Abstract
Background:
Neurofilament light chain (NfL) is essential for axonal maintenance and reflects neuronal damage. Extracellular vesicles (EVs), especially exosomes, secreted by cells into the blood, are emerging as novel biomedical research platforms of physiological and pathological processes. The present study investigated the possible association between plasma EV NfL and Parkinson’s disease (PD).
Methods:
One hundred and sixteen patients with mild to moderate PD and 46 non-PD, neurological controls were recruited, and their clinical motor symptoms and cognitive function were evaluated. Plasma EVs were isolated using an exoEasy kit, and immunomagnetic reduction assay was used to assess EV NfL level. Statistical analysis was performed using SPSS 25.0, and p < 0.05 was considered significant.
Results:
The isolated plasma EVs were validated according to size and the presence of specific surface markers. Compared with the neurological control group, the levels of plasma EV NfL in patients with PD were not significantly different (PD: 9.42 ± 3.89, control: 9.53 ± 3.62 pg/mL plasma, p = 0.71). On the other hand, plasma EV NfL in patients with PD trendwise correlated with the severity of akinetic rigidity (p = 0.05). PD patients with optimal EV NfL (lowest quartile) had 6.66 ± 2.08 lower Unified Parkinson’s Disease Rating Scale-III score after adjustment for age, sex, and disease duration.
Conclusion:
Plasma EV NfL levels did not distinguish patients with PD from the neurological control group. The possible correlation between plasma EV NfL with the severity of motor symptoms within the PD patients, especially with akinetic rigidity, was noted. Further clinical validation of the blood EV NfL by a longitudinal follow-up study of PD patients is warranted.
Keywords: akinetic rigidity, exosome, extracellular vesicle, neurofilament light chain, NfL, motor symptom, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease.1 The diagnosis of PD is mainly based on the onset of motor symptoms, and its progression is determined by clinical manifestations such as cognitive impairment, gait imbalance, and motor fluctuation.2 The lack of precise biomarkers limits the early diagnosis and prognosis of PD, thus beleaguering the anticipated success of numerous clinical trials.3 For neurodegenerative diseases, cerebrospinal fluid (CSF) is considered the best bio-sample for identifying and validating novel biomarkers because it directly reflects the condition of neurons in the central nervous system. However, lumbar puncture, which is the only method of obtaining CSF, is a moderately invasive procedure and is unpopular in many countries. Given the ease of access, peripheral blood is an ideal biomarker source; however, the blood–brain barrier (BBB) restricts the release of the cellular contents of neurons into the bloodstream, thus underscoring the incongruity of blood-borne and neuron-specific biomarkers.4 This is more so as detection of circulating PD-specific proteins or nucleotides is difficult owing to their low levels in the blood.
Extracellular vesicles (EVs) are cell-released, non-replicating, lipid bi-layered small particles containing numerous bio-materials, including proteins and nucleotides.5 Recently, a type of EV called exosome (30–100 nm in diameter), found in most human bio-fluids, has been the focus of increased interest and research, with accruing evidence of exosomes’ potential role in intercellular communication and/or the clearance of cellular waste.5 Encapsulated with the lipid bi-layer membrane, exosomes can cross the BBB, establishing a communications link between the neurons and blood.6,7 Furthermore, their high structural integrity, functional stability, and low thermolability prevent degradation.8 There is increasing documentation of the association of varied concentrations of plasma and serum exosomal cargoes, including proteins and microRNA, with several neurological diseases, thus highlighting the promise of exosomes as neuropathological biomarkers.8–13
Neurofilament light chain (NfL) is a neuronal cytoplasmic protein responsible for maintaining the cytoskeletons of axons. An increase of NfL levels in the CSF and blood reflects the degree of axonal damage in neurological diseases of different etiologies, including inflammatory, traumatic, degenerative, and cerebrovascular.14 For patients with PD, blood and CSF NfL are not always significantly elevated compared with the general population; this may likely be because the loss of myelinated axons is not pertinent to the early stages of the disease.15 Against the background of its limited diagnostic role, CSF NfL can predict progressive supranuclear palsy progression, a Parkinson-plus syndrome conferring a worse prognosis than typical PD.16 Interestingly, exosomes are rich in NfL. Circulating neuron-derived exosomes from individuals with HIV infection and cognitive impairments exhibited significantly higher levels of NfL.17 However, there is a paucity of studies investigating blood EVs in patients with PD. In the present study, we investigated and document the association between EV NfL in the peripheral blood and PD.
Materials and methods
Study participants
This study was approved by the Joint Institutional Review Board of Taipei Medical University (Approval numbers N201609017 and N201801043). In total, 162 participants (116 patients with PD and 46 non-PD controls) were enrolled in this study. Diagnosis of PD was based on the UK Parkinson’s Disease Society Brain Bank Diagnostic Criteria.18 Only patients with mild to moderate PD, defined as stage I to III PD according to the Hoehn and Yahr scale, were included. The non-PD, neurological controls were recruited from the neurology outpatient clinic during the same period as PD patients. During recruitment, age/sex match was neither intended nor anticipated. The inclusion criteria for controls were: (i) patients above 45 years old, (ii) undergoing regular neurological outpatient clinic follow-up, and (iii) willing and able to sign the informed consent. The exclusion criteria were (i) subjects without dementia [defined as mini-mental state test (MMSE) <26], (ii) without other neurodegenerative diseases, and (iii) without psychiatric or major systemic diseases such as malignancies and chronic kidney disease. Their major neurological diagnosis included history of transient ischemic attack or minor ischemic stroke without neurological sequelae (n = 20), chronic dizziness and vertigo (n = 13), chronic low back pain and sciatica (n = 8), chronic headache, (n = 3), and diabetic polyneuropathy, (n = 2). Their conventional vascular risk factors included hypertension (n = 25), hyperlipidemia (n = 23), and diabetes (n = 9).
Clinical assessments
All participants were interviewed to obtain their baseline demographic data. The cognitive function of all study participants was assessed by trained nurses using the Taiwanese versions of the MMSE and Montreal Cognitive Assessment (MoCA). All PD patients were evaluated using Parts I, II, and III of the Unified Parkinson’s Disease Rating Scale (UPDRS) during outpatient visits. The time between the most recent dose of anti-PD medication and the assessment of UPDRS Part III was not recorded; patients with PD were assumed to be in their “on” time. Within the UPDRS-III, akinetic rigidity and tremor scores were calculated based on the previous literature.19
Isolation, concentration, and validation of EVs
For the isolation of EVs, venous blood samples were collected from all study participants. Whole blood was centrifuged at 13,000 × g for 20 min to isolate plasma. Next, EV isolation and purification from 1 mL of plasma from each participant was performed using the exoEasy Maxi Kit (#76064; QIAGEN Inc., Germantown, MD, USA) strictly following the manufacturer’s instructions. The last step of EV isolation was elution of the EV from the column. Typically, 400 μL of eluent was obtained.
The isolated EVs were confirmed to be exosomes based on the detection of tetraspanins, cluster of differentiation (CD)-9, CD-63, CD-81, multivesicular body synthesis proteins, tumor susceptibility gene (TSG)-101, heat shock protein (HSP)-70, and the absence of mitochondrial protein and cytochrome c. The size distribution of isolated EVs was determined by nanoparticle tracking. For the Western blot analyses, antibodies against CD9 (ab92726; Abcam plc, Cambridge, UK), CD63 (ab59479; Abcam plc, Cambridge, UK), anti-CD81 antibody (ab109201; Abcam), TSG-101 (GTX118736; GeneTex Inc., Irwine, CA, USA), cytochrome c (ab110325; Abcam plc, Cambridge, UK), and HSP-70 (NBP1-77456, Novus Biologicals LLC, CO, USA) were used at a 1:1000 dilution. Nanoparticle tracking and characterization were performed using the NanoSight NS300 Instrument (Malvern Panalytical Ltd, Malvern, UK), following the manufacturer’s instructions.
NfL level assessment by immunomagnetic reduction assay
The reagents used to assay the biomarkers were antibody-biofunctionalized magnetic nanoparticles dispersed in phosphate-buffered saline solution. The core of magnetic nanoparticles was iron (II, III) oxide (Fe3O4). NfL immunomagnetic reduction (IMR) reagent (#MF-NFL-0060; MagQu Co., Ltd, New Taipei City, Taiwan) was conjugated with the specific antibody against NfL protein (sc-20012; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The averaged values of the hydrodynamic diameters of the antibody-functionalized magnetic nanoparticles were approximately 55 nm. The concentration of each reagent was about 10 mg Fe/mL.
For NfL IMR assay, 60 μL of reagent was mixed with 60 μL of 10-fold diluted EV solution sample. After that, each mixture was placed into a superconducting quantum interference device (SQUID)-based Immuno-Magnetic Analyzer (Model: XacPro-S361, MagQu Co., Ltd, New Taipei City, Taiwan) to determine the time-dependent alternating current (AC) magnetic susceptibility. The association between the magnetic nanoparticles and the targeted protein molecule resulted in reduced magnetic susceptibility, which was detected by the high-superconducting transition temperature(Tc) SQUID magnetometer as the IMR signal. The IMR signal thus reflects the concentration of the targeted protein. Measurements were conducted in duplicates for each biomarker per sample. The reported concentration of each biomarker was the mean value of duplicated measurements. Consistent with the instruction from MagQu Co., Ltd, the IMR assay detection limit was 3.3 fg/mL.
Statistical analysis
All statistical analyses were performed using IBM SPSS (IBM Corp.; released 2017; IBM SPSS Statistics for Windows, Version 25.0; IBM Corp., Armonk, NY, USA). Chi-square (χ²) test was used to compare the gender distribution between patients with PD and the neurological control group. Pearson’s χ² test was used to compare PD patients’ sex distribution within different groups of plasma EV NfL. A non-parametric Mann–Whitney U test was used to compare the plasma EV NfL levels and other continuous variables between PD and non-PD, neurological control patients. Spearman’s rank correlation was used to investigate the associations between the clinical scale scores and plasma EV NfL level in PD patients. One-way analysis of variance and analysis of covariance with Dunnett’s post-hoc analysis were used to compare the severity of motor symptoms between PD patients with low to high plasma EV NfL (first, second, third, and fourth quartiles). Multivariable logistic regression with partial regression analysis was used to investigate the association between optimal plasma EV NfL (lowest quartile) with the severity of PD symptoms adjusted for age, sex, and disease duration. A p-value < 0.05 was considered statistically significant.
Results
Characterization of plasma EVs
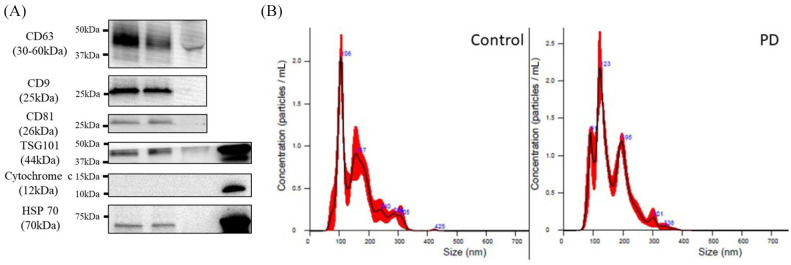
For characterization of the isolated plasma EVs and confirmation of their exosomal nature, their expression of widely recognized exosome markers CD63, CD9, CD81, TSG101, HSP-70, and cytochrome c was probed. At the same time, we found the non-expression of mitochondrial protein cytochrome c in both PD and neurological control samples confirmatory (Figure 1A). These results indicated the exosomal nature of the isolated EVs and revealed the relative abundance of the exosomes in patients with PD and their control counterparts. Furthermore, the exosomal nature of the isolated plasma EVs was validated using IMR-aided nanoparticle tracking. In both the non-PD neurological controls and patients with PD, the sizes of the harvested EVs were approximately 100 nm (Figure 1B), thus corroborating earlier results indicating that the harvested plasma EVs were predominantly exosomes.
Figure 1.
Characterization of isolated plasma extracellular vesicles (EVs). (A) Representative western blot images of the differential expression of EV markers CD63, CD9, CD81, and TSG101 in the isolated EVs from controls and patients with Parkinson’s disease (PD). Serum and SH-SY5Y cell lysate were set as the controls. Cytochrome c and HSP-70 served as negative and loading control of EV, respectively; and (B) nanoparticle tracking analysis of size distribution of the isolated EVs from controls and patients with PD.
EV, extracellular vesicle; NfL, neurofilament light chain.
Clinico-demographic data of study participants
The baseline clinico-demographic data is presented in Table 1. The recorded inter-group difference in age (67.04 ± 7.03 versus 69.66 ± 8.41) or number of female participants (18 versus 62) between the non-PD neurological control group and patients with PD was statistically non-significant. Consistent with the exclusion of persons with dementia (MMSE <26) from the neurological control group, patients with PD had lower MMSE and MoCA scores, thus exhibiting significantly worse cognitive function than their non-PD neurological control counterparts (p < 0.001). The mean duration of PD was 2.71 ± 2.48 years in the PD patients. Compared with the non-PD neurological control group, the plasma EV NfL in patients with PD was not significantly different (PD, 9.42 ± 3.89 pg/mL versus control, 9.53 ± 3.62 pg/mL plasma, p = 0.71). The detailed distribution of blood EV NfL in the non-PD neurological control and PD patients is presented in Supplemental material Figure S1 online.
Table 1.
Clinico-demographic data and plasma extracellular vesicle (EV) neurofilament light chain (NfL) level in patients with Parkinson’s disease (PD) and neurological-control group. Chi-square test was used to compare the gender distribution between non-PD neurological control with PD patients, and non-parametric Mann–Whitney U test was used to compare the continuous variables between PD and non-PD neurological control patients.
| Non-PD neurological control | PD | p value | |
|---|---|---|---|
| Number of patients | 46 | 116 | – |
| Age (years) | 67.04 ± 7.03 | 69.66 ± 8.41 | 0.11 |
| Female | 18 (39.1%) | 62 (53.4%) | 0.12 |
| Disease duration (years) | – | 2.71 ± 2.48 | – |
| MMSE | 28.41 ± 1.23 | 24.18 ± 6.36 | <0.001* |
| MoCA | 24.13 ± 3.07 | 20.43 ± 6.02 | <0.001* |
| UPDRS-I | – | 2.48 ± 2.00 | – |
| UPDRS-II | – | 7.92 ± 5.82 | – |
| UPDRS-III | – | 22.47 ± 9.85 | – |
| EV NfL (pg/mL plasma) | 9.42 ± 3.89 | 9.53 ± 3.62 | 0.71 |
p < 0.05.
MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; UPDRS, Unified Parkinson’s Disease Rating Scale.
Plasma EV NfL level is positively associated with the akinetic rigidity score
Probing for likely clinical relevance of plasma EV NfL, we further investigated the associations between plasma EV NfL level and several clinico-demographic parameters in patients with PD, namely, age, disease duration, severity of motor symptoms (UPDRS-III scores, akinetic rigidity score, and tremor score), and cognitive function. Plasma EV NfL exhibited mild positive correlation with disease duration (r = 0.20, p = 0.04) but no correlation with age (r = 0.05, p = 0.62) or cognitive function (MMSE: r = 0.06, p = 0.56; MoCA: r = 0.04, p = 0.70). Regarding the severity of motor symptoms, plasma EV NfL was trendwise associated with the akinetic rigidity score (r = 0.18, p = 0.05) but not the tremor score (Table 2 and Supplemental Figure S2). When adjusted for age, the association between plasma EV NfL with disease duration was non-significant (p = 0.09, Supplemental Table S1), and the trendwise association with akinetic rigidity score remained (p = 0.05, Supplemental Table S2).
Table 2.
Correlations between plasma extracellular vesicle (EV) neurofilament light chain (NfL) level and clinical manifestations in patients with Parkinson’s disease. Statistical analysis was conducted by Spearman’s rank correlation.
| Age (years) | Disease duration (years) | UPDRS-III | Akinetic rigidity score | Tremor score | MMSE | MoCA | ||
|---|---|---|---|---|---|---|---|---|
| EV NfL level | Correlation coefficient | 0.047 | 0.202 | 0.16 | 0.183 | 0.075 | 0.056 | 0.037 |
| p value | 0.62 | 0.04* | 0.09 | 0.05 | 0.42 | 0.56 | 0.70 |
p < 0.05.
MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; UPDRS, Unified Parkinson’s Disease Rating Scale.
Optimal plasma EV NfL is associated with fewer motor symptoms in patients with PD
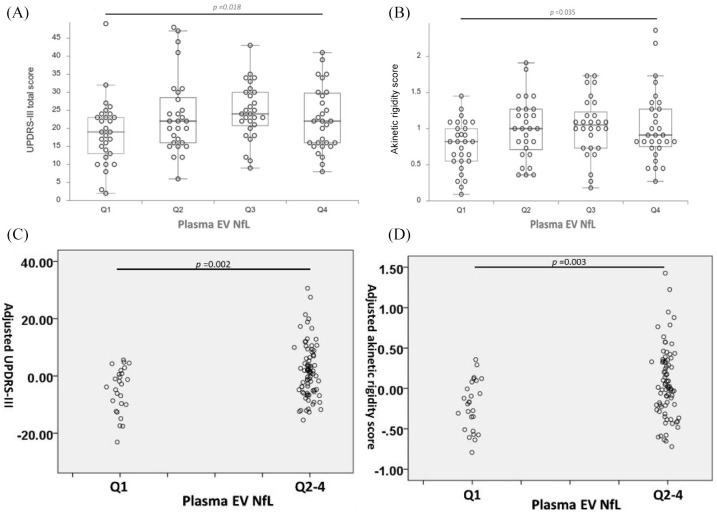
Having demonstrated an association between plasma EV NfL and motor symptoms, we divided the patients with PD into four groups based on the quartiles of the plasma EV NfL levels (Q1–Q4) and performed inter-quartile analyses. Analysis of the UPDRS-III total score revealed the significant variation in the overall UPDRS-III total score across the quartile groups after adjusting age, sex, and disease duration (p for trend = 0.018) (Figure 2A). In the post-hoc analysis, a trendwise difference of the UPDRS-III score between Q1 to Q2, Q3 and Q4 was noted (Supplemental Table S3). In parallel analysis, the overall variations in the akinetic rigidity score for the four groups were significant after adjusting age, sex, and disease duration (p for trend = 0.035) (Figure 2B). Post-hoc analysis revealed that compared with the concurrent lowest plasma EV NfL (Q1), PD patients within plasma EV NfL in Q2, Q3, and Q4 were associated with a trend of higher mean akinetic rigidity scores (Supplemental Table S3). The age, sex, and disease duration were not significantly different for the four groups (Supplemental Tables S4 and S5). After adjustment for these confounding factors of motor symptoms, the total UPDRS-III and akinetic rigidity score for PD patients with optimal plasma EV NfL (lowest quartile, Q1) were 6.66 ± 2.08 (p = 0.002) and 0.28 ± 0.09 (p = 0.003) lower than in the Q2–Q4 group, respectively (Figure 2C and D; also see Supplemental Tables S6 and S7).
Figure 2.
Plasma extracellular vesicles (EVs) neurofilament light chain (NfL) quartile-stratified motor symptoms severity in patients with Parkinson’s disease (PD). Box and dot plots of the differential (A) Unified Parkinson’s Disease Rating Scale (UPDRS)-III total scores; and (B) mean akinetic rigidity scores, for Q1, Q2, Q3, and Q4 plasma EV NfL. Statistical analysis was conducted by the analysis of covariance with the adjustment of age, sex, and disease duration. The partial residual plots demonstrate the relationship between the levels of EV NfL (Q1 or Q2–4) and the UPDRS-III (C) and akinetic rigidity (D) scores given that age, sex and disease duration are adjusted by the multivariable regression model.
Discussion
EVs are secreted by most mammalian cells. There is accruing evidence that the EV content, especially exosome cargoes, often mirrors the intracellular environment. For instance, the phosphorylation level of exosomal insulin receptor substrate-1 was strongly associated with cellular insulin resistance,20 and elevated levels of exosomal pathogenic β-amyloid, tau, and α-synuclein proteins has been implicated in the pathogenesis and progression of neurodegenerative diseases.21 The intracellular accumulation of disease-causing proteins and ensuing pathogenic signals often correlate with the disease onset; this is even more so with exosomes which share their origin with and cargo disease-causing proteins, thus positioning exosomes as early indicators of disease.22 Concordant with the above, it was recently shown that phosphorylated tau and Aβ1-42 in circulating neuron-derived exosomes predict conversion from mild cognitive impairment to Alzheimer’s disease (AD).23
NfL is a neuron-specific protein responsible for the maintenance of the axonal structure. Axonal lesions, serving as indicators of neuronal damage, characterize various neurological diseases, including stroke, trauma, and multiple sclerosis.24 In neurodegenerative diseases, the progressive loss of central nervous system neurons results in the loss of myelinated axons. Neurofilament degradation causes NfL to enter the CSF and blood. Consistent with this, compared with normal controls, higher blood NfL levels have been documented in individuals with AD.25 The last two decades have been characterized by continued investigation of NfL in the CSF of PD patients since it was first found to be elevated in patients with atypical parkinsonism (multiple system atrophy and progressive supranuclear palsy) compared with PD.26 The use of CSF NfL to distinguish PD from atypical parkinsonism has subsequently been confirmed by other large-scale studies.27,28 Compared with controls, CSF NfL from the PD patients was significantly higher.28,29 Although the diagnostic accuracy is mild, the CSF NfL is a promising predictor of PD progression.29,30 Regarding blood NfL, plasma NfL is also increased in PD patients compared with controls. Setting the cut-off value of plasma NfL at 12.34 pg/mL, the sensitivity and specificity of the PD prediction model were 53.2% and 90.5%, respectively.31 Similar to CSF NfL, plasma NfL also correlates with the disease progression, especially the postural instability gait disorder subtype.31–33 The present study found no significant difference between plasma EV NfL in PD patients and their control counterparts. For the PD patients, this study demonstrated that plasma EV NfL level is associated with the severity of motor symptoms, where optimal plasma EV NfL level (lowest quartile) is associated with less severe motor symptoms, especially akinetic rigidity. The akinetic rigidity symptoms of PD, compared with tremor, indicate rapid progress of disease and exhibit more severe α-synuclein pathology.34 Recently, there was report of patients with PD of the akinetic rigidity subtype exhibiting higher CSF NfL than those of the tremor-dominant subtype.30 This association possibly stems from the greater CNS pathology in the akinetic rigidity subtype of PD, which impairs the integrity of neurons and increases the release of NfL though EV. The apparent overlap between the plasma EV NfL levels in PD patients and the control group undermines its potential implications. However, our findings demonstrate its correlation with disease severity, providing a basis for the future study of EV NfL in PD patients.
The limited success of most PD trials attributable to the lack of biomarkers of disease progression35 accentuates another strength of the present study – identifying a functional association between plasma EV NfL and the severity of motor symptoms in patients with PD, especially the akinetic rigidity symptoms. Akinetic rigidity, rather than tremor, has been shown to correlate more with striatal neurodegeneration.29 The association between the optimal plasma EV NfL level and mild severity of motor symptoms, especially akinetic rigidity, indicates the possible association of plasma EV NfL with the severity of neurodegeneration in PD. Compared with CSF, the harvest of plasma EVs requires minimally invasive venipuncture, which is generally tolerable for the population. Compared with peripheral blood NfL, the EV NfL is directly associated with the neuronal cell-of-origin, which contrasts the damaged/destroyed axon origin of blood NfL. Since the destruction of the axon is characteristic of late stage neurodegeneration, the assessment of EV NfL may provide early intracellular information, and inform medical management decisions in advance. Other EV proteins have also been assessed as potential biomarkers of PD. As with the application of CSF NfL,27,28 the EV NfL, in conjunction with other pathognomonic proteins, may form a panel for diagnosis and prediction of PD and its progression, respectively.
The present study has some limitations. First, owing to the small number of cases and the single-center nature of the study, for generalizability, a larger multi-center cohort may be required to validate further the association between plasma EV NfL and PD demonstrated herein. Second, plasma total EVs were used in the present study instead of neuron-derived exosomes. However, as NfL is neuron-specific, a neuronal origin is assumed for the detected NfL. Third, the present study investigated only a selected panel of PD-associated proteins. However, other contents of EVs, especially the PD-pathognomonic proteins such as α-synuclein in the blood EVs, were not investigated.
Nevertheless, it is noteworthy that α-synuclein is abundant in red blood cells,36 and may lead to a confounding bias in measurement. Although the statistical significance was found between the severity of motor symptoms, especially the akinetic rigidity, the correlation was low; the heterogenicity of PD and the small case number are possible reasons. In addition, the assessment of the motor symptoms during the outpatients clinic visit without medication withdrawal also introduces a probable confounder, namely, the effects of dopaminergic agents. The present study adjusted for age, sex, and disease duration in the regression model to minimize their impact and obtain the net association. Another limitation is the lack of association with cognitive function, another indicator of PD progression. As the present study recruited individuals with early-stage PD (mean disease duration <3 years), their cognitive impairment may not meet the detection threshold even as assessed by MoCA. Finally, some of the controls may have already fallen into the category of mild cognitive impairment (MCI), although their MMSE scores were ⩾26. Such MCI status probably results from the early stage of neurodegeneration, which causes the alteration of plasma EV NfL.
In conclusion, there was no statistically significant difference between the plasma EV NfL level in PD patients and controls. For the PD patients, plasma EV NfL level was slightly correlated with the severity of PD motor symptoms, and the optimal plasma EV NfL was associated with mild motor symptoms severity. Longitudinal studies of plasma EV NfL in patients with PD are warranted to further validate the correlation of NfL with the neurodegeneration in PD.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_1756286420975917 for Neurofilament light chain level in plasma extracellular vesicles and Parkinson’s disease by Chen-Chih Chung, Lung Chan, Jia-Hung Chen, Oluwaseun Adebayo Bamodu and Chien-Tai Hong in Therapeutic Advances in Neurological Disorders
Footnotes
Author contributions: Study conception and design: C-CC, LC, JHC, C-TH. Data acquisition and analysis: C-CC, LC, C-TH. Data interpretation: C-CC, OAB, C-TH. Manuscript writing and revision: C-CC, OAB, C-TH. Provision of resources and administrative oversight: C-TH. All authors read and approved the final version of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics approval and consent to participate: This study was approved by the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB approval no. N201609017 and N201801043)
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Ministry of Science and Technology (MOST 107-2314-B-038-043) and Taipei Medical University - Shuang Ho Hospital (108YSR-02).
ORCID iD: Chien-Tai Hong  https://orcid.org/0000-0002-7448-1041
https://orcid.org/0000-0002-7448-1041
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Chen-Chih Chung, Department of Neurology, Shuang Ho Hospital, Taipei Medical University, New Taipei City Department of Neurology, School of Medicine, College of Medicine, Taipei Medical University, Taipei City Graduate Institute of Biomedical Informatics, Taipei Medical University, Taipei City.
Lung Chan, Department of Neurology, Shuang Ho Hospital, Taipei Medical University, New Taipei City Department of Neurology, School of Medicine, College of Medicine, Taipei Medical University, Taipei City.
Jia-Hung Chen, Department of Neurology, Shuang Ho Hospital, Taipei Medical University, New Taipei City.
Oluwaseun Adebayo Bamodu, Department of Hematology & Oncology, Shuang Ho Hospital, Taipei Medical University, New Taipei City Department of Medical Research & Education, Shuang Ho Hospital, Taipei Medical University, New Taipei City Department of Urology, Shuang Ho Hospital, Taipei Medical University, New Taipei City.
Chien-Tai Hong, Department of Neurology, Shuang Ho Hospital, Taipei Medical University, No. 291, Zhongzheng Rd, Zhonghe District, New Taipei City 23561 Department of Neurology, School of Medicine, College of Medicine, Taipei Medical University, Taipei City.
References
- 1. de Lau LML, Breteler MMB. Epidemiology of Parkinson’s disease. Lancet Neurol 2006; 5: 525–535. [DOI] [PubMed] [Google Scholar]
- 2. Poewe W. The natural history of Parkinson’s disease. J Neurol 2006; 253(Suppl. 7): VII2–VII6. [DOI] [PubMed] [Google Scholar]
- 3. Stephenson D, Hill D, Cedarbaum JM, et al. ; Critical Path for Parkinson’s Consortium. The qualification of an enrichment biomarker for clinical trials targeting early stages of Parkinson’s disease. J Parkinsons Dis 2019; 9: 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chahine LM, Stern MB. Parkinson’s disease biomarkers: where are we and where do we go next? Mov Disord Clin Pract 2017; 4: 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem 2019; 88: 487–514. [DOI] [PubMed] [Google Scholar]
- 6. Chen CC, Liu L, Ma F, et al. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng 2016; 9: 509–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–345. [DOI] [PubMed] [Google Scholar]
- 8. Kalra H, Adda CG, Liem M, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 2013; 13: 3354–3364.24115447 [Google Scholar]
- 9. Cerri S, Ghezzi C, Sampieri M, et al. The exosomal/total α-synuclein ratio in plasma is associated with glucocerebrosidase activity and correlates with measures of disease severity in PD patients. Front Cell Neurosci 2018; 12: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goetzl EJ, Ledreux A, Granholm A-C, et al. Neuron-derived exosome proteins may contribute to progression from repetitive mild traumatic brain injuries to chronic traumatic encephalopathy. Front Neurosci 2019; 13: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Si X, Tian J, Chen Y, et al. Central nervous system-derived exosomal alpha-synuclein in serum may be a biomarker in Parkinson’s disease. Neuroscience 2019; 413: 308–316. [DOI] [PubMed] [Google Scholar]
- 12. Goetzl EJ, Mustapic M, Kapogiannis D, et al. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J 2016; 30: 3853–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winston CN, Romero HK, Ellisman M, et al. Assessing neuronal and astrocyte derived exosomes from individuals with mild traumatic brain injury for markers of neurodegeneration and cytotoxic activity. Front Neurosci 2019; 13: 1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaetani L, Blennow K, Calabresi P, et al. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 2019; 90: 870–881. [DOI] [PubMed] [Google Scholar]
- 15. Parnetti L, Gaetani L, Eusebi P, et al. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol 2019; 18: 573–586. [DOI] [PubMed] [Google Scholar]
- 16. Rojas JC, Bang J, Lobach IV, et al. CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology 2018; 90: e273–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun B, Dalvi P, Abadjian L, et al. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS 2017; 31: F9–F17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eggers C, Kahraman D, Fink GR, et al. Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of FP-CIT single photon emission computed tomography. Mov Disord 2011; 26: 416–423. [DOI] [PubMed] [Google Scholar]
- 20. Kapogiannis D, Boxer A, Schwartz JB, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J 2015; 29: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim Y-J, Lee S-J. Are exosomes the vehicle for protein aggregate propagation in neurodegenerative diseases? Acta Neuropathol Commun 2017; 5: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D’Anca M, Fenoglio C, Serpente M, et al. Exosome determinants of physiological aging and age-related neurodegenerative diseases. Front Aging Neurosci 2019; 11: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winston CN, Goetzl EJ, Akers JC, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer’s Dementia (Amst) 2016; 3: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaetani L, Blennow K, Calabresi P, et al. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 2019; 90: 870–881. [DOI] [PubMed] [Google Scholar]
- 25. Mattsson N, Andreasson U, Zetterberg H, et al. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2017; 74: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holmberg B, Rosengren L, Karlsson JE, et al. Increased cerebrospinal fluid levels of neurofilament protein in progressive supranuclear palsy and multiple-system atrophy compared with Parkinson’s disease. Mov Disord 1998; 13: 70–77. [DOI] [PubMed] [Google Scholar]
- 27. Hall S, Öhrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 2012; 69: 1445–1452. [DOI] [PubMed] [Google Scholar]
- 28. Magdalinou NK, Paterson RW, Schott JM, et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry 2015; 86: 1240–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bäckström DC, Eriksson Domellöf M, Linder J, et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol 2015; 72: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 30. Bäckström D, Linder J, Jakobson Mo S, et al. NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology 2020; 95: e827–e838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin C-H, Li C-H, Yang K-C, et al. Blood NfL: a biomarker for disease severity and progression in Parkinson disease. Neurology 2019; 93: e1104–e1111. [DOI] [PubMed] [Google Scholar]
- 32. Ng ASL, Tan YJ, Yong ACW, et al. Utility of plasma neurofilament light as a diagnostic and prognostic biomarker of the postural instability gait disorder motor subtype in early Parkinson’s disease. Mol Neurodegener 2020; 15: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilke C, Dos Santos MCT, Schulte C, et al. Intraindividual neurofilament dynamics in serum mark the conversion to sporadic Parkinson’s disease. Mov Disord 2020; 35: 1233–1238. [DOI] [PubMed] [Google Scholar]
- 34. Zaidel A, Arkadir D, Israel Z, et al. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol 2009; 22: 387–393. [DOI] [PubMed] [Google Scholar]
- 35. Charvin D, Medori R, Hauser RA, et al. Therapeutic strategies for Parkinson disease: beyond dopaminergic drugs. Nat Rev Drug Discov 2018; 17: 804–822. [DOI] [PubMed] [Google Scholar]
- 36. Barbour R, Kling K, Anderson JP, et al. Red blood cells are the major source of alpha-synuclein [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_1756286420975917 for Neurofilament light chain level in plasma extracellular vesicles and Parkinson’s disease by Chen-Chih Chung, Lung Chan, Jia-Hung Chen, Oluwaseun Adebayo Bamodu and Chien-Tai Hong in Therapeutic Advances in Neurological Disorders