Abstract
Background:
Non-alcoholic fatty liver disease (NAFLD) affects up to 30% of the population. Clinical trials have questioned the role of vitamin E in the treatment of NAFLD with or without other interventions, with still no firm conclusion reached. This study aims to examine the efficiency of vitamin E alone or combined in the management of NAFLD.
Methods:
We performed a systematic literature search on PubMed, Scopus, Embase, Ovid, EBSCO host, Science Direct, Web of Science, and Cochrane CENTRAL for randomized controlled trials (RCTs) of the role of vitamin E alone or combined in NAFLD patients. Extracted manuscripts reported data on biochemical, histological, anthropometric, and metabolic outcomes. Baseline characteristics, settings, dosage, and frequency were also collected.
Research:
A total of 1317 patients from 15 RCTs were included in our systematic review and meta-analysis. Vitamin E was superior at improving alanine aminotransferase (ALT), aspartate aminotransferase (AST), NAFLD activity score (NAS), and fibrosis in short- and long-term follow up in the adult population, and long-term follow up in the pediatric population. Improvements in metabolic outcomes were best noticed in pediatric patients. Results from multiple regression models showed a significant association between ALT-AST levels and vitamin E dose. AST levels had a significant effect on NAS, and patients with a baseline AST > 50 IU/l showed more promising results. Changes in weight and body mass index (BMI) were strongly associated with changes in NAS.
Conclusion:
Current evidence affirms that vitamin E – whether alone or combined – improves biochemical and histological outcomes in adults and pediatric patients.
Keywords: meta-analysis, NAFLD, NASH, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, vitamin E
Introduction
Liver disorders are a principal cause of mortality and morbidity worldwide.1 Since 1980, mortality related to liver disorders has increased by 46%.1 In addition, non-alcoholic fatty liver disease (NAFLD) affects 20–30% of the population, and has become the most common liver disease worldwide. NAFLD is the process of lipid deposition within hepatocytes in the complete absence of excessive alcohol consumption or any other known cause of hepatic steatosis.2–4 The disease progresses from simple steatosis to steatohepatitis, with potential development of fibrosis and cirrhosis in up to 15% of patients.5,6 Insulin resistance is one of the most frequent findings associated with NAFLD, together with features of metabolic syndrome such as obesity, central fat distribution, diabetes, dyslipidemia, and atherosclerosis, assigning NAFLD as the hepatic manifestation of metabolic syndrome.7,8
Reports in the literature record NAFLD as an interaction between metabolic abnormality and oxidative stress that triggers inflammation and subsequent damage to hepatocytes.9 The increased production of reactive oxygen species (ROS) is known to affect cellular functions and hemostasis in all organisms and to provoke impaired nucleotide and protein synthesis, leading to the activation of hepatic stellate cells.10 The stress of endoplasmic reticulum (ER) also modifies the development of NAFLD, as disruption in pathological conditions such as inflammation, cardiovascular diseases, and metabolic disturbance implicates ER activity.10 Empirical data revealed that oxidative stress induces ER stress, which further weakens the vital role of ER in maintaining cellular calcium hemostasis, biosynthesis of sterols, carbohydrate, and lipids, leading to cellular damage by ROS.11 Further, the ER stress reduces hepatic lipogenesis and induces lipid droplets accumulation in hepatocytes.12 These events outline the first step in the development of hepatic steatosis.12
Meanwhile, there is no approved primary intervention for NAFLD, but suggested approaches include lifestyle modification, physical exercise, and weight loss.13 These approaches show effectiveness in some patients yet require a combined therapy, strict compliance, and long-term effort, which is not compatible with most patients.3,14 With the increased understanding of NAFLD pathogenesis, antioxidant agents have become promising remedies to resist the effects of ROS.10 Among many antioxidants, vitamin E is the most evaluated agent in NAFLD management, with promising results, as it stabilizes the cell membrane by protecting unsaturated fatty acids from lipid peroxidation and subsequent ROS.15 Clinical trials have investigated the role of vitamin E in the treatment of NAFLD with or without other interventions, but no firm conclusions have yet been reached.16
A previous systematic review concluded that adjuvant vitamin E is beneficial for the treatment of NAFLD exclusively in the adult population.17 However, the conclusion did not offer confirmed implications; with a relatively small size of only five trials and unreliable statistical analyses, the meta-analysis showed no statistical significance for any outcome.
Accordingly, our study aims to compare the efficacy of vitamin E with a placebo, a lifestyle modification, or a no-intervention in NAFLD patients. We consider short-, intermediate-, and long-term follow ups, the effect of co-interventions, baseline variations, and potential associations between the underlying pathogenesis and the drug’s mechanism of action or vital markers that could affect drug efficacy.
Methods
To conduct this systematic review and meta-analysis: we followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guidelines (Supplementary), as well as the standards of the Cochrane Handbook for Systematic Reviews of Interventions.18
Literature search strategy
A comprehensive literature search was conducted in eight electronic databases including PubMed, Scopus, Embase, Ovid, EBSCO host, Science Direct, Web of Science, and Cochrane CENTRAL. The following keywords were used: “NASH”, “NAFLD”, “nonalcoholic steatohepatitis”, “nonalcoholic fatty liver disease”, “fatty liver”, “vitamin E”, “alpha-tocopherol”, “alpha-tocotrienol”.
All published manuscripts (full-text and conference) were considered, with no language or publication period restrictions. The bibliography of the included studies was searched manually to identify additional relevant records that were not retrieved during the literature search.
Eligibility criteria and study selection
We included all studies meeting the following criteria: (1) population: patients diagnosed with NAFLD, regardless of age and gender, (2) intervention: vitamin E (all doses) alone or combined with any other co-interventions, (3) comparator: placebo, lifestyle modifications or no intervention, (4) outcomes: trials reporting the impact of vitamin E on at least one of the following treatment outcomes: (I) biochemical outcomes [alanine aminotransferase (ALT), aspartate aminotransferase (AST)]; (II) histological outcomes [NAFLD activity score (NAS) – covering steatosis, lobular inflammation, hepatocellular ballooning – and fibrosis Score]; (III) anthropometric outcomes [body mass index (BMI), weight, and waist circumference); (IV) metabolic outcomes [fasting blood glucose (FBG) and fasting blood insulin (FBI) levels, homeostatic model assessment of insulin resistance (HOMA-IR), total cholesterol, triglycerides, low-density lipoprotein (LDL) and high-density lipoprotein (HDL)]; were considered for inclusion, and (5) study design: randomized controlled trials (RCTs). We excluded the following: (1) observational and non-randomized trials, (2) in vitro and animal studies, and (3) studies whose data were unreliable for extraction and analysis. Duplicates were removed using EndNote X9.3.3 software. Retrieved references were screened in two steps: the first step was to screen titles/abstracts for matching our inclusion criteria, and the second step was to screen the retrieved full-text articles for eligibility to meta-analysis. Each step was performed by three independent reviewers.
Data extraction
Each type of dataset was extracted independently by two authors. Discrepancies were reconciled through full discussion and consensus among the reviewers. The extracted data involved the following: (I) summary of patients included in our study including: study ID (name of the author, year and setting of the publication), study design, major inclusion criteria, various intervention groups (intervention group, number of participants, dosage, frequency per day and co-intervention), study duration period, follow up in months and the conclusion of each study; (II) baseline characteristics of each intervention arm of the enrolled patients including: sex, age, anthropometric parameters (BMI, weight and waist circumference), metabolic parameters (triglycerides, total cholesterol, HDL, LDL, FBG, FBI, HOMA-IR, AST, ALT), and histological parameters (NAS, steatosis grade, ballooning grade, fibrosis grade, lobular inflammation, and portal inflammation); (III) risk of bias (ROB) domains including: randomization, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias; and (IV) treatment outcome measures. The following outcome measures were extracted at various week endpoints as mean and standard deviations (SDs) to indicate the short- and long-term efficacy related to the treatment groups: (1) Biochemical outcomes (ALT, AST); (2) Histological outcomes (NAS – covering steatosis, lobular inflammation, hepatocellular ballooning – fibrosis score); (3) anthropometric outcomes (BMI, weight, and waist circumference); (4) metabolic outcomes (FBG, FBI, HOMA-IR, total cholesterol, triglycerides, LDL, HDL).
ROB assessment
The risk of bias within each included study was assessed by two independent authors using the Cochrane ROB assessment tool -adequately described in chapter 8.5 of the Cochrane handbook of systematic reviews of interventions.18 ROB domains included randomization (selection bias); allocation concealment (selection bias); blinding of participants (performance bias); blinding of outcome assessment(detection bias); incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias including extreme baseline irregularity, unreliable study design, or trial termination shortly due to data-dependent considerations. We classified RCTs in each domain as low, high, or unclear ROB. Any discrepancies were settled through discussion and consent. The assessments of publication bias using funnel plots and Egger’s test were performed. We also considered the Grading of Recommendations Assessment Development and Evaluation (GRADE) framework (Table 1).
Table 1.
The GRADE framework for the major outcomes.
| Question: should vitamin E versus Control be used for NAFLD? | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||||
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Vitamin E | Control | Relative (95% CI) |
Absolute (95% CI) |
||||
| ALT (follow up: 24 months; scale from: −17.493 to −5.367) | ||||||||||||||
| 11 | Randomized trials | Not serious | Seriousa | Not serious | Seriousb | Very strong association dose response gradient | 382 | 572 | – | MD 11.43 1000 lower
(17.493 lower to 5.367 lower) |
⨁⨁⨁⨁ HIGH |
IMPORTANT | ||
| AST (follow up: 24 months; scale from: −11.686 to −1.846) | ||||||||||||||
| 10 | Randomized trials | Not serious | Seriousa | Not serious | Seriousb | Strong association dose response gradient |
350 | 576 | – | MD 6.766 1000 lower
(11.686 lower to 1.846 lower) |
⨁⨁⨁⨁ HIGH |
IMPORTANT | ||
| Fibrosis (follow up: 24 months; scale from: −0.426 to −0.023) | ||||||||||||||
| 7 | Randomized trials | Not serious | Not serious | Not serious | Seriousb | Strong association | 261 | 428 | – | MD 0.224 1000 lower
(0.426 lower to 0.023 lower) |
⨁⨁⨁⨁ HIGH |
CRITICAL | ||
| NAS (follow up: 24 months; scale from: −2.495 to −0.510) | ||||||||||||||
| 7 | Randomized trials | Not serious | Not serious | Not serious | Seriousb | Strong association | 256 | 446 | – | MD 1.503 1000 lower
(2.495 lower to 0.51 lower) |
⨁⨁⨁⨁ HIGH |
CRITICAL | ||
Eight included studies reported superiority of vitamin E alone or combined compared with control. Five other trials demonstrated that the control was more efficient in reducing NAFLD relative to Vitamin E. Another two trials reported that the two arms did not differ markedly in terms of their effects in improving hepatic and metabolic outcomes.
Wide 95% CI was present at some endpoints.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; GRADE, grading of recommendations assessment development and evaluation; MD, mean difference; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score.
Data synthesis
Statistical analyses were performed using Open Meta (Analyst) and STATA version 16.0. We employed the random-effects model with the Der-Simonian Liard method. All data were continuous (means of change and standard deviations “SD”) and were pooled as weighted mean differences (MD) with 95% confidence intervals (CI). Missing SD of changes from baseline was calculated from the standard error or 95% CI or range according to Wan et al.19 or obtained from SD of baseline and SD of final endpoint according to Cochrane 16.1.3.2.18 Heterogeneity between trials was examined visually and statistically through Chi-square and I2 tests: values of 0−40%, 30−60%, 50−90%, and 75−100% represented low, moderate, substantial, and considerable heterogeneity; respectively. p < 0.1 was set as a level of significant heterogeneity. When considerable heterogeneity was detected: we performed a sensitivity analysis to determine the source of heterogeneity by excluding one study at a time. Subgroup analysis was employed according to follow-ups and study population. Further, a meta-regression was conducted to examine: whether dose, sex, age, co-interventions, anthropometric and metabolic parameters may predict alterations in biochemical and histological outcomes.
Results
Search results and characteristics of included studies
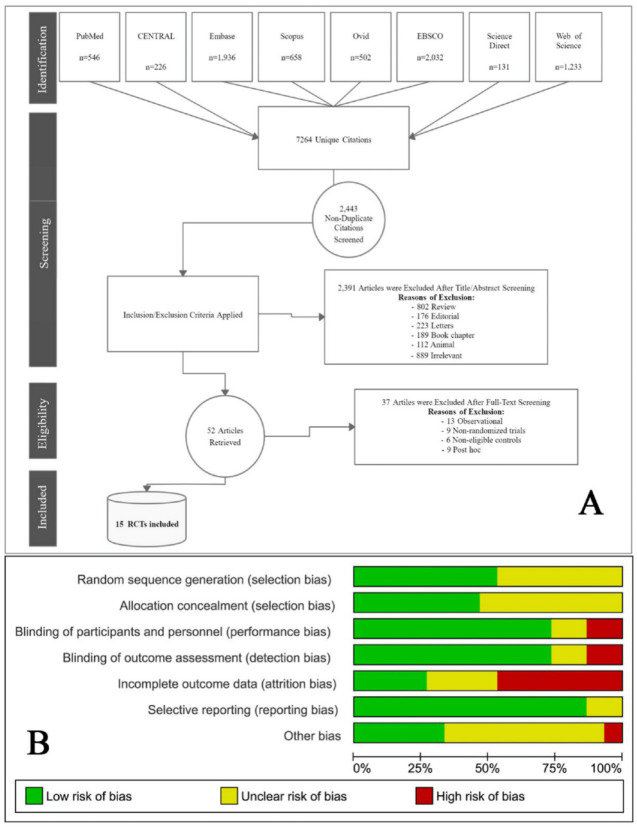
Our search retrieved 7264 unique citations from searching electronic databases. Following title and abstract screening, 52 full-text articles were retrieved and screened for eligibility. Of them: 37 articles were excluded, and 15 RCTs of 13 articles and two conferences (n = 1317 patients) were reviewed in detail and included in this meta-analysis (PRISMA flow diagram; Figure 1A).
Figure 1.
(A) PRISMA flow diagram illustrates the search strategy, screening and the selection process. (B) Risk of bias graph according to Cochrane risk of bias assessment tool.
PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCT, randomized controlled trial.
The references of the included RCTs were searched manually, but no further records were added. All the included studies were performed between 2003 and 2020; eight studies in Europe,20–27 four studies in North America,9,28–30 and three studies in Asia.31–33 A total of 12 studies compared vitamin E with placebo,9,20–25,27–29,30,31 and three studies compared vitamin E with lifestyle modifications or no intervention.26,32,33 Six trials included lifestyle modifications as a co-intervention to vitamin E, four trials involved vitamin C, two trials involved ursodeoxycholic acid (UDCA), one trial involved docosahexaenoic acid (DHA) plus choline, one trial involved hydroxytyrosol, and one trial involved Silymarin (Table 2 in Supplemental Material).
The majority of the studies reported a frequency of one dose per day, three trials considered a frequency of two doses daily; eight trials reported a dosage of 400 IU or below, six trials involved 600–1000 IU. The follow-up period ranged from 1 month to 24 months. Half of the trials included adult populations (n = 8 RCTs), and the other half included pediatric populations (n = 7 RCTs) (Table 2).
Table 2.
Summary data of patients in included studies.
| Author, setting and country | Study design | Population | Treatment | Study duration | Follow up | Conclusion | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Number | Dosage | Frequency | Co-Intervention | ||||||
| Mosca et al.23
(Italy) |
Randomized double-blinded, placebo-controlled
trial (Conference Paper) |
Adolescents (age range, 4−16 years) with liver biopsy proven NAFLD and without other causes of liver disease. | vitamin E | 40 | __ | __ | Hydroxytyrosol | NA | 4 months | - Vitamin E and Hydroxytyrosol reduced the
systemic inflammation with a significantly decrease of
IL-6. - The combination increased the expression of IL-10, which is able to inhibit the synthesis of pro-inflammatory cytokines. |
| Placebo | 40 | __ | __ | None | ||||||
| Khachidze et al.32
(Georgia) |
Randomized double-blinded, placebo-controlled
trial (Conference Paper) |
Patients with elevated aminotransferase levels and drinking less than 40 g alcohol per week with a diagnosis of NASH. | vitamin E | 52 | 400 IU | Once daily | vitamin C 500 mg/day + lifestyle modification | NA | 12 months | Vitamin E plus vitamin C combination is an effective, safe and inexpensive treatment option in patients with NASH and may be useful to reduce damage from oxidative stress and slow the process leading to cirrhosis. |
| lifestyle modification | 20 | __ | __ | None | ||||||
| UDCA | 35 | 15 mg/kg | Once daily | lifestyle modification | ||||||
| Anushiravani et al.31
(Iran) |
Randomized double-blinded, placebo-controlled trial | Patients aged between 18 and 65 years with a probable diagnosis of NAFLD in liver sonography (grades II and III steatosis) with or without increased levels of liver enzymes AST and ALT (above 20 mg/dl for women and 30 mg/dl for men). | vitamin E | 30 | 400 IU | Once daily | lifestyle | April 2016 − October 2017 | 3 months | - Vitamin E shows a significant benefit in improving liver aminotransferases in patients with NAFLD after only 3 months, without exerting any specific side effects. |
| Placebo | 30 | __ | __ | lifestyle | ||||||
| Metformin | 30 | 500 mg | Once daily | lifestyle | ||||||
| Silymarin | 30 | 140 mg | Once daily | lifestyle | ||||||
| pioglitazone | 30 | 15 mg | Once daily | lifestyle | ||||||
| Bril et al.30
(United States) |
Randomized, double-blind, placebo-controlled trial | Patients aged between 18 and 70 years with a diagnosis of type 2 diabetes mellitus, based on prior medical history, results from prior laboratories (hemoglobin A1C or fasting plasma glucose), and with a diagnosis of NASH based on a liver biopsy, and defined as: zone 3 accentuation of macrovesicular steatosis (any grade), hepatocellular ballooning (any degree) and lobular inflammatory infiltrates (any amount). | vitamin E | 36 | 400 IU | twice day | None | June 2010– September 2016 | 18 months | - Combination therapy was better than placebo in
improving liver histology in patients with NASH and
T2DM. - Vitamin E alone did not significantly change the primary histological outcome. |
| Placebo | 32 | __ | __ | None | ||||||
| vitamin E | 37 | 400 IU | twice day | pioglitazone 45 mg/day | ||||||
| Zöhrer et al.20
(Italy) |
Randomized, double-blind, placebo-controlled trial | Children or adolescents (age range, 4–16 years) with liver biopsy-proven NASH and without other causes of liver disease. | vitamin E | 20 | 39 IU | Once daily | choline 201 mg + DHA 250 mg | NA | 12 months | - Combination of DHA, vitamin E and choline could improve steatosis and reduce ALT and glucose levels in children with NASH. |
| Placebo | 20 | __ | __ | None | ||||||
| Aller et al.26
(Spain) |
Randomized clinical pilot study | Patients with diagnosis of NAFLD confirmed by percutaneous liver biopsy. | vitamin E | 18 | 80 IU | Once daily | silymarin + hypocaloric diet + exercise | NA | 3 months | - Vitamin E plus silymarin and a hypocaloric
diet ameliorate function hepatic test, and non-invasive
NAFLD index. - Silymarin can be an alternative valid therapeutic option particularly when other drugs are not indicated or have failed or as a complementary treatment associated with other therapeutic programs. |
| hypocaloric diet | 18 | __ | __ | None | ||||||
| Lavine et al.9
(United States) |
Randomized, double-blind, double-dummy, placebo controlled clinical trial | Patients aged 8−17 years with NAFLD by a liver biopsy demonstrating more than 5% steatosis within a 6-month period before randomization and persistently elevated levels of ALT was defined by a value greater than 60 U/L for 1−6 months before and at the time of randomization. | vitamin E | 58 | 800 IU | Once daily | diet + exercise | September 2005−March 2010. |
24 months | - Neither vitamin E nor metformin was superior to placebo in attaining the primary outcome of sustained reduction in ALT level in patients with pediatric NAFLD. |
| placebo | 58 | __ | __ | diet + exercise | ||||||
| metformin | 57 | 1000 mg | __ | diet + exercise | ||||||
| Sanyal et al.29
(United States) |
Phase III, multicenter, randomized, double-blind, placebo controlled, clinical trial |
adults without diabetes who had nonalcoholic steatohepatitis by a liver biopsy within 6 months before randomization. | vitamin E | 84 | 800 IU | Once daily | None | January 2005−January 2007 | 24 months | - Vitamin E was superior to placebo for the
treatment of NASH in adults without diabetes. - There was no benefit of pioglitazone over placebo for the primary outcome; however, significant benefits of pioglitazone were observed for some of the secondary outcomes. |
| placebo | 83 | __ | Once daily | None | ||||||
| pioglitazone | 80 | 30 mg | Once daily | None | ||||||
| Balmer et al.27
(Switzerland) |
Randomized, placebo-controlled, double-blind study | Patients 18−75 years of age with histologically proven NASH by a liver biopsy. | vitamin E | 14 | 400 IU | twice day | UDCA 12–15 mg/kg/day | NA | 24 months | - UDCA + Vit E improves not only
aminotransferase levels and liver histology of patients with NASH, but also decreases hepatocellular apoptosis and restores circulating levels of adiponectin. - UDCA1VitE combination has metabolic effects in addition to its beneficial cytoprotective properties. |
| placebo | 13 | __ | __ | None | ||||||
| UDCA | 14 | 12−15 mg/kg | Once daily | None | ||||||
| Wang et al.33
(China) |
Randomized, Single-blind study | Obese children, according to the criteria that a child is obese when the BMI exceeded the 95th BMI percentage for age and sex. The patients age ranged from 10 to 17 years (mean 13.7 ± 1.9 years). They were all obese with liver fatty infiltration in ultrasonic appearance and abnormal liver function with higher ALT by at least 1.5 times over the upper normal limit which was diagnosed as NASH. | vitamin E | 19 | 150 IU | Once daily | None | NA | 1 month | - Short-term lifestyle intervention and vitamin
E therapy have an effect on NAFLD in obese
children. - Compared with vitamin E, lifestyle intervention is more effective. Therefore, lifestyle intervention should represent the first step in the management of children with NAFLD. |
| lifestyle intervention | 19 | __ | __ | None | ||||||
| no intervention | 38 | __ | __ | None | ||||||
| Nobili et al.21
(Italy) |
Randomized, placebo-controlled, double-blind study | children or adolescents with diagnosis of NAFLD by a liver biopsy and diffusely echogenic liver on imaging studies. Patients had persistently elevated serum aminotransferase levels. | vitamin E | 25 | 600 IU | Once daily | vitamin C 500 mg/day + diet + exercise | January 2003 − October 2006 | 24 months | - Lifestyle intervention with diet and increased
physical activity induces weight loss and is associated with
a significant improvement in liver histology and laboratory
abnormalities in pediatric NAFLD. - Vitamin E plus ascorbic acid does not seem to increase the efficacy of lifestyle intervention alone. |
| placebo | 28 | __ | __ | diet + exercise | ||||||
| Nobili et al.22
(Italy) |
Randomized, placebo-controlled, double-blind study | children or adolescents (aged 3−18 years) with biopsy-proven NAFLD and diffusely echogenic liver in imaging studies. Patients had persistently elevated serum aminotransferase levels. | vitamin E | 45 | 600 IU | Once daily | vitamin C 500 mg/day | January 2003 − March 2005 | 12 months | Diet and physical exercise in NAFLD children seem to lead to a significant improvement of liver function and glucose metabolism beyond any antioxidant therapy. |
| placebo | 43 | __ | __ | diet + exercise | ||||||
| Dufour et al.25
(Switzerland) |
Multicenter randomized, prospective, double-blind, placebo-controlled trial | Patients 18−75 years of age with a persistent elevation of serum ALT levels of at least 1.5 times the upper limit of normal for at least 6 months and a weekly alcohol consumption of less than 40 g were eligible. Patients had a liver biopsy showing macrovesicular steatosis of more than 10% of the hepatocytes, hepatocellular injury (ballooning, dropout), and lobular inflammation. | vitamin E | 15 | 400 IU | twice day | UDCA 12−15 mg/kg/day | January 1999 − December 2002 | 24 months | - Vitamin E in combination with UDCA improved
laboratory values and hepatic steatosis of patients with NASH. |
| placebo | 15 | __ | __ | None | ||||||
| UDCA | 18 | 12−15 mg/kg | Once daily | placebo | ||||||
| Vajro et al.24
(Italy) |
Randomized, placebo-controlled, Single-blind study | Patients with a probable diagnosis of NAFLD in liver sonography with increased levels of liver enzymes AST and ALT ⩾ 1.5 times above normal values for more than 6 months. | vitamin E | 14 | 600 IU × 2 months | Once daily | diet | January 1999 − June 2001 | 5 months | Oral vitamin E warrants consideration in obesity related liver dysfunction for children unable to adhere to low-calorie diets. |
| 150 IU × 3 months | Once daily | |||||||||
| placebo | 14 | __ | __ | diet | ||||||
| Harrison et al.28
(United States) |
Prospective, randomized, double-blind, placebo-controlled trial | Patients with a probable diagnosis of NASH 18 years of age or older and had a liver biopsy within the past 6 months for elevated aminotransferases. Hb values of at least 12 g/dl for women and 13 g/dl for men, white blood cell count of greater than 3000/mm3, neutrophil count of greater than 1500/mm3, platelets greater than 70,000/mm3, serum albumin greater than 3 g/dl, and a serum creatinine less than 1.4 mg/dl. | vitamin E | 23 | 1000 IU | Once daily | vitamin C 1000 mg/day + Diet + exercise | August 2000 – June 2002. | 6 months | - Vitamin E and vitamin C were well tolerated
and were effective in improving fibrosis scores in NASH
patients. - No improvement in necroinflammatory activity or ALT was seen with this combination of drug therapy. |
| placebo | 22 | __ | __ | Diet + exercise |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DHA, docosahexaenoic acid; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, nonalcoholic steatohepatitis; UDCA, ursodeoxycholic acid.
Males and females were represented equally between studies. A summary of the characteristics of included patients and studies is available in Table 2 in the Supplemental Material.
Potential sources of bias
Following the Cochrane ROB tool, the quality of the included studies ranged from moderate to high. The main concern was incomplete outcome data (loss to follow up), which was detected in Bril et al.,30 Harrison et al.,28 Lavine et al.,9 Nobili et al.,22 Sanyal et al.,29 and Zöhrer et al.20 A summary of quality assessment domains is presented in Figure 1B. while authors’ judgments with justifications are shown in Supplemental File X and Supplemental Figure S4.
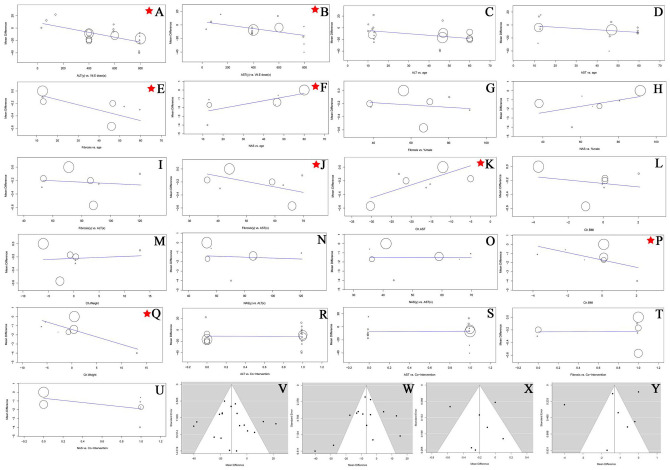
Funnel plots of the standard errors versus the mean differences were examined for all major outcomes; no publication bias was detected visually for ALT, AST, NAS, and Fibrosis (Figure 2V−Y). Further, Egger’s regression revealed no small study effects.
Figure 2.
The overall meta-regression mean difference of the interaction between dose/age on x-axis and ALT/AST on y-axis. (E–H) The overall meta-regression mean difference of the interaction between age/sex on x-axis and Fibrosis/NAS on y-axis. (I–Q) The overall meta-regression mean difference of the interaction between ALT/AST/BMI/weight on x-axis and Fibrosis/NAS on y-axis. (R–U) The overall meta-regression mean difference of the interaction between co-interventions on x-axis and ALT/AST/Fibrosis/NAS on y-axis. The stars indicates significant predictions. (V–Y) Funnel plots of ALT/AST/Fibrosis/NAS showing no evidence of publication bias.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score.
Outcomes
Biochemical outcomes
Alanine aminotransferase
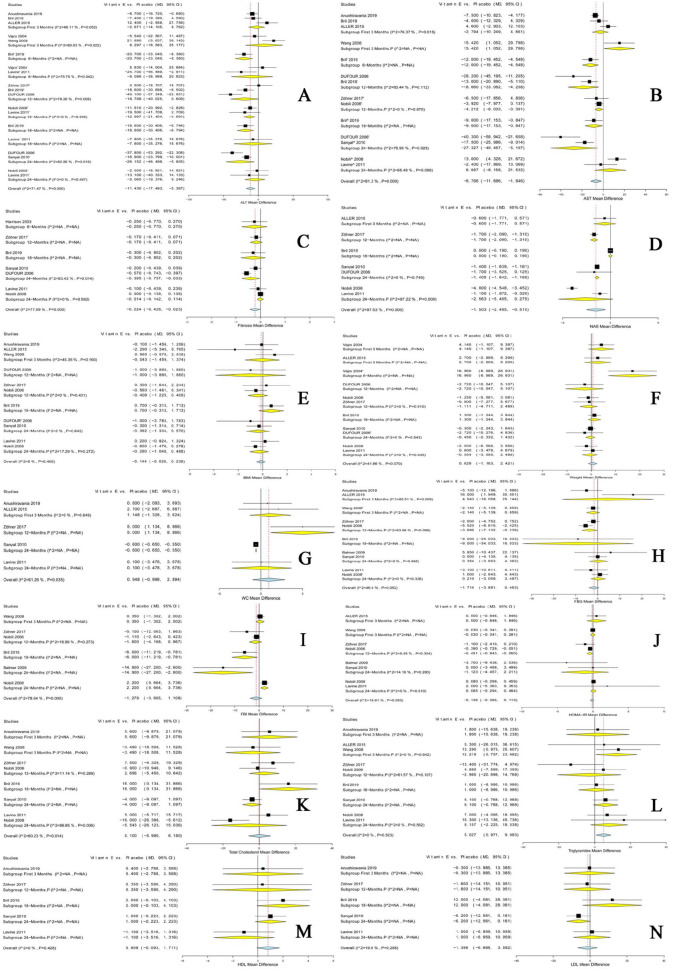
The overall effect showed a significant difference between the two groups in ALT levels [MD = −11.430, 95% CI (−17.493, −5.367)] favoring vitamin E (Figure 3A).
Figure 3.
Forest plots show the MD in each outcome along with the associated 95% CI in the two arms at 3, 6, 12, 18, and 24 months; p indicates pediatric population. Outcomes: (A) ALT, (B) AST, (C) fibrosis, (D) NAS, (E) BMI, (F) weight, (G) waist-circumference, (H) FBG, (I) FBI, (J) HOMA-IR, (K) total-cholesterol, (L) triglycerides, (M) to HDL, (N) LDL.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; FBG, fasting blood glucose; FBI, fasting blood insulin; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score.
In the adult population, vitamin E significantly reduced ALT levels after 6 months [MD = −20.700, 95% CI (−33.040, −8.360)], 18 months [MD = −18.600, 95% CI (−30.406, −6.794)], and 24 months [MD = −26.152, 95% CI (−46.498, −5.805)]; pooled analyses were homogenous, and reductions were not significant at the other endpoints.
In the pediatric population, vitamin E significantly reduced ALT levels after 12 months [MD = −12.997, 95% CI (−21.404, −4.591)]; pooled analyses were homogenous, and reductions were not significant at the other endpoints.
Aspartate aminotransferase
The overall effect showed a significant difference between the two groups in AST levels [MD = −6.766, 95% CI (−11.686, −1.846)] favoring vitamin E (Figure 3B).
In the adult population, vitamin E significantly reduced AST levels after 6 months [MD = −12.000, 95% CI (−19.452, −4.548)], 12 months [MD = −18.660, 95% CI (−33.062, −4.258)], 18 months [MD = −9.000, 95% CI (−17.153, −0.847)], and 24 months [MD = −27.327, 95% CI (−49.457, −5.197)]; pooled analyses were homogenous, heterogeneity was not significant, and reductions were not significant at the other endpoints.
In the pediatric population, vitamin E significantly reduced AST levels after 12-months [MD = −4.212, 95% CI (−8.033, −0.391)]; pooled analyses were homogenous, and reductions were not significant at the other endpoints.
Histological outcomes
NAFLD activity score
The overall effect showed a significant difference between the two groups in NAS levels [MD = −1.503, 95% CI (−2.495, −0.510)] favoring vitamin E (Figure 3D).
In the adult population, vitamin E significantly reduced NAS levels after 12 months [MD = −1.700, 95% CI (−2.090, −1.310)] and 24 months [MD = −1.405, 95% CI (−1.642, −1.168)]; pooled analyses were homogenous, and reductions were not significant at the other endpoints.
In the pediatric population, reductions were not significant.
Fibrosis score
The overall effect showed a significant difference between the two groups in fibrosis levels [MD = −0.224, 95% CI (−0.426, −0.023)] favoring vitamin E (Figure 3C).
In the adult population, vitamin E significantly reduced fibrosis levels after 24 months [MD = −0.395, 95% CI (−0.757, −0.033)]; pooled analyses were homogenous, and reductions were not significant at the other endpoints.
In the pediatric population, reductions were not significant.
Anthropometric outcomes
The pooled analysis revealed no significant differences between the two groups in BMI, weight, and waist circumference, either in the adult or the pediatric populations (Figure 3E–G).
Metabolic outcomes
In the adult populations, reductions were significant in FBI levels after 18 months [MD −6.000, 95% CI (−11.219, −0.781)], whereas, in the pediatric populations, reductions were significant in HOMA-IR levels after 12 months [MD = −0.451, 95% (−0.843, −0.060)] and FBG levels after 12 months [MD = −3.686, 95% (−7.132, −0.239)].
Otherwise, the pooled analysis revealed no significant differences between the two groups in total cholesterol, triglycerides, LDL, and HDL (Figure 3H−N).
Meta-regression models
Results from multiple regression models showed a significant association of a 49% R2 between ALT levels and vitamin E dose (coefficient −0.039679; p = 0.002), and AST levels and vitamin E dose (coefficient ‒0.0245566; p = 0.01). It also showed no significant effect of age on ALT (p = 0.06) or AST (p = 0.1), and no significant effect of sex on fibrosis (p = 0.7) or NAS (p = 0.1). However, the regression showed a strong effect of age on fibrosis (coefficient −0.008; p = 0.013; R2 = 64.57) and NAS (Coefficient 0.042; p = 0.019; R2 = 70.09) (Figure 2A–H).
Double interaction regression of fibrosis versus ALT, AST, BMI, and weight revealed that patients with a baseline AST > 50 IU/l show more promising results (coefficient ‒0.0093526; p = 0.03, R2 = 47%), changes in AST were strongly associated with changes in fibrosis (coefficient 0.029093; p = 0.000, R2 = 100%), neither changes in weight nor BMI were associated with changes in fibrosis (p = 0.8, p = 0.7; respectively). Double interaction regression of NAS versus ALT, AST, BMI, and weight revealed that: changes in weight and BMI were associated strongly with changes in NAS; a weight loss by 5−10 kg was associated with a reduction in NAS by 1 degree (coefficients ‒0.1618272; p = 0.0233, R2 = 40%) (Figure 2I–Q).
We performed additional meta-regression to determine whether these favorable outcomes were due to the effects of vitamin E or attributed to the co-interventions. According to our regression model, co-interventions had no significant modification on the changes of ALT (p = 0.927), AST (p = 0.897), fibrosis (p = 0.960), or NAS (p = 0.174) (Figure 2R–U).
Discussion
In this systematic review and meta-analysis of 15 RCTs and 1317 patients, eight of our included studies reported superiority of vitamin E alone or combined over placebo or lifestyle modifications.23–27,29,31,32 However, five other trials demonstrated that the comparable intervention was more efficient in reducing NAFLD or nonalcoholic steatohepatitis (NASH) relative to Vitamin E.9,21,22,30,33 To complicate this even further, two other trials reported that the two arms did not differ markedly in terms of their effects in improving hepatic and metabolic parameters.20,28 In a previous systematic review of nine RCTs, the authors concluded that adjuvant vitamin E might produce significant biochemical and histological improvements only in the adult population.17 The conclusion was not conclusive, as the meta-analysis included only five RCTs and showed no statistically significant difference at any outcome.
Nonetheless, the relatively small sample size, the short-term follow ups, the absence of dose consideration, non-comprehensive literature search, the inclusion of post hoc analyses, the unreliable statistical combination of mean change with final endpoint mean, and the significant heterogeneity left the question unsettled. Whether the intervention is more efficient remains a valid debate. And what type of patient can benefit the most remains a critical clinical question. Thus, we performed this meta-analysis to compare the efficacy of vitamin E with a placebo, lifestyle modification, or no-intervention comparison in NAFLD and NASH patients. We considered short, intermediate, and long-term follow ups, the effect of co-interventions, baseline variations, possible associations between the underlying pathogenesis and the drug’s mechanism of action, and significant markers that could indicate drug efficacy.
Our analysis revealed that the group who received vitamin E had a statistically significant improvement in ALT, AST, fibrosis, and NAS at early and late follow up. This improvement was prominent in the adult population. In the pediatric population, the significant change in biochemical parameters started to appear at long-term follow-up. This delay could explain the negative results of short-term studies.22,33 Further, the regression model revealed a strong negative association between age and histological changes. This finding justifies the minimal improvement in pediatric histological parameters, and also the negative results of previous studies.9,30 Otherwise, vitamin E showed more favorable metabolic changes in the pediatric population, although the histological changes were minimal. A possible explanation for this negative association between age and histological changes may be attributed to the increased oxidative stress in pediatric populations.34–36
Interestingly, the regression revealed no significant association between metabolic changes and histological changes. This finding softens the traditional hypothesis that weight changes affect fibrosis.37,38 However, a strong association was present between change in weight or BMI and change in NAS. Double interaction regression revealed no significant overlap between fibrosis and NAS. This diversion implies that no single medication can guarantee both metabolic and all-histological improvement at the same time. Hence, this finding supports the idea of combined or multiple interventions.39 We also examined whether these favorable outcomes were due to the effect of vitamin E or attributed to the co-interventions. According to our regression model: co-interventions did not significantly modify the changes in ALT, AST, fibrosis, or NAS. This finding implies that vitamin E alone can improve these outcomes, which lessens the general attitude that considers it as merely an adjuvant.40,41
The proper dose of vitamin E has long been debated, with considerable variations among trials.42,43 Results from multiple regression models showed a significant negative association between ALT, AST levels, and vitamin E dosage – more favorably between 400 and 800 IU. We also considered the clinical question of what type of patient and what kind of prognosis. According to our analysis, the most promising patient is an obese male or female aged between 15 and 50 years, with baseline AST > 50 IU/l, daily intake of 400–800 IU vitamin E, and liability to lose 5–10 kg. The interaction regression of this combination yields an R2 of 100% (p = 0.000). We propose this as a score from 1 to 5 where 3 points or above leads to a good prognosis. Previous analyses considered NAFLD as a predictor for Type-II diabetes mellitus (DM) or cardiovascular events.44,45 In contrast, this score can help predict the course of NAFLD itself with vitamin E.
Previous cumulative analyses support our results.16,17 Amanullah and colleagues examined the effects of vitamin E among different k population groups, but data concerning vitamin E impact on hepatic histology were insufficient. Sato et al. reported significant results of liver function improvement due to vitamin E. The mechanism of action of vitamin E in NAFLD and NASH patients involves anti-oxidant, anti-free-radical, anti-apoptotic, and anti-fibrotic roles.46,47 In NAFLD: mitochondrial malfunctions and pathological cytokines increase the production of ROS leading to lipid peroxidation and oxidative stress, which plays a vital role in the progression of the disease from NAFLD to NASH.48,49 While this mechanism justifies the biochemical and histological effects, it does not explain its metabolic actions.
Meanwhile, a previous meta-analysis indicated that a high dose of vitamin E could increase the risk of mortality,50 though the association was not well established.51,52 Other reports raised concerns about prostate cancer risks, though the data are insufficient for a conclusive answer.53,54 Further, a more extensive meta-analysis of 57 RCTs revealed that vitamin E doses up to 5500 IU/day did not affect all-cause mortality.55 Also, another meta-analysis reported a possible increased risk of hemorrhagic stroke by 22% (1 in every 1250 patients), and reduced risk of ischemic stroke by 10% with vitamin E (1 in every 476 patients).56 However, the study included only five blinded and two open-label trials in the analysis with different baseline morbidities and without emphasis on follow-up duration. The safety profile of vitamin E remains a highly important clinical question, with an urgent call for further investigations, especially on long-term follow up.
The quality of a systematic review and meta-analysis rests upon its included studies. Our included studies presented relatively high quality. Our findings settle a group of assumptions and advocate a reliable reference for prospective clinical decisions. To our knowledge, this the first systematic review and meta-analysis to analyze the short-, intermediate-, and long-term outcomes of vitamin E in pediatric and adult populations over 24 months with meta-regression considerations. Even so, there were some limitations to our work. The results of the metabolic outcomes were limited by the heterogeneity of the included studies. The variations in the clinical definitions of NAFLD may contribute to the clinical heterogeneity. Additionally, most of the trials had moderately small sample sizes.
Overall, the current evidence indicates that vitamin E is superior in improving biochemical outcomes in adult and pediatric patients – whether alone or combined. It also favors additional histological improvements for adults and metabolic improvements for pediatric populations. Further multi-center, large sample RCTs are needed to investigate the safety of daily vitamin E alone or combined with other anti-oxidants. Future studies should also consider our prognosis score in the management of NAFLD and NASH patients.
Highlights
In the pediatric population: the significant change in biochemical parameters appears at long-term follow up.
Data from RCTs reveal no significant association between metabolic changes and fibrosis changes.
A strong association was present between change in weight or BMI and change in NAS.
The most promising patient is an obese male or female aged between 15 and 50 years, with a baseline AST > 50 IU/l, daily intake of 400–800 IU vitamin E, and liability to lose 5–10 kg.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_1756284820974917 for The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression by Mohamed Abdel-Maboud, Amr Menshawy, Esraa Menshawy, Amany Emara, Mohamed Alshandidy and Muhammad Eid in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-2-tag-10.1177_1756284820974917 for The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression by Mohamed Abdel-Maboud, Amr Menshawy, Esraa Menshawy, Amany Emara, Mohamed Alshandidy and Muhammad Eid in Therapeutic Advances in Gastroenterology
Footnotes
Author contributions: M.A. and A.M. conceptualized the question and designed the study. M.A., E.M, A.M., and AE. performed the literature search. A.M., E.M., AE., MA., M.E., and M.A. extracted the data from eligible studies and performed the quality assessment. M.A. conducted the statistical analysis and interpreted data. M.A., A.M., M.A., A.E., M.E., and E.M. drafted the manuscript, revised it critically, and approved the version to be published. All authors read and agreed on the final version of the manuscript.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
ORCID iD: Mohamed Abdel-Maboud  https://orcid.org/0000-0002-7746-524X
https://orcid.org/0000-0002-7746-524X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mohamed Abdel-Maboud, Al-Azhar Medical School, Al- Hussein University Hospitals, Cairo, 11651, Egypt.
Amr Menshawy, Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
Esraa Menshawy, Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
Amany Emara, Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
Mohamed Alshandidy, Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
Muhammad Eid, Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
References
- 1. Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med 2014; 12: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson EL, Howe LD, Jones HE, et al. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One 2015; 10: e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019; 7: 313–324. [DOI] [PubMed] [Google Scholar]
- 4. Chen F, Esmaili S, Rogers GB, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology 2020; 71: 1213–1227. [DOI] [PubMed] [Google Scholar]
- 5. Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong MJ, Houlihan DD, Rowe IA. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 363: 1185–1186. [DOI] [PubMed] [Google Scholar]
- 7. Hardy T, Anstee QM, Day CP. Nonalcoholic fatty liver disease: new treatments. Curr Opin Gastroenterol 2015; 31: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foster T, Budoff MJ, Saab S, et al. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the st francis heart study randomized clinical trial. Am J Gastroenterol 2011; 106: 71–77. [DOI] [PubMed] [Google Scholar]
- 9. Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin e or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents the tonic randomized controlled trial. JAMA 2011; 305: 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hickman I, Macdonald G. Is vitamin E beneficial in chronic liver disease? Hepatology 2007; 46: 288–290. [DOI] [PubMed] [Google Scholar]
- 11. Ashraf NU, Sheikh TA. Endoplasmic reticulum stress and oxidative stress in the pathogenesis of non-alcoholic fatty liver disease. Free Radic Res 2015; 49: 1405–1418. [DOI] [PubMed] [Google Scholar]
- 12. Lee JS, Zheng Z, Mendez R, et al. Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model. Toxicol Lett 2012; 211: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018; 24: 908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015; 149: 367–378e5. [DOI] [PubMed] [Google Scholar]
- 15. Sarkhy A, Al-Hussaini A, Nobili V. Does vitamin E improve the outcomes of pediatric nonalcoholic fatty liver disease? a systematic review and meta-analysis. Saudi J Gastroenterol 2014; 20: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato K, Gosho M, Yamamoto T, et al. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition 2015; 31: 923–930. [DOI] [PubMed] [Google Scholar]
- 17. Amanullah I, Khan YH, Anwar I, et al. Effect of vitamin E in non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomised controlled trials. Postgrad Med J 2019; 95: 601–611. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Thomas J, Chandler J, et al. (eds). Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons, 2019. [Google Scholar]
- 19. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zöhrer E, Alisi A, Jahnel J, et al. Efficacy of docosahexaenoic acid-choline-vitamin E in paediatric NASH: a randomized controlled clinical trial. Appl Physiol Nutr Metab 2017; 42: 948–954. [DOI] [PubMed] [Google Scholar]
- 21. Nobili V, Manco M, Devito R, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology 2008; 48: 119–128. [DOI] [PubMed] [Google Scholar]
- 22. Nobili V, Manco M, Devito R, et al. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2006; 24: 1553–1561. [DOI] [PubMed] [Google Scholar]
- 23. Mosca A, Crudele A, Tozzi G, et al. The anti-inflammatory effects of hydroxytyrosol and vitamin e on paediatric NAFLD. Dig Liver Dis 2020; 52: e42–e43. [Google Scholar]
- 24. Vajro P, Mandato C, Franzese A, et al. Vitamin E treatment in pediatric obesity-related liver disease: a randomized study. J Pediatr Gastroenterol Nutr 2004; 38: 48–55. [DOI] [PubMed] [Google Scholar]
- 25. Dufour JF, Oneta CM, Gonvers JJ, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin E in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2006; 4: 1537–1543. [DOI] [PubMed] [Google Scholar]
- 26. Aller R, Izaola O, Gómez S, et al. Effect of silymarin plus Vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur Rev Med Pharmacol Sci 2015; 19: 3118–3124. [PubMed] [Google Scholar]
- 27. Balmer ML, Siegrist K, Zimmermann A, et al. Effects of ursodeoxycholic acid in combination with vitamin E on adipokines and apoptosis in patients with nonalcoholic steatohepatitis. Liver Int 2009; 29: 1184–1188. [DOI] [PubMed] [Google Scholar]
- 28. Harrison SA, Torgerson S, Hayashi P, et al. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2003; 98: 2485–2490. [DOI] [PubMed] [Google Scholar]
- 29. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bril F, Biernacki DM, Kalavalapalli S, et al. Role of Vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2019; 42: 1481–1488. [DOI] [PubMed] [Google Scholar]
- 31. Anushiravani A, Haddadi N, Pourfarmanbar M, et al. Treatment options for nonalcoholic fatty liver disease: a double-blinded randomized placebo-controlled trial. Eur J Gastroenterol Hepatol 2019; 31: 613–617. [DOI] [PubMed] [Google Scholar]
- 32. Khachidze T. Comparison of the efficacy of Ursodeoxycholic acid (UDCA) versus vitamin E plus vitamin C in non-diabetic patients with nonalcoholic Steatohepatitis (NASH). HPB 2019; 21: S398–S399. [Google Scholar]
- 33. Wang CL, Liang L, Fu JF, et al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol 2008; 14: 1598–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Friel JK, Friesen RW, Harding SV, et al. Evidence of oxidative stress in full-term healthy infants. Pediatr Res 2004; 56: 878–882. [DOI] [PubMed] [Google Scholar]
- 35. Nasca MM, Zhang R, Super DM, et al. Increased oxidative stress in healthy children following an exercise program: a pilot study. J Dev Behav Pediatr 2010; 31: 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soto-Méndez MJ, Aguilera CM, Mesa MD, et al. Correction: strong associations exist among oxidative stress and antioxidant biomarkers in the circulating, cellular and urinary anatomical compartments in guatemalan children from the western highlands. PLoS One 2016; 11: e0149740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoofnagle JH, Van Natta ML, Kleiner DE, et al. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2013; 38: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hino K, Murakami Y, Nagai A, et al. Alpha-tocopherol [corrected] and ascorbic acid attenuates the ribavirin [corrected] induced decrease of eicosapentaenoic acid in erythrocyte membrane in chronic hepatitis C patients. J Gastroenterol Hepatol 2006; 21: 1269–1275. [DOI] [PubMed] [Google Scholar]
- 40. Suzuki A, Lindor K, St Saver J, et al. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol 2005; 43: 1060–1066. [DOI] [PubMed] [Google Scholar]
- 41. Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 42. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55: 2005–2023. [DOI] [PubMed] [Google Scholar]
- 43. Socha P, Horvath A, Vajro P, et al. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review. J Pediatr Gastroenterol Nutr 2009; 48: 587–596. [DOI] [PubMed] [Google Scholar]
- 44. Jaruvongvanich V, Wirunsawanya K, Sanguankeo A, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification: a systematic review and meta-analysis. Dig liver Dis 2016; 48: 1410–1417. [DOI] [PubMed] [Google Scholar]
- 45. Lallukka S, Yki-Järvinen H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract Res Clin Endocrinol Metab 2016; 30: 385–395. [DOI] [PubMed] [Google Scholar]
- 46. Dufour J-FF. Vitamin E for nonalcoholic steatohepatitis: ready for prime time? Hepatology 2010; 52: 789–792. [DOI] [PubMed] [Google Scholar]
- 47. Rajendran P, Li F, Manu KA, et al. γ-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent. Br J Pharmacol 2011; 163: 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mantena SK, King AL, Andringa KK, et al. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med 2008; 44: 1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gambino R, Musso G, Cassader M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: mechanisms and therapeutic opportunities. Antioxid Redox Signal 2011; 15: 1325–1365. [DOI] [PubMed] [Google Scholar]
- 50. Miller ER, III, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142: 37–46. [DOI] [PubMed] [Google Scholar]
- 51. Berry D, Wathen JK, Newell M. Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality. Clin Trials 2009; 6: 28–41. [DOI] [PubMed] [Google Scholar]
- 52. Jiang S, Pan Z, Li H, et al. Meta-analysis: low-dose intake of vitamin E combined with other vitamins or minerals may decrease all-cause mortality. J Nutr Sci Vitaminol (Tokyo) 2014; 60: 194–205. [DOI] [PubMed] [Google Scholar]
- 53. Klein EA, Thompson IMJ, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the selenium and Vitamin E cancer prevention trial (SELECT). JAMA 2011; 306: 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang FF, Barr SI, McNulty H, et al. Health effects of vitamin and mineral supplements. BMJ 2020; 369: m2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abner EL, Schmitt FA, Mendiondo MS, et al. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 2011; 4: 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schürks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ 2010; 341: c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_1756284820974917 for The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression by Mohamed Abdel-Maboud, Amr Menshawy, Esraa Menshawy, Amany Emara, Mohamed Alshandidy and Muhammad Eid in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-2-tag-10.1177_1756284820974917 for The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression by Mohamed Abdel-Maboud, Amr Menshawy, Esraa Menshawy, Amany Emara, Mohamed Alshandidy and Muhammad Eid in Therapeutic Advances in Gastroenterology