Abstract
Vinpocetine (Vinp), a natural compound extracted from the leaves of Phyllostachys pubescens with apoptosis modulatory properties in variety of disorders. In the present study, we investigated the possible mechanism of Vinp in alleviating of the progress of osteonecrosis of the femoral head (ONFH) both in vitro and in vivo experiments. The results showed that treatment with Vinp suppressed the dexamethasone (Dex) induced over-regulation of ROS level and apoptotic factors. Mechanistically, the Vinp activated Akt signaling pathway in osteoblast. Moreover, Vinp exerted a protective role in animal ONFH model. To summarize, this work illustrated Vinp possessed a new potential therapeutic drug in ONFH.
Keywords: Vinpocetine, osteoblast, apoptosis, Akt, ONFH
Introduction
Osteonecrosis of the femoral head (ONFH) is a chronic malady may lead to the collapse of the femoral head after severe osteoarthritis [1]. Chronic alcoholism, thingfection, long-term glucocorticoids (GCs) therapy are thought to be the principal factors of nontraumatic ONFH [2]. GCs is the most common element among them. Nowadays, numerous hypothesis have been proposed to explain the pathological process of ONFH [3,4]. Among these, apoptosis and oxidative stress in osteoblast are considered to be the vital factors of the molecular mechanism of ONFH. At present, it is generally believed that ONFH is a multi-origin disorder, which need the development of more in-depth study and better therapeutic drugs.
The imbalance between the activity of osteoblasts and osteoclasts, by promoting osteoblasts apoptosis, will eventually result in osteonecrosis of femoral head [5,6]. Osteoblast, which is the principal target cells of GCs, plays an important part in promoting osteogenesis [7,8]. Using dexamethasone (Dex)-induced osteoblast apoptosis to establish an ONFH cellular model has been proved been feasible [9]. Previous study showed that the level of ROS was increased by Dex treatment and excessive ROS lead to cell apoptosis via the BAX/Bcl2/caspase3 apoptotic pathway [10].
Vinpocetine (Vinp), a small molecule natural compound extracted from the leaves of Phyllostachys pubescens with apoptosis modulatory properties in variety of disorders [11]. Numerous studies have illustrated that Vinp has a wide range of protective activities such as anti-inflammation [12], apoptosis [13], an antioxidant [14] Zhao et al. found that Vinp inhibited the apoptosis in the rat cerebral cortex after cerebral I/R Injury via PI3K/AKT pathway [15]. The PI3K/Akt signaling pathway is a vital pathway associated with numerous cellular processes, such as proliferation and differentiation [16]. Currently, a research demonstrated that the inhibition of the PI3K/Akt signaling pathway resulted in osteoblast apoptosis. To our knowledge, the anti-apoptosis effect of Vinp in osteoblasts and ONFH is still limited. In the present study, we detected the anti-apoptosis effect of Vinp in Dex induced rat osteoblast and explored the mechanism.
Materials and methods
Cell culture
As previously reported [9], osteoblasts were isolated from neonatal rats’ calvariae. Cells were cultured inα-MEM contained 10% serum and antibiotics. All operations are approved by the Animal Care and Use Committee of this institution and comply with the guidelines of international regulations.
Cell viability
To determine the cytotoxicity of Vinp on osteoblast, CCK-8 was performed. Briefly, 5 × 103 osteoblasts were seeded in 96-well plates for 24 h and then incubated in different concentration of Vinp for 24 h or 48 h. The absorbance at 450 nm was measured with a spectrophotometer.
Western blotting
In vitro experiments, the total protein extracted from osteoblast was separated using RIPA lysis buffer with proteinase inhibitor. In vivo experiments, the unilateral femoral heads of all rats were washed twice in ice-cold PBS, and then dissolved in a RIPA, and then ground in liquid nitrogen for about half an hour followed by 10 min microcentrifugation at 12000 rpm for 10 minutes at 4°C. The protein concentration was measured by BCA assay kit. 40 ng of protein was separated by SDS PAGE and transferred to a PVDF membrane. After blocking with 5% BSA for 2 h, the membranes were incubated with the primary antibody overnight at 4°C, and followed by incubation with secondary antibodies for 2 h at room temperature. Finally, the expression of protein was quantified with Image Lab 3.0 software (Bio-Rad).
ALP staining
After 7 days cultured, cells were fixed with 4% paraformaldehyde. Then, they were stained with 5-Bromo-4-chloro-3-indolyl phosphate and Nitroblue tetrazolium chloride. Digital images were captured through a stereomicroscope.
Reactive oxygen species (ROS)
ROS in osteoblast was measured by using a ROS assay kit. In breif, osteoblasts were washed with HBSS. Cells were then loaded with 1 μM DCFH-DA in α-MEM and cultured for 15 min in darkness at room temperature. Then, Osteoblasts were washed with PBS and photographed in fluorescent microscope.
ROS quantification by flow cytometry
To determine the oxidation of the intracellular fluorophore DCFH-DA, we used Reactive Oxygen Assay Kit according to its protocols [17].
Apoptosis analysis
Apoptosis was determined by using an PE Annexin V/7AAD kitaccording to its protocols [18]. Osteoblasts were harvested and resuspended in 200 μL of 1 × binding buffer and stained with PE/7AAD. After incubated in dark at room temperature for 30 min, we analyzed it by a flow cytometer (EpicsAltra; Beckman Coulter, Fullerton, CA, United States).
Μicro-ct scanning
The specimens were scanned with a micro-CT scaning (Bruker, Billerica). The diagnostic standard for femoral head necrosis is as reported [19]. BMD, BV/TV, Tb.N, and Tb.Sp as previously reported [20].
Rat model in vivo
12-weeks-old adult male SD rats were choosed to build ONFH animal model. We divided 45 rats into three groups (the control group, the MPS group and the MPS+Vinp group). The ONFH group and the MPS+Vinp group were received intraperitoneal injection with LPS on first two days and got intramuscular injection of 40 mg/kg MPS on the next 3 days. Afterwards, the rats in the MPS+Vinp group received Vinp (10 mg/kg/d) for seven days. The rest of the rats were injected with NS. 8 weeks later, all the rats were sacrificed, and bilateral femoral heads were collected.
Histological analysis
The samples were decalcified in 12.5% EDTA solution for 2 weeks and embedded in paraffin blocks. Lanteral sections (4 µm thickness) were stained with HE. Under the microscope, empty bone lacuna of bone cells, bone marrow cell necrosis and trabecular bone fracture were observed. Record the percentage of empty lacunae.
Statistical analysis
Data are expressed as means ± SD. One-way ANOVA analysis was used for the statistical comparison of multiple groups. Nonparametric data were analyzed by the Kruskal-Wallis H test. Values of P < 0.05 were considered statistically significant. SPSS 21.0 software was used for statistical analysis.
Results
Effect of Vinp on osteoclast viability
The morphology of primary osteoblast is illustrated in Figure 1A. As shown in Figure 1B, the phenotype was determined by ALP staining. Figure 1C illustrated the chemical formula of Vinp. Figure 1D showed that Vinp was cytotoxic to mice osteoblast at 20 µM after 24 h and 48 h, but cell viability was not affected by Vinp (≤ 10 µM). Thus, the concentrations of Vinp (5 and 10 µM) were used in subsequent experiments.
Figure 1.
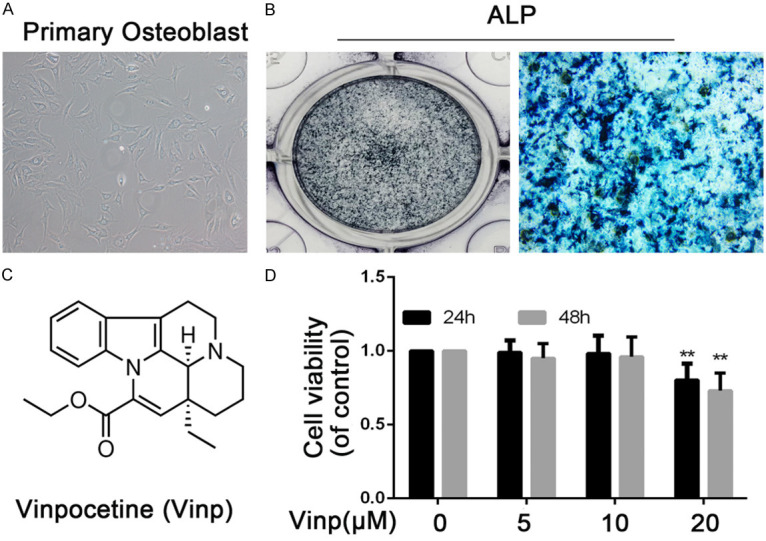
Phenotype determination and viability of rat primary osteoblast. A. The morphology of normal passage two primary osteoblast. B. ALP stain was performed to stain the proteoglycan in osteoblast. C. Chemical structure of Vinp. D. The cytotoxic effect of Vinp on osteoblast was determined at various concentrations for 24 and 48 hours using a CCK8 assay. The values presented are the means ± S.D. of three independent experiments. **P < 0.01 vs. control group.
Vinp attenuates Dex-induced osteoblasts apoptosis via activating the Akt pathway
The flow cytometry results revealed that apoptotic cells signifcantly elevated when treated with Dex (Figure 2A, 2B). Moreover, treatment with Vinp (5, 10 μM) significantly decreased apoptotic cells compared with the Dex group. To investigate the mechanism, WB was performed to explore changes in the Akt pathway. Compared with the control group, Dex signifcantly decreased the p-Akt expression (P < 0.01). Interestingly, treatment with Vinp obviously reversed p-Akt level in Dex-induced osteoblasts apoptosis (P < 0.01).
Figure 2.
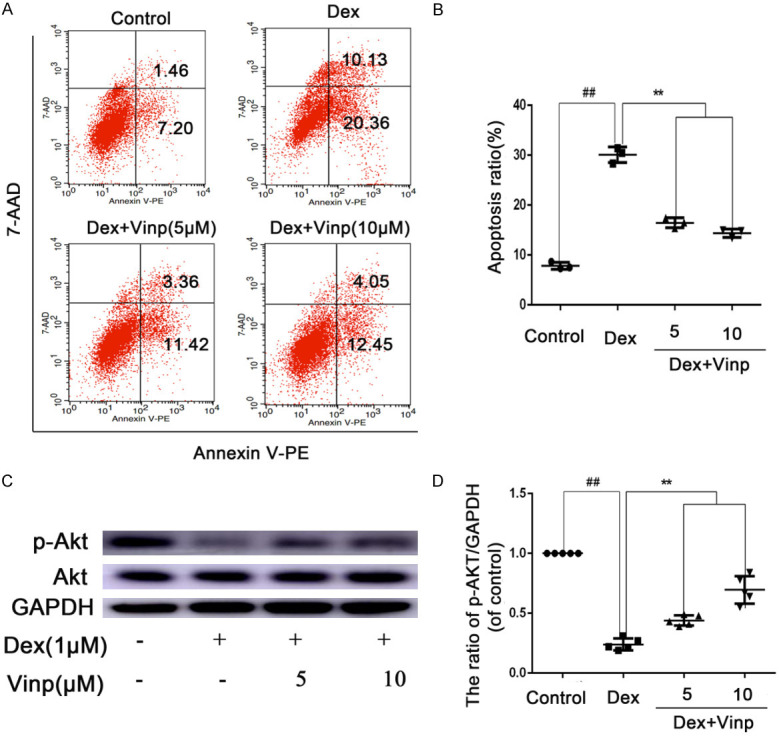
Vinp attenuates Dex-induced osteoblasts apoptosis via activating the Akt pathway. Osteoblasts were treated with various concentrations of Vinp (5 and 10 μM) and with or without stimulated with Dex (10 μM) for 24 h or 2 h. (A) Apoptotic osteoblasts detection by flow cytometry. Quantitative analyze of Apoptosis rate was conducted (B). The protein expressions of Akt and p-Akt in osteoblasts were visualized by western blotting (C), and quantified in (D). The data in the figures represent the averages ± S.D. ##P < 0.01 compared with Control group. **P < 0.01 compared with Dex group. n = 3.
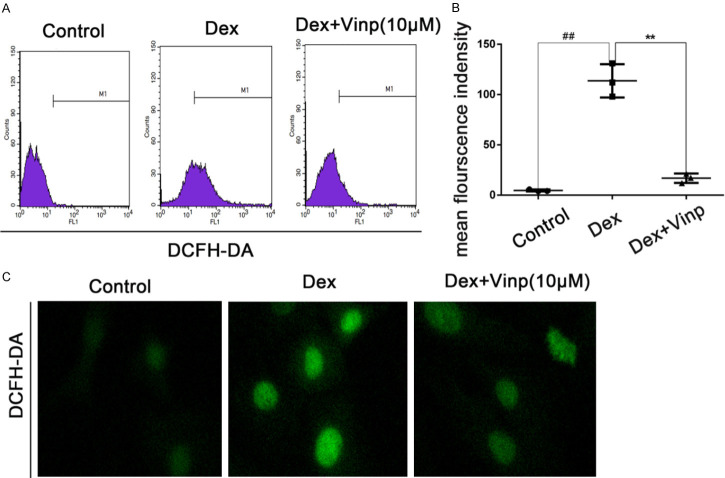
Vinp reduces ROS levels in osteoblast
Osteoblasts were treated with Vinp, in a manner with 10 μM, suppressed the upregulation of ROS stimulated by Dex (Figure 3A-C).
Figure 3.
Vinp reduces ROS Levels in osteoblast. Osteoblasts were stimulated with Dex in the presence or absence of Vinp at the concentrations of 10 μM for 24 h. A. Reactive oxygen species (ROS) detection by flow cytometry. B. Quantitative analyze of ROS mean flourscence was conducted. C. Intracellular ROS was determined by DCFH-DA staining; Data are expressed as mean ± SD. All experiments were repeated three times.
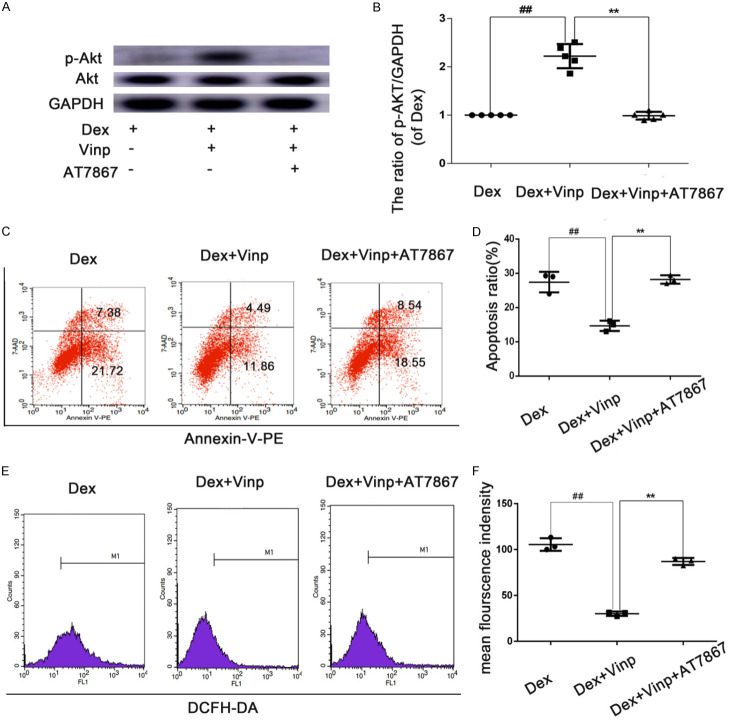
Effects of Dex, Vinp with Dex, and AT7867 with Dex on the Akt signaling pathway, apoptosis and ROS level
To figure out whether the Akt pathway plays an important part in Dex-induced apoptotic response, we used AT7867, an Akt inhibitor. As shown in Figure 4A, 4B, AT7867 partially suppressed the phosphorylation of Akt after treatment with Dex. Consistent with the above data, the number of apoptotic cells (Figure 4C, 4D) and the level of ROS were also rebounded (Figure 4E, 4F).
Figure 4.
Effects of Dex, Vinp with Dex, and AT7867 with Dex on the Akt signaling pathway, apoptosis and ROS level. (A, B) Vinp significantly increased levels of p-Akt, an effect which could be partially reversed through the addition of Vinp or by treatment with AT7867. The effects of Vinp on apoptosis and ROS were abolished (C-F). The values are mean ± SD of five independent experiments. ##P < 0.01 compared with Dex group. **P < 0.01 compared with Dex+Vinp group.
Vinp protected against ONFH in rats by decreasing the percentage of empty lacunae
As shown in Figure 5A, 5B, the percentage of empty lacunae increased signifcantly in the MPS group. Moreover, MPS+Vinp decreased the percentage of empty lacunae compared with the MPS group.
Figure 5.
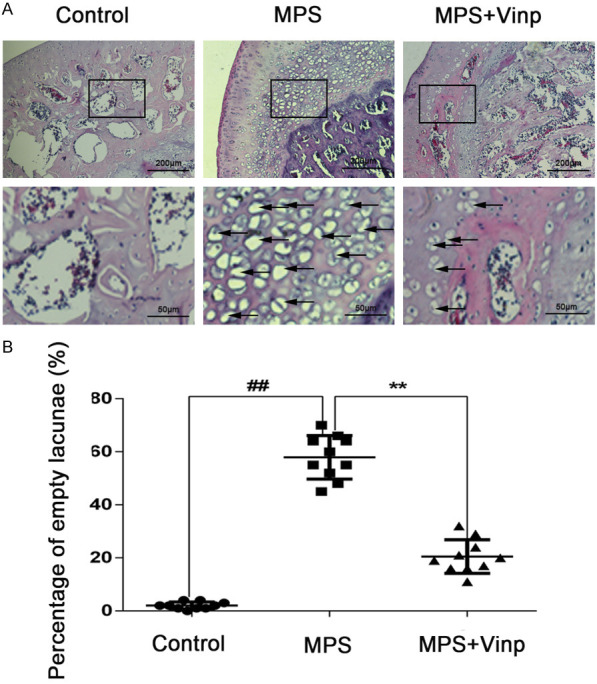
Morphological changes in the femoral head as seen on H&E staining in the control, MPS, and MPS+Vinp groups. In the femoral head of control group, no empty bone lacunae could be found (A). Massive empty lacunae (black arrows) surrounded by necrotic marrow cells are seen in the MPS group, with fewer empty bone lacunae in the MPS+Vinp group. The ratio of empty lacunae was visibly higher in the model group than in either the control or Vinp group (B). ##P < 0.01 compared with Control group. **P < 0.01 compared with MPS group.
Vinp prevents bone loss in ONFH rats
Micro-CT was taken to detect the osteonecrosis. The femoral heads in control group was smooth and integrated. However, in the MPS group, the subchondral trabeculae were severely destroyed. Compared with MPS group, the subchondral trabeculae in MPS+Vinp group were reversed. The quantitative analysis of BMD, BV/TV, Tb.N, and Tb.Sp were signifcantly higher in the MPS+Vinp group compared to the MPS group (Figure 6A, 6B).
Figure 6.
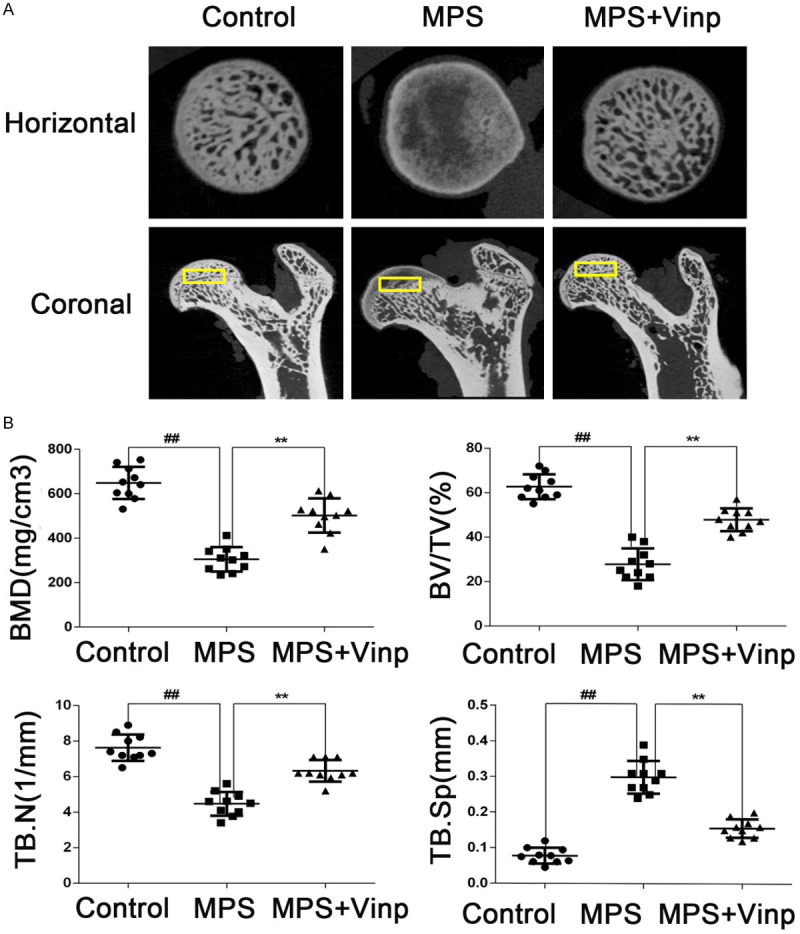
Vinp prevents bone loss. Yellow blocks indicate the region of interest (ROI, 1.5 mm × 1.5 mm × 0.5 mm) in the rat femoral heads of the three groups (A). Horizontal and coronal CT images show damage to the subchondral trabeculae MPS group. Quantitative analysis of trabeculae revealed that bone mineral density, bone volume/total volume, trabecular number, and trabecular separation were significantly higher in the Flu group compared to the model group (B). Data represent the average ± S.D. ##P < 0.01 compared with Control group. **P < 0.01 compared with MPS group.
The in vivo protective effect of Vinp against apoptosis
To explore whether the protective effect of Vinp in MPS-induced ONFH involves osteoclast differentiation and apoptosis, expression of the proteins related to osteoclast differentiation and apoptosis was examined by WB analysis. However, Vinp had no effect on osteoclast differentiation. In contrast, Vinp obviously inhibited Dex-stimulated upregulation of protein expression, including Cleaved-caspase3 and Bax, while significantly inhibiting the downregulation of Bcl-xl and Bcl2 (Figure 7A-D).
Figure 7.
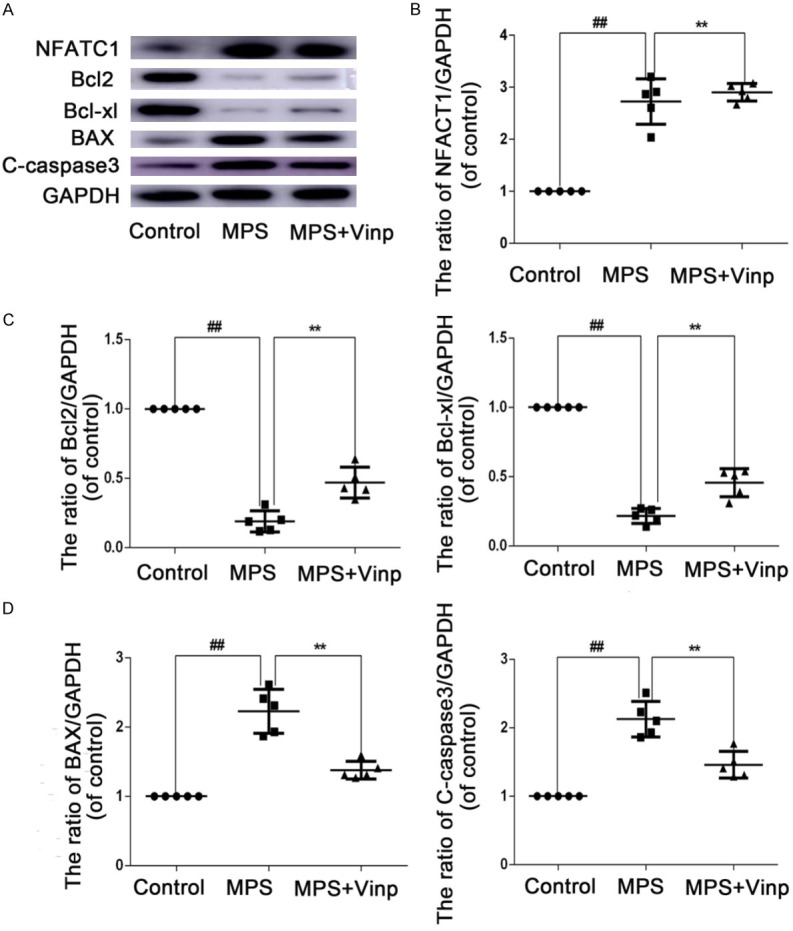
The in vivo protective effect of Vinp against apoptosis. Protein expression was assessed by Western blot analysis (A) and quantification of the resulting bands (B-D). The data in the figures represent the averages ± S.D. Significant differences between the treatment and control groups are indicated as **P < 0.01. n = 3.
Discussion
ONFH is one of the most widespread chronic joint diseases [21]. The specific molecular mechanism of ONFH remains unsuspected and without any effective drug. Among various hypothesis, apoptosis of osteoblast is one of the most studied mechanisms. PI3K/Akt-Bax/Bcl-2/caspase3 pathway has been accounted as an apoptotic signal in several orthopedic diseases [20,22]. Vinp, extracted from the leaves of Phyllostachys pubescens, has been reported relevant to a large range of anti-apoptosis activities in numerous apoptosis-associated disorders [21,23]. Nevertheless, its specific mechanisms involved in ONFH remain unknown. In this study, we evaluated whether Vinp was protective against Dex stimulated apoptosis in ONFH.
The balance between osteoblasts and osteoclasts determines the fate of the femoral head, which has been widely accepted [6]. Contrary to Zhu’s research [24], we found that Vinp didn’t inhibit osteoclast differentiation in ONFH model. Numerous researches have concentrated on osteoblast apoptosis [9,10]. Consequently, we focus on the osteoblasts. In our flow cytometry result illustrated that Vinp inhibited Dex-induced apoptosis in ostoblast. Additionally, activation of Akt pathway can inhibit osteoblast apoptosis [25]. Moreover, activation of Akt pathway is considered to be an option for treatment of ONFH. In our study, we found that Vinp activated Akt phosphorylation in osteoblast, which was partly in accordance to previous study showing that Vinp exerted inhibitory effects on RANKL-induced osteoclastogenesis via inhibition PI3K/Akt signaling pathway [24]. Previous study showed that Vinp attenuated the Osteoblastic Differentiation of Vascular Smooth Muscle Cells [26]. From our perspectives, if vinpocetine simply inhibits osteoblast differentiation, there will be a phenotype that promotes necrosis of the femoral head. Our animal experiments showed that Vinp inhibited cell apoptosis and inhibited the progress of ONFH. Therefore, we believed that osteoblast differentiation may involved in ONFH but not the dominant mechanism. Osteoblast apoptosis might be the dominant factor.
Dex can increase the expression of reactive oxygen species (ROS). Excess ROS may lead to apoptosis through the mitochondrial caspase apoptosis pathway [22]. Moreover, the accumulation of ROS will cause oxidative stress and activate the JNK pathway in osteoblasts, thereby inactivating the Akt pathway and promoting osteoblast apoptosis [2]. Deng et al. demonstrated that reducing ROS is effective for ONFH [25]. Our results illustrated that Vinp reduced Dex-induced increased levels of ROS, thus demonstrating the potential value of Vinp in down-regulating oxidative stress. Our experiments showed that AT7867, an Akt inhibitor, partially suppressed the Dex-induced Akt activation. The effects of Vinp on apoptosis and ROS were abolished. A previous study showed that caspase3, an important member of the caspase family, is activate in ONFH [3]. Meanwhile, some researchers have found that the level of caspase-3 is dramatically elevated in ONFH [27]. Xue et al. reported that phosphorylation of Akt in osteoblasts to can enhance the anti-apoptotic function of Bcl2 [28]. The Bcl2 protein family includes the pro-apoptotic protein Bax and the apoptosis-inhibiting proteins Bcl-xl and Bcl2 [29]. In our in vivo experiments, MPS stimulation obviously elevated the level of Bax and cleaved caspase3 while decreasing the levels of Bcl2 and Bcl-xl. All changes above were reversed in Vinp-treated rats.
To further explore the protective effect of Vinp in vivo, we established an animal model of ONFH in this study. The scheme of LPS and MPS is a highly efficient method to make an animal ONFH model for this in vivo analysis. The empty lacunae represent the degree of osteonecrosis and reflect the effect of bone repair to a certain extent. However, all the histological changes were attenuated by the pre-treatment of Vinp. Compared with the MPS group, the Vinp group had lower empty lacunae. These in vivo experiments above were in keeping with the in vitro studies and suggested that Vinp was safe and effective in vivo.
Taken together, we demonstrate that Vinp impeded the Dex-stimulated apoptosis via activating the inhibition of Akt signaling via blocking the activation of ROS level in osteoblast. All these data suggest that the use of Vinp as a promising regimen in the treatment of ONFH.
Acknowledgements
The study was supported by Jiaxing Public Welfare Science and Technology Project, Zhejiang Province, China (Grant No. 2019AD32194).
Disclosure of conflict of interest
None.
References
- 1.Petek D, Hannouche D, Suva D. Osteonecrosis of the femoral head: pathophysiology and current concepts of treatment. EFORT Open Rev. 2019;4:85–97. doi: 10.1302/2058-5241.4.180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cakir E, Yilmaz A, Demirag F, Oguztuzun S, Sahin S, Yazici UE, Aydin M. Prognostic significance of micropapillary pattern in lung adenocarcinoma and expression of apoptosis-related markers: caspase-3, bcl-2, and p53. Apmis. 2011;119:574–580. doi: 10.1111/j.1600-0463.2011.02778.x. [DOI] [PubMed] [Google Scholar]
- 3.Dai WW, Wang LB, Jin GQ, Wu HJ, Zhang J, Wang CL, Wei YJ, Lee JH, Lay YE, Yao W. Beta-ecdysone protects mouse osteoblasts from glucocorticoid-induced apoptosis in vitro. Planta Med. 2017;83:888–894. doi: 10.1055/s-0043-107808. [DOI] [PubMed] [Google Scholar]
- 4.Gao Y, Zhu H, Wang Q, Feng Y, Zhang C. Inhibition of PERK signaling prevents against glucocorticoid-induced endotheliocyte apoptosis and osteonecrosis of the femoral head. Int J Biol Sci. 2020;16:543–552. doi: 10.7150/ijbs.35256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HJ. New understanding of glucocorticoid action in bone cells. BMB Rep. 2010;43:524–529. doi: 10.5483/bmbrep.2010.43.8.524. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, Chi JT, Salter AB, Lento WE, Reya T, Chao NJ, Chute JP. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souttou B, Raulais D, Vigny M. Pleiotrophin induces angiogenesis: involvement of the phosphoinositide-3 kinase but not the nitric oxide synthase pathways. J Cell Physiol. 2001;187:59–64. doi: 10.1002/1097-4652(2001)9999:9999<00::AID-JCP1051>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Feng Z, Zheng W, Tang Q, Cheng L, Li H, Ni W, Pan X. Fludarabine inhibits STAT1-mediated up-regulation of caspase-3 expression in dexamethasone-induced osteoblasts apoptosis and slows the progression of steroid-induced avascular necrosis of the femoral head in rats. Apoptosis. 2017;22:1001–1012. doi: 10.1007/s10495-017-1383-1. [DOI] [PubMed] [Google Scholar]
- 10.Nie Z, Deng S, Zhang L, Chen S, Lu Q, Peng H. Crocin protects against dexamethasone-induced osteoblast apoptosis by inhibiting the ROS/Ca2+-mediated mitochondrial pathway. Mol Med Rep. 2019;20:401–408. doi: 10.3892/mmr.2019.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, Yan C, Wei C, Yao Y, Ma X, Gong Z, Liu S, Zang D, Chen J, Shi FD, Hao J. Vinpocetine inhibits NF-κB-dependent inflammation in acute ischemic stroke patients. Transl Stroke Res. 2018;9:174–184. doi: 10.1007/s12975-017-0549-z. [DOI] [PubMed] [Google Scholar]
- 12.Zhang P, Cao Y, Chen S, Shao L. Combination of vinpocetine and dexamethasone alleviates cognitive impairment in nasopharyngeal carcinoma patients following radiation injury. Pharmacology. 2020:1–8. doi: 10.1159/000506777. [DOI] [PubMed] [Google Scholar]
- 13.Sönmez MF, Ozdemir Ş, Guzel M, Kaymak E. The ameliorative effects of vinpocetine on apoptosis and HSP-70 expression in testicular torsion in rats. Biotech Histochem. 2017;92:92–99. doi: 10.1080/10520295.2016.1259499. [DOI] [PubMed] [Google Scholar]
- 14.Fattori V, Borghi SM, Guazelli CFS, Giroldo AC, Crespigio J, Bussmann AJC, Coelho-Silva L, Ludwig NG, Mazzuco TL, Casagrande R, Verri WA Jr. Vinpocetine reduces diclofenac-induced acute kidney injury through inhibition of oxidative stress, apoptosis, cytokine production, and NF-κB activation in mice. Pharmacol Res. 2017;120:10–22. doi: 10.1016/j.phrs.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Hou S, Feng L, Shen P, Nan D, Zhang Y, Wang F, Ma D, Feng J. Vinpocetine protects against cerebral ischemia-reperfusion injury by targeting astrocytic connexin43 via the PI3K/AKT signaling pathway. Front Neurosci. 2020;14:223. doi: 10.3389/fnins.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinz N, Jücker M. Distinct functions of AKT isoforms in breast cancer: a comprehensive review. Cell Commun Signal. 2019;17:154. doi: 10.1186/s12964-019-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oksvold MP, Skarpen E, Widerberg J, Huitfeldt HS. Fluorescent histochemical techniques for analysis of intracellular signaling. J Histochem Cytochem. 2002;50:289–303. doi: 10.1177/002215540205000301. [DOI] [PubMed] [Google Scholar]
- 18.Tints K, Prink M, Neuman T, Palm K. LXXLL peptide converts transportan 10 to a potent inducer of apoptosis in breast cancer cells. Int J Mol Sci. 2014;15:5680–5698. doi: 10.3390/ijms15045680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Y, Li Y, Huang C, Gao K, Weng X. Systemic application of teriparatide for steroid induced osteonecrosis in a rat model. BMC Musculoskelet Disord. 2015;16:163. doi: 10.1186/s12891-015-0589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Qian W, Weng X, Wu Z, Li H, Zhuang Q, Feng B, Bian Y. Glucocorticoid receptor and sequential P53 activation by dexamethasone mediates apoptosis and cell cycle arrest of osteoblastic MC3T3-E1 cells. PLoS One. 2012;7:e37030. doi: 10.1371/journal.pone.0037030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Wen L, Peng W, Li H, Zhuang J, Lu Y, Liu B, Li X, Li W, Xu Y. Vinpocetine attenuates neointimal hyperplasia in diabetic rat carotid arteries after balloon injury. PLoS One. 2014;9:e96894. doi: 10.1371/journal.pone.0096894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravindran J, Gupta N, Agrawal M, Bala Bhaskar AS, Lakshmana Rao PV. Modulation of ROS/MAPK signaling pathways by okadaic acid leads to cell death via, mitochondrial mediated caspase-dependent mechanism. Apoptosis. 2011;16:145–161. doi: 10.1007/s10495-010-0554-0. [DOI] [PubMed] [Google Scholar]
- 23.Huang EW, Xue SJ, Zhang Z, Zhou JG, Guan YY, Tang YB. Vinpocetine inhibits breast cancer cells growth in vitro and in vivo. Apoptosis. 2012;17:1120–1130. doi: 10.1007/s10495-012-0743-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhu M, Liu H, Sun K, Liu J, Mou Y, Qi D, Zhou C, Abudunaibi M, Tasiken B, Li J, Cheng H, Huang H. Vinpocetine inhibits RANKL-induced osteoclastogenesis and attenuates ovariectomy-induced bone loss. Biomed Pharmacother. 2020;123:109769. doi: 10.1016/j.biopha.2019.109769. [DOI] [PubMed] [Google Scholar]
- 25.Deng S, Zhou JL, Fang HS, Nie ZG, Chen S, Peng H. Sesamin protects the femoral head from osteonecrosis by inhibiting ros-induced osteoblast apoptosis in rat model. Front Physiol. 2018;9:1787. doi: 10.3389/fphys.2018.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma YY, Sun L, Chen XJ, Wang N, Yi PF, Song M, Zhang B, Wang YZ, Liang QH. Vinpocetine attenuates the osteoblastic differentiation of vascular smooth muscle cells. PLoS One. 2016;11:e0162295. doi: 10.1371/journal.pone.0162295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Wen H, Hu Y, Yu H, Zhang Y, Chen C, Pan X. STAT1-caspase 3 pathway in the apoptotic process associated with steroid-induced necrosis of the femoral head. J Mol Histol. 2014;45:473–485. doi: 10.1007/s10735-014-9571-6. [DOI] [PubMed] [Google Scholar]
- 28.Xue XH, Feng ZH, Li ZX, Pan XY. Salidroside inhibits steroid-induced avascular necrosis of the femoral head via the PI3K/Akt signaling pathway: in vitro and in vivo studies. Mol Med Rep. 2018;17:3751–3757. doi: 10.3892/mmr.2017.8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16:203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]