Abstract
Hyperbilirubinemia accounts for about 60% of full-term and 80% of preterm neonates globally, which is characterized by physiologically elevated unconjugated bilirubin in serum, but abnormally high levels of bilirubin have potential neurotoxic effects. Several factors contribute to the development of neonatal hyperbilirubinemia, including isoimmunization, dysregulated gut flora, genetic alteration and environmental factors. Animal studies have pinpointed the causal roles of several bacteria in bilirubin metabolism. Human studies have revealed microbiota composition in hyperbilirubinemia and found that gut microbiota affect newborns with different severity of hyperbilirubinemia. However, dysbiosis and subsequent changes in microbiota-related metabolic processes are not always considered. This review aims to describe the critical microbiota signatures for neonatal hyperbilirubinemia and focus on the underlying pathogenetic mechanism. These scientific bases give a new and accurate therapeutic strategy for the application of gut microbiota.
Keywords: Gut microbiota, neonatal hyperbilirubinemia, bilirubin metabolism, bilirubin encephalopathy, probiotics, prebiotics
Introduction
Neonatal jaundice is a common condition currently affecting up to 60% of term and 80% of preterm babies and carrying a high health burden [1]. Globally, the risk of severe neonatal hyperbilirubinemia decreases with the increase of useful tools for screening, diagnosis and treatment of neonatal hyperbilirubinemia [2]. However, hazardous neonatal hyperbilirubinemia is still reported worldwide, and the situation in some developing countries is even worse. In these areas, the incidence of severe neonatal jaundice is about 100 times higher than that in developed countries [3,4]. Neonatal jaundice is defined as an elevated serum bilirubin level, which usually develops in the first week of life. Neonatal jaundice is generally benign and physiologic within 3-5 days without significant complications. Severe hyperbilirubinemia, although rare, has potentially neurotoxic [5-7]. The etiology of neonatal hyperbilirubinemia is multifactorial with physiology, isoimmunization, genetic alteration and environmental factors [8]. Among the environmental factors, the dysbiosis of the intestinal microbiota is increasingly considered to be one of the pathogenic factors for neonatal hyperbilirubinemia [9-15]. However, the underlying mechanism of the link between gut microbiota and hyperbilirubinemia remains unclear.
Animal and human studies have supported the interaction between microbiota and hyperbilirubinemia, including the metabolic pathway in the gut-liver axis, immune changes and effects on the therapy of jaundice [16]. In view of the important role of microflora in the pathogenesis of neonatal jaundice, it provides a broad prospect for the diagnosis and treatment of neonatal hyperbilirubinemia. Microbiota-induced effects of neonatal bilirubin are not fully understood, leaving a key gap in understanding how they influence serum bilirubin in the neonate and how the gut balances intimately contact with the host. In this review, we aim to address the question, summarise the changes of microbiota in neonate hyperbilirubinemia, describe the pathophysiological basis of these associations and identify future targets for microbial therapy.
Intestinal microbiota community structure in newborns
The gut microbiota (that is, intestinal symbiotic microorganisms) is regarded as an “organ” of the human body, which plays an important role in pathogen protection, nutrition, metabolism and immune maturation of newborns [17,18]. Understanding the first bacteria seeded in early life is essential to understand the formation of gut microbiota and the relationship between them. Prior studies have demonstrated that the uterus is considered sterile and that the initial microbiota is involved in the neonatal gut during delivery [19]. On the contrary, a multitude of evidence recently quests the dogma of gut microbiota acquisition [20,21]. These studies suggest that the low abundance bacteria genomic DNA is isolated from meconium [21,22], placenta [21,23], fetal membranes [21], amniotic fluid [24], umbilical cord samples [25], and amniotic fluid samples [21]. However, these researches employing more sensitive molecular techniques lack conclusive evidence that live, persistent, functional and cultured microbial colonies co-exist with the fetus during the fetus [26]. Regardless, intestinal microbes colonize rapidly from the maternal and environmental microflora within minutes of birth. In the first few days, facultative anaerobes are mostly in aerobic environments, such as Enterobacteriaceae [27]. After intestinal hypoxia, dominant colonization is transferred to anaerobic bacteria, such as Bifidobacterium, Clostridium, and Bacteroides [28]. In the first four weeks, the diversity of gut microbiota gradually increased at the phylum and genus level, forming a bacterial community composed of Enterococcaceae, Streptococcaceae, Lactobacillaceae, Clostridiaceae, and Bifidobacteriaceae [27,29,30].
The gut, a dynamic environment of microbiota, is influenced by various endogenous and exogenous factors [19,31,32]. The mode of delivery is considered to be an essential determinant of early colonization [33]. Infants born vaginally are rich in Lactobacillus, Bifidobacterium and Bacteroides, which is caused by microbial inoculation in the vagina and fecal microbiota [34]. In contrast, the microbiota of cesarean-born newborns is more likely to be settled by Staphylococcus, Streptococcus and Propionibacteria, especially from maternal skin and environmental microorganisms [35]. Postnatal factors also affect the development of microbiota in multiple ways, including diet, antibiotics and other environmental factors. Bifidobacterium was found in breast-fed infants because of bacteria engaged in breast milk and human milk oligosaccharides (HMOs) [36]. The microbiota of formula-feeding is characterized by low levels of less beneficial bacteria species, such as Clostridium diciffile [36]. Antibiotics, used prescription drugs in newborns, can lead to microbial imbalance and low abundance of bacterial diversity, and delay the colonization of the Bacteroidetes [37].
Microbiota and neonatal hyperbilirubinemia
Many studies on faecal microflora of neonatal hyperbilirubinemia, including different types of neonatal jaundice, are carried out by means of culture and metagenome. In this part, we will elluminate the metabolism of bilirubin from the perspective of microbiota, summarize several animal and clinical studies and find out the mixed mechanisms of hyperbilirubinemia and microbiota.
Gut microbiota and bilirubin metabolism in the neonate
Neonates produce more bilirubin than adults because of high hemoglobin levels and short erythrocyte lifespan [38]. Approximately 80-85% of bilirubin derives from aging senescent and hemolytic erythrocytes in the spleen, liver, or bone marrow and a relatively small portion comes from circulation [39]. Before hepatocellular uptake, this pigment is called unconjugated bilirubin (UCB), a yellow non-polar pigment that is transported to the liver through albumin. UCB entering into hepatocytes through organic anion transporter (OATP) is synthesized into bilirubin-glucuronoside with solubility by the UGT 1A1 (UDP-glucuronosyltransferase enzyme) in the endoplasmic reticulum [40]. In the neonate, the activity of enzyme UGT 1A1 in hepatic is significantly lower than that of adults [38]. Then, conjugated bilirubin is released into the intestine lumen by multidrug-resistant protein (MRP2) for r clearance and transferred back into the bloodstream by MRP3 [39,41]. Following secretion, water-soluble conjugated bilirubin is hydrolyzed into UCB by rich β-glucuronidase at the brush margin of the newborn intestine. Subsequently, intestinal microbiota reduced it to a series of colorless urobilinogen mainly in distal intestine ileum and colon, where the total bacterial load is higher than that of the small intestine [42,43]. Here, higher activity of mucosal β-glucuronidase and lower concentration of gut flora lead to more effective deconjugated responses in the neonate. The non-polar unconjugated bilirubin is reabsorbed via passive diffusion, mainly at colonic level through the portal vein. Furthermore, high levels of serum unconjugated bilirubin increased the permeability of epithelium and reduced the expression of epithelial tight junction protein, which may increase the outflow of bilirubin to the portal vein [38]. The reabsorbed bilirubin is conjugated in the liver, subsequently re-excreted into either bile flow or urine through the kidneys [39]. The whole process constitutes a cycle of the enterohepatic circulation (Figure 1A). However, to date, we know little about the neonatal bilirubin metabolic process in the context of microflora.
Figure 1.
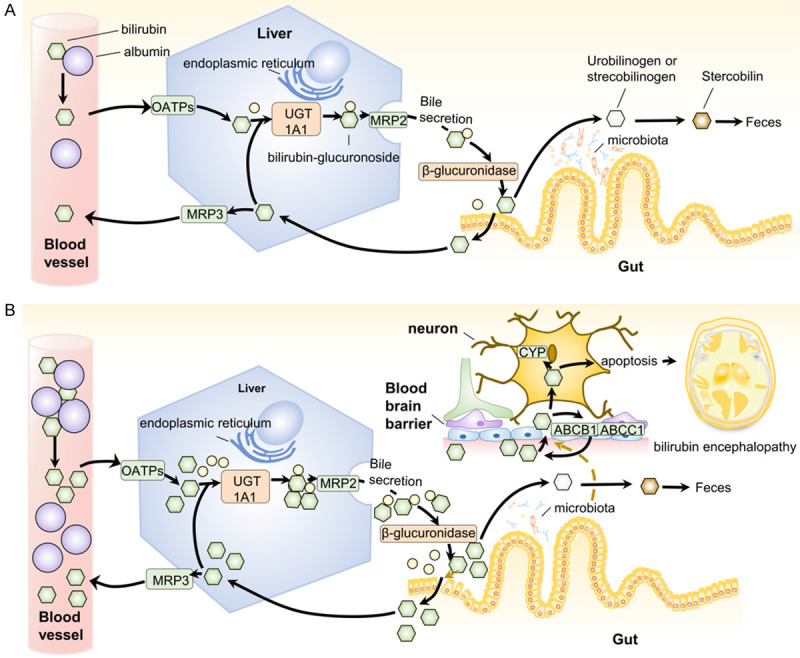
A. Enterohepatic circulation of bilirubin. B. The relationship between microbiota and bilirubin metabolism in neonatal hyperbilirubinemia. A. Enterohepatic circulation of bilirubin. Unconjugated bilirubin mainly derived from heme binds to albumin in the blood, which is transfered to hepatocyte through OATPs and separateed from albumin. Bilirubin-glucuronide is synthesized by UGT1A1 on the endoplasmic reticulum and excreted into the intestinal tract with bile through MRP2. The secreted conjugated bilirubin is deconjugated by β-glucuronidase at the brush edge of the intestinal tract, partly decomposed into urinary bilirubin or stylcholin through the microflora, and then oxidized into stylcholin by feces. Extra bilirubin is reabsorbed to the liver through the portal vein and then transferred to the bloodstream by MRP3 or re-secreted into bile by MRP2. B. The relationship between microbiota and the metabolism process of bilirubin in neonatal hyperbilirubinemia. The production of bilirubin in neonatal circulation increases, followed by an increase in the catabolism and secretion of bilirubin in hepatocytes. Newborns lack microbiota, and their feces contain more bilirubin in the intestines, in which the bilirubin is more easily reabsorbed. The microflora that synthesizes fewer short fat chains in newborns has a low level, which will affect the permeability of the blood-brain barrier. At the same time, bilirubin can cross the blood-brain barrier, some of which efflux from brain through ABCB1 or ABCB2, whereas the rest enter neurons. In nerve cells, bilirubin is catabolized by cytochrome P-450 (CYP) and bilirubin oxidase, but excessive bilirubin will cause oxidative stress and mitochondrial changes, and eventually cause neuronal apoptosis. This injury in newborns eventually leads to bilirubin encephalopathy.
Clinical and basic studies have shown that bilirubin is an effective endogenous antioxidant factor, which has a cytoprotective effect, regulates lipid peroxidation, cholesterol metabolism, adipokines and ppar γ levels, and participates in inflammation [44,45]. A number of studies have shown that elevated circulating bilirubin levels help slow the progression of chronic liver disease, coronary heart disease, diabetes and obesity in animal models and human experiments [45-48]. Low levels of serum unconjugated bilirubin up-regulate the expression of UDP-glucuronosyltransferases 1 family polypeptide A1 (UGT1A1), thereby increasing bilirubin metabolism in circulation [49]. Furthermore, bilirubin may be used as a means to negatively influence substrate utilization of Streptococcus agalactiae, which is the most common bacteria involved in neonatal early-onset sepsis [50]. Therefore, mild jaundice has a protective effect on newborns and may have antioxidation and antibacterial effects.
Conversely, excessive bilirubin is a nuisance and can even cause neurotoxicity and cytotoxicity to neurons, astrocytes, microglia and oligodendrocytes [51]. High levels of bilirubin in neonates destroy the tight junction of intestinal epithelial cells, followed by an increase in enterohepatic circulation. Unbound circulating bilirubin diffuses into the central nervous system via the blood-brain barrier, resulting in neurologic damage, especially in acidosis, premature, infection, hypoxia, ischemia and so on. Capillary endothelial cells scavenge part of bilirubin through ATP-binding cassette transporter B1 (ABCB1) and ATP-binding cassette transporter C1 (ABCC1) to maintain bilirubin equilibrium [52]. Whereas others bind tightly to myelin-rich membranes of central neurons, enhancing the activity of acid-sensing ion channels (ASICs) (Figure 2) [51], increasing the overload of intracellular calcium ion, upregulating cytochrome P-450 (CYP) [53], causing oxidative stress and mitochondrial changes and eventually leading to autophagy activity or ultimately neuronal apoptosis (Figure 1B) [51,54]. Irreversible neurogenesis causes early cerebral injury, prolonging learning and memory impairment and then damage to complex sensorimotor functional areas, such as the brainstem, cerebellum, vestibular and auditory nuclei, hippocampus [51]. Additionally, neonatal hyperbilirubinemia is associated with childhood type 1 diabetes [55], visual impairment [56], asthma [57] and other mental developmental disorders in childhood [58]. To sum up, whether bilirubin is a curse or boon for neonate depends on different levels of bilirubin, regional specificity and cellar specificity.
Figure 2.
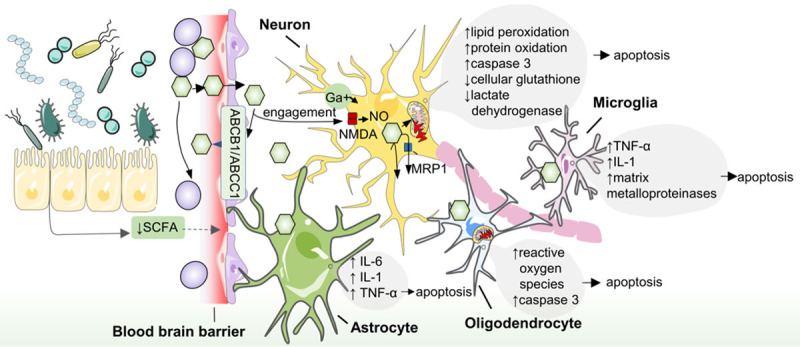
Associations between gut microbiota and bilirubin encephalopathy. The microbiota in gut produce less SCFA which increases the permeability of blood brain barrier, causing unbound bilirubin entry into the central nervous system. The effect of high bilirubin in cerebrospinal fluid is neuronal damage. In neurons, bilirubin causes intracellular calcium increase, lipid peroxidation, protein oxidation, nitric oxide and mitochondrial membrane damage. There are also inflammatory injuries in non-neuronal cells of the central nervous system. In hazardous levels of bilirubin, oligodendrocytes secrete more reactive oxygen species and caspase 3, and cause damage to mitochondrial function. Astrocyte are also susceptible to high levels of bilirubin resulting in an increase in intracellular IL-6, IL-1 and TNF-α. Microglia respond to bilirubin with high level of TNF-α, IL-1 and matrix metalloproteinases. At the same time, MRP1, ABCB1 or ABCC1 on the membrane also have the protective mechanism of clearing unconjugated bilirubin in the brain. Bilirubin oxidase and CYP450 enzyme are metabolic enzymes of intracellular bilirubin. SCFA: short chain fatty acid. ABCB1: ATP-binding cassette transporter B1 (ABCB1). ABCC1: ATP-binding cassette transporter C1. NMDA: N-methyl-D-aspartic acid receptor. MRP1: multidrug resistance-associated protein. IL: interleukin. TNF: tumor necrosis factor.
Cross talk between microbiota and bilirubin
Regulation of bilirubin by intestinal microbiota
An increasing large body of studies have demonstrated that gut microbiota regulates bilirubin metabolism in enterohepatic circulation through dihydroxylation and dehydrogenation. Early evidence from germ-free animals elucidates that gut microbiota was a necessary condition for bilirubin catabolism in the gut as early as the 1960s. It was found that the average fecal urobilin production of the germ-free rodents is 0 mmol, while that of conventional rats was 3.4 mmol [59]. In a research, when rats were fed with clindamycin or neomycin to fight anaerobic bacteria, the urobilin disappeared, while serum bilirubin continued to rise [60]. In addition, subsequent studies have shown that the use of antibiotics to clear intestinal microflora in healthy people has a similar effect on bilirubin [61]. These data demonstrate that intestinal microbiota alternation not only decreases the eradication of bilirubin, but also increases plasma levels, posing a potential threat to some organs of the human body.
Several studies have confirmed specific bacterial strains that can hydrolyze bilirubin, containing Clostridium ramosum [59,62], Clostridium perfringens and difficile [60,63-65], and Bacteriodes fragilis [66]. These core species could reduce urobilinogen mixtures, including dihydrobilirubin, mesobilirubin, dihydromesobilirubin, urobilinogen, half-stercobilinogen, and stercobilinogen to oxidant urobilin respectively in vivo and in vitro conditions. Escherichia coli has a synergistic effect on urobilin production in vitro [59]. Although there is no evidence showing that Bifidobacterium can metabolize bilirubin directly, it is found that Bifidobacterium can directly metabolize bilirubin in rat model, which is related to the activity of oral β-glucuronidase [67]. A study demonstrated that supplementation of Lactobacillus Plantarum to activate PKC pathway can inhibit dysfunction of tight junction protein in intestine epithelium induced by unconjugated bilirubin [49]. Human gut contains 100 trillion microorganisms, forming a complex bacterial network [68]. The bilirubin catabolism may be accomplished by combined action of bacteria of the human body. Notably, although we know their omnipresent occurrence in the gut and irreplaceable function, the bilirubin reductase and complex mechanisms present in bacteria have not been identified.
Bilirubin as a regulator of intestinal microbiota
In turn, it is well evidenced that bilirubin can regulate the structure and metabolism of the microbiota. There are two studies showing that bilirubin is potentially toxic towards Gram-positive bacterium, such as Enterococcus faecalis, Bacillus cereus, Staphylococcus aureus and Streptococcus agalactiae, because their sensitivity to bilirubin destroys the integrity of the bacterial membranes, respiratory metabolism and carbohydrate metabolism [50,69]. Conversely, bilirubin can promote the proliferation of Gram-negative bacterium Enterococcus faecalis under neutralizing free radicals attacking bacteria [69].
Association between gut microbiota and hyperbilirubinemia: clinical data
It has been recognized that microbiota is vital in bilirubin metabolism in the previous discussion. Human studies have shown that the microorganism communities associated with hyperbilirubinemia are statistically different from those in the control group. Neonatal gut, a nearly germ-free environment, is colonized by very few bacteria at birth. Urobilinogen was not detected in feces until the fifth day after delivery, and the amount of bilirubin is only one-tenth of that of adults, due to the low bacterial conversion [65]. The imbalance of the microbial community is considered to be one of the causes of neonatal hyperbilirubinemia and has attracted much attention in recent years. In this section, we summarize the interaction between neonatal hyperbilirubinemia and gut microbiota in different states (Table 1) [9-12,14].
Table 1.
Summary of studies linking microbiota and hyperbilirubinemia in neonate
| Type | Author | NO of: Infants | Analysis techniques | Age | Abundance in infants with NH | Diversity |
|---|---|---|---|---|---|---|
| Year | samples | region, achine | ||||
| Design | case, control | database | ||||
| Meconium | Dong [14] | 301 | 16S rRNA | 1 d | Genus level | ↑α-diversity in cesarean infants (Shannon index) (P<0.05) |
| 2018 | 301 | V3 | ↑Bifidobacterium pseudolongum (P=0.03) | |||
| PCS/RCT | 141, 160 | HiSeq2500 PE250 | ||||
| PICRUSt/KEGG | ||||||
| Beast Milk Jaundice | Duan [9] | 34 | 16S rRNA | 14-35 d | Genus level | ↓α-diversity (Simpson index) |
| 2018 | 34 | V3-V4 | ↓Escherichia/Shigella (P=0.01) | |||
| PCS/RCT | 12, 22 | MiSeq Illumina | ||||
| KEGG | ||||||
| Li [10] | 11 | 16S rRNA | 7-14 d | Family level | α-diversity (P>0.05) | |
| 2019 | 11 | V3-V4 | ↑Escherichia (P=0.01) | ↓β-diversity (P<0.05) | ||
| PCS/RCT | 6, 5 | MiSeq Illumina | ↓Staphylococcus (P=0.016) | |||
| ↓Klebsiella (P=0.009) | ||||||
| ↓Akkermansia (P=0.02) | ||||||
| Genus level | ||||||
| ↑Escherichia (P<0.05) | ||||||
| Ru [12] | 152 | qPCR | 0-28 d | Genus level | ||
| 2015 | 152 | Bifidobacterium adolescentis | ↓Bifidobacterium adolescentis (P=0.000) | |||
| PCS/RCT | 76, 76 | Bifidobacterium bifidum | ↓Bifidobacterium bifidum (P=0.000) | |||
| Bifidobacterium longum | ↓Bifidobacterium longum (P=0.000) | |||||
| Lactobacillus fermentans | ||||||
| Lactobacillus carinii | ||||||
| Lactobacillus plantarum | ||||||
| Bilirubin encephalopathy | Li [11] | 54 | 16S rRNA | 0-28 d | Genus level | ↓α-diversity (numbers of OTUs, Chao1 index and Shannon index) at family level (P<0.05) |
| 2018 | 54 | V3-V4 | ↓Fusobacterium (P<0.05) | |||
| PCS/RCT | 26, 28 | MiSeq Illumina | ↓Catabacter (P<0.05) | |||
| ↓Succinivibrio (P<0.05) | ||||||
| ↓Clostridium (P<0.05) | ||||||
| ↓Bacteroides (P<0.05) | ||||||
| Yang [15] | 108 | 16S rRNA | 3-38 d | Genus level | ||
| 2019 | 108 | V3-V4 | ↓Catabacter (P<0.05) | |||
| PCS/RCT | 52, 56 | MiSeq Illumina | ↓Fusobacterium (P<0.05) | |||
| ↓Bacteroides (P<0.05) | ||||||
| ↓Succinivibrio (P<0.05) | ||||||
| ↓Clostridium (P<0.05) |
NH, neonate hyperbilirubinemia; 16S rRNA, 16S rRNA gene sequencing; PCS, prospective cohort study; qPCR, quantitative polymerase chain reaction.
Neonate hyperbilirubinemia and meconium
Meconium is the neonatal first stool sample containing the initial gut microbiota associated with profound effects on the various diseases during every period of life, including NEC [70], respiratory problems and allergic diseases [71]. Dong [14] investigated the role of microbiota in hyperbilirubinemia by a nested case-control design. The results showed that newborns with jaundice had lower Shannon index (representing lower α diversity and proportion in microflora) in the cesarean section, but there was no similar result in vaginal delivery. Besides, the research found that Clostridium perfringens was significantly upregulated in the jaundice group [14], which is a bit surprising because it has been found that Clostridium perfringens can reduce intestinal bilirubin to urinary bilirubin [63,64]. This result was considered to be feedback on hyperbilirubinemia in the neonate [14]. It is suggested that the microorganisms in meconium are the basis of the formation of microflora and have a far-reaching influence on the occurrence of jaundice.
Breast milk jaundice and microbiota
Breast milk received increased attention because of its beneficial function [72], and breast milk jaundice occurs more frequently in four-week-old newborns who are mainly breast-fed, up to 20% and 30% [8]. The etiology of breast milk jaundice is unclear, and the usually compelling theory is the increase in enterohepatic circulation, although it may be caused by several factors, such as β-glucuronidase and pregnane-3a, 20β-diol contained in breast milk, UGT 1A1 enzyme activity and gut microbiota. As we all know, breast milk contains a large number of living microorganisms, including Staphylococcus, Streptococcus, Lactobacillus, Bifidobacterium, Proteobacteria, which can regulate the development of microbiome and affect the functional metabolites of gastrointestinal tract in newborns [73,74].
The relationship between microbiota and breast milk jaundice has been consistent in several studies, mainly focusing on the characteristic changes and richness of intestinal flora. In general, breast milk jaundice occurs in 5-7 days of life [38]. In newborns, one preliminary study uses real-time PCR to study the difference of microbial composition between neonates (2 weeks) with the breast milk jaundice group (n=76) and healthy control group (n=76). The fecal levels of Bifidobacterium adolescentis, Bifidobacterium bifidum and Bifidobacterium longum in the breast milk jaundice group were significantly lower than those in the control group, and negatively correlated with serum bilirubin [12]. These findings are consistent with two other studies on infants under three months old, who also found a negative correlation between Bifidobacterium in feces and serum bilirubin in infants with breast milk jaundice [75,76]. Furthermore, Zhou [76] performing shotgun metagenomic sequencing and metagenome-wide association revealed that the fecal microbiota profile of breast milk jaundice was characterized by decreased microbial diversity and decreased abundance of bifidobacteria, Bacteroides Fragiles, and Bacteroides Thetaiotaomicron. The research also found that the expression of metabolic genes in microbiota was related to breast milk jaundice, especially the expression of galactose metabolism-related genes. On the one hand, the decrease of Bifidobacterium was positively associated with the decrease of the expression of galactose metabolism pathway-related genes, which reduces the upstream production of UDP-glucose, leads to the shortage of UDPGA, and affects the transformation of bilirubin [77]. Bifidobacterium reduces the activity of β-glucuronidase [78], thus inhibiting bilirubin enterohepatic circulation. In another study, the fecal microbiota composition of newborns with normal and severe breast milk jaundice was compared by 16S rRNA sequencing. There was no difference in α-diversity and Bifidobacterium in the samples, but it was found that the abundance of Staphylococcaceae, Staphylococcus, Klebsiella, and bacillales was negatively correlated with the severity of the disease [10]. These studies show that the imbalance is associated with the severity of breast milk jaundice, and provide strong evidence for the application of Bifidobacterium in breast milk jaundice.
Bilirubin encephalopathy and microbiota
Microbiota-gut-brain axis is a relatively new concept, which reflects the bidirectional, effective communication between the central nervous system and intestinal microbiota in the gut via signals of immune, nervous, endocrine and metabolites [79]. The accumulation of bilirubin in brain tissue may cause neuronal damage, which in turn leads to the cascade effect of neonatal brain cells. It is well established due to the down-regulation of tight junction protein, the blood-brain barrier of germ-free rats is more permeable as compared to normal rats at birth and adulthood [80]. The relationship between intestinal microbiota and encephalopathy in neonatal hyperbilirubinemia is an attractive topic. Li found that the microbial abundance in bilirubin encephalopathy group was lower than that in the non-brain injury group, and the abundance of some bacteria that mainly produced short-chain fatty acids, such as Fusobacterium, Catabacter, Succinivibrio, Clostridium and Bacteroides, were significantly lower than those in the control group [11]. Another study using 16S ribosomal DNA sequence also found that bacteria reduction in bilirubin encephalopathy is associated with short-chain fatty acid metabolism [15]. Several studies have demonstrated that the supplement of short-chain fatty acids can reduce the permeability of the blood brain barrier by histone acetylation [81], and increase hippocampal neurogenesis and hippocampal metabolic activation [82]. Therefore, the dysbiosis of gut microbiota indirectly affects the blood brain barrier and brain regions via short chain fatty acid (Figure 2).
Multiple evidence suggests the crucial role for bacteria in neonatal hyperbilirubinemia but further experiments are still under way to fully understand the complex relationship between microflora and hyperbilirubinemia. Although microbiota-associated metabolism of microbiota has been described, there may be many other molecules that affect the development of hyperbilirubinemia. Future research should pay more attention to molecular relationship between microbiota and hyperbilirubinemia.
Modulating microbiota for neonatal hyperbilirubinemia therapy
Probiotics
Probiotics are live microorganisms that provide benefits on host health after being administered in amounts dose [83]. Several lines of research have evidenced that probiotic is the preferred therapy target for neonatal hyperbilirubinemia. Given the key role of microflora disorders in neonatal jaundice and enterohepatic circulation [9-14,75], microbiota intervention is expected to pave the way for the treatment and prevention of neonatal hyperbilirubinemia, but its benefits remain controversial (Table 2) [84-92]. In several population-based studies, numerous probiotics have shown beneficial effects in the treatment of neonatal hyperbilirubinemia. Two meta-analyses involving the randomized control trials (RCT) recently found that the single-strain or multi-strain probiotics with standard treatment were more effective in reducing total serum bilirubin, jaundice regression time, duration of phototherapy and hospitalization [93,94]. However, a subsequent intervention comprising saccharomyces boulardii in 119 neonates (58 in the case group and 61 in the placebo group) failed to reduce the clinical course of hyperbilirubinemia [84,95]. Despite the experimental therapeutic date, the meta-analysis of Deshmukh [93] found that oral probiotics did not significantly reduce the incidence of neonates with jaundice for hyperbilirubinemia prophylaxis. Although probiotics increased the frequency of fecal emptying to reduce enterohepatic circulation compared with the control group, supplementation of probiotics did not continuously reduce serum bilirubin more effectively [88].
Table 2.
The therapeutic effects and safety of probiotics in neonatal hyperbilirubinemia
| Probiotic strain | Author | No of Case Control | Involved requirement (Gastational age Birth weight) | Primary Outcomes (case VS. control) | Adverse effect |
|---|---|---|---|---|---|
| Year | |||||
| Study design | |||||
| Saccharomyces boulardii | Serce [84] | 58 | 35-42 weeks | TCB (mg/dl) | NO |
| 2015 | 61 | 24 h: 4.1 vs. 13.5 P=0.085 | |||
| RCT | 96 h: 14 vs. 13.4 P=0.24 | ||||
| Demirel [85] | 81 | ≤32 weeks | Duration of phototherapy (days) | NO | |
| 2013 | 98 | ≤1.5 kg | 1.9 vs. 2.6 P=0.000 | ||
| RCT | |||||
| Suganthi [86] | 86 | ≥32 weeks | Incidence of clinical jaundice | NO | |
| 2016 | 95 | >2.5 kg | 66% vs. 77 P=0.047 | ||
| RCT | |||||
| Bacillus clausii | Chandrashekhar [92] | 510 | >35 weeks | Duration of phototherapy (hours) | NO |
| 2017 | 533 | 18 vs. 24 P=0.027 | |||
| RCT | |||||
| Bacillus licheniformis | Tian [91] | 35 | ≥37 weeks | STB (umol/l) | No |
| 2016 | 34 | 5 d: 116.38 vs. 140.84 P<0.01 | |||
| RCT | |||||
| Lactobacillus reuteri | Shadkam [87] | 30 | 28-34 weeks | Incidence of jaundice | NEC |
| 2015 | 30 | 1000-1800 g | 96.6% vs. 86.7% P<0.35 | Spesis | |
| RCT | |||||
| Lactobacillus rhamnosus | Mutlu [88] | 30 | 35 to 42 weeks | STB (umol/l) | NO |
| 2019 | 30 | 24 h: 9.46 vs. 9.67 P=0.478 | |||
| RCT | 48 h: 10.63 vs. 11.04 P=0.221 | ||||
| Lactobacillus bulgaricus | Liu [90] | 34 | 38-42 weeks | STB (umol/l) | NO |
| Bifidobacterium | 2015 | 34 | 1 d: 312 vs. 309 P>0.05 | ||
| Streptococcus thermophilus | RCT | 4 d: 195 vs. 155 P=0.002 | |||
| 7 d: 108 vs. 179 P=0.007 | |||||
| Bifidobacterium lactis | Torkaman [89] | 45 | ≥35 weeks | TCB (mg/dl) | NO |
| Bifidobacterium bifidum | 2017 | 47 | ≥2500 g | 24 h: 12.12 vs. 13.71 P=0.001 | |
| Lactobacillus acidophilus | RCT | 96 H: 9.8 vs. 10.29 P=0.74 | |||
| Lactobacillus rhamnosus |
STB: serum total bilirubin. TCB: transcutaneous bilirubin.
Probiotics affect neonatal hyperbilirubinemia via various potential mechanisms to balance microbiota dysbiosis and reduce bilirubin (Figure 3). The most popular probiotics strains, including (but not limited to) Saccharomyces boulardii, Bifidobacterium, Lactobacillus reuteri, Bacillus clausii, Bacillus subtilis, Clostridium butyricum, have been evaluated for their efficacy in the treatment of hyperbilirubinemia [88,93,94]. Investigators found that supplementation of therapeutic probiotics in newborns was beneficial to promoting intestinal colonization and inhibiting pathogens, and different probiotics showed strain-specific functions [96]. In neonatal jaundice, the relative abundance of Bifidobacterium in patients receiving breast milk was lower than that in the control group [12,75,76]. Bifidobacterium can increase stool frequency, decrease enterohepatic circulation and intestinal pH and inhibit the activity of the enzyme β-glucuronidase [67,93]. In vitro, Lactobacillus could enhance the tight junction protein of intestinal epithelium, after high concentration of bilirubin increased the intestinal permeability [49]. Saccharomyces boulardii has been found to increase intestinal polyamines to promote intestinal maturity, which is a potential mechanism for decreasing enterohepatic circulation [84].
Figure 3.
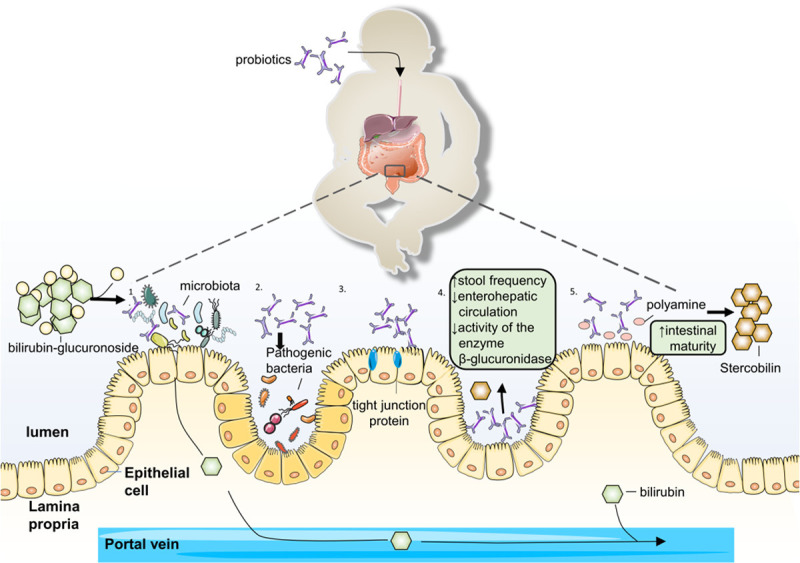
The potential mechanism of probiotic in neonate jaundice. 1. Promote colonization course. 2. Supress pathogenic. 3. Increase stool frequency and reduce enterohepatic circulation and inhibit the activity of the enzyme β-glucuronidase. 4. Enhance the tight junction protein. 5. Increase polyamines in the gut to improve the intestinal maturity.
A fundamental risk of probiotic strategies is that newborns are vulnerable to infections caused by live microbes. Although the infections mostly occur in premature infants and surgical conditions, neonatal jaundice has a potential risk of sepsis with oral probiotics [97,98]. Meta-analysis showed that among the 207 cases, 20 cases of probiotics had adverse reactions such as fever, diarrhea, rash and fatigue [94]. Therefore, using probiotics in the vulnerable neonate with jaundice should weigh up the pros and cons, and the heightened scrutiny is necessary [93].
Prebiotics
Prebiotics are non-digestible food supplement which stimulates microbiota in the gut and confers health benefits to the host [99]. Oligosaccharides in breast milk serve as prebiotics to increase the stool frequency and growth of Lactobacillus and Bifidobacterium [100]. Armenian [101] investigated the effectiveness of prebiotics including short-chain galacto-oligosacarids and long-chain fructo-oligosacarids in preterm neonates and found during phototherapy, prebiotics increased fecal frequency and decreased serum bilirubin. Similarly, a clinical study has shown that the serum bilirubin was reduced with the administration of prebiotic in term neonates [102]. Recently, a systematic review and meta-analysis including 154 subjects found that prebiotics supplementation reduced the risk of hyperbilirubinemia, duration of hospital stay and increased the fecal frequencies, whereas there was no significant difference in maximum serum bilirubin and phototherapy time [96]. The underlying mechanisms may be to promote intestinal growth, regulate intestinal microflora, remove the regulation of β-glucuronidase in the middle colon to reduce the enterohepatic circulation of bilirubin [101,103,104]. There was no negative consequence found in the study of neonatal jaundice, but the profound effect is poorly understood.
With many therapeutic targets for altering the intestinal microbiome of neonate hyperbilirubinemia, it is worth mentioning that probiotic is not the only way to reduce enterohepatic circulation, which is essential to weigh advantages and disadvantages. Prebiotics are potential administration that influence the microbiota community in reducing the risk of hyperbilirubinemia. Continued studies are needed to explore more effective ways to evaluate the long-effects of probiotics and prebiotics in the treatment of neonatal hyperbilirubinemia.
Conclusions and perspectives
The combination of technology, observation data and experimental insights is elucidating the role of microbiota in the pathogenesis of neonatal hyperbilirubinemia. Microbiota closely interact with gut epithelial cells to regulate intestinal bilirubin, play a role in convergence, and promote the metabolic pathway of bilirubin. Though microbiota plays an important role in the progression of hyperbilirubinemia, the detailed bioactive molecular mechanisms are not fully understood. Several advanced tools which are helpful to clarify the potential pathological mechanism of neonatal hyperbilirubinemia are humanized animal models, metagenome technology, metabonomic technology. Although the bioinformatics spectrum and metabolic pathways of bioactive molecules related to bacteria have been explored, further research on fungi, viruses and archaea will promote the development of research in this field. Notably, the etiological study of specific bacteria ignores the interaction between different microbial communities. Further detailed research and design can ameliorate this problem, and technology is needed to find out the links between different types of organisms. Although there are problems with the landscape of microflora, it is still a scientific challenge to identify biomarkers of kernicterus in newborns. Therefore, more multicenter, randomized clinical trials are worth studying to select a peculiar microbial signature associated with neonatal hyperbilirubinemia or kernicterus.
Neonatal hyperbilirubinemia is the most common clinical disorder, and its pathogenesis is related to the imbalanced of microbial community. Our proposed bilirubin-intestinal microbiota-neonatal hyperbilirubinemia cycle is an important step in understanding neonatal jaundiced and bilirubin encephalopathy (Figure 4). Modulating microbiota dysbiosis in neonatal hyperbilirubinemia has proved to be an effective strategy to reduce serum bilirubin in several studies. However, even though probiotics and prebiotics have a significant effect on the treatment of hyperbilirubinemia, efficacy is still controversial. It is necessary to study the mechanism in detail in order to provide a persuasive new treatment strategy for neonatal hyperbilirubinemia or kernicterus. Furthermore, ongoing research on the therapeutic efficacy of the hyperbilirubinemia should compare the effects of probiotics and prebiotics. Research is also necessary to examine the risks and benefits of multiple and specific strains, and additional criteria should be involved to limit the use of probiotics to avoid safety problems. Additionally, further strong research designs are needed to cover the long-term benefits or risks of probiotics on neonatal jaundice later in life. A novel approach in the complex field of microflora and neonatal hyperbilirubinemia will make a breakthrough in treatment and, more importantly, in the prevention of encephalopathy.
Figure 4.
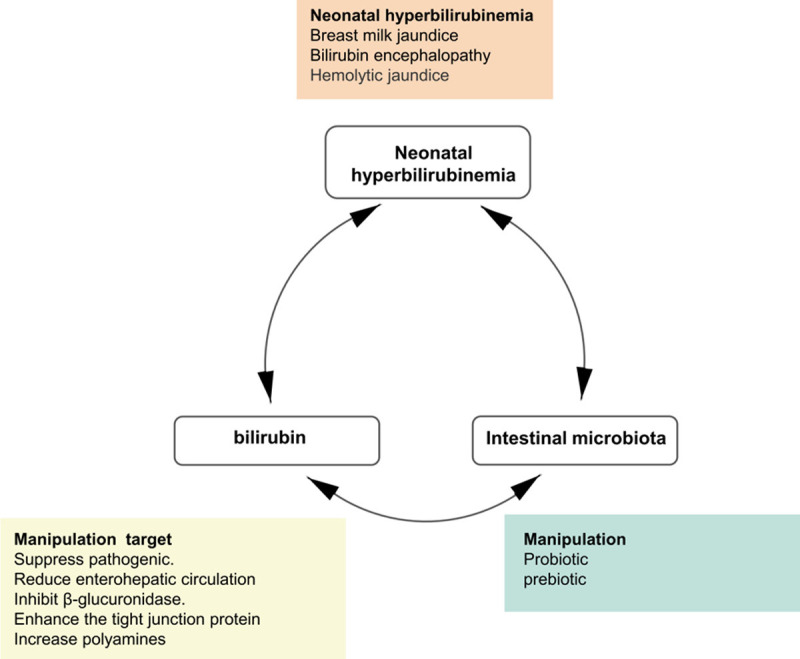
A bilirubin-intestinal microbiota-neonatal hyperbilirubinemia cycle. The relationship between bilirubin, intestinal microbiota and neonatal hyperbilirubinemia is not unidirectional but actually interacts with each other through different mechanisms. This cycle provides exciting new perspective for the pathogenesis and treatment of neonatal hyperbilirubinemia.
Acknowledgements
The work of the authors is supported by Grants from National Natural Science Foundation of China (81571466).
Disclosure of conflict of interest
None.
References
- 1.Corujo-Santana C, Falcon-Gonzalez JC, Borkoski-Barreiro SA, Perez-Plasencia D, Ramos-Macias A. The relationship between neonatal hyperbilirubinemia and sensorineural hearing loss. Acta Otorrinolaringol Esp. 2015;66:326–331. doi: 10.1016/j.otorri.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Watchko JF. TcB, FFR, phototherapy and the persistent occurrence of kernicterus spectrum disorder. J Perinatol. 2020;40:177–179. doi: 10.1038/s41372-019-0583-7. [DOI] [PubMed] [Google Scholar]
- 3.Slusher TM, Zamora TG, Appiah D, Stanke JU, Strand MA, Lee BW, Richardson SB, Keating EM, Siddappa AM, Olusanya BO. Burden of severe neonatal jaundice: a systematic review and meta-analysis. BMJ Paediatr Open. 2017;1:e000105. doi: 10.1136/bmjpo-2017-000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olusanya BO, Kaplan M, Hansen TWR. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc Health. 2018;2:610–620. doi: 10.1016/S2352-4642(18)30139-1. [DOI] [PubMed] [Google Scholar]
- 5.Wu YW, Kuzniewicz MW, Wickremasinghe AC, Walsh EM, Wi S, McCulloch CE, Newman TB. Risk for cerebral palsy in infants with total serum bilirubin levels at or above the exchange transfusion threshold: a population-based study. JAMA Pediatr. 2015;169:239–246. doi: 10.1001/jamapediatrics.2014.3036. [DOI] [PubMed] [Google Scholar]
- 6.Rawat V, Bortolussi G, Gazzin S, Tiribelli C, Muro AF. Bilirubin-induced oxidative stress leads to DNA damage in the cerebellum of hyperbilirubinemic neonatal mice and activates DNA double-strand break repair pathways in human cells. Oxid Med Cell Longev. 2018;2018:1801243. doi: 10.1155/2018/1801243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gale C, Statnikov Y, Jawad S, Uthaya SN, Modi N Brain Injuries expert working group. Neonatal brain injuries in England: population-based incidence derived from routinely recorded clinical data held in the National Neonatal Research Database. Arch Dis Child Fetal Neonatal Ed. 2018;103:F301–F306. doi: 10.1136/archdischild-2017-313707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ullah S, Rahman K, Hedayati M. Hyperbilirubinemia in neonates: types, causes, clinical examinations, preventive measures and treatments: a narrative review article. Iran J Public Health. 2016;45:558–568. [PMC free article] [PubMed] [Google Scholar]
- 9.Duan M, Yu JL, Feng JX, He Y, Xiao S, Zhu DP, Zou ZH. 16S ribosomal RNA-based gut microbiome composition analysis in infants with breast milk jaundice. Open Life Sciences. 2018;13:208–216. doi: 10.1515/biol-2018-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YX, Mo X, Sun JH, Shen N, Xu LQ, Hu R, Yang LF, Li J. Characteristics of intestinal flora in breast-fed neonates with severe hyperbilirubinemia. J Chin Pediatr. 2019;37:351–355. [Google Scholar]
- 11.Li YB, Zhou W, Yuan WM, Tang J, Chen XW, Tao L, Wei M, Su H, Zhao N, Huang XH, Zhang Z. Composition of gut microbiome in neonates with severe hyperbilirubinemia and its effect on bilirubin brain injury. Chin J Appl Clin Pediatr. 2018;33:103–107. [Google Scholar]
- 12.Ru CW, Qin T, Wang L, Jiang SS, Wu FG, Chen H. The correlation between bacteria in breast milk and newborn feces and breast milk jaundice. Chin J Microecol. 2015;27:325–327. [Google Scholar]
- 13.Zhou JL, Zhou SM, Wang MB, Cheng YW, Dai DL, Cai HB, Zhu ZS, Zou Y, Ding J. Metagenome of gut microbiota of patients with breast milk jaundice. Chin J Microecol. 2016;28:893–898. [Google Scholar]
- 14.Dong T, Chen T, White RA 3rd, Wang X, Hu W, Liang Y, Zhang Y, Lu C, Chen M, Aase H, Xia Y. Meconium microbiome associates with the development of neonatal jaundice. Clin Transl Gastroenterol. 2018;9:182. doi: 10.1038/s41424-018-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang N, Yang RX, Wang AH, Zhang YQ. The effect of intestinal flora on the neural development of severe hyperbilirubinemia neonates. Eur Rev Med Pharmacol Sci. 2019;23:1291–1295. doi: 10.26355/eurrev_201902_17024. [DOI] [PubMed] [Google Scholar]
- 16.Hamoud AR, Weaver L, Stec DE, Hinds TD Jr. Bilirubin in the liver-gut signaling axis. Trends Endocrinol Metab. 2018;29:140–150. doi: 10.1016/j.tem.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Monaco MH, Donovan SM. Impact of early gut microbiota on immune and metabolic development and function. Semin Fetal Neonatal Med. 2016;21:380–387. doi: 10.1016/j.siny.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5:48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stinson LF, Payne MS, Keelan JA. Planting the seed: origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit Rev Microbiol. 2017;43:352–369. doi: 10.1080/1040841X.2016.1211088. [DOI] [PubMed] [Google Scholar]
- 21.Liu CJ, Liang X, Niu ZY, Jin Q, Zeng XQ, Wang WX, Li MY, Chen XR, Meng HY, Shen R, Sun SY, Luo YY, Yang E, Geng JW, Li XR. Is the delivery mode a critical factor for the microbial communities in the meconium? EBioMedicine. 2019;49:354–363. doi: 10.1016/j.ebiom.2019.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapiainen T, Paalanne N, Tejesvi MV, Koivusaari P, Korpela K, Pokka T, Salo J, Kaukola T, Pirttila AM, Uhari M, Renko M. Maternal influence on the fetal microbiome in a population-based study of the first-pass meconium. Pediatr Res. 2018;84:371–379. doi: 10.1038/pr.2018.29. [DOI] [PubMed] [Google Scholar]
- 23.Seferovic MD, Pace RM, Carroll M, Belfort B, Major AM, Chu DM, Racusin DA, Castro ECC, Muldrew KL, Versalovic J, Aagaard KM. Visualization of microbes by 16S in situ hybridization in term and preterm placentas without intraamniotic infection. Am J Obstet Gynecol. 2019;221:146.e1–146.e23. doi: 10.1016/j.ajog.2019.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bello-Lopez JM, Nogueron-Silva J, Castaneda-Sanchez JI, Rojo-Medina J. Molecular characterization of microbial contaminants isolated from Umbilical Cord Blood Units for transplant. Braz J Infect Dis. 2015;19:571–577. doi: 10.1016/j.bjid.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald B, McCoy KD. Maternal microbiota in pregnancy and early life. Science. 2019;365:984–985. doi: 10.1126/science.aay0618. [DOI] [PubMed] [Google Scholar]
- 27.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore RE, Townsend SD. Temporal development of the infant gut microbiome. Open Biol. 2019;9:190128. doi: 10.1098/rsob.190128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’Shea CA, Watkins C, Dempsey E, Mattivi F, Tuohy K, Ross RP, Ryan CA, O’Toole PW, Stanton C. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi: 10.1186/s40168-016-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timmerman HM, Rutten NBMM, Boekhorst J, Saulnier DM, Kortman GAM, Contractor N, Kullen M, Floris E, Harmsen HJM, Vlieger AM, Kleerebezem M, Rijkers GT. Intestinal colonisation patterns in breastfed and formula-fed infants during the first 12 weeks of life reveal sequential microbiota signatures. Sci Rep. 2017;7:8327. doi: 10.1038/s41598-017-08268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, Lieber AD, Wu F, Perez-Perez GI, Chen Y, Schweizer W, Zheng X, Contreras M, Dominguez-Bello MG, Blaser MJ. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8:343ra382. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahl C, Stigum H, Valeur J, Iszatt N, Lenters V, Peddada S, Bjornholt JV, Midtvedt T, Mandal S, Eggesbo M. Preterm infants have distinct microbiomes not explained by mode of delivery, breastfeeding duration or antibiotic exposure. Int J Epidemiol. 2018;47:1658–1669. doi: 10.1093/ije/dyy064. [DOI] [PubMed] [Google Scholar]
- 33.Aagaard KM. Mode of delivery and pondering potential sources of the neonatal microbiome. EBioMedicine. 2020;51:102554. doi: 10.1016/j.ebiom.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesa MD, Loureiro B, Iglesia I, Fernandez Gonzalez S, Llurba Olive E, Garcia Algar O, Solana MJ, Cabero Perez MJ, Sainz T, Martinez L, Escuder-Vieco D, Parra-Llorca A, Sanchez-Campillo M, Rodriguez Martinez G, Gomez Roig D, Perez Gruz M, Andreu-Fernandez V, Clotet J, Sailer S, Iglesias-Platas I, Lopez-Herce J, Aras R, Pallas-Alonso C, de Pipaon MS, Vento M, Gormaz M, Larque Daza E, Calvo C, Cabanas F. The evolving microbiome from pregnancy to early infancy: a comprehensive review. Nutrients. 2020;12:133. doi: 10.3390/nu12010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, Hoashi M, Rivera-Vinas JI, Mendez K, Knight R, Clemente JC. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22:250–253. doi: 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M, Li M, Wu S, Lebrilla CB, Chapkin RS, Ivanov I, Donovan SM. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J Pediatr Gastroenterol Nutr. 2015;60:825–833. doi: 10.1097/MPG.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eck A, Rutten NBMM, Singendonk MMJ, Rijkers GT, Savelkoul PHM, Meijssen CB, Crijns CE, Oudshoorn JH, Budding AE, Vlieger AM. Neonatal microbiota development and the effect of early life antibiotics are determined by two distinct settler types. PLoS One. 2020;15:e0228133. doi: 10.1371/journal.pone.0228133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen TWR, Wong RJ, Stevenson DK. Molecular physiology and pathophysiology of bilirubin handling by the blood, liver, intestine, and brain in the newborn. Physiol Rev. 2020;100:1291–1346. doi: 10.1152/physrev.00004.2019. [DOI] [PubMed] [Google Scholar]
- 39.Vitek L. Bilirubin as a signaling molecule. Med Res Rev. 2020;40:1335–1351. doi: 10.1002/med.21660. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien L, Hosick PA, John K, Stec DE, Hinds TD Jr. Biliverdin reductase isozymes in metabolism. Trends Endocrinol Metab. 2015;26:212–220. doi: 10.1016/j.tem.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundararaghavan VL, Sindhwani P, Hinds TD Jr. Glucuronidation and UGT isozymes in bladder: new targets for the treatment of uroepithelial carcinomas? Oncotarget. 2017;8:3640–3648. doi: 10.18632/oncotarget.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dani C, Poggi C, Pratesi S. Bilirubin and oxidative stress in term and preterm infants. Free Radic Res. 2019;53:2–7. doi: 10.1080/10715762.2018.1478089. [DOI] [PubMed] [Google Scholar]
- 45.Gazzin S, Vitek L, Watchko J, Shapiro SM, Tiribelli C. A novel perspective on the biology of bilirubin in health and disease. Trends Mol Med. 2016;22:758–768. doi: 10.1016/j.molmed.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Inoue T, Sonoda N, Hiramatsu S, Kimura S, Ogawa Y, Inoguchi T. Serum bilirubin concentration is associated with left ventricular remodeling in patients with type 2 diabetes mellitus: a cohort study. Diabetes Ther. 2018;9:331–338. doi: 10.1007/s13300-018-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stec DE, John K, Trabbic CJ, Luniwal A, Hankins MW, Baum J, Hinds TD Jr. Bilirubin binding to PPARalpha inhibits lipid accumulation. PLoS One. 2016;11:e0153427. doi: 10.1371/journal.pone.0153427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Dong H, Zhang Y, Cao M, Song L, Pan Q, Bulmer A, Adams DB, Dong X, Wang H. Bilirubin increases insulin sensitivity by regulating cholesterol metabolism, adipokines and PPARgamma levels. Sci Rep. 2015;5:9886. doi: 10.1038/srep09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Qin H, Zhang M, Shen T, Chen H, Ma Y, Chu Z, Zhang P, Liu Z. Lactobacillus plantarum inhibits intestinal epithelial barrier dysfunction induced by unconjugated bilirubin. Br J Nutr. 2010;104:390–401. doi: 10.1017/S0007114510000474. [DOI] [PubMed] [Google Scholar]
- 50.Hansen R, Gibson S, De Paiva Alves E, Goddard M, MacLaren A, Karcher AM, Berry S, Collie-Duguid ESR, El-Omar E, Munro M, Hold GL. Adaptive response of neonatal sepsis-derived Group B Streptococcus to bilirubin. Sci Rep. 2018;8:6470. doi: 10.1038/s41598-018-24811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai K, Song XL, Shi HS, Qi X, Li CY, Fang J, Wang F, Maximyuk O, Krishtal O, Xu TL, Li XY, Ni K, Li WP, Shi HB, Wang LY, Yin SK. Bilirubin enhances the activity of ASIC channels to exacerbate neurotoxicity in neonatal hyperbilirubinemia in mice. Sci Transl Med. 2020;12:eaax1337. doi: 10.1126/scitranslmed.aax1337. [DOI] [PubMed] [Google Scholar]
- 52.Bockor L, Bortolussi G, Vodret S, Iaconcig A, Jasprova J, Zelenka J, Vitek L, Tiribelli C, Muro AF. Modulation of bilirubin neurotoxicity by the Abcb1 transporter in the Ugt1-/- lethal mouse model of neonatal hyperbilirubinemia. Hum Mol Genet. 2017;26:145–157. doi: 10.1093/hmg/ddw375. [DOI] [PubMed] [Google Scholar]
- 53.Gambaro SE, Robert MC, Tiribelli C, Gazzin S. Role of brain cytochrome P450 mono-oxygenases in bilirubin oxidation-specific induction and activity. Arch Toxicol. 2016;90:279–290. doi: 10.1007/s00204-014-1394-4. [DOI] [PubMed] [Google Scholar]
- 54.Qaisiya M, Mardesic P, Pastore B, Tiribelli C, Bellarosa C. The activation of autophagy protects neurons and astrocytes against bilirubin-induced cytotoxicity. Neurosci Lett. 2017;661:96–103. doi: 10.1016/j.neulet.2017.09.056. [DOI] [PubMed] [Google Scholar]
- 55.Liao PF, Tsai JD, Chen HJ, Pan HH, Hung TW, Chang HY, Sheu JN. Neonatal hyperbilirubinaemia is associated with a subsequent increased risk of childhood-onset type 1 diabetes. Paediatr Int Child Health. 2020;40:35–43. doi: 10.1080/20469047.2019.1600854. [DOI] [PubMed] [Google Scholar]
- 56.Preel M, Rackauskaite G, Larsen ML, Laursen B, Lorentzen J, Born AP, Langhoff-Roos J, Uldall P, Hoei-Hansen CE. Children with dyskinetic cerebral palsy are severely affected as compared to bilateral spastic cerebral palsy. Acta Paediatr. 2019;108:1850–1856. doi: 10.1111/apa.14806. [DOI] [PubMed] [Google Scholar]
- 57.Kuzniewicz MW, Niki H, Walsh EM, McCulloch CE, Newman TB. Hyperbilirubinemia, phototherapy, and childhood asthma. Pediatrics. 2018;142:e20180662. doi: 10.1542/peds.2018-0662. [DOI] [PubMed] [Google Scholar]
- 58.Wusthoff CJ, Loe IM. Impact of bilirubin-induced neurologic dysfunction on neurodevelopmental outcomes. Semin Fetal Neonatal Med. 2015;20:52–57. doi: 10.1016/j.siny.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gustafsson BE, Lanke LS. Bilirubin and urobilins in germfree, ex-germfree, and conventional rats. J Exp Med. 1960;112:975–981. doi: 10.1084/jem.112.6.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vitek L, Zelenka J, Zadinova M, Malina J. The impact of intestinal microflora on serum bilirubin levels. J Hepatol. 2005;42:238–243. doi: 10.1016/j.jhep.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Saxerholt H, Carlstedt-Duke B, Hoverstad T, Lingaas E, Norin KE, Steinbakk M, Midtvedt T. Influence of antibiotics on the faecal excretion of bile pigments in healthy subjects. Scand J Gastroenterol. 1986;21:991–996. doi: 10.3109/00365528608996410. [DOI] [PubMed] [Google Scholar]
- 62.Midtvedt T, Gustafsson BE. Microbial conversion of bilirubin to urobilins in vitro and in vivo. Acta Pathol Microbiol Scand B. 1981;89:57–60. doi: 10.1111/j.1699-0463.1981.tb00152_89b.x. [DOI] [PubMed] [Google Scholar]
- 63.Vitek L, Majer F, Muchova L, Zelenka J, Jiraskova A, Branny P, Malina J, Ubik K. Identification of bilirubin reduction products formed by Clostridium perfringens isolated from human neonatal fecal flora. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;833:149–157. doi: 10.1016/j.jchromb.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 64.Konickova R, Jiraskova A, Zelenka J, Leseticky L, Sticha M, Vitek L. Reduction of bilirubin ditaurate by the intestinal bacterium Clostridium perfringens. Acta Biochim Pol. 2012;59:289–292. [PubMed] [Google Scholar]
- 65.Vitek L, Kotal P, Jirsa M, Malina J, Cerna M, Chmelar D, Fevery J. Intestinal colonization leading to fecal urobilinoid excretion may play a role in the pathogenesis of neonatal jaundice. J Pediatr Gastroenterol Nutr. 2000;30:294–298. doi: 10.1097/00005176-200003000-00015. [DOI] [PubMed] [Google Scholar]
- 66.Fahmy K, Gray CH, Nicholson DC. The reduction of bile pigments by faecal and intestinal bacteria. Biochim Biophys Acta. 1972;264:85–97. doi: 10.1016/0304-4165(72)90119-5. [DOI] [PubMed] [Google Scholar]
- 67.An HM, Park SY, Lee DK, Kim JR, Cha MK, Lee SW, Lim HT, Kim KJ, Ha NJ. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011;10:116. doi: 10.1186/1476-511X-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 69.Nobles CL, Green SI, Maresso AW. A product of heme catabolism modulates bacterial function and survival. PLoS Pathog. 2013;9:e1003507. doi: 10.1371/journal.ppat.1003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dobbler PT, Procianoy RS, Mai V, Silveira RC, Corso AL, Rojas BS, Roesch LFW. Low microbial diversity and abnormal microbial succession is associated with necrotizing enterocolitis in preterm infants. Front Microbiol. 2017;8:2243. doi: 10.3389/fmicb.2017.02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen X, Wang M, Zhang X, He M, Li M, Cheng G, Wan C, He F. Dynamic construction of gut microbiota may influence allergic diseases of infants in Southwest China. BMC Microbiol. 2019;19:123. doi: 10.1186/s12866-019-1489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verhasselt V. Is infant immunization by breastfeeding possible? Philos Trans R Soc Lond B Biol Sci. 2015;370:20140139. doi: 10.1098/rstb.2014.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger K, Bailey A, Bushman FD, Sleasman JW, Aldrovandi GM. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171:647–654. doi: 10.1001/jamapediatrics.2017.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fitzstevens JL, Smith KC, Hagadorn JI, Caimano MJ, Matson AP, Brownell EA. Systematic review of the human milk microbiota. Nutr Clin Pract. 2017;32:354–364. doi: 10.1177/0884533616670150. [DOI] [PubMed] [Google Scholar]
- 75.Tuzun F, Kumral A, Duman N, Ozkan H. Breast milk jaundice: effect of bacteria present in breast milk and infant feces. J Pediatr Gastroenterol Nutr. 2013;56:328–332. doi: 10.1097/MPG.0b013e31827a964b. [DOI] [PubMed] [Google Scholar]
- 76.Zhou S, Wang Z, He F, Qiu H, Wang Y, Wang H, Zhou J, Zhou J, Cheng G, Zhou W, Xu R, Wang M. Association of serum bilirubin in newborns affected by jaundice with gut microbiota dysbiosis. J Nutr Biochem. 2019;63:54–61. doi: 10.1016/j.jnutbio.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 77.Thongaram T, Hoeflinger JL, Chow J, Miller MJ. Prebiotic galactooligosaccharide metabolism by probiotic lactobacilli and bifidobacteria. J Agric Food Chem. 2017;65:4184–4192. doi: 10.1021/acs.jafc.7b00851. [DOI] [PubMed] [Google Scholar]
- 78.Rocha Martin VN, Lacroix C, Killer J, Bunesova V, Voney E, Braegger C, Schwab C. Cutibacterium avidum is phylogenetically diverse with a subpopulation being adapted to the infant gut. Syst Appl Microbiol. 2019;42:506–516. doi: 10.1016/j.syapm.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Quigley EMM. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17:94. doi: 10.1007/s11910-017-0802-6. [DOI] [PubMed] [Google Scholar]
- 80.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyas B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin C, Lim Y, Lim H, Ahn TB. Plasma short-chain fatty acids in patients with Parkinson’s disease. Mov Disord. 2020;35:1021–1027. doi: 10.1002/mds.28016. [DOI] [PubMed] [Google Scholar]
- 82.Arnoldussen IAC, Wiesmann M, Pelgrim CE, Wielemaker EM, van Duyvenvoorde W, Amaral-Santos PL, Verschuren L, Keijser BJF, Heerschap A, Kleemann R, Wielinga PY, Kiliaan AJ. Butyrate restores HFD-induced adaptations in brain function and metabolism in mid-adult obese mice. Int J Obes (Lond) 2017;41:935–944. doi: 10.1038/ijo.2017.52. [DOI] [PubMed] [Google Scholar]
- 83.Hojsak I, Fabiano V, Pop TL, Goulet O, Zuccotti GV, Cokugras FC, Pettoello-Mantovani M, Kolacek S. Guidance on the use of probiotics in clinical practice in children with selected clinical conditions and in specific vulnerable groups. Acta Paediatr. 2018;107:927–937. doi: 10.1111/apa.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serce O, Gursoy T, Ovali F, Karatekin G. Effects of Saccharomyces boulardii on neonatal hyperbilirubinemia: a randomized controlled trial. Am J Perinatol. 2015;30:137–142. doi: 10.1055/s-0034-1376390. [DOI] [PubMed] [Google Scholar]
- 85.Demirel G, Celik IH, Erdeve O, Dilmen U. Impact of probiotics on the course of indirect hyperbilirubinemia and phototherapy duration in very low birth weight infants. J Matern Fetal Neonatal Med. 2013;26:215–218. doi: 10.3109/14767058.2012.725115. [DOI] [PubMed] [Google Scholar]
- 86.Suganthi V, Das AG. Role of saccharomyces boulardii in reduction of neonatal hyperbilirubinemia. J Clin Diagn Res. 2016;10:SC12–SC15. doi: 10.7860/JCDR/2016/20115.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shadkam MN, Jalalizadeh F, Nasiriani K. Effects of probiotic lactobacillus reuteri (DSM 17938) on the incidence of necrotizing enterocolitis in very low birth weight premature infants. Iran J Neonatology. 2015;6:15–20. [Google Scholar]
- 88.Mutlu M, Aslan Y, Kader S, Akturk Acar F. Preventive effects of probiotic supplementation on neonatal hyperbilirubinemia caused by isoimmunization. Am J Perinatol. 2020;37:1173–1176. doi: 10.1055/s-0039-1692690. [DOI] [PubMed] [Google Scholar]
- 89.Torkaman M, Mottaghizadeh F, Khosravi MH, Najafian B, Amirsalari S, Afsharpaiman S. The effect of probiotics on reducing hospitalization duration in infants with hyperbilirubinemia. Iran J Pediatr. 2017;27:e5096. [Google Scholar]
- 90.Liu W, Liu H, Wang T, Tang X. Therapeutic effects of probiotics on neonatal jaundice. Pak J Med Sci. 2015;31:1172–1175. doi: 10.12669/pjms.315.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian J, Guo YX. Efficacy of Bacillus licheniformis in the treatment of breast milk jaundice. Chin J Microecol. 2016;28:1172–1174. [Google Scholar]
- 92.Chandrasekhar J, Varghese TP, Gopi A, Raj M, Sudevan R, Jayakumar H. Treatment effect of probiotic bacillus clausii on neonatal jaundice in late preterm and term newborn babies: an experimental study. Pediatr Ther. 2017;7:326. [Google Scholar]
- 93.Deshmukh J, Deshmukh M, Patole S. Probiotics for the management of neonatal hyperbilirubinemia: a systematic review of randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:154–163. doi: 10.1080/14767058.2017.1369520. [DOI] [PubMed] [Google Scholar]
- 94.Chen Z, Zhang LL, Zeng LN, Yang XY, Jiang LC, Gui G, Zhang ZJ. Probiotics supplementation therapy for pathological neonatal jaundice: a systematic review and meta-analysis. Front Pharmacol. 2017;8:432. doi: 10.3389/fphar.2017.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pasha YZ, Ahmadpour-Kacho M, Jazi AA, Gholinia H. Effect of probiotics on serum bilirubin level in term neonates with jaundice: a randomized clinical trial. Int J Pediatr-Mashhad. 2017;5:5925–5930. [Google Scholar]
- 96.Armanian AM, Jahanfar S, Feizi A, Salehimehr N, Molaeinezhad M, Sadeghi E. Prebiotics for the prevention of hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2019;8:CD012731. doi: 10.1002/14651858.CD012731.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brecht M, Garg A, Longstaff K, Cooper C, Andersen C. Lactobacillus sepsis following a laparotomy in a preterm infant: a note of caution. Neonatology. 2016;109:186–189. doi: 10.1159/000441965. [DOI] [PubMed] [Google Scholar]
- 98.Dani C, Coviello CC, Corsini II, Arena F, Antonelli A, Rossolini GM. Lactobacillus sepsis and probiotic therapy in newborns: two new cases and literature review. AJP Rep. 2016;6:e25–29. doi: 10.1055/s-0035-1566312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sohn K, Underwood MA. Prenatal and postnatal administration of prebiotics and probiotics. Semin Fetal Neonatal Med. 2017;22:284–289. doi: 10.1016/j.siny.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Leoz ML, Kalanetra KM, Bokulich NA, Strum JS, Underwood MA, German JB, Mills DA, Lebrilla CB. Human milk glycomics and gut microbial genomics in infant feces show a correlation between human milk oligosaccharides and gut microbiota: a proof-of-concept study. J Proteome Res. 2015;14:491–502. doi: 10.1021/pr500759e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Armanian AM, Barekatain B, Hoseinzadeh M, Salehimehr N. Prebiotics for the management of hyperbilirubinemia in preterm neonates. J Matern Fetal Neonatal Med. 2016;29:3009–3013. doi: 10.3109/14767058.2015.1113520. [DOI] [PubMed] [Google Scholar]
- 102.Bisceglia M, Indrio F, Riezzo G, Poerio V, Corapi U, Raimondi F. The effect of prebiotics in the management of neonatal hyperbilirubinaemia. Acta Paediatr. 2009;98:1579–1581. doi: 10.1111/j.1651-2227.2009.01387.x. [DOI] [PubMed] [Google Scholar]
- 103.Barszcz M, Taciak M, Skomial J. The effects of inulin, dried Jerusalem artichoke tuber and a multispecies probiotic preparation on microbiota ecology and immune status of the large intestine in young pigs. Arch Anim Nutr. 2016;70:278–292. doi: 10.1080/1745039X.2016.1184368. [DOI] [PubMed] [Google Scholar]
- 104.Scott KP, Grimaldi R, Cunningham M, Sarbini SR, Wijeyesekera A, Tang MLK, Lee JC, Yau YF, Ansell J, Theis S, Yang K, Menon R, Arfsten J, Manurung S, Gourineni V, Gibson GR. Developments in understanding and applying prebiotics in research and practice-an ISAPP conference paper. J Appl Microbiol. 2020;128:934–949. doi: 10.1111/jam.14424. [DOI] [PubMed] [Google Scholar]


