Abstract
Purpose: To investigate the therapeutic effects of β-ecdysterone on osteoarthritis (OA) and the underlying mechanism. Methods: OA model was established on rats by injecting MIA. ELSA was used to determine the concentration of IL-1β, IL-6, NO and TNF-α in the chondrocytes and cartilage tissues. Immunofluorescence assay was used to determine the expression of collagen II in the chondrocytes. The survival rate of chondrocytes was evaluated by MTT assay. The apoptosis of chondrocytes was checked by AO/PI staining and flow cytometry assay. The expression level of Atg7, PI3K and caspase-3 was evaluated by qRT-PCR. Western Blot was used determine the expression of PI3K, p-AKT1, AKT1, p-mTOR, mTOR, p70S6K, p-p70S6K, LC3I, LC3II and caspase-3. HE staining was used to check the pathological state of cartilage tissues. Results: Chondrocytes were tolerable to rapamycin, 3-methyladenine and β-ecdysterone at the concentration of 10 mM, 100 nM and 40 μM, respectively. The apoptosis of chondrocytes was inhibited by rapamycin and β-ecdysterone, and induced by 3-methyladenine. PI3K, p-AKT1, p-mTOR, p-p70S6K and caspase-3 were down-regulated by rapamycin and β-ecdysterone, and up-regulated by 3-methyladenine in both the chondrocytes and the cartilage tissues. The expression of Atg7 and LC3II/LC3I were regulated in a opposite way. The inflammation state was improved by rapamycin and β-ecdysterone both the chondrocytes and the cartilage tissues. HE staining results showed that the pathological state of cartilage tissues was alleviated by β-ecdysterone. Conclusion: β-ecdysterone might alleviate osteoarthritis by activating autophagy in chondrocytes through regulating PI3K/AKT/mTOR signal pathway.
Keywords: β-ecdysterone, osteoarthritis, autophagy, chondrocytes, mTOR
Introduction
Osteoarthritis (OA), an inflammatory disease widely diagnosed in elderly population. In USA, the age of OA patients is mostly over 60. Overall, 10% of male and 13% female in America are diagnosed with OA [1,2]. The physiological symptoms of OA include stiffness, loss of joint range of motion and pain. The quality of the patients is significantly affected by OA. Main pathogenesis for OA is the wear of cartilage over time, which is reported lacking of self-repair capacity [3,4].
Plenty of pharmaceutical mechanisms have been reported for achieving effective drugs to treat OA. Inhibition of the activity of COX proteins [5], the synthesizing of prostaglandin [6], the activity of cyclooxygenase enzymes [7], the pain signal pathway in the central nervous system [8], the reuptake of serotonin-norepinephrine [9], the over-expression of inflammatory factors [10], the activity of catabolic enzymes [11] are effective approaches to relieve the symptoms of OA. More mechanisms include promoting of embryogenesis [12], chondrogenesis [13], cartilage repair [14], extracellular matrix degradation among chondrocytes [15] and the thickness of cartilage [16] et al.
Chondrocytes are the main components of cartilage tissues, the dysfunction of which is reported to be related to the development and process of OA. By maintaining the stabilization of cartilage tissues and extracellular matrix, chondrocytes play an important role in keeping the metabolic balance of cartilage [17,18]. It is reported that apoptosis, hypertrophy and swelling are observed in the chondrocytes isolated from OA cartilage tissues, which provides a novel idea for the early diagnosis of OA [19]. Apoptotic rates at 5% and 22% evaluated by flow cytometry are observed on chondrocytes isolated from normal cartilage tissues and OA cartilage tissues, respectively [20]. Autophagy is one of the main physiological processes that involved in the apoptosis of chondrocytes. On the early stage of OA, the autophagy in the chondrocytes and cartilage tissue is observed to be promoted verified by the up-regulation of LC3 and Beclin, which are the autophagy related proteins. However, the transitory activation of autophagy is proved to be only the compensation response to cellular stress. Structural damage will be induced when the compensation response is not able to defense the stress, which is accompanied with the suppression of autophagy [21]. The expression level of LC3 and Beclin is declined as the deceasing of glycosaminoglycan, which contributes to the apoptosis of chondrocytes. It is reported that the aging of knee joint in OA mouse was related to the inactivation of autophagy and activation of apoptosis in chondrocytes [22], which was could be alleviated by activating the PI3K/AKT/mTOR signal pathway [23]. Therefore, improving autophagy and suppressing apoptosis in the chondrocytes through activating PI3K/AKT/mTOR signal pathway may be a novel therapeutic method to treat OA.
Currently, no ideal treatment for OA is conformably introduced. A couple of treatments are developed mainly for relief of the pain caused by OA or improvement of the patients’ functional abilities. Exercise, weight management, physical therapy, medications and surgery are the main suggested treatments for OA according to the guidelines of the American Academy of Orthopaedic Surgeons (AAOS) and the Osteoarthritis Research Society International (OARSI) [24]. Regarding the medical treatment, non-steroidal anti-inflammatory drugs (NSAIDs) are chosen as the first-choice for the pharmacological treatment of OA by many physicians [25]. However, drug resistance arises from chronic administration of NSAIDs. β-ecdysterone is an active ingredient isolated from radix achyranthis bidentatae and reported to exert anti-arrhythmia, anti-fatigue, proliferation promoting and hypolipidemic effects [26]. Current reports showed that the effects on the differentiation, the proliferation and apoptosis of osteoblast of dexamethasone could be reversed by β-ecdysterone [27]. In the present study, the effects of β-hydroxyecdysone on the autophagy and apoptosis in the chondrocytes will be investigated, by which a potential medical treatment for clinical OA might be available.
Methods and materials
The establishment of OA model in rats
50 rats were purchased from Beijing Charles River Experimental Animal Technology Co. LTD. After 1 week of acclimation, rats were anesthetized with isoflurane and administered an intra-articular injection of 3 mg/kg MIA (Sigma-Aldrich) to the right knee using a 30 G needle in 50 μL volume. The normal control group was injected with 0.9% saline (JW Pharmaceutical Co., Seoul, Korea). After 2 weeks of MIA injection, the cartilage tissue and chondrocytes were isolated from 5 rats for verification of the OA model. DMEM medium with 10% fetal bovine serum was used to culture the isolated chondrocytes at 37°C with 5% CO2.
Enzyme-linked immunosorbent assay (ELISA)
According to the instruction of the manufacturer (Sigma), the concentration of IL-1β, IL-6, NO and TNF-α in the chondrocytes and cartilage tissues was determined by ELISA. Basically, the operation includes: sample adding, enzyme adding, incubation, working solution preparing, washing, dyeing, terminating and detecting. Linear regression equation was described based on the concentration of standards and OD value. The concentrations of the samples were calculated according to the equation, detected OD value and dilution factor.
Immunofluorescence assay
The chondrocytes were incubated with primary rabbit anti-collagenII (OmnimAbs, 1:1000) overnight at 4°C. After three washes with PBS, cells were incubated with secondary Cy3-conjugated anti-ribbit IgG (Abcam, 1:200) for an additional 30 min at room temperature. The DAPI was added to dye the nuclear for 5 min and 50% glycerinum was used to block the medium. Stained cells were photographed under a fluorescence microscope (Olympus, Tokyo, Japan).
MTT assay
The appropriate concentration of rapamycin, 3-methyladenine and β-ecdysterone was determined by MTT assay on the chondrocytes. Cells were seeded into plates (Corning, Corelle City, NY, USA) at 37°C for 24 h and incubated with rapamycin (10, 20, 40, 60, 80, 100, 120, 140 nM), 3-methyladenine (1, 2, 4, 6, 8, 10, 12, 14 mM) and β-ecdysterone (1, 2, 5, 10, 20, 40, 60, 80, 100 μM), respectively, for 24 h, 48 h and 72 h. Then, 10 μL of 5 mg/mL MTT solution (Thermo Fisher Scientific, Waltham, USA) was mixed in the medium. 4 h later, the formazan was added in about 200 μL of dimethylsulfoxide (DMSO, Genview, Beijing, China). OD value at 490 nm was recorded by a microplate reader (BMG LABTECH). The value (ODcontrol-ODtreatment)/ODcontrol was used to represent suppressive rate.
In-vitro and in-vivo grouping
6 groups were divided in the in-vitro and in-vivo experiments, respectively. The chondrocytes isolated from the OA rat model were treated with blank medium, 10 mM 3-methyladenine, 100 nM rapamycin, 10 μM β-ecdysterone, 20 μM β-ecdysterone and 40 μM β-ecdysterone, respectively, and collected for subsequent study after incubation for 24 hours. The OA rats were treated with 30 mg/kg 3-methyladenine, 1 mg/kg rapamycin, 0.6 mg/kg β-ecdysterone, 0.8 mg/kg β-ecdysterone and 1 mg/kg β-ecdysterone, respectively, with model rats without dosing as control. The administration route for 3-methyladenine and rapamycin was intraperitoneal injection and that for β-ecdysterone was subcutaneous injection. The dosing frequency for all the 5 groups was twice a week for 4 weeks.
Acridine orange (AO)/propidium iodide (PI) staining
The cultural medium of treated chondrocytes was removed and washed with 1 × PBS at a ratio of 1:1 (v/v). Subsequently, the cells were resuspended with 1 mL PBS containing 1 mg/mL acridine orange and 1 mg/mL propidium iodide (AO/PI), followed by incubating in the dark for 15 min. The dyed cells were dropped to the sliding glass and imaged with a Nikon-C2 laser scanning confocal microscope.
Flow cytometer assay
Approximately 10 μL fluorescently-labeled Annexin V reagent and 5 μL PI reagent were added into the 1.5 mL centrifuge tubes, following collecting the chondrocytes by centrifugation at 300 g for 10 min. After incubating for 10 min at room temperature in the dark, approximately 200 μL of solution containing cells was mixed with 2 mL PBS in the flow tubes, which was tested by the flow cytometry (Thermo Fisher Scientific, Waltham, USA). Three independent assays were performed.
Western blot
Tissues or cells were collected and lysed in RIPA lysis buffer (Thermo Fisher Scientific, Massachusetts, USA). 15% SDS-PAGE was used to segregate the proteins. Subsequently, the isolated proteins were transfer to the PVDF membranes (Millipore, Massachusetts, USA) by semi-dry transfer. The membranes were incubated with 5-10% BSA solution for 1-2 h to remove non-specific binding. Then the membranes were incubated with primary antibody against PI3K (1:1000), RunX2 (1:1000), AKT1 (1:1000), p-AKT1 (1:1000), mTOR (1:1000), p-mTOR (1:1000), p70S6K (1:1000), p-p70S6K (1:1000), LC3I (1:1000), LC3II (1:1000), Caspase-3 (1:1000) or GAPDH (1:1000) (Abcam, Cambridge, UK). Following 3 times washing, horseradish peroxidase-conjugated secondary antibody (1:3000, Abcam, Cambridge, UK) was used to incubate with the membranes at 25°C. One to two hours later, Blots were incubated with the ECL reagents (Amersham, UK) and exposed under Amersham Imager 600 (GE). The relevant blots were quantified by densitometry by using a computerized image analysis program.
Real-time RT-PCR
An RNA Extraction kit (Takara, Tokyo, Japan) was used according to the manufacture’s instruction. The NanoDrop spectrophotometer (Thermo Fisher Scientific, MA, USA) was used to quantifying the extracted RNA. Subsequently, the complementary DNA was reverse-transcribed by a specific RT primer. In the present study, SYBR Premix Ex TaqTM II (Takara, Tokyo, Japan) was used to perform the amplification reaction. The expression level of Arg7, PI3K and RunX2 was determined by the threshold cycle (Ct) and the relative expression levels were determined by 2-ΔΔCt method. GADPH in the tissues was used as a negative control. Three independent assays were performed. The sequence of the primers was shown in Table 1.
Table 1.
The sequences of primers for Atg7, PI3K and caspase-3 and GAPDH
| primer name | primer sequence (5’-3’) |
|---|---|
| Atg7 forward | CAGTCCGTTGAAGTCCTC |
| Atg7 reverse | TCAGTGTCCTAGCCACATTAC |
| PI3K forward | CATCACTTCCTCCTGCTCTAT |
| PI3K reverse | CAGTTGTTGGCAATCTTCTTC |
| caspase-3 forward | GGTATTGAGACAGACAGTGG |
| caspase-3 reverse | CATGGGATCTGTTTCTTTGC |
| GAPDH forward | CAATGACCCCTTCATTGACC |
| GAPDH reverse | GAGAAGCTTCCCGTTCTCAG |
Hematoxylin staining
Cartilage tissue of each animal was collected and washed over by sterile water for a couple of hours. The tissue was dehydrated by 70%, 80% and 90% ethanol solution successively and mixed with equal quality of ethanol and xylene. After 15 min incubation, the tissue was mixed with equal quality of xylene for 15 min. Repeat the step until the tissue looked transparent. Subsequently, the tissue was embedded in paraffin, sectioned, stained with hematoxylin and eosin. Pictures were taken by inverted microscope (Olympus).
Statistical analysis
The results were presented as mean ± SD for experiments with triplicate measurements. Multiple samples were analyzed by one-way analysis of variance (ANOVA), followed by pairwise comparisons using the t-test. All differences with P-value < 0.05 were considered statistically significant.
Ethic statements
We declare that all animal experiments involved in this manuscript were authorized by the ethical committee of Xiaoshan Hospital of Traditional Chinese Medicine and carried out according to the guidelines for care and use of laboratory animals and as well as to the principles of laboratory animal care and protection.
Results
OA was successfully established on rats and isolated chondrocytes were verified
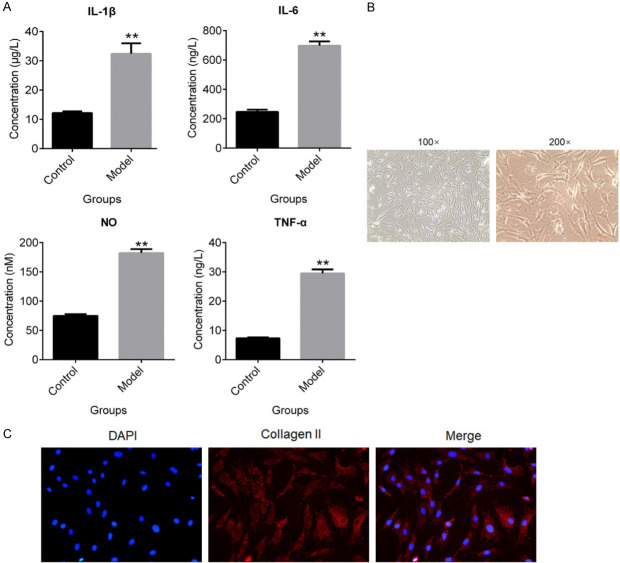
To verify that whether OA model was successfully established on rats, the concentration of inflammation related factors in the cartilage tissues was evaluated by ELISA. As shown in Figure 1A, IL-1β, IL-6, NO and TNF-α were significantly up-reregulated in the cartilage tissues by OA modeling (**P < 0.01, vs. Control). To evaluate the effects of β-ecdysterone on injured chondrocytes, the chondrocytes were isolated from OA rats. The cellular morphology of chondrocytes was shown in Figure 1B. The results of immunofluorescence assay showed that collagen II was highly expressed in the isolated chondrocytes, which indicated that the chondrocytes was successfully isolated from OA rats.
Figure 1.
OA was established on rats and the chondrocytes were isolated. A. The concentration of IL-1β, IL-6, NO and TNF-α was evaluated by ELISA (**P < 0.01, vs. Control). B. The morphology of chondrocytes was pictured by microscope. C. The expression level of collagen II was determined by immunofluorescence assay.
Apoptosis of chondrocytes was significantly inhibited by β-ecdysterone
To exclude the impact of the cytotoxicity effects of drugs on chondrocytes, different concentrations of rapamycin, 3-methyladenine and β-ecdysterone were used to incubate with isolated chondrocytes, respectively, and MTT assay was used to evaluate the survival rate of cells. As shown in Figure 2, when the concentration of rapamycin, 3-methyladenine and β-ecdysterone exceeded 100 nM, 10 mM and 40 μM, respectively, the survival rate of chondrocytes decreased greatly. In this way, the maximal concentration of rapamycin, 3-methyladenine and β-ecdysterone for the incubation with chondrocytes were settled as 100 nM, 10 mM and 40 μM, respectively. To evaluate the effects of β-ecdysterone on the apoptosis of OA chondrocytes, the AO/PI staining and flow cytometry assay were performed. As shown in Figure 3A, higher apoptotic rate was observed in chondrocytes treated with 10 mM 3-methyladenine, compared with control (*P < 0.05, vs. Control). The apoptotic rate decreased greatly in chondrocytes after incubating with 100 nM rapamycin, 20 μM and 40 μM β-ecdysterone, respectively (*P < 0.05, vs. Control, **P < 0.01, vs. Control). Figure 3B showed the results of flow cytometry. The apoptotic rate of chondrocytes in control, 10 mM 3-methyladenine, 100 nM rapamycin, 10 μM, 20 μM and 40 μM β-ecdysterone groups was 30.39%, 34.9%, 12.95%, 19.91%, 15.63% and 6.4%, respectively. These data indicated that the apoptosis of OA chondrocytes was significantly suppressed by β-ecdysterone in a dose dependent manner. As shown in Figure 3C, 3D, the expression level of caspase-3 was inhibited greatly by rapamycin and β-ecdysterone and elevated by 3-methyladenine (*P < 0.05, vs. Control, **P < 0.01, vs. Control).
Figure 2.
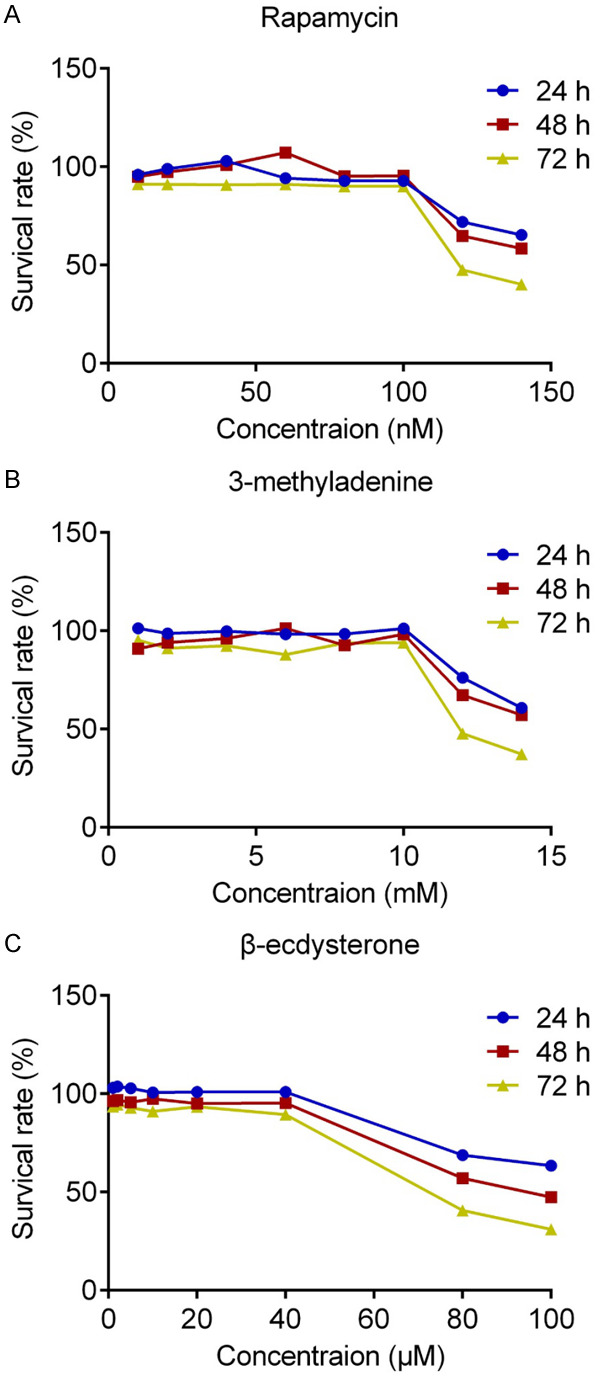
The tolerable concentration of rapamycin (A), 3-methyladenine (B) and β-ecdysterone (C) against chondrocytes was evaluated by MTT assay.
Figure 3.
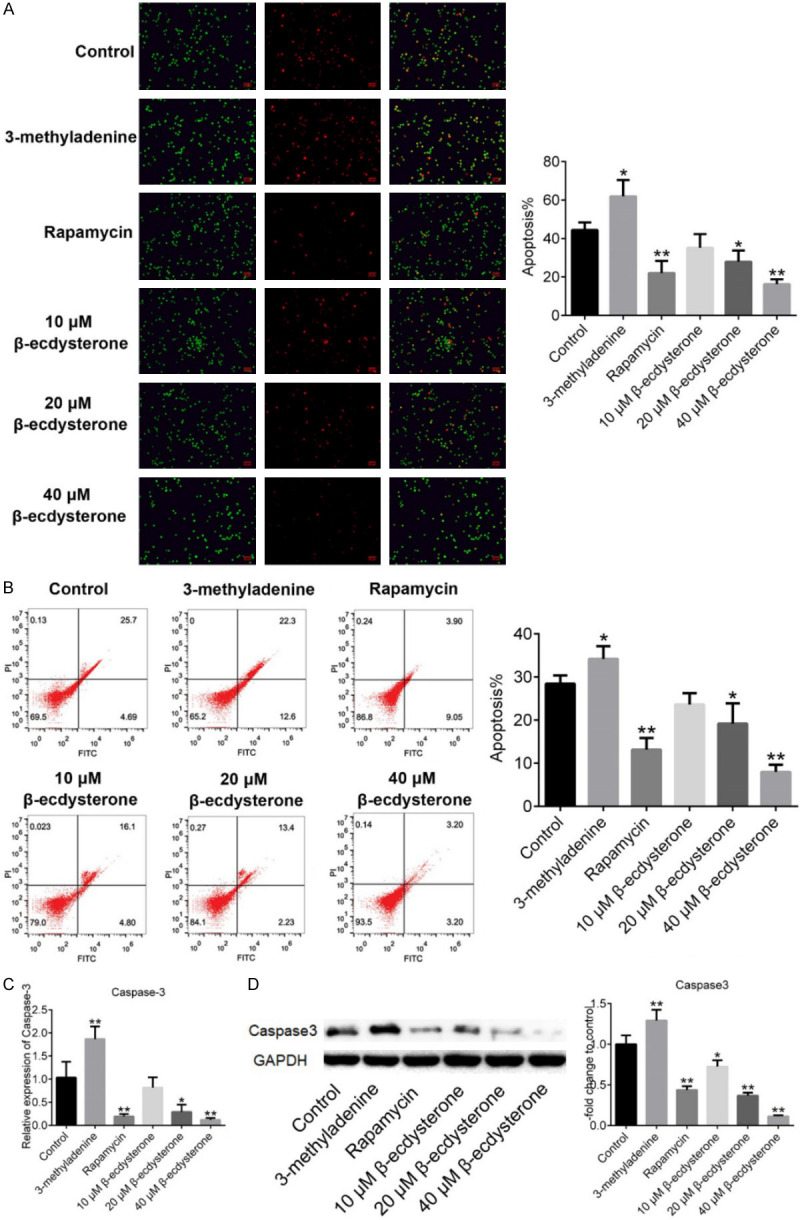
The apoptosis of chondrocytes threated with 10 mM 3-methyladenine, 100 nM rapamycin, 10 μM, 20 μM and 40 μM β-ecdysterone was determined by AO/PI staining (A) and flow cytometry assay (B). The gene expression level (C) and protein expression level (D) were evaluated by qRT-PCR and Western Blot, respectively. (*P < 0.05, vs. Control, **P < 0.01, vs. Control).
Autophagy in OA chondrocytes were activated by β-ecdysterone
As shown in Figure 4A, the results of qRT-PCR showed that, the expression level of Arg7, a autophagy related protein, was inhibited greatly by 100 nM rapamycin, 20 μM and 40 μM β-ecdysterone and promoted by 10 mM 3-methyladenine (*P < 0.05, vs. Control, **P < 0.01, vs. Control). The Western Blot results (Figure 4B) shows that the expression ratio of LC3II/LC3I was promoted greatly by rapamycin and β-ecdysterone and suppressed by 3-methyladenine.
Figure 4.
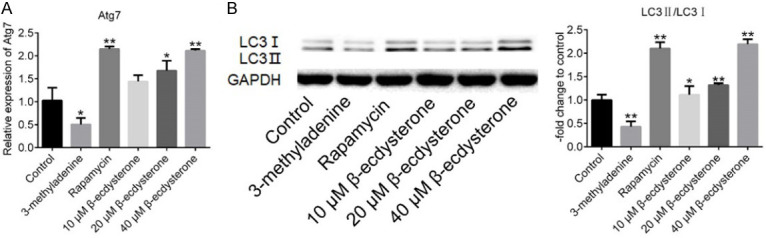
The autophagy state of chondrocytes threated with 10 mM 3-methyladenine, 100 nM rapamycin, 10 μM, 20 μM and 40 μM β-ecdysterone, respectively. A. The expression level in chondrocytes of Atg7 was evaluated by qRT-PCR. B. The expression level in chondrocytes of LC3I, LC3II, and GAPDH was determined by Western Blot (*P < 0.05, vs. Control, **P < 0.01, vs. Control).
PI3K/AKT/mTOR signal pathway OA chondrocytes were inhibited by β-ecdysterone
As shown in Figure 5A, the results of qRT-PCR showed that, PI3K was significantly down-regulated by 100 nM rapamycin, 20 μM and 40 μM β-ecdysterone and up-regulated by 10 mM 3-methyladenine (*P < 0.05, vs. Control, **P < 0.01, vs. Control). The Western Blot results were shown in Figure 5B. PI3K, p-AKT1, p-mTOR and p-p70S6K were significantly down-regulated by rapamycin and β-ecdysterone and up-regulated by 3-methyladenine, with the expression level of AKT1, mTOR and p70S6K unchanged (*P < 0.05, vs. Control, **P < 0.01, vs. Control).
Figure 5.
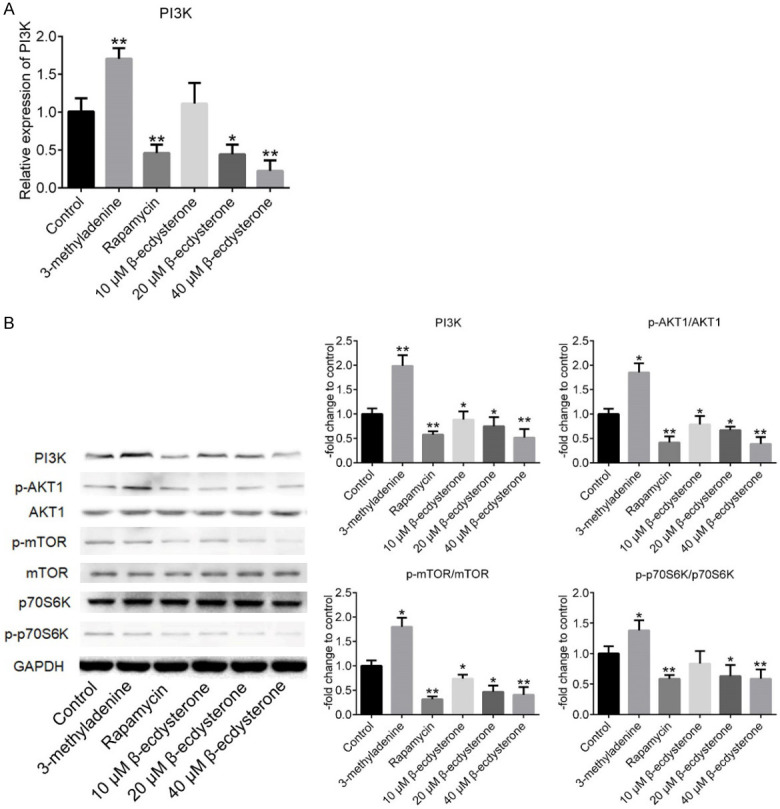
The effects of β-ecdysterone on the PI3K/AKT signal pathway in the chondrocytes. A. The expression level in chondrocytes of PI3K was evaluated by qRT-PCR. B. The expression level in chondrocytes of PI3K, p-AKT1, AKT1, p-mTOR, mTOR, p70S6K, and GAPDH was determined by Western Blot (*P < 0.05, vs. Control, **P < 0.01, vs. Control).
OA was alleviated by β-ecdysterone on rats
To evaluate the therapeutic effects of β-ecdysterone on OA rats, HE staining was performed on the cartilage tissues to determine the pathological state and the concentration of inflammation related factors in the cartilage tissues were evaluated by ELISA. As shown in Figure 6A, significant cartilage degeneration, with proteoglycan depletion, loss of surface lamina and fibrillations were observed on OA model rats and OA rats treated with 3-methyladenine. These OA-like symptoms were greatly improved in OA rats treated with rapamycin and β-ecdysterone, especially with 100 nM rapamycin and 40 μM β-ecdysterone. As shown in Figure 6B, the concentration of IL-1β, IL-6, NO and TNF-α were found to be significantly decreased in rapamycin and β-ecdysterone treated rats, compared with control. On the contrary, IL-1β, IL-6, NO and TNF-α were excessively secreted in the cartilage tissue in OA rats treated with 3-methyladenine (*P < 0.05, vs. Control, **P < 0.01, vs. Control). These data indicated that the pathological and inflammatory states of OA rats were remarkably improved by β-ecdysterone in a dose dependent manner.
Figure 6.
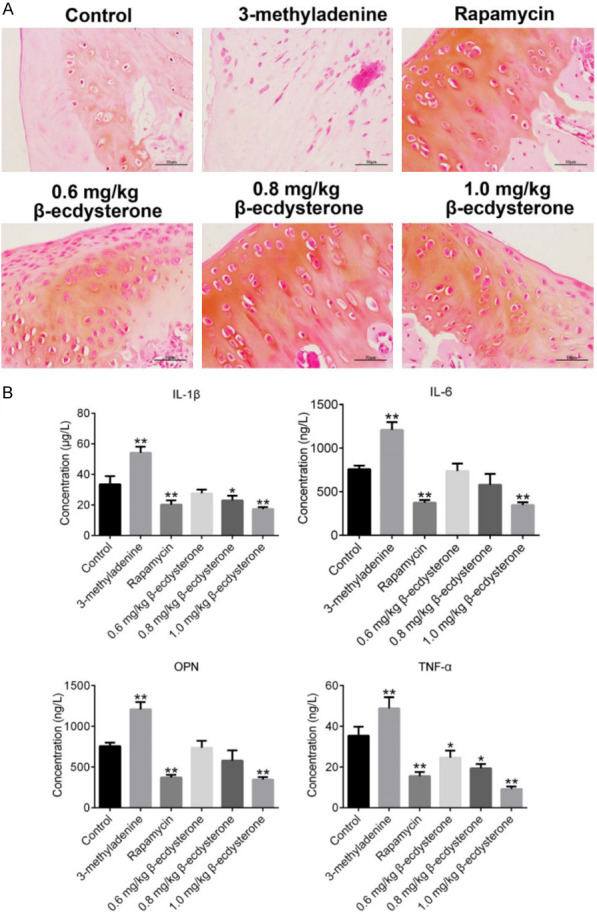
OA-like symptom was alleviated by β-ecdysterone. A. The pathological of cartilage tissues was evaluated by HE staining. B. The expression level of IL-1β, IL-6, NO and TNF-α in the cartilage tissues was determined by ELISA (*P < 0.05, vs. Control, **P < 0.01, vs. Control).
Autophagy was activated and apoptosis was inhibited by β-ecdysterone in OA rats
To verify the effects of β-ecdysterone on autophagy and apoptosis, Western Blots assay was performed on the cartilage tissues. As shown in Figure 7, the expression ratio of LC3II/LC3I was promoted greatly by rapamycin and β-ecdysterone and suppressed by 3-methyladenine. On the contrary, the expression level of caspase-3 was inhibited greatly by rapamycin and β-ecdysterone and elevated by 3-methyladenine (*P < 0.05, vs. Control, **P < 0.01, vs. Control).
Figure 7.
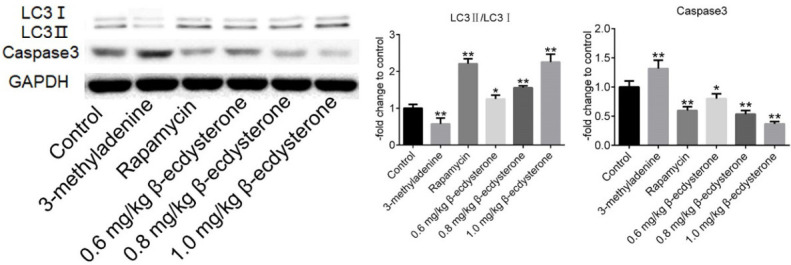
The expression level in cartilage tissues of LC3I, LC3II, caspase-3 and GAPDH was determined by Western Blot (*P < 0.05, vs. Control, **P < 0.01, vs. Control).
The PI3K/AKT/mTOR signal pathway was inhibited by β-ecdysterone in OA rats
As shown in Figure 8, PI3K, p-AKT1, p-mTOR and p-p70S6K were significantly down-regulated by rapamycin and β-ecdysterone and up-regulated by 3-methyladenine, with the expression level of AKT1, mTOR and p70S6K unchanged (*P < 0.05, vs. Control, **P < 0.01, vs. Control).
Figure 8.
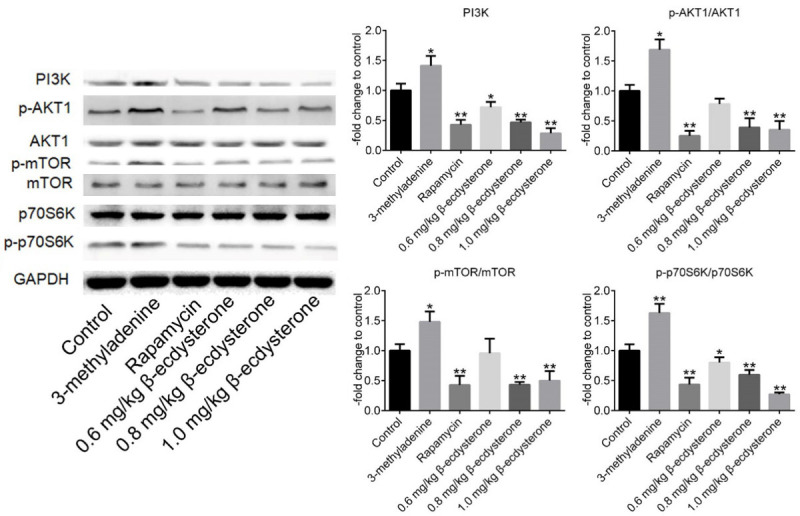
The expression level in cartilage tissues of PI3K, p-AKT1, AKT1, p-mTOR, mTOR, p70S6K, p-p70S6K, and GAPDH was determined by Western Blot (*P < 0.05, vs. Control, **P < 0.01, vs. Control).
Discussion
OA is common aging skeletal muscle disease mainly caused by joint dysfunction, which is one of the global factors resulting in disability [28]. Articular cartilage degeneration is regarded as the main inducement for OA and currently this pathological state could not be reversed by any drug. Excessive apoptosis of chondrocytes is reported to be an important factor that induce articular cartilage injury and degeneration, accompanied with overflow of inflammatory factors, which in turns to aggravate the severity of injury [29,30]. In the present study, the rats were injected with MIA to establish the OA model, which was reported to be the classic way to stimulate the symptom of OA in rats [31,32]. The concentration of inflammatory factors, which were represented with IL-1β, IL-6, NO and TNF-α, in the cartilage tissues of the rats were determined to verify the successful establishment of OA model. And prominent inflammation was observed on OA rats. Chondrocytes are the amin components of cartilage tissues and the pathological state of chondrocytes are regarded as the representative of OA [33]. In the present study, the chondrocytes were isolated from OA rats and verified by the immunofluorescence results of collagen II, which was reported to be mainly produced by chondrocytes and involved in such tissues as bones, joint and tendons [34].
Autophagy is a physiological process functioned as maintaining the cellular homeostasis, which is dependent on the degradation effects of lysosome on dysfunctional organelles and biomacromolecules under the regulation of autophagy related genes (ATG) [35]. By response to the cellular stress, autophagy functioned as the protective mechanism for apoptosis. For example, the essential cellular function can be maintained by the ATP derived from the intracellular components degraded by autophagy, which protects the cells from injury by decreasing the unnecessary or dysfunctional components [36]. It is reported that autophagy is involved in the function of maintaining endochondral balance. The autophagy regulators were observed to be down-regulated in the articular cartilage isolated from OA animals or human beings, accompanied by the aggravation of chondrocytes apoptosis [17,37]. In the present study, to claim the effects of β-ecdysterone on autophagy, rapamycin, an autophagy activator, and 3-methyladenine, and autophagy inhibitor, were taken as positive control and negative control, respectively. Chondrocytes can be injured by high dosage of chemistry materials, by which the effects of chemistry materials on chondrocytes will be impacted. The cytotoxic effects of rapamycin, 3-methyladenine and β-ecdysterone on chondrocytes were evaluated by MTT assays after incubating for 24, 48 and 72 hours. We found that chondrocytes were tolerable to rapamycin, 3-methyladenine and β-ecdysterone at the concentration of 10 mM, 100 nM and 40 μM, respectively. Apoptosis was significantly suppressed in chondrocytes by the introduction of rapamycin and β-ecdysterone, and was promoted by incubating with 3-methyladenine based on the results of AO/PI staining and flow cytometry. These data indicated that the declined apoptosis in chondrocytes could be induced by activating the autophagy and β-ecdysterone exerted the same effects as rapamycin, which was consistent with the opinion proposed by Yu that novel therapeutic methods against OA could be achieved by activating autophagy to suppress apoptosis [38]. By determining the expression level of Atg7 and LC3II/LC3I, we found that promising autophagy activation effects were observed on β-ecdysterone, especially on the high dosage, indicating that the anti-apoptotic effects of β-ecdysterone might be related with its autophagy activation effects. Further investigations were explored on the therapeutic effects of rapamycin, 3-methyladenine and β-ecdysterone against OA rats. We found that the pathological state of cartilage tissues was significantly improved by rapamycin and β-ecdysterone, and was aggravated by the treatment of 3-methyladenine. The inflammation in the cartilage tissues was suppressed by rapamycin and β-ecdysterone, and was activated by 3-methyladenine. These data indicated that OA-like symptom has been greatly alleviated by β-ecdysterone and it was the activation of autophagy induced by β-ecdysterone that contributed to the therapeutic effects, which was proved by the up-regulation of LC3II/LC3I in the cartilage tissues.
Rapamycin is a specific mammalian target of rapamycin (mTOR) inhibitor and mTOR is regarded as an important autophagy regulator. mTOR complex 1 (mTORC1), which is mediated by growth factors, energy level and nutrition state, regulates the process of autophagy by PI3K/AKT/mTOR signal pathway. ATG13 is highly phosphorylated by mTORC1 under nutritional sufficiency and the affinity between high phosphorylated ATG13 and ATG1 kinase is decreased, which contributes to the decline of the activity of ATG1 kinase and finally inhibits the process of autophagy. On the contrary, ATG13 is dephosphorylated as the function of mTORC1 is suppressed under nutrient scarcity and the ATG1 kinase is closely bound with ATG13, which promotes the activity of ATG1 kinase and finally in-duces the process of autophagy [29]. In the present study, similar the effects of rapamycin, the PI3K/AKT/mTOR signal pathway was significantly inhibited by β-ecdysterone in a dose dependent manner, which indicated that the activation effects of β-ecdysterone on autophagy process might be related to the inhibition of PI3K/AKT/mTOR signal pathway. However, the inhibition of PI3K/AKT/mTOR signal pathway was accompanied with the down-regulation of caspase-3 and suppress of apoptosis. We suspected that the anti-apoptotic effect brought by autophagy activation was more significant than the pro-apoptotic effect brought by the inhibition of PI3K/AKT/mTOR signal pathway, which deserved to be further investigated for verification in the future work.
Taken together, our data indicated that β-ecdysterone might alleviate OA-like symptom by inducing autophagy in chondrocytes through regulating PI3K/AKT/mTOR signal pathway.
Acknowledgements
This work was supported by grants from the Hangzhou Xiaoshan District major Science and Technology project (Grant No. 2018211) and the Hangzhou Science and Technology Planning Guide Project (Grant No. 20171226Y97).
Disclosure of conflict of interest
None.
References
- 1.Kraus VB, Karsdal MA. Osteoarthritis: current molecular biomarkers and the way forward. Calcif Tissue Int. 2020 doi: 10.1007/s00223-020-00701-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Pearson SH. Proactive wellness care for patients with osteoarthritis. Nurs Clin North Am. 2020;55:133–147. doi: 10.1016/j.cnur.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Guerrero EM, Bullock GS, Helmkamp JK, Madrid A, Ledbetter L, Richard MJ, Garrigues GE. The clinical impact of arthroscopic vs. open osteocapsular debridement for primary osteoarthritis of the elbow: a systematic review. J Shoulder Elbow Surg. 2020;29:689–698. doi: 10.1016/j.jse.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Billesberger LM, Fisher KM, Qadri YJ, Boortz-Marx RL. Procedural treatments for knee osteoarthritis: a review of current injectable therapies. Pain Res Manag. 2020;2020:3873098. doi: 10.1155/2020/3873098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsueh MF, Bolognesi MP, Wellman SS, Kraus VB. Anti-inflammatory effects of naproxen sodium on human osteoarthritis synovial fluid immune cells. Osteoarthritis Cartilage. 2020;28:639–645. doi: 10.1016/j.joca.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rausch-Derra L, Huebner M, Wofford J, Rhodes L. A prospective, randomized, masked, placebo-controlled multisite clinical study of grapiprant, an ep4 prostaglandin receptor antagonist (PRA), in dogs with osteoarthritis. J Vet Intern Med. 2016;30:756–763. doi: 10.1111/jvim.13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park MH, Jung JC, Hill S, Cartwright E, Dohnalek MH, Yu M, Jun HJ, Han SB, Hong JT, Son DJ. FlexPro MD(R), a combination of krill oil, astaxanthin and hyaluronic acid, reduces pain behavior and inhibits inflammatory response in monosodium iodoacetate-induced osteoarthritis in rats. Nutrients. 2020;12:956. doi: 10.3390/nu12040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steen Pettersen P, Neogi T, Magnusson K, Berner Hammer H, Uhlig T, Kvien TK, Haugen IK. Peripheral and central sensitization of pain in individuals with hand osteoarthritis and associations with self-reported pain severity. Arthritis Rheumatol. 2019;71:1070–1077. doi: 10.1002/art.40850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa S, Natsume T, Takamatsu H. Pharmacological profile of a novel nonhuman primate model of knee osteoarthritis. Nihon Yakurigaku Zasshi. 2018;152:132–138. doi: 10.1254/fpj.152.132. [DOI] [PubMed] [Google Scholar]
- 10.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, Lee G, Rhee J, Ryu JH, Chun CH, Chun JS. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156:730–743. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 12.McCulloch K, Litherland GJ, Rai TS. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017;16:210–218. doi: 10.1111/acel.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9:247. doi: 10.1186/s13287-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zbyn S, Mlynarik V, Juras V, Szomolanyi P, Trattnig S. Evaluation of cartilage repair and osteoarthritis with sodium MRI. NMR Biomed. 2016;29:206–215. doi: 10.1002/nbm.3280. [DOI] [PubMed] [Google Scholar]
- 15.Bai Y, Chen K, Zhan J, Wu M. miR-122/SIRT1 axis regulates chondrocyte extracellular matrix degradation in osteoarthritis. Biosci Rep. 2020;40:BSR20191908. doi: 10.1042/BSR20191908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochberg MC, Guermazi A, Guehring H, Aydemir A, Wax S, Fleuranceau-Morel P, Reinstrup Bihlet A, Byrjalsen I, Ragnar Andersen J, Eckstein F. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the forward randomized clinical trial. JAMA. 2019;322:1360–1370. doi: 10.1001/jama.2019.14735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carames B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HA, Blanco FJ. Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets. 2007;8:333–345. doi: 10.2174/138945007779940025. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi N, Carames B, Ronfani L, Ulmer U, Komiya S, Bianchi ME, Lotz M. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106:1181–1186. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson EO, Charchandi A, Babis GC, Soucacos PN. Apoptosis in osteoarthritis: morphology, mechanisms, and potential means for therapeutic intervention. J Surg Orthop Adv. 2008;17:147–152. [PubMed] [Google Scholar]
- 21.Portal-Nunez S, Esbrit P, Alcaraz MJ, Largo R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem Pharmacol. 2016;108:1–10. doi: 10.1016/j.bcp.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Barranco C. Osteoarthritis: activate autophagy to prevent cartilage degeneration? Nat Rev Rheumatol. 2015;11:127. doi: 10.1038/nrrheum.2015.12. [DOI] [PubMed] [Google Scholar]
- 23.Huang X, Ni B, Xi Y, Chu X, Zhang R, You H. Protease-activated receptor 2 (PAR-2) antagonist AZ3451 as a novel therapeutic agent for osteoarthritis. Aging (Albany NY) 2019;11:12532–12545. doi: 10.18632/aging.102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160–167. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou F, Roos EM, Underwood M. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Xu T, Niu C, Zhang X, Dong M. beta-Ecdysterone protects SH-SY5Y cells against beta-amyloid-induced apoptosis via c-Jun N-terminal kinase- and Akt-associated complementary pathways. Lab Invest. 2018;98:489–499. doi: 10.1038/s41374-017-0009-0. [DOI] [PubMed] [Google Scholar]
- 27.Tang YH, Yue ZS, Li GS, Zeng LR, Xin DW, Hu ZQ, Xu CD. Effect of betaecdysterone on glucocorticoidinduced apoptosis and autophagy in osteoblasts. Mol Med Rep. 2018;17:158–164. doi: 10.3892/mmr.2017.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiligsmann M, Cooper C, Arden N, Boers M, Branco JC, Luisa Brandi M, Bruyere O, Guillemin F, Hochberg MC, Hunter DJ, Kanis JA, Kvien TK, Laslop A, Pelletier JP, Pinto D, Reiter-Niesert S, Rizzoli R, Rovati LC, Severens JL, Silverman S, Tsouderos Y, Tugwell P, Reginster JY. Health economics in the field of osteoarthritis: an expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Semin Arthritis Rheum. 2013;43:303–313. doi: 10.1016/j.semarthrit.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 31.Molinet M, Alves N, Vasconcelos A, Deana NF. Comparative study of osteoarthritis (OA) induced by monoiodoacetate (MIA) and papain in rabbit temporomandibular joints: macroscopic and microscopic analysis. Folia Morphol (Warsz) 2019;79:516–527. doi: 10.5603/FM.a2019.0104. [DOI] [PubMed] [Google Scholar]
- 32.Kim JE, Song DH, Kim SH, Jung Y, Kim SJ. Development and characterization of various osteoarthritis models for tissue engineering. PLoS One. 2018;13:e0194288. doi: 10.1371/journal.pone.0194288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh P, Marcu KB, Goldring MB, Otero M. Phenotypic instability of chondrocytes in osteoarthritis: on a path to hypertrophy. Ann N Y Acad Sci. 2019;1442:17–34. doi: 10.1111/nyas.13930. [DOI] [PubMed] [Google Scholar]
- 34.Hu N, Gong X, Yin S, Li Q, Chen H, Li Y, Li F, Qing L, Yang J, Zhu S, Wang J, Li J. Saxagliptin suppresses degradation of type II collagen and aggrecan in primary human chondrocytes: a therapeutic implication in osteoarthritis. Artif Cells Nanomed Biotechnol. 2019;47:3239–3245. doi: 10.1080/21691401.2019.1647223. [DOI] [PubMed] [Google Scholar]
- 35.Villarejo-Zori B, Boya P. Autophagy and vision. Med Sci (Paris) 2017;33:297–304. doi: 10.1051/medsci/20173303017. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Hou R, Zou Z, Luo T, Zhang Y, Wang L, Wang B. Mechanically induced autophagy is associated with ATP metabolism and cellular viability in osteocytes in vitro. Redox Biol. 2018;14:492–498. doi: 10.1016/j.redox.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinatier C, Dominguez E, Guicheux J, Carames B. Role of the inflammation-autophagy-senescence integrative network in osteoarthritis. Front Physiol. 2018;9:706. doi: 10.3389/fphys.2018.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YS, Zhang FJ, Zeng C, Luo W, Xiao WF, Gao SG, Lei GH. Autophagy in osteoarthritis. Joint Bone Spine. 2016;83:143–148. doi: 10.1016/j.jbspin.2015.06.009. [DOI] [PubMed] [Google Scholar]