Abstract
Objectives
Molecular assays on nasopharyngeal swabs remain the cornerstone of COVID-19 diagnostics. The high technicalities of nasopharyngeal sampling and molecular assays, as well as scarce resources of reagents, limit our testing capabilities. Several strategies failed, to date, to fully alleviate this testing process (e.g. saliva sampling or antigen testing on nasopharyngeal samples). We assessed the clinical performances of SARS-CoV-2 nucleocapsid antigen (N-antigen) ELISA detection in serum or plasma using the COVID-19 Quantigene® (AAZ, France) assay.
Methods
Performances were determined on 63 serum samples from 63 non-COVID patients and 227 serum samples (165 patients) from the French COVID and CoV-CONTACT cohorts with RT-PCR-confirmed SARS-CoV-2 infection, including 142 serum samples (114 patients) obtained within 14 days after symptom onset.
Results
Specificity was 98.4% (95% CI 95.3–100). Sensitivity was 79.3% overall (180/227, 95% CI, 74.0–84.6) and 93.0% (132/142, 95% CI, 88.7–97.2) within 14 days after symptom onset. Ninety-one of the included patients had serum samples and nasopharyngeal swabs collected in the same 24 hr. Among those with high nasopharyngeal viral loads, i.e. Ct value below 30 and 33, only 1/50 and 4/67 tested negative for N-antigenaemia, respectively. Among those with a negative nasopharyngeal RT-PCR, 8/12 presented positive N-antigenaemia; the lower respiratory tract was explored for six of these eight patients, showing positive RT-PCR in five cases.
Discussion
This is the first evaluation of a commercially available serum N-antigen detection assay. It presents a robust specificity and sensitivity within the first 14 days after symptoms onset. This approach provides a valuable new option for COVID-19 diagnosis, only requiring a blood draw and easily scalable in all clinical laboratories.
Keywords: Antigen, Antigenaemia, Blood, COVID-19, Diagnostic, Plasma, SARS-CoV-2, Serum
Introduction
Molecular assays on nasopharyngeal swabs remain the cornerstone of COVID-19 diagnostics. Despite massive efforts, the high technicalities of nasopharyngeal sampling and molecular assays, as well as scarce resources of reagents, limit our testing capabilities. Several strategies have failed, to date, to fully alleviate this testing process, e.g. saliva sampling [1,2] or antigen testing on nasopharyngeal samples [3,4]. Nucleocapsid-antigen (N-antigen) has been detected in the serum of SARS-CoV-infected patients and, recently, it has been demonstrated in a single study of SARS-CoV-2-infected patients to have a global sensitivity of 41/64 patients [5,6].
In this work, we assessed the performances of N-antigen sera detection in a large patients' population using the first commercially available assay, the COVID-19 Quantigene® (AAZ France) providing a low limit of detection at 2.98 pg/mL.
Materials and methods
Patients and ethics
Negative samples comprised 50 pre-pandemic samples (collected between 2 December 2019 and 13 January 2020) and 13 pandemic samples from SARS-CoV-2 non-infected patients that tested positive for other microbial antigens (i.e. NS1 antigen, HBs antigen, HIV-1 p24 antigen, HKU1 coronavirus or malaria antigens). Positive samples were collected between 25 January 2020 and 2 September 2020 from study participants included in the French COVID (clinicaltrials.gov NCT04262921) and CoV-CONTACT cohorts (clinicaltrials.gov NCT04259892). We selected the first serum samples available after COVID-19 diagnosis (cf. Fig. S2). The following serum samples of those patients, when collected at the physician's discretion, were also included. They provided written informed consent for the use of their samples for research. Ethics approval was given by the French Ethics Committee CPP-Ile-de-France 6 (ID RCB: 2020-A00256-33 and ID RCB: 2020-A00280-39) and the French National Data Protection Commission (approval #920102).
For COVID-19 patients, available serum samples were classified into different categories according to the delay since symptom onset: serum collected ≤14 days post-symptom onset (142 serum samples from 114 patients), serum samples collected >14 days post-symptom onset (81 serum samples from 72 patients), serum samples collected from asymptomatic patients (three serum samples from three patients) and patient without date of symptom onset (one serum sample from one patient). Distribution of serum samples according to date of sampling and hospitalization status is detailed in Fig. S2.
N-antigen level assessment
Prior to analysis, serum samples were stored at –80°C. N-antigenaemia levels were determined with a CE-IVD ELISA microplate assay, COVID-Quantigene® (AAZ), according to manufacturer's recommendations. Briefly, in each well of 96-well microplates previously coated with anti-SARS-CoV-2 N-antibodies, 50 μL of a solution containing biotinylated anti-SARS-CoV-2 N-antibodies and 50 μL of serum were added. After incubation at 37°C for 60 min, plates were washed five times with a phosphate buffer solution. Then, 100 μL of a solution containing a Horseradish peroxidase-conjugated streptavidin was added, followed by incubation for 30 min at 37°C. Plates were washed five times with the phosphate buffer solution, then 50 μL of a solution containing the substrate (3,3′,5,5′-tetramethylbenzidine (TMB)) and 50 μL of a second solution containing urea were added. After 15 min at 37°C, the colorimetric reaction was stopped by adding 50 μL of H2SO4. Absorbance values were measured at 450 nm, with the reference set at 630 nm. In each plate, standards made of recombinant N-antigens were tested, to quantify the N-antigenaemia levels for each patient's sample. As the purpose of this study was to assess the sensitivity of this new assay, samples with titres above 180 pg/mL were not diluted for precise quantification.
RT-PCR assays
For all patients included in this study, diagnosis of SARS-CoV-2 infection was performed in the virology department of Bichat-Claude Bernard University Hospital by RT-PCR on nasopharyngeal swabs, as recommended. Different techniques were performed throughout the study period for nasopharyngeal samples, due to frequent shortages issues and requirements for fast turnaround time: RealStar® SARS-CoV-2 (Altona, Hamburg, Germany), Cobas® SARS-CoV-2 (Roche Diagnostics, Branchburg, NJ, USA), Simplexa® COVID-19 Direct (DiaSorin, Gerenzano, Italy), BioFire® SARS-CoV-2 (BioMerieux, Salt Lake City, UT, USA), QIAstat-Dx® Respiratory SARS-CoV-2 (Qiagen, Hilden, Germany) and NeumoDX® (QIAgen, Hilden, Germany) using IP2 Institute Pasteur and WHO E gene primers [7]. E gene cycle threshold (Ct) values, available for all techniques except Simplexa® COVID-19 Direct and BioFire® SARS-COV-2, were used as a proxy for viral load for 104 samples from 91 patients with paired nasopharyngeal swabs and sera (i.e. collected in the same 24 hr).
For a subset of 146 samples, corresponding to 89 patients included in the French COVID-19 cohort, paired sera and plasma samples were available, allowing one to determine the presence of viral RNA in plasma. Briefly, viral nucleic acids were extracted from 200 μL of plasma with the MagNA Pure LC Total Nucleic Acid Isolation Kit – Large Volume (Roche Diagnostics, Branchburg, NJ, USA) and eluted in 50 μL. RT-PCR was performed on 10 μL of eluate using the RealStar® SARS-CoV-2 assay (Altona, Germany), according to the manufacturer's recommendations. Samples with RT-PCR cycle threshold values above 40 were considered negative.
Detection of anti-SARS-CoV-2 nucleocapsid IgG
For a subset of 85 serum samples, corresponding to 80 patients (ICU patients: n = 21, ward patients: n = 36 and outpatients: n = 23), we performed a chemiluminescent microparticle immunoassay detecting anti-N immunoglobulin G (Architect SARS-CoV-2 IG Assay, Abbott). Results were reported as a signal to cut-off (S/Co) value. The positivity threshold was set to 1.4, as recommended by the manufacturer.
Data availability
A file compiling all data used in this article is available on Mendeley Data public repository (https://data.mendeley.com/datasets/fjz6zbkxvm/1).
Results
Specificity of the COVID-19 Quantigene® was 98.4% (95% confidence interval (CI) 95.3–100), as N-antigenaemia was negative for 62 samples out of 63 non-COVID-19 patients.
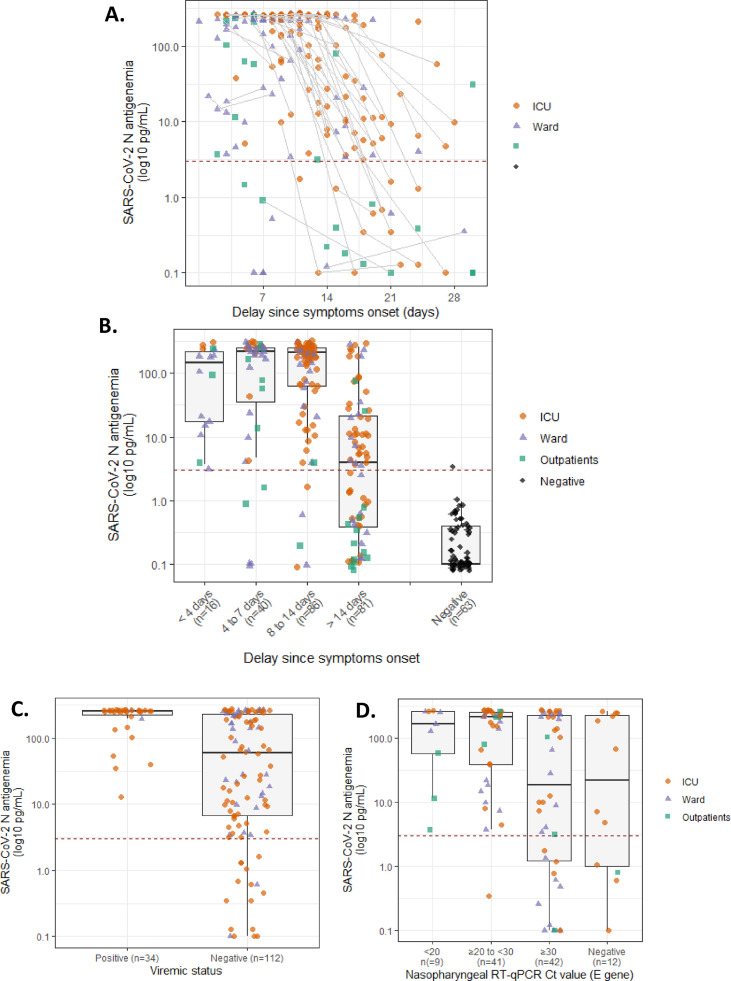
N-antigenaemia sensitivity was determined on 227 serum samples, obtained from 165 patients included in the French COVID and CoV-CONTACT cohorts with RT-PCR confirmed SARS-CoV-2 infection. Among them, 180/227 serum samples tested positive, leading to a sensitivity of 79.3% (95% CI 74.0–84.6). When restricting sensitivity analysis to samples collected in the first 2 weeks after symptom onset, 132 out of 142 samples tested positive for N-antigenaemia, leading to a sensitivity of 93.0% (95% CI 88.7–97.2) (Fig. 1 A,B). Patients with positive RNAaemia (viraemic patients) exhibited higher N-antigen sera levels (Fig. 1C). In serum samples collected more than 14 days after symptom onset, N-antigenaemia frequently declined and was undetectable in 84.6% (11/13), 42.1% (8/19) and 32.7% (16/49) samples of outpatients, ward and ICU patients, respectively. The lower detection in late-stage samples appears linked with the apparition of anti-N IgG (Fig. 1A and supplementary material).
Fig. 1.
(A) Evolution of N-antigen sera levels in SARS-CoV-2-infected patients according to hospitalization status (n = 227 serum samples from 165 patients); sequential samples are connected with a grey line, while the positivity threshold value for N-antigen (2.97 pg/mL) is indicated with a dashed red line. (B) N-antigenaemia levels according to delay since symptoms onset. (C) N-antigen sera levels according to positive and negative RNAaemia status (n = 146 sera, n = 89 patients). (D) N-antigen sera levels according to E-gene cycle threshold value of 104 nasopharyngeal swabs collected within 24 hr (n = 91 patients).
For 91 patients, 104 paired serum samples and nasopharyngeal swabs collected in the same 24 hours were available, allowing us to compare N-antigen detection with E gene Ct values for those patients (Fig. 1D). Among patients with E gene Ct value below 30 and below 33 on their nasopharyngeal swab, only 1/50 and 4/67 tested negative for N-antigenaemia, respectively (Fig. S1B). For patients with positive nasopharyngeal samples with Ct values ≥ 33, only 15/25 (60%) were positive for N-antigenaemia. Interestingly, eight out of 12 patients with a negative nasopharyngeal RT-PCR presented positive N-antigenaemia. The lower respiratory tract was explored for 6 of these 8 patients either the same day or in the 5 following days. RT-PCR on the lower respiratory tract sample was positive in five of these six patients.
Discussion
This is the first evaluation of a commercially available SARS-CoV-2 N-antigen serum or plasma detection. This assay presented a low detection limit at 2.98 pg/mL and a sensitivity above 90% during the acute phase of the disease (i.e. <14 days after symptoms onset in PCR confirmed COVID-19 patients). In the first 2 weeks, N-antigen negativity was associated with anti-N IgG detection (6/10) and/or low nasopharyngeal viral load in the same 24 hr (7/7, Ct value > 30). This sensitivity could allow its use for COVID-19 diagnostic and is in line with RT-PCR on nasopharyngeal samples whose reported sensitivity rates ranged between 71% and 98%, based on negative RT-PCR tests which were positive on repeat testing [8].
Detection of viral antigens in the blood of COVID-19 patients has been recently described by Ogata and collaborators, who detected N and S1 antigens in the blood of 41 out of 64 COVID-19 patients [5]. Antigen circulation in blood is not uncommon in infectious diseases, antigenaemia tests usually target blood-borne pathogens, notably Dengue, CMV, HBV or HIV. In respiratory diseases, antigen circulation into non-respiratory body fluids is usually not considered, even, if likely, because of the focal nature of the infection or possible pre-existence of antibodies. Antigen detection in non-respiratory fluids is still used for two respiratory bacteria: Streptococcus pneumonia and Legionella pneumophila. Interestingly, it has also been reported in SARS-CoV-1 infection [6]. Whether the circulation of free viral antigens has an impact on disease physiopathology should be assessed in future studies.
N-antigenaemia was also detectable in outpatients but the decrease seems to occur earlier in our study. Detection of viral antigens in this population was not evaluated in the study by Ogata and collaborators. Detection of N-antigenaemia was higher for patients with either high nasopharyngeal viral loads, i.e. Ct below 30, or active replication in the lung, i.e. high Ct values (>30) in nasopharyngeal samples but positive RT-PCR in lower respiratory tract samples.
This innovative marker may also be of help for prognostic evaluation of patients. An association between high N-antigen levels and higher ICU admission rates has been reported by Ogata et al. [5]. In our study, we observed higher N-antigen levels in serum of viraemic patients, in line with the possible association of viraemia, or RNAaemia, with disease severity and/or immunosuppression [[9], [10], [11]].
Our study presents several limitations. We included a very small number of outpatients and this population needs to be explored in larger cohorts, ideally with longitudinal samples of paucisymptomatic and asymptomatic patients. The case–control design is another limitation and the test performances could be slightly deteriorated in real life condition.
In conclusion, sensitive N-antigen detection in serum or plasma provides a valuable new marker for COVID-19 diagnosis, only requiring a blood draw, that is scalable in all clinical laboratories. It allows potential new developments to design rapid antigen blood test or combined ELISA assays, detecting both antigens and antibodies. It also raises new questions about the physiological mechanisms at play explaining blood circulation of this antigen and its potential correlation with disease severity.
Transparency declaration
Benoit Visseaux reports grants, personal fees and non-financial support from Qiagen, personal fees from BioMérieux, personal fees from Hologic, personal fees from Gilead, all outside the submitted work. Xavier Duval reports grants from Sanofi Pasteur, grants from Pfizer, all outside the submitted work. Charles Burdet reports personal fees and non-financial support from Da Volterra, personal fees from Mylan, all outside the submitted work. Jean-François Timsit reports grants and personal fees from MSD, grants and personal fees from Pfizer, grants and personal fees from Beckton Dickinson, personal fees from Biomerieux, personal fees from Medimune, personal fees from Gilead, all outside the submitted work. Jade Ghosn reports grants and personal fees from Gilead Sciences, grants and personal fees from ViiV Healthcare, personal fees from MSD, all outside the submitted work. Charlotte Charpentier reports personal fees from ViiV Healthcare, personal fees from Gilead, personal fees from Theratechnologie, all outside the submitted work. Diane Descamps reports personal fees from ViiV Healthcare, personal fees from Gilead, all outside the submitted work. Yazdan Yazdanpanah has been a board member receiving consultancy fees from Abbvie, BMS, Gilead, MSD, J&J, Pfizer, and ViiV Healthcare, all outside the submitted work and all these activities have been stopped in the 3 past years. The other authors have nothing to disclose. This study has been funded in part by the REACTing (REsearch & ACTion emergING infectious diseases) consortium, by a grant from the French Ministry of Health (PHRC n° 20-0424) and by the AC43 group of the ANRS (Agence Nationale de la Recherche sur le SIDA et les hépatites virales). The study was supported by AAZ (Boulogne-Billancourt, France) in the form of free consumables and they had no role in conceptualization, design, data collection and analyses, decision to publish and manuscript preparation.
Author contributions
Q.L.H., B.V., C.Cha., D.D., N.H.F. conceptualized the study and its methodology. Q.L.H., H.I., F.D., N.B., M.B. performed the experiments. C.L., S.T., C.B., C.Cho., X.D., J.F.T., L.B., J.G., Y.Y. collected data and participated to the validation of the study. Q.L.H., B.V. wrote the first draft. All authors reviewed and edited the final manuscript.
Acknowledgement
The French COVID cohort was sponsored by Inserm. We wish to thank the ANRS (Agence Nationale de la Recherche sur le SIDA et les hépatites virales).
Members of the French-COVID cohort study group (alphabetical order by group): Alpha Diallo, Soizic Le Mestre, Noémie Mercier, Christelle Paul, Ventzislava Petrov-Sanchez (ANRS, Paris); Denis Malvy (Bordeaux – SMIT); Catherine Chirouze (CHRU Jean Minjoz, Besançon); Claire Andrejak (CHU Amiens); François Dubos (CHU Lille), Patrick Rossignol (CHU Nancy); Olivier Picone (Colombes – Louis Mourier – Gynécologie); François Bompart (Drugs for Neglected Diseases initiative, Geneva, Switzerland); Tristan Gigante, Morgane Gilg, Bénédicte Rossignol, Claire Levy-Marchal (F-CRIN INI-CRCT, Paris); Marine Beluze (F-CRIN Partners Platform, Paris); Jean Sébastien Hulot (HEGP, Paris); Delphine Bachelet, Krishna Bhavsar, Lila Bouadma, Anissa Chair, Camille Couffignal, Charlene Da Silveira, Marie Pierre Debray, Diane Descamps, Xavier Duval, Philippine Eloy, Marina Esposito-Farese, Nadia Ettalhaoui, Nathalie Gault, Jade Ghosn, Isabelle Gorenne, Isabelle Hoffmann, Ouifiya Kafif, Sabrina Kali, Antoine Khalil, Cédric Laouénan, Samira Laribi, Minh Le, Quentin Le Hingrat, François-Xavier Lescure, Jean Christophe Lucet, France Mentré, Jimmy Mullaert, Nathan Peiffer-Smadja, Gilles Peytavin, Carine Roy, Marion Schneider, Nassima SI Mohammed, Lysa Tagherset, Coralie Tardivon, Marie Capucine Tellier, Jean-François Timsit, Théo Trioux, Sarah Tubiana, Benoit Visseaux (Hôpital Bichat, Paris); Noémie Vanel (Hôpital la Timone, Marseille); Romain Basmaci (Hôpital Louis Mourrier, Colombes); François Angoulvant (Hôpital Necker, Paris); Florentia Kaguelidou, Justine Pages (Hôpital Robert Debré, Paris); Christelle Tual, Aurélie Veislinger (Inserm CIC-1414, Rennes); Sandrine Couffin-Cardiergues, Hélène Esperou, Ikram Houas, Salma Jaafoura, Aurélie Papadopoulos (Inserm sponsor, Paris), Alexandra Coelho, Alphonsine Diouf, Alexandre Hoctin, Marina Mambert (Inserm UMR 1018, Paris); Maude Bouscambert, Alexandre Gaymard, Bruno Lina, Manuel Rosa-Calatrava, Olivier Terrier (Inserm UMR 1111, Lyon); Dehbia Benkerrou, Céline Dorival, Amina Meziane, François Téoulé (Inserm UMR 1136, Paris); Jérémie Guedj, Hervé Le Nagard, Guillaume Lingas, Nadège Neant (Inserm UMR 1137, Paris); Laurent Abel (Inserm UMR 1163, Paris); Mathilde Desvallée, Coralie Khan (Inserm UMR 1219, Bordeaux); Dominique Deplanque (Lille Calmette – SMIT); Yazdan Yazdanpanah (Paris – Bichat – SMIT); Sylvie Behilill, Vincent Enouf, Hugo Mouquet, Sylvie Van Der Werf (Pasteur Institute, Paris); Minerva Cervantes-Gonzalez, Eric D'ortenzio, Oriane Puéchal, Caroline Semaille (REACTing, Paris); Marion Noret (RENARCI, Annecy); Manuel Etienne (Rouen – SMIT); Yves Levy, Aurélie Wiedemann (Vaccine Research Insitute (VRI), Inserm UMR 955, Créteil, France).
Members of the CoV-CONTACT study group. Principal investigator: Duval Xavier; Steering Committee: Burdet Charles, Duval Xavier, Lina Bruno, Tubiana Sarah, Van Der Werf Sylvie; CoV-CONTACT Clinical Centres: Abad Fanny, Abry Dominique, Alavoine Loubna, Allain Jean-Sébastien, Amiel-Taieb Karline, Audoin Pierre, Augustin Shana, Ayala Sandrine, Bansard Hélène, Bertholon Fréderique, Boissel Nolwenn, Botelho-Nevers Elisabeth, Bouiller Kévin, Bourgeon Marilou, Boutrou Mathilde, Brick Lysiane, Bruneau Léa, Caumes Eric, Chabouis Agnès, Chan Thien Eric, Chirouze Catherine, Coignard Bruno, Costa Yolande, Costenoble Virginie, Cour Sylvie, Cracowski Claire, Cracowski Jean Luc, Deplanque Dominique, Dequand Stéphane, Desille-Dugast Mireille, Desmarets Maxime, Detoc Maelle, Dewitte Marie, Djossou Felix, Ecobichon Jean-Luc, Elrezzi Elise, Faurous William, Fortuna Viviane, Fouchard Julie, Gantier Emilie, Gautier Céline, Gerardin Patrick, Gerset Sandrine, Gilbert Marie, Gissot Valérie, Guillemin Francis, Hartard Cédric, Hazevis Béatrice, Hocquet Didier, Hodaj Enkelejda, Ilic-Habensus Emila, Jeudy A, Jeulin Helene, Kane Maty, Kasprzyk Emmanuelle, Kikoine John, Laine Fabrice, Laviolle Bruno, Lebeaux David, Leclercq Anne, Ledru Eric, Lefevre Benjamin, Legoas Carole, Legrand Amélie, Legrand Karine, Lehacaut Jonathan, Lehur Claire, Lemouche Dalila, Lepiller Quentin, Lepuil Sévérine, Letienne Estelle, Lucarelli Aude, Lucet Jean-Christophe, Madeline Isabelle, Maillot Adrien, Malapate Catherine, Malvy Denis, Mandic Milica, Marty-Quinternet Solène, Meghadecha Mohamed, Mergeay-Fabre Mayka, Mespoulhe Pauline, MEUNIER Alexandre, Migaud Maria-Claire, Motiejunaite Justina, Gay Nathalie, Nguyen Duc, Oubbea Soumaya, Pagadoy Maïder, Paris Adeline, Paris Christophe, Payet Christine, Peiffer-Smadja Nathan, Perez Lucas, Perreau Pauline, Pierrez Nathalie, Pistone Thierry, Postolache Andreea, Rasoamanana Patrick, Reminiac Cécile, Rexah Jade, Roche-Gouanvic Elise, Rousseau Alexandra, Schoemaecker Betty, Simon Sandrine, Soler Catherine, Somers Stéphanie, Sow Khaly, Tardy Bernard, Terzian Zaven, Thy Michael, Tournier Anne, Tyrode Sandrine, Vauchy Charline, Verdon Renaud, Vernet Pauline, Vignali Valérie, Waucquier Nawal; Coordination and statistical analyses: Burdet Charles, Do Thi Thu Huong, Laouénan Cédric, Mentre France, Pauline Manchon, Tubiana Sarah, Dechanet Aline, Letrou Sophie, Quintin Caroline, Frezouls Wahiba; Virological Lab: Le Hingrat Quentin, Houhou Nadhira, Damond Florence, Descamps Dianes, Charpentier Charlotte, Visseaux Benoit, Vabret Astrid, Lina Bruno, Bouscambert Maud, Van Der Werf Sylvie, Behillil Sylvie, Gaillanne Laurence, Benmalek Nabil, Attia Mikael, Barbet Marion, Demeret Caroline, Rose Thierry, Petres Stéphane, Escriou Nicolas, Barbet Marion, Petres Stéphane, Escriou Nicolas, Goyard Sophie; Biological Centre: Kafif Ouifiya, Piquard Valentine, Tubiana Sarah; Partners: RECOVER, REACTING, Santé Publique France (Coignard Bruno, Mailles Alexandra), Agences régionales de santé (Simondon Anne, Dreyere Marion, Morel Bruno, Vesval Thiphaine); Sponsor: Inserm, Amat Karine, Ammour Douae, Aqourras Khadija, Couffin-Cadiergues Sandrine, Delmas Christelle, Desan Vristi, Doute Jean Michel, Esperou Hélène, Hendou Samia, Kouakam Christelle, Le Meut Guillaume, Lemestre Soizic, Leturque Nicolas, Marcoul Emmanuelle, Nguefang Solange, Roufai Layidé; Genetic: Laurent Abel, Caillat-Zucman Sophie.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.11.025.
Contributor Information
French COVID Cohort Management Committee:
Alpha Diallo, Soizic Le Mestre, Noémie Mercier, Christelle Paul, Ventzislava Petrov-Sanchez, Denis Malvy, Catherine Chirouze, Claire Andrejak, François Dubos, Patrick Rossignol, Olivier Picone, François Bompart, Tristan Gigante, Morgane Gilg, Bénédicte Rossignol, Claire Levy-Marchal, Marine Beluze, Jean Sébastien Hulot, Delphine Bachelet, Krishna Bhavsar, Lila Bouadma, Anissa Chair, Camille Couffignal, Charlene Da Silveira, Marie Pierre Debray, Diane Descamps, Xavier Duval, Philippine Eloy, Marina Esposito-Farese, Nadia Ettalhaoui, Nathalie Gault, Jade Ghosn, Isabelle Gorenne, Isabelle Hoffmann, Ouifiya Kafif, Sabrina Kali, Antoine Khalil, Cédric Laouénan, Samira Laribi, Minh Le, Quentin Le Hingrat, François-Xavier Lescure, Jean Christophe Lucet, France Mentré, Jimmy Mullaert, Nathan Peiffer-Smadja, Gilles Peytavin, Carine Roy, Marion Schneider, Nassima Si Mohammed, Lysa Tagherset, Coralie Tardivon, Marie Capucine Tellier, Jean-François Timsit, Théo Trioux, Sarah Tubiana, Benoit Visseaux, Noémie Vanel, Romain Basmaci, François Angoulvant, Florentia Kaguelidou, Justine Pages, Christelle Tual, Aurélie Veislinger, Sandrine Couffin-Cardiergues, Hélène Esperou, Ikram Houas, Salma Jaafoura, Aurélie Papadopoulos, Alexandra Coelho, Alphonsine Diouf, Alexandre Hoctin, Marina Mambert, Maude Bouscambert, Alexandre Gaymard, Bruno Lina, Manuel Rosa-Calatrava, Olivier Terrier, Dehbia Benkerrou, Céline Dorival, Amina Meziane, François Téoulé, Jérémie Guedj, Hervé Le Nagard, Guillaume Lingas, Nadège Neant, Laurent Abel, Mathilde Desvallée, Coralie Khan, Dominique Deplanque, Yazdan Yazdanpanah, Sylvie Behilill, Vincent Enouf, Hugo Mouquet, Sylvie Van Der Werf, Minerva Cervantes-Gonzalez, Eric D'ortenzio, Oriane Puéchal, Caroline Semaille, Marion Noret, Manuel Etienne, Yves Levy, and Aurélie Wiedemann
CoV-CONTACT Study Group:
Xavier Duval, Charles Burdet, Xavier Duval, Bruno Lina, Sarah Tubiana, Sylvie Van Der Werf, Fanny Abad, Dominique Abry, Loubna Alavoine, Jean-Sébastien Allain, Karline Amiel-Taieb, Pierre Audoin, Shana Augustin, Sandrine Ayala, Hélène Bansard, Fréderique Bertholon, Nolwenn Boissel, Elisabeth Botelho-Nevers, Kévin Bouiller, Marilou Bourgeon, Mathilde Boutrou, Lysiane Brick, Léa Bruneau, Eric Caumes, Agnès Chabouis, Eric Chan Thien, Catherine Chirouze, Bruno Coignard, Yolande Costa, Virginie Costenoble, Sylvie Cour, Claire Cracowski, Jean-Luc Cracowski, Dominique Deplanque, Stéphane Dequand, Mireille Desille-Dugast, Maxime Desmarets, Maelle Detoc, Marie Dewitte, Felix Djossou, Jean-Luc Ecobichon, Elise Elrezzi, William Faurous, Viviane Fortuna, Julie Fouchard, Emilie Gantier, Céline Gautier, Patrick Gerardin, Sandrine Gerset, Marie Gilbert, Valérie Gissot, Francis Guillemin, Cédric Hartard, Béatrice Hazevis, Didier Hocquet, Enkelejda Hodaj, Emila Ilic-Habensus, Jeudy A., Helene Jeulin, Maty Kane, Emmanuelle Kasprzyk, John Kikoine, Fabrice Laine, Bruno Laviolle, David Lebeaux, Anne Leclercq, Eric Ledru, Benjamin Lefevre, Carole Legoas, Amélie Legrand, Karine Legrand, Jonathan Lehacaut, Claire Lehur, Dalila Lemouche, Quentin Lepiller, Sévérine Lepuil, Estelle Letienne, Aude Lucarelli, Jean-Christophe Lucet, Isabelle Madeline, Adrien Maillot, Catherine Malapate, Denis Malvy, Milica Mandic, Solène Marty-Quinternet, Mohamed Meghadecha, Mayka Mergeay-Fabre, Pauline Mespoulhe, Alexandre Meunier, Maria-Claire Migaud, Justina Motiejunaite, Nathalie Gay, Duc Nguyen, Soumaya Oubbea, Maïder Pagadoy, Adeline Paris, Christophe Paris, Christine Payet, Nathan Peiffer-Smadja, Lucas Perez, Pauline Perreau, Nathalie Pierrez, Thierry Pistone, Andreea Postolache, Patrick Rasoamanana, Cécile Reminiac, Jade Rexah, Elise Roche-Gouanvic, Alexandra Rousseau, Betty Schoemaecker, Sandrine Simon, Catherine Soler, Stéphanie Somers, Khaly Sow, Bernard Tardy, Zaven Terzian, Michael Thy, Anne Tournier, Sandrine Tyrode, Charline Vauchy, Renaud Verdon, Pauline Vernet, Valérie Vignali, Nawal Waucquier, Charles Burdet, Huong Do Thi Thu, Cédric Laouénan, France Mentre, Manchon Pauline, Sarah Tubiana, Aline Dechanet, Sophie Letrou, Caroline Quintin, Wahiba Frezouls, Quentin Le Hingrat, Nadhira Houhou, Florence Damond, Dianes Descamps, Charlotte Charpentier, Benoit Visseaux, Astrid Vabret, Bruno Lina, Maud Bouscambert, Sylvie Van Der Werf, Sylvie Behillil, Laurence Gaillanne, Nabil Benmalek, Mikael Attia, Marion Barbet, Caroline Demeret, Thierry Rose, Stéphane Petres, Nicolas Escriou, Marion Barbet, Stéphane Petres, Nicolas Escriou, Sophie Goyard, Ouifiya Kafif, Valentine Piquard, Sarah Tubiana, Bruno Coignard, Alexandra Mailles, Anne Simondon, Marion Dreyere, Bruno Morel, Thiphaine Vesval, Inserm, Karine Amat, Douae Ammour, Khadija Aqourras, Sandrine Couffin-Cadiergues, Christelle Delmas, Vristi Desan, Jean-Michel Doute, Hélène Esperou, Samia Hendou, Christelle Kouakam, Guillaume Le Meut, Soizic Lemestre, Nicolas Leturque, Emmanuelle Marcoul, Solange Nguefang, Layidé Roufai, Abel Laurent, and Sophie Caillat-Zucman
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rao M., Rashid F.A., Sabri F.S.A.H., Jamil N.N., Zain R., Hashim R. Comparing nasopharyngeal swab and early morning saliva for the identification of SARS-CoV-2. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M., Vijayakumar P. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flower B., Brown J.C., Simmons B., Moshe M., Frise R., Penn R. Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey. Thorax. 2020;75:1082–1088. doi: 10.1136/thoraxjnl-2020-215732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinnes J., Deeks J.J., Adriano A., Berhane S., Davenport C., Dittrich S. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogata A.F., Maley A.M., Wu C., Gilboa T., Norman M., Lazarovits R. Ultra-sensitive serial profiling of SARS-CoV-2 antigens and antibodies in plasma to understand disease progression in COVID-19 patients with severe disease. Clin Chem. 2020;66:1562–1572. doi: 10.1093/clinchem/hvaa213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Che X., Di B., Zhao G., Wang Y., Qiu L., Hao W. A Patient with asymptomatic severe acute respiratory syndrome (SARS) and antigenemia from the 2003–2004 community outbreak of SARS in Guangzhou, China. Clin Infect Dis. 2006;43:e1–e5. doi: 10.1086/504943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . WHO; Paris: 2020. Protocol: Real-time RT-PCR assays for the detection of SARS-CoV-2 Institut Pasteur.https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2 [Google Scholar]
- 8.Arevalo-Rodriguez I., Buitrago-Garcia D., Simancas-Racines D., Zambrano-Achig P., del Campo R., Ciapponi A. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. MedRxiv. 2020 doi: 10.1101/2020.04.16.20066787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veyer D., Kernéis S., Poulet G., Wack M., Robillard N., Taly V. Highly sensitive quantification of plasma SARS-CoV-2 RNA sheds light on its potential clinical value. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buetti N., Patrier J., Le Hingrat Q., Loiodice A., Bouadma L., Visseaux B. Risk factors for SARS-CoV-2 detection in blood of critically ill patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020;71:1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A file compiling all data used in this article is available on Mendeley Data public repository (https://data.mendeley.com/datasets/fjz6zbkxvm/1).