Abstract
Photons, electrons and protons have therapeutic use however positrons have only been used for diagnostic imaging purposes. The energies of positrons (β+) from F-18 (0.633 MeV) and electrons (β-) from I-131 (0.606 MeV) are very close and have similar equilibrium dose constants. Since [18F]-fluorodeoxyglucose (18F-FDG) clears rapidly from circulation, administration of 37-74 GBq (1-2 Ci) of 18F-FDG is relatively safe from an internal radiation dosimetry point of view. We initiated a phase I dose escalation study to assess the safety, toxicity, and potential therapeutic utility of administering 100-200 mCi/m2 18F-FDG delivered over a 1 to 5 day period in patients with advanced lymphomas and solid tumors refractory to standard of care treatment (SCT). Here we report the results of the first four patients treated. Four patients with advanced cancers received a single dose of 3.7-7.4 GBq/m2 (100-200 mCi/m2) 18F-FDG. We monitored the patients for adverse effects and for response. No treatment-related toxicities were observed. There was no increased radiation exposure to personnel. Two patients showed decrease in the index lesions’ SUVs by 17-33% (Day 1) and 25-31% (Day 30) post treatment. The two other patients showed stable disease on 18F-PET-CT. Interestingly, responses were seen at low radiotherapy doses (below 1 Gy). This exploratory study demonstrated the safety of therapeutic administration of up to 14.2 GBq (385 mCi) 18F-FDG. In patients with 18F-FDG-avid cancers, targeted radionuclide 18F-FDG therapy appears safe and may offer clinical benefit.
Keywords: Cancer, lymphoma treatment, 18F-FDG, hypermetabolic tumors, radionuclide therapy, 18F-PET-CT scan
Introduction
Patients with metastatic disease have limited treatment options, which often involve chemotherapeutic agents that are poorly tolerated and toxic to normal organs. Radionuclide electron-based cancer therapy has been in use for decades such as in the treatment of thyroid cancer with I-131. Tumoricidal effects of such therapy occur through the loss of kinetic energy as the isotope decays.
18F-Fluorodeoxyglucose (18F-FDG) is routinely used for diagnostic tumor imaging however as it is not a substrate in glycolysis and remains trapped in cells also makes 18F-FDG a candidate for targeted radionuclide therapy [1]. Meyer et al. demonstrated tumor shrinkage by locally injecting 18F-FDG into glioma xenograft mice [2]. Subsequently, several groups found that systemic administration of 18F-FDG at maximally tolerated dose in a murine model of breast cancer has tumoricidal effects and improved survival in mice with aggressive disease however end-organ toxicity could limit significant further dose escalation in humans to achieve the same effects [3].
There are several theoretic considerations that make 18F-FDG a potential ideal candidate for treatment of solid tumors:
The ubiquity of its uptake in human tumors: 18F-FDG uptake being was documented in several types of tumors, non-small cell lung cancer (NSCLC), esophageal cancer, colorectal cancer, head and neck cancers, breast cancers, melanoma, Hodgkin and non-Hodgkin lymphoma.
The relatively high tolerance of normal organs to radioactivity: Normal organs that exhibit the highest 18F-FDG uptake are the brain, the heart and the bladder. However, these organs have a relatively high tolerance to radioactivity, thus limiting the possibility for toxicity [4].
The minimal potential of toxicity to normal organs: 18F-FDG has a short half-life (110 minutes) and should have a minimal toxicity on normal tissues.
The lack of immunogenic potential: 18F-FDG is not an antibody and thus, in case of response, can be administered repeatedly, without the body mounting an immune response to it.
The direct visualization of radioisotope uptake in organ tissues: The effect can be monitored closely with an 18F-FDG-PET scan.
The potential for tumoricidal activity: Given the fact that positrons are positively charged electrons that lose their kinetic energy immediately, in theory, they should behave similarly to electrons and have the same destructive effect on tumors. It is also possible that positrons may affect the vasculature of the tumors and so they may damage the tumor in an indirect way.
The effect is localized to tumor tissue: The mean range of the positrons from 18F decay is close to 2 mm (approximately 200 cells diameter) which is intermediate between the range of 131-Iodine (1 mm) and that of 90-Yttrium (11 mm). The decay energy of the 18F (average of approximately 249.8 KeV) is also less than the decay energy of the 90-Yttrium (average of approximately 936.5 KeV) which should result in lower levels of non-specific radiation of the normal tissue with 18F and thus less toxicity to the normal tissues than with 90-Yttrium. Both 131-Iodine and 90-Yttrium have been used successfully in the treatment of human cancer.
We can see from the Table 1 that, in theory, even if we administered 100 times the diagnostic 18F-FDG dose (which, usually is up to a maximum of 23 millicuries), the uptake in the marrow would be below the MTD of the bone marrow (200 cGy) [4].
Table 1.
Comparison of radiation dosimetry of 131I-tositumomab and 18F-FDG, The delivered doses are in cGy
| Organ | 131I-Bexxar | 18F-FDG | |||
|---|---|---|---|---|---|
|
|
|
||||
| 100 mCi | 10 mCi | 100 mCi | 1.0 Ci | 2.0 Ci | |
| Brain | 48 | 0.7 | 7 | 70 | 140 |
| Kidneys | 725 | 0.74 | 7.4 | 74 | 148 |
| Heart wall | 462 | 2.2 | 22 | 220 | 440 |
| Spleen | 421 | 1.4 | 14 | 140 | 280 |
| Liver | 303 | 0.58 | 5.8 | 58 | 116 |
| Lungs | 292 | 0.64 | 6.4 | 64 | 128 |
| Bone marrow | 240 | 0.48 | 4.8 | 48 | 96 |
| Testes | 307 | 0.42 | 4.2 | 42 | 84 |
| Ovaries | 93 | 0.54 | 5.4 | 54 | 108 |
| Urinary Bladder Wall | 237 | 2.7 | 27 | 270 | 540 |
| Total body | 88 | 0.44 | 4.4 | 44 | 88 |
Therefore, to assess the safety, toxicity and potential therapeutic utility of low dose 18F-FDG, we designed a phase I study (NCT #02130492, IND #103704) in patients with advanced treatment-refractory malignancies. We report here our results with the first four patients treated.
Materials and methods
From February 2015 to April 2016, adult patients ages 21 and over with advanced solid tumors and lymphomas refractory to two or more standard of care therapies, were screened from the outpatient oncology clinic of Monter Cancer Center from Lake Success, New York. The study was approved by the Institutional Review Board and all subjects signed an informed consent form. Pre-treatment baseline screening 18F-PET/CT scans were conducted on all participants. Patients were selected based on a required target SUVmax tumor/SUVmean liver ratio > 5. Patient with a bladder SUV > 100 at baseline were excluded. Additional key exclusion criteria included tumor bone marrow involvement of > 25%, radiation resistant tumors (i.e. melanoma), as well as primary or untreated metastatic cancer to the brain.
The complete list of eligibility criteria is available on line at: https://clinicaltrials.gov/ct2/show/NCT02130492.
Study design
This was a first-in-human radiopharmaceutical study using 18F-FDG for treatment.
Special considerations of using 18F-FDG as a treatment:
-Tumor tissue is non-homogeneous: clusters of normal cells alternate with clusters of (pre)malignant cells. This phenomenon occurs at a microscopic scale far beyond the resolution of the gamma camera. Consequently, scintigraphy with 18F-FDG measures an average energy demand in the tumor that does not fully reflect the metabolic status of the malignancy. We therefore decided to include patients that have a high 18F-FDG uptake in the tumor (SUVmax tumor/SUVmean liver ratio > 5) and/or distal metastasis and physiologic uptake in the normal organs.
-Second, as a result of repair processes induced by physiological reactions or after tumor therapy, macrophages will replace tumor cells and these cells will also demonstrate 18F-FDG uptake. It has even been demonstrated that these macrophages show a higher 18F-FDG uptake than do viable tumor cells. The confusing 18F-FDG uptake in the inflammatory response to tumor cells has been studied by Kubota et al. who demonstrated that surrounding macrophages and newly formed granulation tissue showed greater 18F-FDG uptake than did viable tumor cells [5]. A maximum of about 25% of the total 18F activity measured in tumor tissue was derived from macrophages and granulation tissues. The clinical consequence of this has been reported by Haberkorn et al. who studied patients with colorectal tumors after radiotherapy [6].
In our trial, we attempted to control for this by excluding patients with documented infectious, inflammatory states and those who had recently had a surgery, chemotherapy or radiation therapy. Secondary inflammation resulting from these conditions may induce false-positive 18F-FDG accumulation in the tumors, hampering the scintigraphic evaluation of therapy.
Third, literature on the time-dependent behavior of 18F-FDG in tumors is very scarce. The uptake with time is most likely dependent on local parameters as mitotic activity and growth rate. Therefore, the interval between 18F-FDG administration and scintigraphy may affect the measured uptake. It may also explain the observed variation in uptake in otherwise similar tumors in different patients [7]. This is the reason why, before the actual treatment with 18F-FDG, we included an extra step in which a scout 18F-FDG-PET scan was done in which diagnostic doses of 18F-FDG were administered and images were obtained at 1 hour, 3 hours and 5 hours post 18F-FDG administration in order to better investigate the 18F-FDG uptake in normal organs and tumor.
We planned an accelerated titration design with one patient per dose level [8]. The reason for choosing the accelerated design was related to the fact that given the relatively low starting dose, toxicities were not expected until much larger doses would be administered. On the other hand, given the fact this was the first study using positrons for cancer treatment in human we proceeded cautiously. This accelerated design ends when one patient experiences Dose Limiting Toxicity (DLT) and the study continues as a classical 3+3 design. Toxicities were graded with CTCAE v4.0. The starting dose was 100 mCi/m2, based on the known threshold DLT for bone marrow (total dose > 4000 mCi) [9]. Patients were monitored weekly for the first month, then monthly for the next 3 months, then every 3 months. If one patient experienced grade 1 toxicity but no DLT toxicities were observed, three more patients would be accrued at the same dose. To capture toxicities, we enrolled only one patient per month. Given the high physiological uptake of 18F-FDG in the heart and brain, there was concern regarding the effect of high dose 18F-FDG to these organs therefore close monitoring with ambulatory EKG’s, echocardiograms and mini-mental exams was performed.
18F-FDG PET/CT imaging
All imaging was performed on an integrated 18F-FDG PET/CT device (GE Discovery 710; GE Medical Systems, Milwaukee, Wisconsin). Within one week prior to administration of therapeutic 18F-FDG, a pre-treatment scout 18F-FDG PET/CT study was carried out with a single intravenous injection of approximately 10 mi 18F-FDG. Images were acquired from the base of skull to mid-thigh at about 1 hour, 3 hours and 5 hours following injection. Post-treatment imaging was performed < 72 hours and again at 30 days following administration of the therapeutic dose of 18F-FDG. Pre- and post-treatment studies were performed with the same imaging parameters. Images were reviewed on a dedicated GE AW Workstation, software version 4.6, using multiplanar reconstruction.
18F-FDG administration
Treatments were administered in a single dose by slow IV push over approximately one minute by board-certified radiation medicine and nuclear medicine physicians. The infusion time was the same for all doses regardless of the amount of radioactivity administered. The patients were treated in a dedicated radiation-shielded room.
Tumor uptake
Scout and post-treatment 18F-FDG PET/CT scans were analyzed by the same board-certified nuclear medicine physician. A target lesion with the highest SUV was identified before treatment in each patient and monitored during the study. Tumor uptake of 18F-FDG was quantified using a volume of interest around the tumor to determine the maximum SUV.
Dosimetry
Radiation dosimetry for 18F-FDG has been published before and the uptake to key normal organs has been calculated (Table 2) [9-12]. Normal organs and tumor dosimetric analysis were performed using OLINDA/EXM 2.0 [13]. Uptake is assumed to reach a maximum value immediately after injection and the retention in the specified source organs is infinite. Tumor volumes were extracted from the 18F-PET/-CT scans and measured activity in the tumor volumes of interest expressed in kBq/cm3. Assuming homogeneous activity distribution in the tumor, total activities were calculated for each time point and normalized to the injected pre-treatment scout activity. These time-activity curves were fit to exponential curves with a biological and a physical decay component. Integration of the exponential fits produced the normalized cumulative activity (NCA) or residence time. NCA values were input into the OLINDA/EXM 2.0 special sphere model. Absorbed dose results for the sphere size closest in value to the measured tumor volume were used; there was no interpolation of absorbed dose results. Total absorbed dose was determined by scaling the sphere model results to the sum of the scout and therapeutic injected activities.
Table 2.
Radiation dosimetry for 1 Curie of 18F
| Organ | ICRP 106 [10] mGy/MBq | Hays et al. [11] cGy/mCi | Cristy & Eckerman [12] cGy/mCi |
|---|---|---|---|
| Bladder | 481 | 270 | Not reported |
| Heart | 248 | 250 | 220 |
| Brain | 141 | 170 | 70 |
| Liver | 78 | 88 | 58 |
| Lungs | 74 | 56 | 64 |
| Kidneys | 63 | 78 | 74 |
| Ovaries | 52 | 41 | Not reported |
| Pancreas | 48 | 52 | 96 |
| Red Marrow | 41 | 40 | 48 |
| Spleen | 41 | 56 | 140 |
| Testes | 41 | 41 | Not reported |
Results
Patient characteristics and dosing
Four patients with median age 70 (57-79) were treated on study with characteristics are summarized in Table 3. The four patients which were treated on study received doses ranging from 170 millicuries to 380 millicuries. All patients were heavily pre-treated before with multiple regimens of chemotherapy. The first patient had a locally advanced tumor of the oropharynx (patient 1), the second patient had metastatic small cell lung cancer (patient 2), the third patient had an advanced diffuse large B cell lymphoma (patient 3) and the fourth patient had an ovarian cancer (patient 4). All four patients had stable disease at the 1-month 18F-FDG PET-CT scan evaluation. They all lived at least 6 months after the 18F-FDG administration (median survival of 8.5 months). Patient 1, who was heavily pretreated and had refractory disease, avoided hospice and stabilized her disease for 6 weeks with the treatment. Also, remarkably, patient 2, whose metastatic disease included several treated brain lesions, lived for 14 months following treatment. Two of the four patients treated showed a substantial decrease in the index lesions SUVs of 17-33% (Day 1) and 25-31% (Day 30) post 18F-FDG treatment. One patient with a rapidly growing diffuse large B cell lymphoma showed a 31% decrease in SUV in the kidney lesion and stable disease at 30 days on CT by RECIST criteria (Figure 2). Also, in the same patient, a lung nodule showed reduction in size and SUV.
Table 3.
Patient characteristics and tumor dosimetry
| Patient | Age | Gender | Primary tumor | Metastatic sites | Index lesion | Tumor dosimetry (site) |
|---|---|---|---|---|---|---|
| 1 | 71 | F | oropharyngeal squamous cell cancer | None [locally advanced, unresectable] | oral | 0.63 Gy (oral lesion) |
| 2 | 70 | F | small cell lung cancer | Adrenal gland | adrenal | 0.76 Gy (adrenal lesion) |
| Brain [treated] | ||||||
| 3 | 79 | M | diffuse large B-cell lymphoma | Lung | renal | 0.8 Gy (renal lesion), 0.44 Gy (lung lesion) |
| Kidney | ||||||
| 4 | 57 | F | ovarian cancer | Adrenal gland | axillary lymph node | 0.78 Gy (axillary LN), 0.44 G (adrenal) |
| Peritoneum | ||||||
| Lymph nodes |
Figure 2.
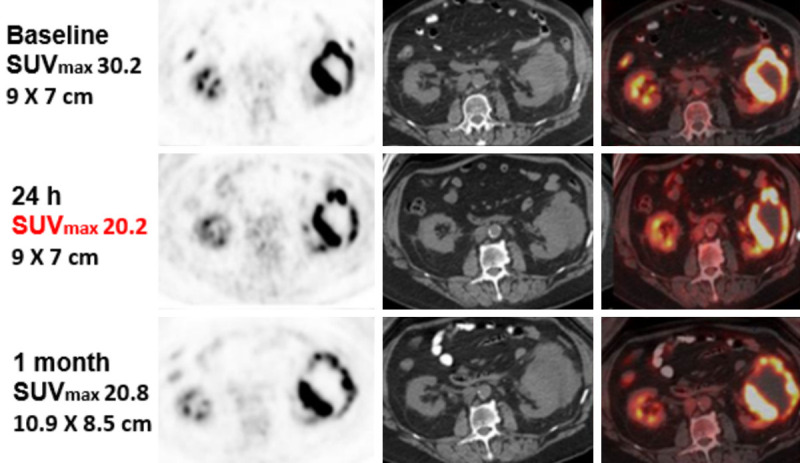
Patient 3 target lesion (renal) at 24 hours and at 1 month post-treatment.
Target lesion location, 18F-FDG doses given and estimated target lesion dose delivery are detailed in Table 4. The calculated radiation to the normal organs was within published accepted limits [4]. Currently, accrual is interrupted due to lack of funding.
Table 4.
Treatment doses and pre- and post-treatment uptake in index lesion
| Patient | Dose mCi/m2/Actual | BSA (m2) | 5-12 weeks pre-treatment SUVmax | 5-11 days pre-treatment SUVmax | 1-day post-treatment SUVmax | 30 days post-treatment SUVmax |
|---|---|---|---|---|---|---|
| 1 | 100/71 | 1.57 | 17.9 | 24.4 | 20.2 (17% decrease) | 18.2 (25% decrease) |
| 2 | 150/220 | 1.60 | 16.3 | 16.3 | 16.9 (4% increase) | 17.7 (8% increase) |
| 3 | 200/385 | 2.00 | 16.9 | 30.2 | 20.2 (33% decrease) | 20.8 (31% decrease) |
| 4 | 200/360 | 1.68 | 15.7 | 18.5 | 18.7 (1% increase) | 16.2 (13% decrease) |
Pharmacokinetics
The decline in 18F-FDG blood activity was similar to the decline observed after a diagnostic dose of 18F-FDG (Figure 1). As an example, for the same patient 3, the recorded SUVs for tumors and normal organs after a diagnostic dose of 11.02 mi 18F-FDG at 1 h, 3 h and 5 h were as following:
Figure 1.
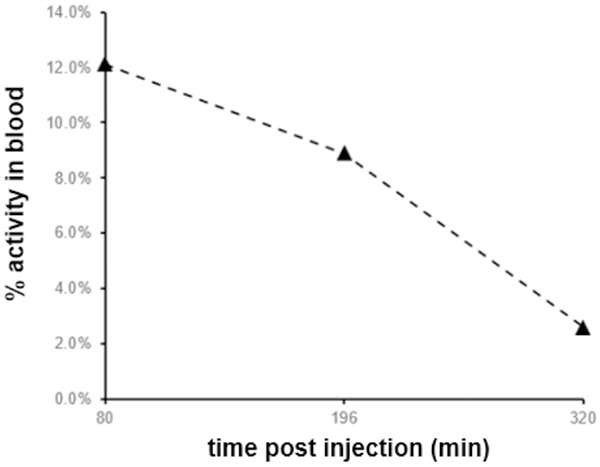
Blood activity curve-Patient 3.
-Right frontal cortex at level of basal ganglia SUVmax: 12.1; 14.0; 11.8.
-Right lower lobe nodule SUVmax: 13.7; 20.8; 18.9.
-Left ventricular myocardium SUVmax: 9.5 (previous SUV 4.0); 11.3; 13.9.
-Liver SUV mean: 3.1 (previous SUV 3.7); 3.0; 3.6.
-Left renal tumor mass SUVmax: 30.2 (previous SUV 16.9); 35.7; 38.1.
-Right upper pole kidney SUVmax: 13.5 (previous SUV 19.2); 13.9; 7.9.
-Bladder SUVmax: 77.0; 63.0; 40.9.
The increase of the SUV’s with time is an artificial phenomenon related to the decrease in the background activity. This is the reason why all the treatment 18F-PET-CT scans were done using the same dose of 18F-FDG as the scout 18F-PET-CT scans and SUV’s measurements were all done at 1 h post diagnostic dose administration.
Due to toxicity precautions we did not perform 18F-PET-CTs after the therapeutic doses of 18F-FDG.
All patients were discharged 8 hours after infusion of 18F-FDG. At that time, the measured radioactivity at 1 meter was less than 2 mR/h in all patients.
Safety and toxicity
Patient toxicities
Treatment was well tolerated in three patients with no toxicities ≥ grade 1. One patient with ovarian cancer developed respiratory arrest after receiving 1 mg of oral and 1 mg of intravenous lorazepam premedication given for claustrophobia. The patient recovered promptly without invasive measures, and the serious adverse event (SAE) was ruled by the Safety Monitoring Board Committee and the IRB as unrelated to the 18F-FDG. No sequelae were observed on long term follow-up.
Radiation safety-toxicities to study personnel
The administration of high dose 18F-FDG was found to be safe and within regulatory limits for study personnel who was equipped with dosimetry badges throughout the study with the highest reading reaching only 31 mrem and observed in the physician caring for the patient with the SAE.
Response assessment
There was preliminary evidence of efficacy during short-term follow-up. Characteristics of response are described in Table 5.
Table 5.
Response characteristics
| Patient | RECIST RESPONSE at 1 month | Survival |
|---|---|---|
| 1 | SD | 6 months |
| 2 | SD | 14 months |
| 3 | SD | 6.7 months |
| 4 | SD | 6.5 months |
18F-FDG PET-CT findings
All patients had stable disease in the index lesion at 1 month after 18F-FDG treatment. The most prominent response was seen in the patient with diffuse large B cell lymphoma whose renal tumor demonstrated increased central necrosis (Figure 2). Unfortunately, a two-month follow-up 18F-FDG PET-CT scan demonstrated tumor progression.
Discussion
Our experience with the first four patients on this study demonstrated the safety of therapeutic 18F-FDG doses up to 14.2 GBq (385 mCi) for both patients and personnel. No renal, cardiac or CNS toxicities were observed. The isolated SAE encountered in the ovarian cancer patient was consistent with lorazepam toxicity and not deemed related to 18F-FDG. Radiation exposure of study personnel was low.
Although the goal of this phase I trial was not to evaluate efficacy, we did obtain intriguing preliminary results in a heavily pretreated and treatment-refractory patient group. The finding of disease stability in all patients at one month after a single low dose of 18F-FDG and evidence of tumor necrosis in a lymphomatous tumor as well as a remarkable survival time in the setting of metastatic SCLC offers a correlate to preclinical findings of 18F-FDG-induced tumor apoptosis [14]. The basis of these findings could reside in a radiation-induced immunomodulatory effect of low dose 18F-FDG exposure [15]. A preclinical study has shown that radionuclide therapy using 177Lu-DOTATATE induces recruitment of antigen presenting cells and NK cells in a murine neuroendocrine tumor model [16]. 18F-FDG-uptake has been shown to be predictive of PD-L1 expression in several tumor types [17]. Low dose 18F-FDG may be uniquely suited to a combined approach with PD-1 and/or PD-L1-targeting therapies such as checkpoint inhibitors, affording a low toxicity “priming” agent which can concurrently target all tumor sites rather than rely simply on a relatively weak abscopal effect or relying on more specific targeted therapies which can prove ineffective when the target is downregulated [18,19]. DLTs and grade ≥ 1 toxicities were not seen and further dose escalation of 18F-FDG via repeat administration in combination with systemic checkpoint inhibitor therapy is planned.
Conclusions
Our pilot experience demonstrates the safety of therapeutic administration of up to 14.2 GBq (385 mi) of 18F-FDG in patients with 18F-FDG avid malignancies. Therapeutic low-dose 18F-FDG appears safe and may offer clinical benefit. Further study of low dose 18F-FDG in combination with systemic immunotherapy is planned.
Acknowledgements
This work was done with the support of a grant from Northwell Health Cancer Institute. Hermes Medical Solutions provided us the OLINDA/EXM 2.0. Software free of charge. We thank Dr. B. Cox and Dr. A. Kapur for their contribution. Special thanks to Jan Stieb, RN and Elizabeth Connelly, NP for their support all throughout the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (include name of committee + reference number) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board of Feinstein Institute IRB #13-132A and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Food and Drug Administration and received the IND number 103704.
Disclosure of conflict of interest
None.
References
- 1.Jaini S, Dadachova E. FDG for therapy of metabolically active tumors. Semin Nucl Med. 2012;42:185–189. doi: 10.1053/j.semnuclmed.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Meyer MA. Positron-emission tomography in cancer therapy. N Engl J Med. 2006;354:1958–1959. doi: 10.1056/NEJMc060556. [DOI] [PubMed] [Google Scholar]
- 3.Moadel RM, Weldon RH, Katz EB, Lu P, Mani J, Stahl M, Blaufox MD, Pestell RG, Charron MJ, Dadachova E. Positherapy: targeted nuclear therapy of breast cancer with 18F-2-deoxy-2-fluoro-D-glucose. Cancer Res. 2005;65:698–702. [PubMed] [Google Scholar]
- 4.Emami B. Tolerance of normal tissue to therapeutic radiation. Int J Radiat Oncol Biol Phys. 1991;1:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 5.Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–1980. [PubMed] [Google Scholar]
- 6.Haberkorn U, Strauss LG, Dimitrakopoulou A, Engenhart R, Oberdorfer F, Ostertag H, Romahn J, Van Kaick G. PET studies of fluorodeoxyglucose metabolism in patients with recurrent colorectal tumors receiving radiotherapy. J Nucl Med. 1991;32:1485–1490. [PubMed] [Google Scholar]
- 7.Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994;35:1308–1312. [PubMed] [Google Scholar]
- 8.Simon R, Rubinstein L, Arbuck SG, Christian MC, Freidlin B, Collins J. Accelerated titration designs for phase I clinical trials in oncology. J Nat Cancer Inst. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 9.Mattsson S, Johansson L, Leide Svegborn S, Liniecki J, Nosske D, Riklund KA, Stabin M, Taylor D, Bolch W, Carlsson S, Eckerman K, Giussani A, Soderberg L, Valind S ICRP. Radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. Ann ICRP. 2015;44:7–321. doi: 10.1177/0146645314558019. [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1016/j.icrp.2008.08.003. ICRP, 2008. Radiation Dose to Patients from Radiopharmaceuticals - Addendum 3 to ICRP Publication 53. ICRP Publication 106. Ann. ICRP 38 (1-2) [DOI] [PubMed] [Google Scholar]
- 11.Hays MT, Watson EE, Thomas SR, Stabin M. MIRD dose estimate report no. 19: radiation absorbed dose estimates from (18)F-FDG. J Nucl Med. 2002;43:210–214. [PubMed] [Google Scholar]
- 12.Cristy M, Eckerman K. Specific absorbed fractions of energy at various ages from internal photon sources. I. Method. 1987 [Google Scholar]
- 13.Stabin MG. MIRDOSE: personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 1996;37:538–546. [PubMed] [Google Scholar]
- 14.Wang Y, Li M, Diao R, Tung B, Zhang D, Li Y. Experimental study on the therapeutic effect and underlining mechanisms of positron in pancreatic cancer cells. Oncotarget. 2017;8:51652. doi: 10.18632/oncotarget.18366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Pfeifer AK, Myschetzky R, Garbyal RS, Rasmussen P, Knigge U, Bzorek M, Kristensen MH, Kjær A. Induction of anti-tumor immune responses by peptide receptor radionuclide therapy with 177Lu-DOTATATE in a murine model of a human neuroendocrine tumor. Diagnostics. 2013;3:344–355. doi: 10.3390/diagnostics3040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Togo M, Yokobori T, Shimizu K, Handa T, Kaira K, Sano T, Tsukagoshi M, Higuchi T, Yokoo S, Shirabe K. Diagnostic value of 18 F-FDG-PET to predict the tumour immune status defined by tumoural PD-L1 and CD8+ tumour-infiltrating lymphocytes in oral squamous cell carcinoma. Br J Cancer. 2020:1–9. doi: 10.1038/s41416-020-0820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Bartelink IH, Jones EF, Shahidi-Latham SK, Lee PRE, Zheng Y, Vicini P, van’t Veer L, Wolf D, Iagaru A, Kroetz DL. Tumor drug penetration measurements could be the neglected piece of the personalized cancer treatment puzzle. Clin Pharmacol Therapeut. 2019;106:148–163. doi: 10.1002/cpt.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]


