beninato, Laura Mazzeo, Carmen G. Rea, Gabriella Mariani, Filippo de Braud, Giulia V. Bianchi, Claudio Vernieri and Giuseppe Capri in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors would like to thank the Associazione Italiana per la Ricerca sul Cancro (AIRC) and the scientific directorate of Fondazione IRCCS Istituto Nazionale dei Tumori (Milan, Italy) for funding our research (MFAG 2019 -22977 PI Dr Claudio Vernieri).
Footnotes
Author contributions: CV and GC conceived and designed the study, with the help of RL and EZ. RL, EZ, FN, MP, FL, MB, PS, FC, GP, AO, TB, CGR, GM, GVB, CV and GC were involved in data collection. Statistical analyses were performed by RL and EZ, with the help of FN and MP. Results of statistical analyses were interpreted by CV, RL and EZ. RL, EZ and CV wrote the paper with input and critical review from GM, GVB, FdB and GC. All authors read and approved the final manuscript.
Conflict of interest: The authors declare that there is no conflict of interest.
Data availability: The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Our research is supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) (MFAG 2019 -22977 PI Dr Claudio Vernieri) and the scientific directorate of Fondazione IRCCS Istituto Nazionale dei Tumori (Milan, Italy).
ORCID iDs: Riccardo Lobefaro  https://orcid.org/0000-0003-3484-9718
https://orcid.org/0000-0003-3484-9718
Laura Mazzeo  https://orcid.org/0000-0001-9226-8861
https://orcid.org/0000-0001-9226-8861
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Riccardo Lobefaro, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Emma Zattarin, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Federico Nichetti, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Michele Prisciandaro, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Francesca Ligorio, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Marta Brambilla, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Pierangela Sepe, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Francesca Corti, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Giorgia Peverelli, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Arianna Ottini, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Teresa Beninato, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Laura Mazzeo, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Carmen G. Rea, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
Gabriella Mariani, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Filippo de Braud, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy.
Giulia V. Bianchi, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
Claudio Vernieri, Fondazione IRCCS Istituto Nazionale dei Tumori, Via Venezian 1, 20133, Milan, Italy; Istituto FIRC di Oncologia Molecolare (IFOM), Via Adamello 16, Milan, 20139, Italy.
Giuseppe Capri, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
References
- 1. Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012; 379: 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Francis P, Crown J, Di Leo A, et al. Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02-98 randomized trial. J Natl Cancer Inst 2008; 100: 121–133. [DOI] [PubMed] [Google Scholar]
- 3. Eiermann W, Pienkowski T, Crown J, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol 2011; 29: 3877–3884. [DOI] [PubMed] [Google Scholar]
- 4. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel Project protocols B-18 and B-27. J Clin Oncol 2008; 26: 778–785. [DOI] [PubMed] [Google Scholar]
- 5. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 2005; 97: 188–194. [DOI] [PubMed] [Google Scholar]
- 6. Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998; 16: 2672–2685. [DOI] [PubMed] [Google Scholar]
- 7. Killelea BK, Yang VQ, Mougalian S, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the national cancer database. J Am Coll Surg 2015; 220: 1063–1069. [DOI] [PubMed] [Google Scholar]
- 8. Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from national surgical adjuvant breast and bowel Project B-18. J Clin Oncol 1997; 15: 2483–2493. [DOI] [PubMed] [Google Scholar]
- 9. Bonadonna G, Valagussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol 1998; 16: 93–100. [DOI] [PubMed] [Google Scholar]
- 10. Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel Project B-18. J Natl Cancer Inst Monogr 2001; 30: 96–102. [DOI] [PubMed] [Google Scholar]
- 11. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 12. Von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–1804. [DOI] [PubMed] [Google Scholar]
- 13. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 14. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376: 2147–2159. [DOI] [PubMed] [Google Scholar]
- 15. Von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019; 380: 617–628. [DOI] [PubMed] [Google Scholar]
- 16. Miller K, Tong Y, Jones DR, et al. Cisplatin with or without rucaparib after preoperative chemotherapy in patients with triple negative breast cancer: final efficacy results of Hoosier Oncology Group BRE09-146. J Clin Oncol 2015; 33 (Suppl. 15): 1082. [Google Scholar]
- 17. Pusztai L, Barlow W, Ganz PA, et al. A randomized, phase III trial to evaluate the efficacy and safety of MK-3475 as adjuvant therapy for triple receptor-negative breast cancer with >1 cm residual invasive cancer or positive lymph nodes (ypN+) after neoadjuvant chemotherapy. Cancer Res 2018; 78: OT1-02. [Google Scholar]
- 18. Bonadonna G, Brusamolino E, Valagussa P, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976; 294: 405–410. [DOI] [PubMed] [Google Scholar]
- 19. Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet 1998; 352: 930–942. [PubMed] [Google Scholar]
- 20. Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 2003; 21: 976–983. [DOI] [PubMed] [Google Scholar]
- 21. Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 2005; 23: 3686–3696. [DOI] [PubMed] [Google Scholar]
- 22. Earl HM, Hiller L, Dunn JA, et al. Adjuvant epirubicin followed by cyclophosphamide, methotrexate and fluorouracil (CMF) vs CMF in early breast cancer: results with over 7 years median follow-up from the randomised phase III NEAT/BR9601 trials. Br J Cancer 2012; 107: 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gianni L, Baselga J, Eiermann W, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol 2009; 27: 2474–2481. [DOI] [PubMed] [Google Scholar]
- 24. Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 2009; 27: 1177–1183. [DOI] [PubMed] [Google Scholar]
- 25. Von Minckwitz G, Raab G, Caputo A, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol 2005; 23: 2676–2685. [DOI] [PubMed] [Google Scholar]
- 26. Steger GG, Greil R, Lang A, et al. Epirubicin and docetaxel with or without capecitabine as neoadjuvant treatment for early breast cancer: final results of a randomized phase III study (ABCSG-24). Ann Oncol 2014; 25: 366–371. [DOI] [PubMed] [Google Scholar]
- 27. Samuel JA, Wilson JW, Bandos H, et al. NSABP B-36: a randomized phase III trial comparing six cycles of 5-fluorouracil (5-FU), epirubicin, and cyclophosphamide (FEC) to four cycles of adriamycin and cyclophosphamide (AC) in patients (pts) with node-negative breast cancer. Cancer Res 2015; 75: abstract S3-02. [Google Scholar]
- 28. Kerbrat P, Desmoulins I, Roca L, et al. Optimal duration of adjuvant chemotherapy for high-risk node-negative (N–) breast cancer patients: 6-year results of the prospective randomised multicentre phase III UNICANCER-PACS 05 trial (UCBG-0106). Eur J Cancer 2017; 79: 166–175. [DOI] [PubMed] [Google Scholar]
- 29. Shulman LN, Berry DA, Cirrincione CT, et al. Comparison of doxorubicin and cyclophosphamide versus single-agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (alliance). J Clin Oncol 2014; 32: 2311–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mariani G, Galli G, Cavalieri S, et al. Single institution trial of anthracycline- and taxane-based chemotherapy for operable breast cancer: the ASTER study. Breast J 2019; 25: 237–242. [DOI] [PubMed] [Google Scholar]
- 31. Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006; 24: 2019–2027. [DOI] [PubMed] [Google Scholar]
- 32. Gianni L, Mansutti M, Anton A, et al. Comparing neoadjuvant nab-paclitaxel vs paclitaxel both followed by anthracycline regimens in women with ERBB2/HER2-negative breast cancer – the Evaluating Treatment with Neoadjuvant Abraxane (ETNA) trial: a randomized phase 3 clinical trial. JAMA Oncol 2018; 4: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Evans TR, Yellowlees A, Foster E, et al. Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: an Anglo-Celtic Cooperative Oncology Group study. J Clin Oncol 2005; 23: 2988–2995. [DOI] [PubMed] [Google Scholar]
- 34. Murphy C, Muscat A, Ashley D, et al. Tailored NEOadjuvant epirubicin, cyclophosphamide and nanoparticle albumin-bound paclitaxel for breast cancer: the phase II NEONAB trial-clinical outcomes and molecular determinants of response. PLoS One 2019; 14: e0210891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuwayama T, Nakamura S, Hayashi N, et al. Randomized multicenter phase II trial of neoadjuvant therapy comparing weekly nab-paclitaxel followed by FEC with docetaxel followed by FEC in HER2. Clin Breast Cancer 2018; 18: 474–480. [DOI] [PubMed] [Google Scholar]
- 36. Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015; 372: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017; 377: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tiff-1-tam-10.1177_1758835920970081 for Antitumor activity and efficacy of shorter versus longer duration of anthracycline-taxane neoadjuvant chemotherapy in stage II–III HER2-negative breast cancer: a 10-year, retrospective analysis by Riccardo Lobefaro, Emma Zattarin, Federico Nichetti, Michele Prisciandaro, Francesca Ligorio, Marta Brambilla, Pierangela Sepe, Francesca Corti, Giorgia Peverelli, Arianna Ottini, Teresa Beninato, Laura Mazzeo, Carmen G. Rea, Gabriella Mariani, Filippo de Braud, Giulia V. Bianchi, Claudio Vernieri and Giuseppe Capri in Therapeutic Advances in Medical Oncology