Abstract
In animal models, hepatocytes can be reprogrammed into insulin-producing cells (IPCs) for a novel antidiabetic treatment. However, the potential for an immunologic reaction and issues with gene integration of the viral vehicle hamper system efficacy. Here, we adopted an Ultrasound Targeted Microbubble Destruction (UTMD) enhanced hydrodynamic gene delivery system in a streptozotocin induced mouse diabetic model to examine its treatment effect. After transfection by combining UTMD and hydrodynamic injection, accumulated luciferase signal was only found in the liver with optimal signal intensity. Liver function tests showed an increase in alanine aminotransferase level followed by a decrease to normal levels. Then this new gene delivery system was used to deliver Pdx1, Neurog3, and MafA plasmids into diabetic mice. We found that glucose levels gradually decreased, and insulin levels increased in transfected diabetic mice compared to controls. Glucose intolerance in transfected mice was alleviated. Gene expression assay confirmed the reprogramming of hepatocytes. We demonstrated the feasibility of repeated plasmid transfection in vivo by UTMD enhanced hydrodynamic gene delivery system.
Keywords: UTMD, hydrodynamic, reprogramming, hepatocyte, insulin producing cells, diabetes
Introduction
Reprogramming of adult non-pancreatic cells into Insulin-Producing Cells (IPCs) has been shown to be a novel molecular way to treat diabetes in animal models [1]. Direct reprogramming refers to inducing trans-differentiation of one type of mature somatic cell into another cell type without dedifferentiation back to multipotent stem cells. Adenovirus vector-mediated transfection of genes encoding multiple pancreatic development-related transcription factors (TFs) including Pdx1, Neurog3 and MafA, effectively reversed hyperglycemia symptoms by reprogramming hepatocytes into IPCs in mouse models [2,3]. Exogenous TFs are able to switch the progression of trans-differentiation from hepatocytes to IPCs. However, viral vectors have certain drawbacks, such as the possibility for immunologic reactions and gene integration issues [4]. Thus, this approach has been restrained from extensive clinical application.
Ultrasound targeted microbubbles destruction (UTMD) has become a promising method of gene delivery due to its non-invasiveness, safety and versatility to concentrate sound energy on specific areas of the organ [5], thus has been applicated into several diseases [6-9]. It is speculated that cavitation plays a major role in ultrasound-induced membrane permeability or acoustic perforation [10]. Acoustic pressure, pulse repetition frequency, duty cycle are the key factors for cavitation activity. However, for efficient ultrasound-mediated gene delivery, plasmids need to be localized at the site of exposure because ultrasound is limited in the process of transporting genetic material across various tissue barriers without causing significant collateral damage. Hydrodynamic gene delivery showed a tissue-specific pattern with a liver transfection efficiency of 40% using a plasmid concentration of less than 50 μg [11]. Increased endothelial and parenchymal cell permeability caused by hydrodynamic pressure in the hepatic sinusoid permitted entrance of plasmid DNA into hepatocytes [12]. Opened cell membranes can be reclosed within minutes, and the enlarged liver returns to normal within 24 hours [13]. Despite this, it has been observed that, following hydroporation, much of the plasmid is bound to the outer surface of the plasma membrane for more than 1 hour, indicating insufficient permeabilization from hydrodynamic pressure [14]. Therefore, UTMD and hydrodynamic injection are complementary. Ultrasound induced cavitation, as additional mechanical forces, is capable of enhancing the entry of DNA molecules into the cytoplasm; thus could further enhance the efficiency of hydrodynamic-induced gene delivery. In this study, we aimed to investigate the efficiency of UTMD enhanced hydrodynamic gene delivery in in vivo hepatocytes and antidiabetic treatment efficacy and safety of Pdx1, Neurog3, and MafA (PNM) transfection by this gene delivery method in a diabetic mouse model.
Materials and methods
Plasmids
The plasmids, containing the human transcription factor Pdx1, Neurog3, MafA gene, driven by the CMV promoter, was constructed and kept in our laboratory [15]. Luciferase reporter plasmid with same vector backbone was used as control. Plasmids were further purified with ZymoPURE II Plasmid Maxiprep Kit (Zymo research, USA).
Microbubbles
SonoVue microbubble contrast agent (Bracco, Milan, Italy) was prepared according to the manufacturer’s instructions. Firstly, microbubble was reconstituted in 5 ml of saline solution, fully mixed and securely capped. SonoVue microbubbles are lipid-shelled and filled with sulfur hexafluoride gas, containing approximately 2-5×108 microbubbles/ml, with an average diameter of 2.5-6.0 μm.
Experimental animals
Male C57BL/6N mice (age: 6 weeks, weight: 18-20 g) were used for this study (Guangdong Medical Laboratory Animal Center, Guangdong, China). All mice were housed under pathogen-free conditions. Animal procedures were approved by the Institutional Animal Care and Use Committee of the Second clinical Medical College, Jinan University (20180226046). The diabetic mouse model was established by streptozotocin (STZ) administration. Mice were fasted overnight for 14 hours, and 2% STZ (Sigma, USA) was injected intraperitoneally at a dosage of 60 mg/kg once a day for 3 consecutive days. Mice with blood glucose level of 300 mg/dl or greater for 3 consecutive days, which was stable for at least 1 week, were considered as diabetic.
UTMD enhanced hydrodynamic gene injection
The ultrasound signal is generated by the Shenzhen Welder Ultrasonic Cavitation Therapy Instrument. The non-focusing probe has a diameter of 2 cm and the ultrasonic depth is 5-6 cm. The default US condition was 1 MHz frequency, an acoustic peak negative pressure amplitude of 1 MPa, 20 cycle pulses, 50 Hz PRF and total ultrasonic time 5 min with an interval of 6 s. Different plasmid dose, acoustic peak negative pressure and duty cycles has been screened to achieve optimal gene delivery efficiency. The parameters were shown in Table 1.
Table 1.
List of ultrasound parameters used in UTMD enhanced hydrodynamic injection
| Group | UTMD Bolus 2.5 | UTMD Bolus 3.0 | UTMD Bolus 3.5 | UTMD Bolus 0.5% | UTMD Bolus 2% | UTMD Bolus 0.5 MPa | UTMD Bolus 1.5 MPa |
|---|---|---|---|---|---|---|---|
| Number of animals | 5 | 6 | 6 | 5 | 4 | 7 | 5 |
| Bolus, 8% | Bolus, 8% | Bolus, 8% | Bolus, 8% | Bolus, 8% | Bolus, 8% | Bolus, 8% | Bolus, 8% |
| Plasmid Dose | 2.5 μg/g | 3.0 μg/g | 3.5 μg/g | 2.5 μg/g | 2.5 μg/g | 2.5 μg/g | 2.5 μg/g |
| Peak negative pressure (MPa) | 1 | 1 | 1 | 1 | 1 | 0.5 | 1.5 |
| Number of cycles | 200 | 200 | 200 | 100 | 400 | 200 | 200 |
| PRF | 50 | 50 | 50 | 100 | 25 | 50 | 50 |
| MBs/sonication | 10 μl/g | 10 μl/g | 10 μl/g | 10 μl/g | 10 μl/g | 10 μl/g | 10 μl/g |
| Time US on (min) | 5 min | 5 min | 5 min | 5 min | 5 min | 5 min | 5 min |
| Total exposure time | 1.5 s | 1.5 s | 1.5 s | 0.75 s | 3.0 s | 1.5 s | 1.5 s |
PRF: Pulse repetition frequency; MBs: Microbubbles; US: Ultrasound.
The hydrodynamic tail vein injection procedure has been reported previously [16], indicated as bolus in the study. Total injection volume was equal to 8% body weight. The microbubble dose was 10% (v/v) of the injection volume. Besides the plasmid dose, the remaining volume was supplemented with physiological saline. Briefly, for a 20-gram weight mouse, 1.6 mL delivery solution containing 50 μg plasmid DNA and 160 μL microbubbles was injected into the tail vein within 5-8 seconds. Then the animal was placed on a constant temperature heating pad for isoflurane anesthesia induction. Within 1 minute, unfocused ultrasound irradiation was performed for 5 minutes with an ultrasonic coupling agent thickness of approximately 2 mm.
Pathology test
On days 7 post-transfection, mouse livers were dissected from mice in the UTMD Bolus group (n = 3). Liver was fixed in 10% formalin, embedded in paraffin, and sectioned into 5-μm slides for hematoxylin and eosin staining. Pathological changes were observed under the microscope (Olympus IX71, Japan).
Immunofluorescence staining
For immunostaining, mouse livers and pancreas were dissected and fixed in 4% paraformaldehyde. Tissue blocks were embedded in O.C.T. compound (SAKURA Tissue-Tek, PA, USA), and 7-μm sections were prepared for immunostaining. Frozen tissue sections were permeabilized and blocked with 0.2% Triton X-100 plus 1% bovine serum albumin. Sections were incubated with guinea pig anti-insulin (1:100; Abcam, Cambridge, UK) or sheep anti-glucagon (1:200; Abcam, Cambridge, UK) at 4°C overnight, followed by incubation with secondary antibodies of Alexa Fluor 568-conjugated goat anti-guinea pig IgG (1:1000; Abcam) or FITC-conjugated rabbit anti-sheep IgG (1:300, Abcam). Nuclei were stained with 4’,6-diamidino-2-phenylindole (1:800, Invitrogen). Images were observed and captured under a Leica microscope (Leica, DMi8) with its software LAS X.
In vivo bioluminescence imaging
Efficiency of hydrodynamic delivery of the luciferase plasmid was assessed by in vivo bioluminescence imaging of luciferase activity using an IVIS Spectrum system (Perkin-Elmer, Waltham, MA) at 24, 48, 72, and 168 hours post-injection. Mice were anesthetized with isoflurane, followed by intraperitoneal injection of 150 mg/kg of D-luciferin (Goldbio, USA). Eight consecutive scans (1 min acquisition time/scan) were taken after 10 min. Images were processed by using Living Image 4.2 software. An ellipsoidal region of interest was drawn over the liver. Data were shown in radiance units (photons/s/cm2/steradian) for statistical analysis. To determine the distribution of luciferase activity, mice were euthanized, and liver, spleen, kidney, pancreas, heart, stomach, gastrointestinal tract, and brain were dissected 10 minutes after D-luciferin injection.
Study design
Male C57BL/6J mice were either induced by STZ to develop diabetes or defined as a healthy control group. Mice were divided into four groups: (1) healthy control mice (n = 8); (2) diabetic control mice (n = 8); (3) hydrodynamic-injection diabetic mice (n = 5); (4) UTMD Bolus diabetic mice (n = 5). Mice were evaluated from the day of completion of transfection (day 0) until the end of the experiment (day 126). Evaluation included measurements of glucose metabolism and gene expression levels. Fasting glucose levels were measured at 4:00 PM every day for the first week, followed by once every 3 days until day 21, and once a week until day 126. Insulin level was measured and an intraperitoneal glucose tolerance test (IPGTT) was performed on days 14, 56, 100, and 126 post-transfection. Gene expression levels were determined on days 126.
Biochemical tests
Fasting blood glucose levels were measured by glucometer (Life Scan Canada Ltd.) every week post-injection at 4:00 PM until day 126. Insulin levels were measured by mouse Insulin ELISA kits (Mercodia, Sweden) according to the manufacturer’s protocol. IPGTT was performed after a 6-hour fast in mice. Glucose solution (2 g/kg) was injected intraperitoneally, and blood glucose was measured from the tail vein at 0, 15, 30, 60, 120, and 180 min after glucose injection.
Real time RT-PCR
Total RNA was isolated from mouse liver by using TRIzol reagent (TaKaRa, Tokyo Japan). A 1-μg aliquot of RNA was reverse-transcribed into cDNA by using a Prime Script RT Reagent Kit with gDNA Eraser (TaKaRa), according to the manufacturer’s protocol. Quantitative real-time PCR using SYBR Green Premix EX Taq (TaKaRa) was performed to determine relative gene expression on a Biorad CFX 96 touch real-time PCR detection system. Ct values were normalized to β-actin, and expression level was calculated by the 2-ΔΔCt method. All primer sequences are listed in Table 1.
Statistical analysis
All data were analyzed using Graphpad Prism software. Continuous variables are expressed as the mean ± standard deviation. Statistical analysis was performed using one-way Analysis of Variance (ANOVA) to compare differences among different groups. T-tests were used to compare differences between two groups. Blood glucose levels and body weight were compared using repeated measures ANOVA. All experiments were performed in technical triplicate. Differences with a P value less than 0.05 were considered statistically significant.
Results
In vivo transfection efficiency
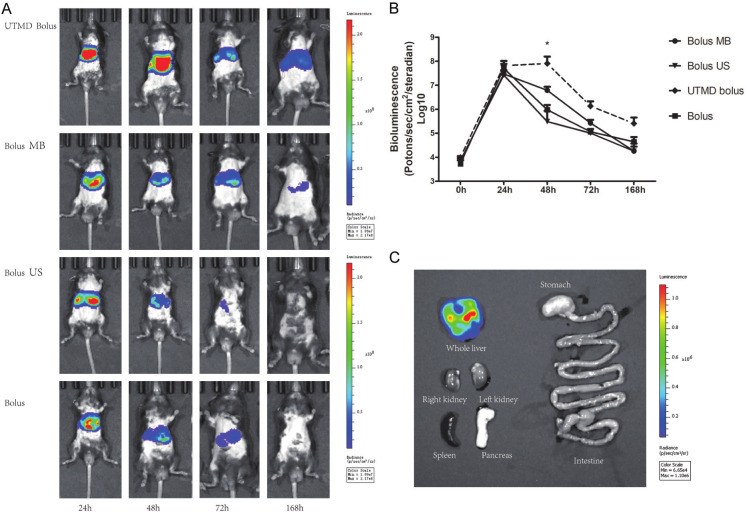
We first assess the transfection efficiency of different gene delivery methods using luciferase vector. We found that both Bolus, bolus with ultrasound (Bolus US), bolus with microbubbles (Bolus MB), and UTMD with bolus (UTMD bolus) effectively induces bioluminescence signals after 24 hours (Figure 1), and no difference was observed between different delivery methods. However, after 24 h, the bioluminescence signals dropped rapidly in bolus, bolus with ultrasound, and bolus with MBs treated mice, while UTMD bolus treated mice maintained high level of bioluminescence signals (Figure 1A, 1B). At 48 hours, the bioluminescence signals in the UTMD bolus treated mice are 8.09±3.82×107 photons/sec/cm2/steradian, comparing to 9.51±5.52×105 photons/sec/cm2/steradian in bolus treated mice, 3.07±2.74×105 photons/sec/cm2/steradian in Bolus US treated mice, and 6.16±2.49×105 photons/sec/cm2/steradian in Bolus MB treated mice. These results indicate that hydrodynamic injection is capable of delivering genes in liver, but gene expression does not last long enough. The application of UTMD can maintain high gene expression up to at least 168 h. Theoretically, gene delivery can be achieved in any regions that are covered by ultrasound signal. Thus, we further examined gene delivery pattern in various tissues, including the liver, gastrointestinal tract, kidneys, and pancreas, which could be covered by ultrasound signal. However, bioluminescence signal was only found in the liver, but not in other tissues examined (Figure 1C), highlighting the specificity of the method.
Figure 1.
In vivo DNA transfection efficiency in the liver of UTMD enhanced hydrodynamic injection. A. Mouse liver bioluminescence images of UTMD enhanced hydrodynamic injection (UTMD bolus) and control groups at different time points. Both Bolus, bolus with ultrasound (Bolus US), bolus with microbubbles (Bolus MB), and UTMD with bolus (UTMD bolus) effectively induces bioluminescence signals after 24 hours. However, at 48 hours, only UTMD bolus treated mice maintained high level of bioluminescence signals, with statistically significant difference vs. Bolus (P < 0.05). *P < 0.05. B. Time-dependent curve of transfection efficacy with different transfection groups (n = 4). UTMD bolus can maintain high gene expression up to at least 168 hours. C. High concentration of bioluminescence signals in mouse liver 48 hours after transfection.
Acoustic pressure [17] and pulse repetition frequency [18] are key factors for ultrasound induced cavitation, and also plasmid concentration has effects on in vivo gene transfection efficiency. Therefore, we tested different parameters of these three factors to achieve optimal in vivo transfection efficiency. When pulse repetition frequency increased to 2%, luciferase expression level in liver increased dramatically (4.19±1.26×107 photons/sec/cm2/steradian), as shown in Figure 2. Gene transfection efficiency tended to increase when plasmid concentration increases. Increasing the peak negative pressure to 1.5 MPa however didn’t enhance gene transfection.
Figure 2.
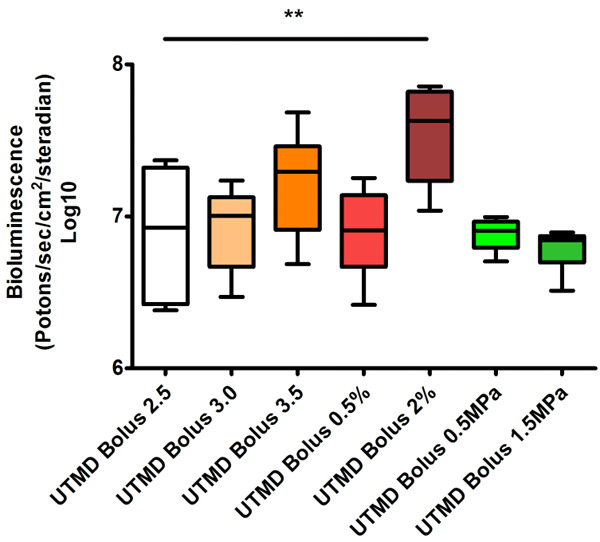
Optimization of ultrasound parameters. Different parameters of acoustic pressure, pulse repetition frequency, and plasmid concentration were tested to achieve optimal in vivo transfection efficiency. Across all groups the statistically significant differences were seen in UTMD Bolus 2% vs. UTMD Bolus 2.5, P < 0.01. There was no statistical difference between UTMD Bolus 3.0 vs. UTMD Bolus 2.5, UTMD Bolus 3.5 vs. UTMD Bolus 2.5, UTMD Bolus 0.5% vs. UTMD Bolus 2.5, UTMD Bolus 0.5 MPa vs. UTMD Bolus 2.5, and UTMD Bolus 1.5 MPa vs. UTMD Bolus 2.5. **P < 0.01. Comparing to UTMD Bolus 2.5, parameters in other groups are the same except one. The details of all groups are listed in Table 1.
Liver functional and pathological damage
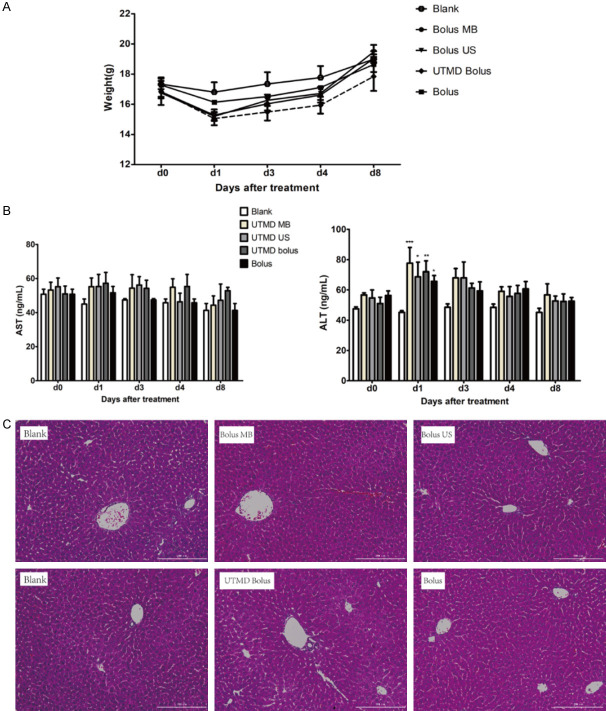
We investigated whether UTMD enhanced hydrodynamic gene delivery would cause any potential damage to the liver damage. As shown in Figure 3A, in the early phase (< 8 days) post transfection, although not significant, body weights of mice received treatments decreased as comparing to control mice without treatment. At day 8, there was not apparent differences in body weight, suggesting that this temporary effect is unlikely caused by liver injury. In agreement with this, we found serum aspartate aminotransferase (AST) activities were unaffected by the treatments (Figure 3B). Mild increase in serum alanine aminotransferase (ALT) activities was found 1 day and 3 days post the treatment (Figure 3B). Serum ALT activities of the mice received treatments were comparable to control mice after 3 days, indicating the liver injury is mild and temporary. Additionally, there were no apparent morphological changes in the liver, revealed by H&E staining (Figure 3C), further excluding the possibility that the treatments cause liver injury.
Figure 3.
Safety investigation after UTMD enhanced hydrodynamic injection. A. Body weights of mice received all treatments decreased as comparing to control mice without treatment in the early days. At day 8, there were not apparent differences in body weight of all groups. B. Liver function after DNA transfection. AST activities were unaffected by all the treatments. Mild increases in ALT activities were found on day 1 post the treatment. AST: aspartate aminotransferase; ALT: alanine aminotransferase. *P < 0.05, **P < 0.01, ***P < 0.001 compared to blank transfection control, as indicated. n = 4 in each group; C. Liver tissue pathology from 8 days mice after transfection (n = 4). There were no apparent morphological changes in the liver of all groups. Scale bar: 100 μm.
Transforming hepatocytes into IPCs alleviates diabetes in mice
We then examined if transforming hepatocytes into IPCs could treat diabetes by monitoring fasting blood glucose levels for up to 126 days. We observed a rapid decreased in fasting blood glucose levels after PNM injection (Figure 4A). Transforming hepatocytes into IPCs using both Bolus and UTMD Bolus reduced the fasting blood glucose levels to a normal level which was maintained until the end of the experiment (Figure 4A). However, the reduction in blood glucose is sharper in mice treated with UTMD Bolus, as compared with mice treated with Bolus only, suggesting that UTMD Bolus is more efficient in transforming hepatocytes. Although reduction in fasting blood glucose is immediate after 7 days, glycemic control, measured by IPGTT at 14th day post transfection, was not improved by both Bolus and UTMD Bolus treatment (Figure 4B). Yet, 56, 100 and 126 days post transfection revealed that UTMD Bolus improves glucose tolerance (Figure 4B). We also measured serum insulin levels in these mice and found that transforming the hepatocytes gradually increased insulin levels (Figure 4C). Again, UTMD Bolus treatment showed higher efficiency than Bolus in rising insulin levels.
Figure 4.
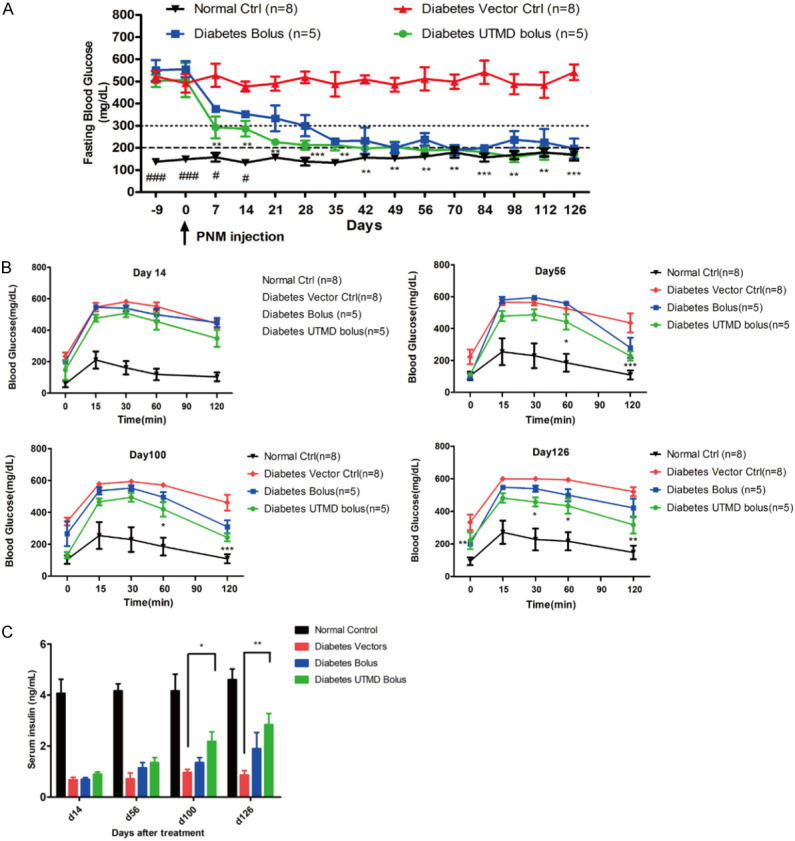
In vivo Pdx1/Neurog3/MafA transfection by UTMD enhanced hydrodynamic injection for antidiabetic treatment. A. Fasting blood glucose level in diabetic mice after transfection. Transforming hepatocytes into IPCs using UTMD Bolus reduced the fasting blood glucose levels to a normal level which was maintained until the end of the experiment. B. Glucose tolerance results in diabetic mice on days 14, 56, 100, and 126 after transfection. UTMD Bolus improves glucose tolerance on day 56, 100 and 126. C. Serum insulin levels in diabetic mice on days 14, 56, 100 and 126 after transfection. Transforming the hepatocytes into IPCs using UTMD Bolus gradually increased serum insulin levels from day 100. Diabetes UTMD Bolus (n = 5), Diabetes Bolus (n = 5), Diabetes vector ctrl (n = 8), Normal ctrl (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 compared to diabetic vector control.
Successful induction of pancreatic β cell specific genes in the liver
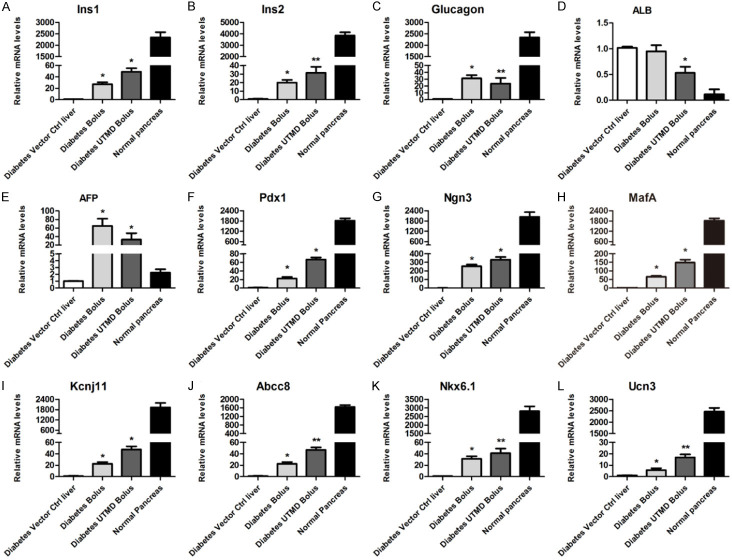
To assess if the transformation of hepatocytes into IPCs are successful, we examined the expression of pancreatic β cell specific genes in the liver (Figure 5). We found that both Bolus and UTMD Bolus treatment induced the expression of Pdx1, Ngn3, and MafA in the liver (Figure 5F-H), validating successful PNM delivery by the treatment. Expression of Ins1 and Ins2 were also significantly increased (Figure 5A, 5B), in accordance with increased serum insulin levels. Interestingly, Gcg, which encodes glucagon, were also induced in the liver (Figure 5C). With the increased expression of pancreatic specific genes, we found the expression of Alb and Afp, two liver-specific genes, were decreased (Figure 5D, 5E). Expression of the key TFs involved in insulin secretion and islet β cell development, including, Kcnj11, Abcc8, and Nkx6-1, were also induced by Bolus and UTMD Bolus mediated PNM delivery (Figure 5I-K). Moreover, expression of Ucn3, a gene only expressed in maturated pancreatic β cell, were highly expressed in UTMD Bolus treated liver, and to a lesser extend in Bolus treated liver (Figure 5L).
Figure 5.
mRNA expression levels of genes relevant to pancreatic β cells and hepatocytes in liver tissue after in vivo transfection. qRT-PCR analysis of pancreatic β cells and hepatocytes marker expression on day 126 (n = 5) after transfection. Normal pancreas was included as positive control for pancreatic β cells marker gene expression. Pancreas-related genes: Ins1: insulin I; Ins2: insulin II; and Gcg: glucagon. Hepatocyte-related genes: Alb: albumin; and Afp: alpha fetoprotein. Key transcription factors related to pancreatic development: Pdx1: pancreatic and duodenal homeobox 1; Neurog3: neurogenin 3; MafA: v-maf musculoaponeurotic fibrosarcoma oncogene family, protein A; Nkx6-1: NK6 homeobox 1; Pancreatic β cell function-related genes: Abcc8: ATP-binding cassette, sub-family C (CFTR/MRP), member 8; Kcnj11: potassium inwardly rectifying channel, subfamily J, member 11; and Ucn3: urocortin 3. *P < 0.05, **P < 0.01 compared to diabetic vector control.
Immunostaining of liver tissue on day 126 showed scatted single IPC in Bolus group (Figure 6A), insulin positive cells were 2.49%±0.15% (Figure S1). IPCs were aggregated in cluster in UTMD Bolus group (Figure 6E), and insulin positive cells were 5.26%±0.33% (Figure S1). However, glucagon was negative in both groups (Figure 6B-F). These results indicate that the hepatocytes were reprogrammed to become IPCs after in vivo gene delivery.
Figure 6.
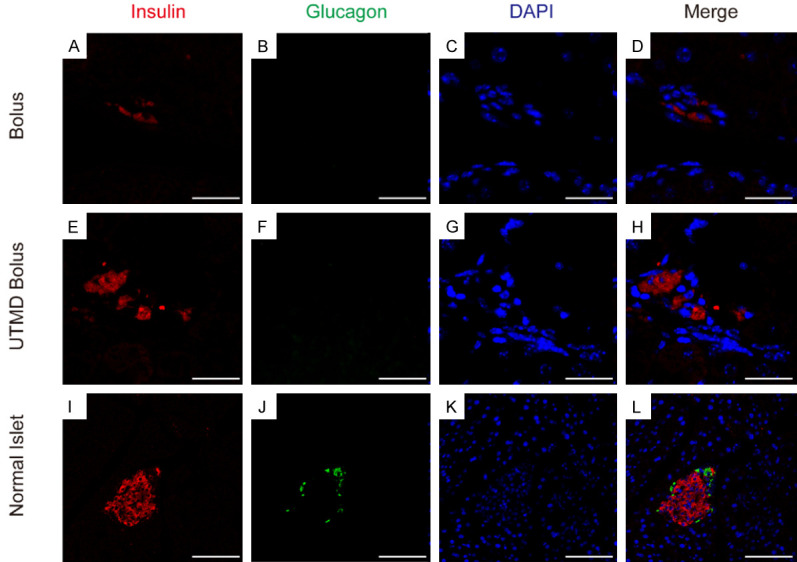
Immunofluorescence images of hepatic insulin-secreting cells. Liver tissue was sectioned and stained on day 126 (n = 5) after transfection. Red fluorescence: insulin; green fluorescence: glucagon; cellular nuclei: blue. Scale bar = 25 μm (A-D), Scale bar = 10 μm (E-H), Scale bar = 100 μm (I-L).
Discussion
This study demonstrated the possibility of reprogramming hepatocytes into IPCs by simultaneously delivery of Pdx1, Ngn3 and MafA using UTMD enhanced hydrodynamic gene delivery. In vivo transfection of PNM plasmids effectively relieved diabetic symptoms, including reduction of glucose levels, alleviation of glucose intolerance, and increase of serum insulin levels. It was without damaging the liver, highlighting its efficacy and safety.
UTMD-mediated gene transfection is a safe non-viral gene transfection method. Multiple studies have shown that ultrasound macrovesicles can achieve gene transmission in vivo in various tissues, including skeletal muscle [19], brain [20], heart [21], liver [22] and kidney [23]. Combination of UTMD with gene delivery methods can increase transfection efficiency. It has been reported that the combination of UTMD and PEI increases the transfection efficiency of plasmid DNA in skeletal muscle cells and solid tumor cells [8]. In addition, the combination of low-intensity ultrasound and hydrodynamics also increases efficient gene transfection in kidneys. Researchers can increase the gene expression level of erythropoietin plasmid in the left kidney of rats by 4.5-fold using ultrasound irradiation with 2 w/cm2 and 10% duty cycle for 15 min [24]. Similarly, our study found that combining UTMD with hydrodynamics can increase the efficiency and duration of gene expression in the liver. We found that increasing ultrasound duty cycle but not the plasmid dose can increase gene transfection efficiency.
Our previous study demonstrated that repeated hydrodynamic gene injection of PNM plasmids can achieve direct reprogramming of liver cells to insulin producing cells [15]. Here, we combine UTMD and hydrodynamic gene injection together, without using transfection reagents, to deliver plasmids to mouse livers in vivo. After the fourth injection and until the later stage of the experiment, we observed steady trends of decreasing glucose level and increasing insulin levels, as demonstrated by gene expression and ELISA assays. Interestingly, we found that the decrease in blood glucose levels did not parallel with the increase in plasma insulin levels. A possibility is that transformed hepatocytes only secrete a small amount of insulin, which did not cause any changes in the insulin levels in the circulation. However, this small amount of insulin may act in a paracrine manner to lower blood glucose levels in the liver. These findings indicate that the transformed hepatocytes were exerting an insulin-producing function. Similarly, Cim et al. reported a transient antidiabetic effect after rat hepatocyte reprogramming by the hydrodynamic method for a week [25]. They speculated that a single injection of PNM plasmids turned on the trans-differentiation of hepatocytes to islet β cells. However, this effect may be reversible [25]. We found that the reprogrammed cells simultaneously express both insulin and glucagon in mRNA level. This phenomenon has been observed in previous studies [3,15]. It is likely that these reprogrammed cells are progenitors of α and β cells and non-terminally differentiated. We also observed an increased expression of beta cells transcripts like Insulin1, Insulin2, Ucn3, Nkx6-1, and potassium channel proteins like Kcnj11 and Abcc8. These indicate that these cells have some properties of pancreatic β cells but are not fully matured.
In conclusion, this study demonstrated the feasibility of repeated transfection of multiple plasmids in mice by a UTMD enhanced hydrodynamic gene delivery system. Hepatocytes can be successfully reprogrammed into functional IPCs to alleviate diabetic symptoms. Further studies in other animal models are warranted to elucidate the treatment effect and safety of hepatocyte reprogramming by the method.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81800685, 81670702), The Natural Science Foundation of Guangdong (No. 2018A030310039, 2020A1515010978), The Science and Technology Project of Shenzhen (JCYJ20170307100154602, JCYJ20190806150001764), and Shenzhen Municipal Health Commission (SZXJ2017019).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Shen J, Cheng Y, Han Q, Mu Y, Han W. Generating insulin-producing cells for diabetic therapy: existing strategies and new development. Ageing Res Rev. 2013;12:469–478. doi: 10.1016/j.arr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banga A, Akinci E, Greder LV, Dutton JR, Slack JM. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci U S A. 2012;109:15336–15341. doi: 10.1073/pnas.1201701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appaiahgari MB, Vrati S. Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther. 2015;15:337–351. doi: 10.1517/14712598.2015.993374. [DOI] [PubMed] [Google Scholar]
- 5.Newman CM, Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 2007;14:465–475. doi: 10.1038/sj.gt.3302925. [DOI] [PubMed] [Google Scholar]
- 6.Fan CH, Lin CY, Liu HL, Yeh CK. Ultrasound targeted CNS gene delivery for Parkinson’s disease treatment. J Control Release. 2017;261:246–262. doi: 10.1016/j.jconrel.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CD, Moisyadi S, Avelar A, Walton CB, Shohet RV. Ultrasound-targeted hepatic delivery of factor IX in hemophiliac mice. Gene Ther. 2016;23:510–519. doi: 10.1038/gt.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Qian J, Yao C, Wan C, Li F. Combined ultrasound-targeted microbubble destruction and polyethylenimine-mediated plasmid DNA delivery to the rat retina: enhanced efficiency and accelerated expression. J Gene Med. 2016;18:47–56. doi: 10.1002/jgm.2875. [DOI] [PubMed] [Google Scholar]
- 9.Shimamura M, Sato N, Taniyama Y, Yamamoto S, Endoh M, Kurinami H, Aoki M, Ogihara T, Kaneda Y, Morishita R. Development of efficient plasmid DNA transfer into adult rat central nervous system using microbubble-enhanced ultrasound. Gene Ther. 2004;11:1532–1539. doi: 10.1038/sj.gt.3302323. [DOI] [PubMed] [Google Scholar]
- 10.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Song YK, Liu D. Long term expression of human alpha1 antitrypsin gene in mouse liver achieved by intravenous administration of plasmid DNA using a hydrodynamic based procedure. Gene Ther. 2000;7:1344–9. doi: 10.1038/sj.gt.3301229. [DOI] [PubMed] [Google Scholar]
- 12.Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11:675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suda T, Gao X, Stolz DB, Liu D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 2007;14:129–137. doi: 10.1038/sj.gt.3302865. [DOI] [PubMed] [Google Scholar]
- 14.Lecocq M, Andrianaivo F, Warnier MT, Wattiaux-De Coninck S, Wattiaux R, Jadot M. Uptake by mouse liver and intracellular fate of plasmid DNA after a rapid tail vein injection of a small or a large volume. J Gene Med. 2003;5:142–156. doi: 10.1002/jgm.328. [DOI] [PubMed] [Google Scholar]
- 15.Yang XF, Ren LW, Yang L, Deng CY, Li FR. In vivo direct reprogramming of liver cells to insulin producing cells by virus-free overexpression of defined factors. Endocr J. 2017;64:291–302. doi: 10.1507/endocrj.EJ16-0463. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 17.Tu J, Matula TJ, Brayman AA, Crum LA. Inertial cavitation dose produced in ex vivo rabbit ear arteries with Optison by 1-MHz pulsed ultrasound. Ultrasound Med Biol. 2006;32:281–288. doi: 10.1016/j.ultrasmedbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Samuel S, Cooper MA, Bull JL, Fowlkes JB, Miller DL. An ex vivo study of the correlation between acoustic emission and microvascular damage. Ultrasound Med Biol. 2009;35:1574–1586. doi: 10.1016/j.ultrasmedbio.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bez M, Foiret J, Shapiro G, Pelled G, Ferrara KW, Gazit D. Nonviral ultrasound-mediated gene delivery in small and large animal models. Nat Protoc. 2019;14:1015–1026. doi: 10.1038/s41596-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CY, Fan CH, Chiu NH, Ho YJ, Lin YC, Yeh CK. Targeted delivery of engineered auditory sensing protein for ultrasound neuromodulation in the brain. Theranostics. 2020;10:3546–3561. doi: 10.7150/thno.39786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Yan F, Ma J, Zhang J, Liu L, Guan L, Zheng H, Li T, Liang D, Mu Y. Ultrasound-targeted microbubble destruction-mediated co-delivery of Cxcl12 (Sdf-1alpha) and Bmp2 genes for myocardial repair. J Biomed Nanotechnol. 2019;15:1299–1312. doi: 10.1166/jbn.2019.2776. [DOI] [PubMed] [Google Scholar]
- 22.Mignet N, Marie C, Delalande A, Manta S, Bureau MF, Renault G, Scherman D, Pichon C. Microbubbles for nucleic acid delivery in liver using mild sonoporation. Methods Mol Biol. 2019;1943:377–387. doi: 10.1007/978-1-4939-9092-4_25. [DOI] [PubMed] [Google Scholar]
- 23.Park DH, Jung BK, Lee YS, Jang JY, Kim MK, Lee JK, Park H, Seo J, Kim CW. Evaluation of in vivo antitumor effects of ANT2 shRNA delivered using PEI and ultrasound with microbubbles. Gene Ther. 2015;22:325–332. doi: 10.1038/gt.2014.120. [DOI] [PubMed] [Google Scholar]
- 24.Xing Y, Pua EC, Lu X, Zhong P. Low-amplitude ultrasound enhances hydrodynamic-based gene delivery to rat kidney. Biochem Biophys Res Commun. 2009;386:217–222. doi: 10.1016/j.bbrc.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cim A, Sawyer GJ, Zhang X, Su H, Collins L, Jones P, Antoniou M, Reynes JP, Lipps HJ, Fabre JW. In vivo studies on non-viral transdifferentiation of liver cells towards pancreatic beta cells. J Endocrinol. 2012;214:277–288. doi: 10.1530/JOE-12-0033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.