Abstract
Atherosclerosis is the most common cause of peripheral artery disease (PAD). We compared the atherosclerotic burden in non-lower extremity arteries in patients with and without PAD using 18F-sodium fluoride (NaF)-PET/CT. We identified five individuals (61.8±6.6 years, one male, four females) with PAD and matched to five individuals without PAD based on age and gender from the unfavorable cardiovascular risk profile group of the CAMONA trial (60±7.2 years, one male, four females). Individuals underwent PET/CT imaging 90 minutes after the injection of NaF (2.2 Mbq/Kg). CT imaging was conducted to account for attenuation correction and anatomic referencing. The NaF uptake was measured by manually defining regions of interest on each axial slice on the following arteries: coronary artery (CA), carotid artery (CR), ascending aorta (AS), arch of aorta (AR), descending aorta (DA), and abdominal aorta (AA). Average SUVmean (aSUVmean) was calculated for each segment. Wilcoxon’s signed rank test was used for statistical analysis. The total aSUVmean was higher in the PAD group compared to the non-PAD group (6.54±0.9 vs. 5.03±0.45, P=0.043). Comparison revealed higher NaF uptake in CR, AS, AR, and DA in the PAD group compared to the non-PAD group (0.93±0.25 vs. 0.54±0.14, P=0.01; 1.28±0.20 vs. 0.86±1.19, P<0.01; 1.18±0.17 vs. 0.90±0.19, P=0.03; 1.32±0.24 vs. 0.91±0.15, P=0.01). The NaF uptake in CA and AA was similar between the two groups (0.77±0.04 vs. 0.71±0.05, P=0.11; 1.07±0.28 vs. 1.12±0.30, P=0.82). We found individuals with PAD had higher atherosclerotic burden in the carotid arteries and thoracic aorta compared to non-PAD subjects.
Keywords: Atherosclerosis, PET/CT imaging, peripheral artery disease, sodium fluoride
Introduction
Atherosclerosis is a systemic vascular disease characterized by progressive buildup of focal plaque resulting in narrowing of the arterial lumen. Advanced age, diabetes, hypertension, hyperlipidemia, and smoking are some of the well-established risk factors for the development of atherosclerosis [1,2]. Its pathogenesis involves a complex interplay of regional and systemic factors resulting in the heterogeneous development of plaque in various specific vascular sites. Due to its systemic nature, the occurrence of symptomatic disease in one vascular site may portend significant subclinical disease in other vascular territories in the affected individual [3]. However, there is a scarcity of available human data on the extent of regional variation of subclinical atherosclerotic burden in individuals with symptomatic disease involving one vascular territory.
Atherosclerotic disease progression in peripheral artery disease (PAD) is particularly of interest for numerous reasons. First, PAD often goes underdiagnosed because its symptoms may align with several other conditions such as ischemic rest pain and limb loss [4]. Furthermore, PAD can be indicative of more severe cardiovascular disease and increases a patient’s risk for rapid vascular decline [5]. Current research is focused on tracking inflammation in PAD vessels; however, we hypothesize that tracking atherosclerotic development in non-lower extremity vessels could also offer insight into disease progression of PAD.
18F-sodium fluoride (NaF) positron-emission tomography (PET)/computed tomography (CT) is a novel non-invasive imaging technology that has shown considerable promise in identifying areas of atherosclerosis by targeting the early stages of plaque calcification [6]. Previous studies have shown the utility of NaF-PET/CT in detecting microcalcification [7-9]. This imaging technique allows for a comprehensive assessment of the atherosclerotic burden in multiple vascular territories simultaneously. In this study, we aimed to compare the regional differences of subclinical atherosclerotic burden in non-lower extremity vascular territories in patients with peripheral artery disease (PAD) and without PAD using 18F-NaF-PET/CT imaging.
Methods
Subjects
This study was conducted in a subset of PAD subjects from the prospective study known as “Cardiovascular Molecular Calcification Assessed by 18F-FDG PET/CT (CAMONA)” in Odense, Denmark. From an initial sample size of 90 healthy controls and 50 subjects with an unfavorable cardiovascular risk profile, we only identified five subjects from the unfavorable group with a positive history of peripheral artery disease and matched them to five individuals without PAD for comparison. The CAMONA study was approved by the Danish National Committee on Biomedical Research Ethics as well as registered at ClinicalTrials.gov (NCT01724749). The study was undertaken in concordance with the Declaration of Helsinki and all subjects provided written informed consent. The subjects in this population were excluded based on the presence of malignancy, immunodeficiency syndrome, autoimmune disease, pregnancy, sarcoidosis, amyloidosis, endocarditis, as well as use of prescription medications.
In this cohort, we identified five individuals (61.8±6.6 years, one male, four females) with PAD and matched them to five individuals without PAD based on age and gender from the unfavorable cardiovascular risk profile group of the CAMONA trial (60±7.2 years, one male, four females). Baseline characteristics are depicted in Table 1.
Table 1.
PAD and non-PAD individual demographics
| PAD (N=5) | Non-PAD (N=5) | p value | |
|---|---|---|---|
| Age | 61.8±6.6 | 60±7.2 | 0.678 |
| Systolic blood pressure (mmHg) | 130.2±21.8 | 129.0±7.5 | 0.919 |
| Diastolic blood pressure (mmHg) | 79.7±6.9 | 74.5±6.7 | 0.418 |
| Homocysteine (umol/L) | 10.2±2.4 | 11.4±2.5 | 0.297 |
| Low density lipoprotein (mmol/L) | 3.4±0.6 | 3.3±1.0 | 0.886 |
| Total cholesterol (mmol/L) | 5.4±0.4 | 5.7±0.6 | 0.509 |
| Triglycerides (mmol/L) | 1.7±1.1 | 0.8±0.1 | 0.124 |
| HDL cholesterol (mmol/L) | 1.3±0.11 | 1.9±0.4 | 0.023 |
| Plasma glucose (mmol/L) | 5.5±0.6 | 5.9±0.8 | 0.514 |
| HbA1c (mmol/L) | 38±3.7 | 37.4±2.7 | 0.732 |
| CRP (mg/L) | 5±7.3 | 1.1±0.2 | 0.302 |
Values are mean ± SD. HbA1c = Glycated hemoglobin.
Quantitative image analysis
All subjects underwent 18F-NaF-PET/CT imaging with an established and uniform protocol (GE Discovery STE, VCT, RX, and 690/710). Patients were made to observe an overnight fast of 6 hours and a blood glucose measurement ensuring a concentration below 8 mmol/L. 18F-NaF-PET/CT imaging was performed 90 minutes following administration of 2.2 MBq of NaF per kilogram of body weight. These images were produced using one of several PET/CT systems (GE Discovery STE, VCT, RX, and 690/710). PET images were corrected for attenuation, scatter, scanner dead time, and random coincidences. Low-dose CT imaging (140 kV, 30-110 mA, noise index 25, 0.8 seconds per rotation, slice thickness 3.75 mm) was performed for attenuation correction and anatomic referencing with PET images.
Quantification of CA, CR, AS, AR, DA, and AA was performed by a trained physician who manually defined a region of interest (ROI) around the structures on each axial PET/CT slice using a DICOM viewer (Osirix MD Software; Pixmeo SARL, Bernex, Switzerland) (Figures 1, 2). This methodology has been tested and verified by numerous other studies and has low interobserver variability (insert references). These vessels were chosen as they are the most common sites for non-lower extremity atherosclerosis in PAD patients [10-13]. For the coronaries, the ROI did not include any parts of the skeleton, cardiac valves, or aortic wall. For every ROI, representing the volume of one arterial slice, the NaF activity was determined as the mean standardized uptake value (SUVmean). Then, these values were added and divided by the sum of the ROI-defined slice volumes to yield a global arterial average SUVmean (aSUVmean) for each vessel.
Figure 1.
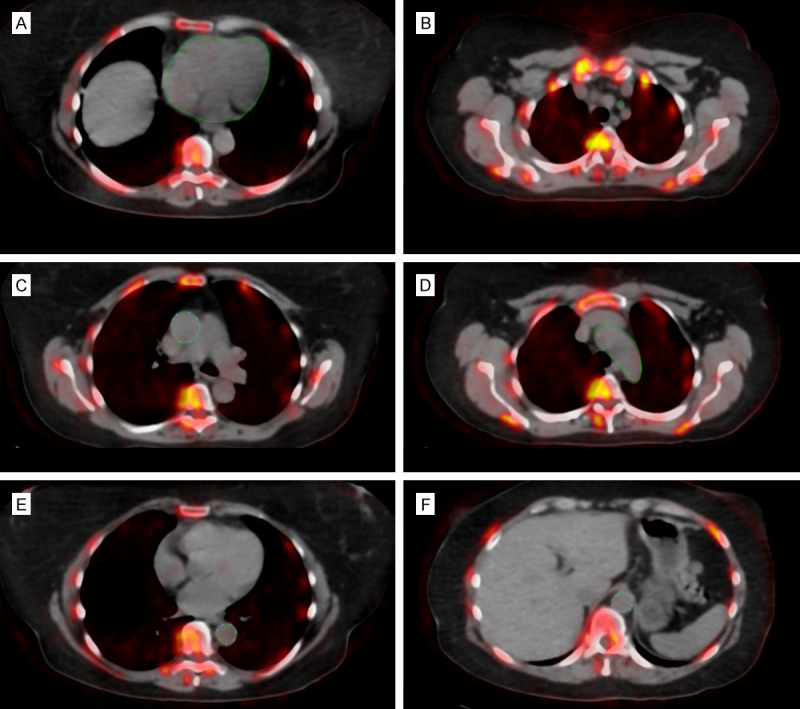
Axial fused NaF-PET/CT with regions of interest depicting non-lower extremity arteries in a PAD individual. Manually-delineated region of interest determined the NaF uptake in the (A) common carotid, (B) coronaries (which did not include the aortic valve, skeletal structures, and aortic wall), (C) ascending aorta, (D) aortic of arch, (E) descending aorta, and (F) abdominal aorta.
Figure 2.
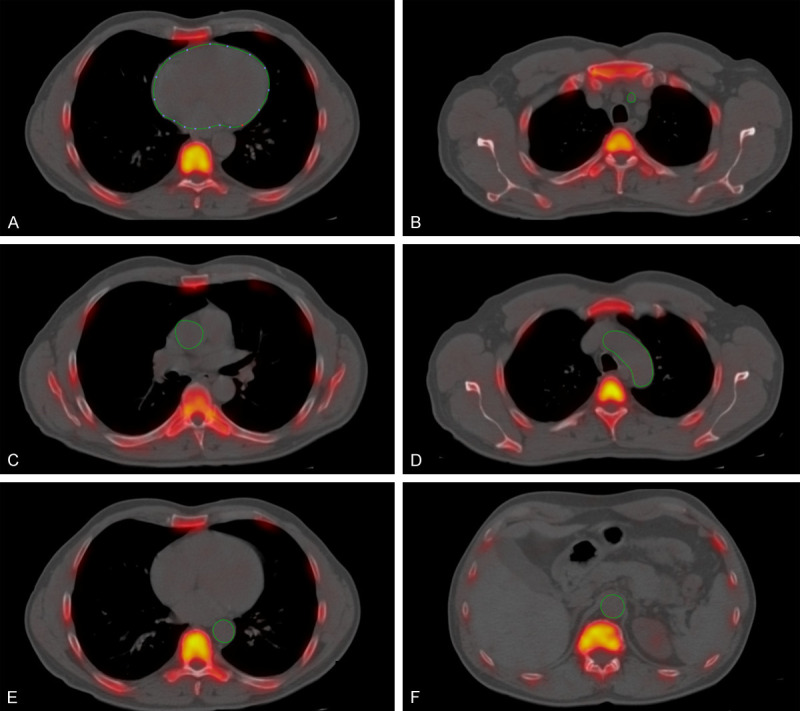
Axial fused NaF-PET/CT with regions of interest depicting non-lower extremity arteries in a non-PAD individual. Manually-delineated region of interest determined the NaF uptake in the (A) common carotid, (B) coronaries (which did not include the aortic valve, skeletal structures, and aortic wall), (C) ascending aorta, (D) aortic of arch, (E) descending aorta, and (F) abdominal aorta.
Statistical analysis
A comparison of the atherosclerotic burden in non-lower extremity arteries between PAD and non-PAD groups were evaluated using a Wilcoxon’s signed rank sum test. Box plots comparing the arterial uptake between the two groups were generated to help with visualization of data. A p value <0.05 was chosen as being statistically significant. We used Statistical software packages SPSS (Version 25.0, IBM), R (R core team 2020), and STATA/MP 16.1 (StataCorp, College Station, Texas 77845 USA) for the statistical analysis and generating figures.
Results
Global arterial uptake (aSUVmean ± SD) was higher in the PAD group compared to the non-PAD group (6.54±0.9 vs. 5.03±0.45, P=0.043). Comparison of each arterial segment revealed a higher NaF uptake on PET/CT in CR, AS, AR, and DA in the PAD group compared to the non-PAD group (0.93±0.25 vs. 0.54±0.14, P=0.01; 1.28±0.20 vs. 0.86±1.19, P<0.01; 1.18±0.17 vs. 0.90±0.19, P=0.03; 1.32±0.24 vs. 0.91±0.15, P=0.01) (Figure 3). The NaF uptake in CA and AA was similar between the two groups (0.77±0.04 vs. 0.71±0.05, P=0.11; 1.07±0.28 vs. 1.12±0.30, P=0.82).
Figure 3.
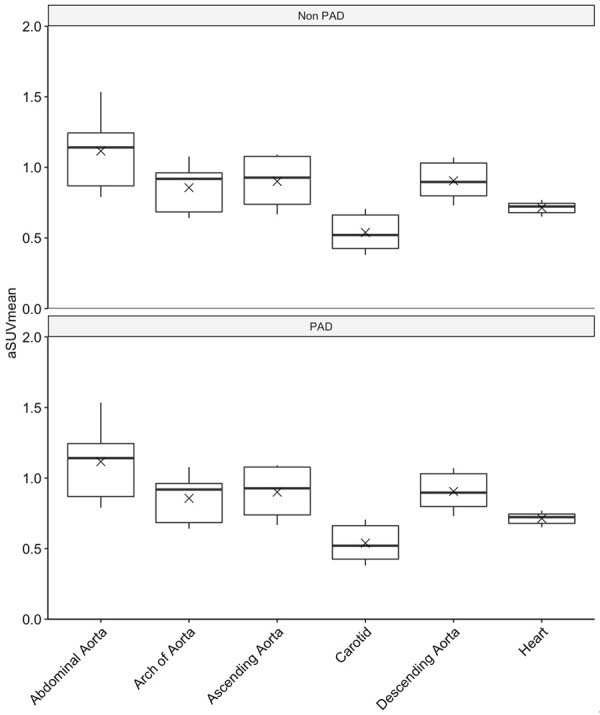
Box plot comparing total uptake of NaF in non-lower extremity vessels of the non-PAD and PAD groups. Wilcoxon’s signed rank test revealed a p value of 0.043. “x” marks delineate mean uptake.
Discussion
Numerous studies have demonstrated the reliability of 18F-NaF-PET/CT imaging for in vivo detection and quantification of vascular calcification as a surrogate marker for the burden of atherosclerosis in various vascular territories [14-17]. To our knowledge, this is the first study utilizing 18F-NaF-PET/CT imaging to analyze subclinical atherosclerosis in multiple vascular sites in the subjects with PAD.
The findings of our study revealed that patients with PAD showed significantly higher NaF uptake on PET/CT in some, but, not all non-lower extremity vascular territories. There was a higher atherosclerotic burden in carotid arteries and thoracic aorta, but not in the coronary vasculature and abdominal aorta compared to age and gender-matched non-PAD subjects. This finding further underscores the heterogeneity in site selectivity of atherosclerosis with exposure to conventional systemic risk factors highlighting the influence of local factors in its pathogenesis. Hemodynamic factors, in particular, differences in flow parameters in specific arterial sites are believed to play a major role. Arterial sites with low shear stress, turbulent or oscillating flow are affected early on due to priming of endothelial cells by alteration of mechanical forces sensed by them, resulting in activation of genes and expression of surface proteins promoting a milieu favorable for the formation of atherosclerotic plaque [3]. Interestingly, in this cohort of patients with unfavorable cardiovascular risk profile, individuals with peripheral arterial disease had higher atherosclerotic burden in non-lower extremity arteries despite having nearly similar exposure to traditional atherosclerotic risk factors. This finding underscores the fact that the development and progression of atherosclerosis goes beyond the exposure of traditional risk factors and the discrepancy noted could possibly be related to one’s own genetic susceptibility in its development.
Conventionally, detection and quantification of calcification in various arterial beds have been achieved with the utility of CT imaging, with a higher burden of calcification being independently predictive of adverse outcomes [18-22]. However, the detection of arterial calcification with CT imaging comes with some limitations. First, CT imaging has limited sensitivity to detect the early stages of calcification. Second, it cannot accurately discriminate metabolically active atherosclerotic lesions from indolent vascular calcification, a possible biomarker for vulnerable and stabilized atherosclerotic plaques, respectively [23-25]. However, these limitations can be overcome by the utilization of hybrid imaging modalities like 18F-NaF-PET/CT as demonstrated in our study. This imaging technique might allow for understanding progression of subclinical atherosclerotic disease in individuals with PAD facilitating the utilization of appropriate screening techniques and therapeutic intervention in a cost-effective manner. Future prospective studies are needed to validate this modality’s prognostic value.
We acknowledge that there are limitations to our study. First, the sample size of analyzed patients is small and as such, future studies need to validate these findings with larger sample sizes. Second, the present study was unable to validate NaF-PET imaging findings with histological data. As NaF utilized for this study aimed to detect arterial wall calcific changes at the molecular level, it would have been useful to confirm these findings histologically. Moreover, the interpretation of our study is limited by its cross-sectional design, which precludes the understanding of temporal associations between PAD and non-PAD in non-lower extremity atherosclerosis relationships. A longitudinal study is required to better understand the relationship and causative outcomes in atherosclerosis in non-lower extremity vessels of PAD.
Acknowledgements
The Jørgen and Gisela Thrane’s Philanthropic Research Foundation, Broager, Denmark, financially supported the CAMONA study.
Disclosure of conflict of interest
None.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, Floyd J, Fornage M, Gillespie C, Isasi C. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger JS, Hochman J, Lobach I, Adelman MA, Riles TS, Rockman CB. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673–681. doi: 10.1016/j.jvs.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 3.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 5.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med. 2010;51:1826–1829. doi: 10.2967/jnumed.110.077933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seraj SM, Raynor WY, Revheim ME, Al-Zaghal A, Zadeh MZ, Arani LS, Rojulpote C, Werner TJ, Gerke O, Høilund-Carlsen PF, Baker JF. Assessing the feasibility of NaF-PET/CT versus FDG-PET/CT to detect abdominal aortic calcification or inflammation in rheumatoid arthritis patients. Ann Nucl Med. 2020;34:424–431. doi: 10.1007/s12149-020-01463-w. [DOI] [PubMed] [Google Scholar]
- 8.Arani L, Zadeh MZ, Oestergaard B, Seraj SM, Al-Zaghal A, Kalboush E, Rojulpote C, Jahangiri P, Werner T, Hoilund-Carlsen PF. Thoracic aorta atherosclerosis in multiple myeloma patients assessed by18F sodium fluoride PET/CT. J Nucl Med. 2019;60:1447–1447. [Google Scholar]
- 9.Rojulpote C, Borja AJ, Zhang V, Aly M, Koa B, Seraj SM, Raynor WY, Kothekar E, Kaghazchi F, Werner TJ. Role of 18F-NaF-PET in assessing aortic valve calcification with age. Am J Nucl Med Mol Imaging. 2020;10:47. [PMC free article] [PubMed] [Google Scholar]
- 10.Patil S, Rojulpote C, Gonuguntla K, Karambelkar P, Bhattaru A, Raynor WY, Borja AJ, Vuthaluru K, Zhang V, Werner TJ, Gerke O. Association of triglyceride to high density lipoprotein ratio with global cardiac microcalcification to evaluate subclinical coronary atherosclerosis in non-diabetic individuals. Am J Cardiovasc Dis. 2020;10:241. [PMC free article] [PubMed] [Google Scholar]
- 11.Rojulpote C, Patil S, Gonuguntla K, Bravo PE, Karambelkar P, Bhattaru A, Zhang V, Werner T, Gerke O, Hoilund-Carlsen P, Alavi A. Association of atherosclerotic cardiovascular risk estimated by pooled cohort equation with coronary plaque burden as assessed by NaF-PET/CT. Arterioscler Thromb Vasc Biol. 2020;40 A274- [Google Scholar]
- 12.Bhattaru A, Rojulpote C, Ghorpade R, Bravo PE, Patil S, Gonuguntla K, Karambelkar P, Vuthaluru K, Zhang V, Werner T, Alavi A. Abstract MP24: correlation between blood pressure and inflammation in the thoracic aorta Of HIV patients with and without cocaine use as assessed by FDG-PET/CT. Hypertension. 2020;76:AMP24. [Google Scholar]
- 13.Rojulpote C, Patil S, Gonuguntla K, Karambelkar P, Bravo PE, Seraj SM, Asadollahi S, Raynor WY, Bhattaru A, Borja AJ, Zhang V. NaF-PET/CT global assessment in detecting and quantifying subclinical cardiac atherosclerosis and its association with blood pressure in non-dyslipidemic individuals. Am J Cardiovasc Dis. 2020;10:101. [PMC free article] [PubMed] [Google Scholar]
- 14.Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, Klutmann S. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med. 2010;51:862–865. doi: 10.2967/jnumed.110.076471. [DOI] [PubMed] [Google Scholar]
- 15.Derlin T, Wisotzki C, Richter U, Apostolova I, Bannas P, Weber C, Mester J, Klutmann S. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: correlation with atherogenic risk factors. J Nucl Med. 2011;52:362–368. doi: 10.2967/jnumed.110.081208. [DOI] [PubMed] [Google Scholar]
- 16.Janssen T, Bannas P, Herrmann J, Veldhoen S, Busch JD, Treszl A, Münster S, Mester J, Derlin T. Association of linear 18 F-sodium fluoride accumulation in femoral arteries as a measure of diffuse calcification with cardiovascular risk factors: a PET/CT study. J Nucl Cardiol. 2013;20:569–577. doi: 10.1007/s12350-013-9680-8. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Li X, Jia Y, Fan J, Wang H, Fan C, Wu L, Si X, Hao X, Wu P. Sodium-fluoride PET-CT for the non-invasive evaluation of coronary plaques in symptomatic patients with coronary artery disease: a cross-correlation study with intravascular ultrasound. Eur J Nucl Med Mol Imaging. 2018;45:2181–2189. doi: 10.1007/s00259-018-4122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS, Mosler TP. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 19.Hou ZH, Lu B, Gao Y, Jiang Sl, Wang Y, Li W, Budoff MJ. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990–999. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor SD, Graffy PM, Zea R, Pickhardt PJ. Does nonenhanced CT-based quantification of abdominal aortic calcification outperform the Framingham Risk Score in predicting cardiovascular events in asymptomatic adults? Radiology. 2019;290:108–115. doi: 10.1148/radiol.2018180562. [DOI] [PubMed] [Google Scholar]
- 21.Desai MY, Cremer PC, Schoenhagen P. Thoracic aortic calcification: diagnostic, prognostic, and management considerations. JACC Cardiovasc Imaging. 2018;11:1012–1026. doi: 10.1016/j.jcmg.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Nandalur KR, Baskurt E, Hagspiel KD, Finch M, Phillips CD, Bollampally SR, Kramer CM. Carotid artery calcification on CT may independently predict stroke risk. AJR Am J Roentgenol. 2006;186:547–552. doi: 10.2214/AJR.04.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 24.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 25.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]


