Abstract
Extranodal NK/T cell lymphoma, nasal type, is a rare type of non-Hodgkin’s lymphoma (NHL), and the aetiology is not fully understood. Although the clinical outcome of anthracycline-based chemotherapy was dismal because of multidrug resistance (MDR). Novel therapeutic strategies including L-asparaginase-containing regimens, radiotherapy, sequential chemotherapy and radiotherapy, and concurrent chemoradiotherapy (CCRT) have remarkably improved outcomes. However, the overall survival (OS) rate of advanced stage patients is not satisfactory compared with patients with non-advanced-stage disease. Immunotherapy is a promising treatment for ENKTCL. Indeed, it has been proven that targeted therapies such as anti-CD30 antibodies and naked anti-CD38 antibodies are effective. In addition to these therapies that target cell surface antigens, therapies targeting intracellular signalling pathways and the microenvironment are considerably beneficial. EBV-driven overexpression of latent membrane proteins [LMP1 and LMP2] activates the pro-proliferation NF-κB/MAPK signalling pathway and leads to high PD-L1 expression. Binding of PD-L1 to PD-1 expressing cytotoxic T cells causes apoptosis and inactivation of T lymphocytes, achieving immune escape. On the basis of this mechanism, a variety of small molecular inhibitors, such as anti-PD-1 antibodies, NF-κB inhibitors, EBV antigens, and LMP1 and LMP2 antigens, can be applied. Via another signalling pathway the JAK/STAT pathway, upregulation and activation and mutation of genes promotes proliferation and ENKTCL lymphomagenesis, and JAK inhibitors have thus been applied. This article reviews recent advances in ENKTCL immunotherapy as a promising treatment for this fatal disease.
Keywords: NK/T cell lymphoma, immunotherapy, targets
Background
Extranodal natural killer/T cell lymphoma (ENKTCL) is an aggressive haematological malignancy that is frequently found in the upper aerodigestive tract but can involve non-nasal sites, such as the gastrointestinal tract, skin, soft tissue and testis. Its incidence in Asia and South America is approximately 10%, though it is as low as 1% in North America and Western Europe [1]. According to Chinese statistics, ENKTCL constitutes 6.4% of non-Hodgkin lymphoma, and more than 20% of mature T- and NK-cell lymphoma [2]. The cause of the regional difference in prevalence is related to environmental and genetic factors, as recent studies based on the SEER registry show that the rate of ENKTCL is much higher in Asian/Pacific Islanders and Hispanics than in other populations [3]. ENKTCL is predominant in young and middle-aged people, with a higher incidence in males than in females. It has been speculated that EBV infection in childhood and pesticides may be risk factors [4].
Known mutations associated with ENKTCL, such as gene amplification, deletion and mutation including in cell cycle regulators Rb, TP53, PRDM1, CDKN and FOXO3, are shared with other subtypes of lymphoma, with no specificity [5]. Therefore, to classify and diagnose ENKTCL, it is necessary to detect expression of CD2, CD56, cytoplasmic CD3 epsilon and cytotoxic molecules such as granzyme B and TIA1 as well as EBV-encoded RNA (EBER) positivity [6].
ENKTCL is usually classified according to the origin of the lesion into upper aerodigestive tract (UAT) and the non-upper aerodigestive tract (non-UAT) type, and the prognosis and treatment response of the latter are significantly worse than those of the former [7]. Non-advanced-stage disease occurs in approximately 70-80% of nasal/paranasal ENKTCL patients, and approximately half of them present with isolated nasal disease. In contrast, advanced disease occurs in 60% of extranasal cases [5]. Chemotherapy, such as CHOP and adriamycin-based regimens, is largely ineffective due to the high expression levels of P-glycoprotein in NK lymphoma cells. However, lack of asparagine synthase renders ENKTCL sensitive to L-asparaginase [8]. and L-asparaginase-containing chemotherapy regimens have led to a dramatic improvement in survival, particularly in relapsed/refractory ENKTCL [9]. Moreover, L-asparaginase-based regimens such as SMILE, AspaMetDex and P-Gemox are recommended by NCCN guidelines. In general, all of these therapies have markedly improved the prognosis of ENKTCL. For example, the 5-year and the 2-year overall survival (OS) of patients with advanced disease were much higher after 2010 than before [10]. Nonetheless, approximately 50% of patients with first-line treatment failure have poor clinical outcomes, with a median progression free survival (PFS) of less than 8 months [11]. In addition, some patients relapse after treatment with an L-asparaginase-based regimen [12]. Thus the best treatment scheme should be further explored. The inherent expression of targeted CD (cluster of differentiation) markers and the intrinsic signs of EBV-induced proliferation, such as overexpression of signalling pathway-related genes and mutation of single genes, have suggested new immunotherapy prospects for patients with L-asparaginase-refractory disease. In this review, we present the available literature and case reports and summarize the latest immunotherapy targets for ENKTCL (Figure 1).
Figure 1.
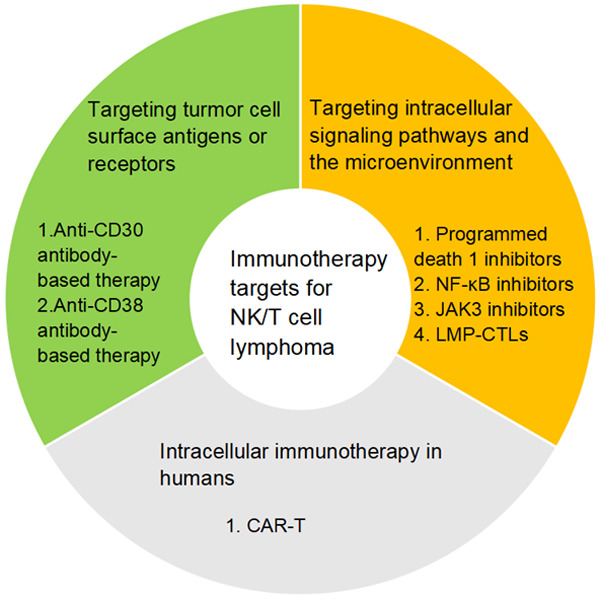
Summary of immunotherapy targets for NK/T cell lymphoma
Targeting tumour cell surface antigens or receptors
Anti-CD30 antibody-based therapy
CD30 is a transmembrane protein belonging to the tumour necrosis factor receptor family (Figure 2) that is reported to be specifically expressed in normal activated (rather than static) B and T cells and NK cells, but not in normal cells. In terms of malignant lymphomas, CD30 is expressed in Hodgkin and Reed-Sternberg cells of Hodgkin’s lymphoma and in almost all neoplastic cells of ALCL. Different studies have reported a CD30 expression rate in ENKTCL of approximately 50-70% [13]. Furthermore, CD30 is more frequently expressed in ENKTCL than in other mature T cells and NK cell lymphoma subtypes, which is most likely explained by the presence of EBV in ENKTCL patients [2].
Figure 2.

Summary of immunotherapy drugs or treatment strategies for NK/T cell lymphoma and their respective targets. Strategies targeting tumour cell surface antigens or receptors include brentuximab vedotin (CD30) and daratumumab (CD38). Strategies targeting intracellular signalling pathways and the microenvironment include anti-pd1 antibodies (such as pembrolizumab and nivolumab), targeted NF-κB drugs (bortezomib), JAK3 inhibitors (PRN371 and tofacitinib) and autologous T cells targeting LMP2 or LMP1 and LMP2 antigens. These strategies bring hope for this fatal disease.
Overall, expression of CD30 varies in ENKTCL is variable and there is no consensus on its clinical significance [14]. One study including 70 patients by Guan-Nan Wang and colleagues showed that compared with CD30-negative ENKTCL, patients with CD30-positive ENKTCL showed significantly worse OS (P = 0.023) and PFS (P = 0.008) [15]. In addition, strong CD30 expression was observed in atypical macronuclear cells in ENKTCL patients with primary skin, prostate and adrenal gland tumours, with poor outcomes, which may suggest that ENKTCL with large cells expresses CD30 and predicts a poor prognosis [16]. In another study, CD30-positive ENKTCL responded better to non-anthracycline therapy than CD30-negative ENKTCL among 72 cases, and the former patients with a COV (critical value) of 25% showed a lower recurrence rate [17]. However, no correlation between CD30 expression and survival outcomes was found in a study of 317 patients with ENKTCL and 91 patients with catalogued CD30 immunohistochemistry tissues (47.3%) [2]. These differences may be attributed to the different cutoff values of CD30 used by different researchers, and studies involving different cohorts have produced conflicting results [18]. Nevertheless, anti-CD30 antibodies are used in therapeutic strategies in many clinical trials, which provide a theoretical and practical basis for the treatment of lymphoma.
A CD30-targeted antibody, brentuximab vedotin (BV) binds with monomethyl auristatin E (MMAE) to exert strong therapeutic effects in recurrent HL and multiple T-cell lymphoma through direct cytotoxicity, bystander effects, [19] and immune augmentation effects [20]. The results of a preliminary phase 2 study showed that SGN-30, a chimeric anti-CD30 monoclonal antibody, had modest efficacy in HL and ALCL [21]. However, responses to BV were observed in patients with all levels of CD30 expression in tumour samples, including 2 T cell lymphoma patients whose CD30 expression could not be detected by IHC in the central evaluation; this suggests that the drug may be dispersed into the tumour microenvironment and subsequently come into contact with tumour cells via the bystander effect [22]. Similar phenomena were also found for diffuse large B cell lymphoma [23]. In addition to the above mechanism of action, mouse models have shown increased activation of T cells, activation of dendritic cells and migration to draining lymph nodes after BV treatment [24]. In general, some results have been achieved with BV similar to the latest phase 3 trial showing that, for patients with CD30-positive peripheral T cell lymphomas, first-line treatment with A CHP (brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone) was better than that with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) as it was associated with significantly improved PFS and OS [25]. Regardless, tumour recurrence and drug resistance, major concerns, occur. Chen and colleagues reported that the percentage of CD30-positive cells in HL patients did not appear to be related to the degree of BV resistance, which may have been due to drug internalization changing the accumulation of MMAE in cells and increasing expression of MDR1 (a known drug exporter) [26].
To date, no clinical trials have been conducted specifically for recurrent/refractory ENKTCL, but CR after BV treatment has been reported for two patients. In one case, a 63-year-old male with CD30-positive non-UADT ENKTCL presented with multiple skin lesions. After four cycles of single-dose brentuximab vedotin treatment, all skin lesions were cleared to complete remission (CR), though therapy was discontinued because of secondary toxic dyspnoea, and the disease recurred three months later [27]. In the other case, a 17-year-old woman with ENKTCL with CD30 expression in approximately 30% of neoplastic cells was treated with a combination regimen of brentuximab vedotin and bendamustine, and complete radiological remission (CR) was achieved after two cycles of treatment; moreover, metabolic CR was obtained on PET/CT after three courses of treatment. Haploidentical haematopoietic stem cells from her father were used for transplantation once remission was achieved and after transplantation, plasma EBV-DNA could not be detected [28] (Table 2). Unfortunately, patients who receive bentuximab will eventually relapse, and the specific mechanism remains unclear. Currently, some clinical trials are using the combination of BV with L-asparaginase and non-L-asparaginase as the first-line regimen, including one study that has completed accrual (NCT01309789) and others that are still being planned (NCT0324750); their results are eagerly awaited. [14] Other ongoing studies are listed in Table 2. (NCT03192202 and NCT04074746) (Table 3).
Table 2.
Select reports of targeted therapy for extranodal NK/T cell lymphoma
| Target/therapy | Patients characteristics | Efficacy | Reference | |
|---|---|---|---|---|
| CD30 | Brentuximab vedotin | Refractory or relapsed HL or CD30(+) NHL patients | Modest efficacy. | [21] |
| A male with ENKTCL and CD30 expression | CR was achieved after four cycles of treatment. | [27] | ||
| Brentuximab vedotin and bendamustine | A woman with ENKTCL and CD30 expression | After two cycles of treatment, CR was achieved. | [28] | |
| Brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone (A+CHP) | Patients with CD30-positive peripheral T cell lymphomas | Significant improvement in progression-free survival and overall survival. | [28] | |
| BV with L-asparaginase and non-L-asparaginase | 26 patients with CD30+ PTCL | All patients (n = 26) achieved an objective response (CR rate, 88%; estimated 1-year PFS rate, 71%). | [14] | |
| CD38 | Daratumumab | A child with CD38+ recurrent B-ALL | Effective. | [36] |
| A patient with stage IV NKTCL | Patients reached a maximum sustained remission period of 21 weeks. | [37] | ||
| PD-1 | Nivolumab | 29 patients with recurrent or refractory lymphatic malignancies | A DBLCL patient and one with FL reached CR. | [48] |
| 3 patients with relapsed/refractory NKTCL | All patients achieved a clinical response. | [44] | ||
| Pembrolizumab | 7 patients with NKTCL | All patients achieved CRs. | [50] | |
| 7 patients with NKTCL | 2 patients reached CR and 2 reached PR. | [45] | ||
| 30 consecutive patients with relapsed/or refractory NHL | 7 patients reached CR and 7reached PR. | [51] | ||
| NF-κB | B-GIFOX (bortezomib, gemcitabine, oxaliplatin and ifosfamide) | 6 ENKTCL patients | The ORR was 42.8%. | - |
| Bortezomib, fludarabine and autologous haematopoietic stem cell transplantation | A patient with relapsed/refractory NKTCL | The patient remained disease-free as of March 2017. | [59] | |
| EBV antigens | Activated/stimulated T cells and LMP1/2- or LMP2-targeted strategies | 52 patients with EBV-related lymphoma | 11 patients with active disease reached CR, 2 patients achieved PR, and 5 ENKTCL patients reached CR. | [63] |
| 8 patients with localized disease and 2 advanced ENKTCL patients | All patients reached CR: OS and PFS were 100% and 90%, respectively. | [65] | ||
| CAR-T | CD19-CAR-T cells | 119 patients with B cell malignancies | Response rates (93%) were higher in all patients than in CLL patients (62%) and lymphoma patients (36%). | [66] |
| CD22-CAR-T cells | 34 patients with B-ALL | 24 patients achieved CR or CRI. | [70] | |
| CD28 co-stimulated anti-CD30 CAR-T cells | 7 patients with relapsed/refractory HL and 2 patients with ALCL | 2 patients with HL reached CR and 3 reached SD; 1 ALCL patients reached CR. | [67] | |
| 4-1BB co-stimulated anti-CD30 CAR-T cells | 17 HL patients and 1 cutaneous ALCL patients | 7 patients achieved PR and 6 patients achieved SD. | [68] |
EBV, Epstein-Barr virus; CR, complete response; PR, partial response; SD, stable disease; HL, Hodgkin’s lymphoma; ALCL, anaplastic large cell lymphoma; ENKTCL, extranodal NK/T cell lymphoma; PTCL, peripheral T cell lymphoma; OS, overall survival; PFS, progression-free survival; B-ALL, B cell acute lymphoblastic leukaemia.
Table 3.
Clinical trials of targeted therapy for various lymphomas, particularly for NK/T cell lymphoma
| Intervention/treatment | Phase | Tumour type | ClinicalTrials.gov Identifier | |
|---|---|---|---|---|
| CD30 | AFM 13 | Phase 1 | Relapsed/Refractory cutaneous lymphomas | NCT 03192202 |
| Phase 2 | ||||
| Modified immune cells (AFM13-NK) | Phase 1 | Recurrent or refractory CD30-positive HL or NHL | NCT 04074746 | |
| CD38 | Daratumumab | Phase 2 | Relapsed or refractory NKTCL | NCT 02927925 |
| PD-1 | Pegaspargase and anti-PD-1 monoclonal antibody | Phase 2 | ENKTCL, nasal type | NCT 04096690 |
| PD-1 antibody, chidamide, lenalidomide and etoposide | Phase 4 | NKTCL | NCT 04038411 | |
| SHR1210 | Phase 2 | NKTCL | NCT 03701022 | |
| Phase 2 | ENKTCL, nasal type | NCT 03363555 | ||
| LEAP regimen | Phase 2 | ENKTCL, nasal and nasal-type | NCT 04004572 | |
| Pembrolizumab | Phase 2 | T cell Lymphoma | NCT 03021057 | |
| NK cell lymphoma | ||||
| Phase 2 | NKTCL of the nasal cavity | NCT 03728972 | ||
| NKTCL of the nasopharynx | ||||
| Phase 1 and phase 2 | ENKTCL and EBV-related DLBCL | NCT 03586024 | ||
| MK-3475 and copanlisib | Phase 1 and phase 2 | NK and T cell non-Hodgkin’s lymphoma | NCT 02535247 | |
| Pembrolizumab and pralatrexate | Phase 1 and phase 2 | T cell lymphomas | NCT 03598998 | |
| Nivolumab | Phase 2 | PTCL | NCT 03075553 | |
| Phase 2 | T cell and NK cell lymphomas, cutaneous squamous cell carcinoma, Merkel cell carcinoma, and other rare skin tumours | NCT 02978625 | ||
| JAK3 | Tofacitinib and chidamide | Phase 2 | ENKTCL | NCT 03598959 |
| CAR-T | CD7 CAR-T cells infusion | Phase 1 | T-lymphoblastic lymphoma and NKTCL | NCT 04004637 |
| Anti-CD30 CAR-T cells | Phase 1 | Relapsed/refractory lymphocyte malignancies | NCT 04008394 | |
| Phase 1 | HL, ALCL, PTCL NOS, DLBCL NOS, PMBCL, grey zone lymphoma, enteropathy-associated T cell lymphoma or ENKTCL | NCT 03049449 | ||
| Phase 1 and phase 2 | Relapsed and refractory CD30-positive lymphomas | NCT 02274584 | ||
| CD7-specific CAR gene-engineered T cells | Phase 1 and phase 2 | T-ALL, TCL, NKTCL and AML | NCT 04033302 | |
| CD19-TriCAR-T/SILK | Early phase 1 | CD19+ children with leukaemia or non-Hodgkin’s lymphoma | NCT 03910842 |
PD-1, programmed death 1; LMP, latent membrane protein; EBV, Epstein-Barr virus; CR, complete response; PR, partial response; SD, stable disease; CAR-T cell, chimeric antigen receptor T cell; HL, Hodgkin’s lymphoma; ALCL, anaplastic large cell lymphoma; ENKTCL, extranodal NK/T cell lymphoma; PTCL NOS, peripheral T cell lymphoma not otherwise specified; DLBCL NOS, diffuse large B cell lymphoma not otherwise specified; PMBCL, primary mediastinal B cell lymphoma; T-ALL, T cell acute lymphoblastic leukaemia; TCL, T cell lymphoma; AML, acute myeloid leukaemia.
Anti-CD38 antibody-based therapy
CD38 is a type II glycosylated protein that acts as a receptor, and is widely expressed in the haematopoietic system, mainly by NK cells, early precursor T cells, activated T cells and mature B cells, but the expression level in normal lymphocytes and myeloid cells is low [29]. CD38 interacts with the ligands CD31 and CD31/CD38, promotes the activation and proliferation of different lymphocyte groups and is expressed in almost all NKTLs. Wang L and colleagues proved that 95% of ENKTCL cases were CD38 positive, with half of them highly expresseing CD38 [30]. Compared with ENKTCL patients with weak expression of CD38, ENKTCL patients with strong expression had significantly poorer outcomes, suggesting the potential role of CD38 as a therapeutic target [31].
To date, several monoclonal antibodies against human CD38 have been successfully developed, such as daratumumab, isatuximab (SAR650984) and MOR202. Daratumumab is a high-affinity therapeutic human mAb that recognizes the unique CD38 epitope. Its complement-dependent cytotoxicity (CDC) and antibody-dependent cytotoxicity are not affected by the presence of bone marrow stromal cells [31]. Daratumumab has been approved in multiple combinations for pretreated and relapsed/refractory multiple myeloma (MM), which has greatly improved the survival outcomes of these patients [14]. However, the complete mechanism of daratumumab is still unclear because even cases with high expression of CD38 may be primary refractory, and thus expression of CD38 in MM does not correlate significantly with response to daratumumab.[32] Nonetheless, reducing the endocytosis of complexed CD38/daratumumab can enhance the efficacy of the anti-tumour mAb [33]. For those who are resistant to daratumumab, all-trans retinoic acid may increase CD38 expression and decrease that of CD55 and CD59 on MM cells, which can restore the CDC mediated by daratumumab [34]. These trials have been registered as NCT00574288 (GEN501) and NCT01985126 (SIRIUS). In leukaemia, daratumumab kills CLL cells through antibody-dependent cell-mediated cytotoxicity and antibody-dependent cell phagocytosis, significantly prolonging the OS rate of CLL animal models [35]. Daratumumab has also been reported to be effective in the treatment of a 14-year-old child with CD38+Ph-positive recurrent B-ALL [36].
The efficacy of daratumumab was reported among 2 patients with relapsed/refractory ENKTCL has been reported [37,38]. In the first case, a 56-year-old female patient with stage IV NK/T cell lymphoma exhibited EBV DNA positivity. The patient relapsed after 6 weeks of radiotherapy, cisplatin therapy and consolidated chemotherapy. Subsequently, she received combined chemotherapy, intrathecal chemotherapy and allogeneic haematopoietic cell transplantation. Three weeks later, the disease recurred in the central nervous system, with systemic recurrence on day 90. However, after six weeks of daratumumab treatment, EBV became undetectable, and the patient reached the longest sustained remission period (21 weeks) since her diagnosis. Daratumumab may also help patients who have undergone extensive pre-treatment [37] (Table 2). Although much research has been conducted on the drug in MM, its unique mechanism in NKTCL remains to be explored. Currently, several Asian countries are conducting phase 2 trials to assess the safety and effectiveness of daratumumab in ENKTCL (NCT02927925). At the 2018 American Society of Hematology (ASH) meeting, preliminary results were reported that daratumumab had a good response rate in the treatment of relapsed and refractory ENKTCL patients (ORR: 35.7%), and the second stage of the study is in progress (Table 3).
Targeting intracellular signalling pathways and the microenvironment
Programmed death 1 inhibitors
As a member of the CD28 costimulatory receptor superfamily, PD-1 is an immunosuppressive receptor expressed by activated T cells, B cells and myeloid cells, and is structurally similar to cytotoxic lymphocyte antigen-4 (CTLA-4). Binding of ligands PD-L1 and PD-L2 by PD-1 leads to T-lymphocyte dysfunction and T-cell-mediated cytotoxicity escape. Programmed death ligand 1 (PD-L1) is a surface glycoprotein of immune regulatory cells (Figure 2) that is mainly expressed at low levels on antigen-presenting cells (APCs), including professional APCs and non-professional APCs. PD-L1 is upregulated in ENKTCL, ranging from 39 to 100%, which allows cells to avoid systemic surveillance [39]. Almost all EBV-related lymphomas are associated with high expression of PD-L1 [4]. However, studies have shown that NKTCL lymph node variants may have higher PD-L1 expression than extranodal variants, which is not related to EBV expression [40]. Based on clinical data, patients with elevated PD-L1 expression had lower serum LDH levels and IPI scores than patients with decreased PD-L1 levels [41]. Several studies have also shown that serum PD-L1 levels are associated with the prognosis of ENKTCL [42]. For instance, Nagato and colleagues reported that elevated levels of PD-L1 in tumour cells are associated with elevated levels of PD-L1 in serum and worsened OS [43], though the exact relationship remains to be elucidated. Overall, the combination of high PD-L1 expression and foreign antigen expression in cancer cells makes the use of checkpoint inhibitors very attractive [44,45].
Anti-PD-1 antibodies such as pembrolizumab and nivolumab can destroy the interaction between PD-L1 and PD-1, thereby restoring the anti-tumour activity of activated T cells [46]. In 2014, the U.S. FDA approved pembrolizumab and nivolumab for the treatment of metastatic melanoma, and in 2015, nivolumab was approved for the treatment of squamous NSCLC. Over time, both drugs have been approved for use in many types of cancer, leading to unprecedented clinical advances. At present, preliminary results published by some institutions support the use of PD-1/PD-L1 signalling pathway inhibitors in a variety of tumours (e.g., digestive tract tumours, liver cancer, triple-negative breast cancer, advanced urothelial cancer, ovarian cancer, advanced Hodgkin’s lymphoma) [47]. Indeed, many large-scale studies of PD-1/PD-L1 monoclonal antibodies are in clinical stages I and II. Among these monoclonal antibodies, nivolumab has been tested in many clinical trials with good results. In one study, 29 patients with recurrent or refractory lymphatic malignancies were evaluated, including one DLBCL patient (9%) and one FL patient (10%) who reached CR [48]; the remaining 27 patients showed some response or tumour shrinkage after treatment. In another independent study, low-dose nivolumab was used in 3 patients with relapsed and refractory ENKTCL, which achieved a clinical response [49]. Pembrolizumab is another completely humanized PD-1 monoclonal antibody, and Kwong and colleagues have confirmed the efficacy of pembrolizumab in patients with ENKTCL [50]. Among seven relapsed and refractory patients with advanced disease who had previously been treated with SMILE or SMILE plus platinum regimens, all displayed a rapid response after 7 cycles of pembrolizumab and 5 achieved CR. Pembrolizumab was effective in 4 of 7 NKTCL patients (2 with complete remission and 2 with partial remission, with a total response rate of 57%) in another study [45]. Seok-Jin Kim and colleagues found that seven EBV-positive NHL patients, including NK/T cell lymphoma patients (6/14, 44%) and one primary mediastinal B cell lymphoma patient (1/4, 25%), responded to pembrolizumab, with patients with EBV-negative subtypes, such as diffuse large B cell lymphoma and PD-L1 expression showing higher responses (4/6, 67%) than those with low PD-L1 expression (1/5, 20%) [51] (Table 2). This phenomenon also has some significance and according to some studies, combination with other immunotherapies such as anti-CD38 antibodies or BV, can enhance efficacy [4]. Overall, there has been a good response to PD-L1 inhibitors, though the short reaction time is the main limitation. A number of clinical trials are currently underway to evaluate the efficacy of anti-PD1 therapy for ENKTCL (NCT04096690, NCT04038411, NCT03701022, NCT03363555, NCT04004572, NCT03021057, NCT03728972, NCT03586024, NCT02535247, NCT03598998, NCT03075553, and NCT02978625) (Table 3).
NF-κB inhibitors
NF-κB (nuclear factor κappa-light-chain-enhancer of activated B cells) is a transcription factor that mediate the development, proliferation and survival of T cells and B cells; changes in NF-κB activity will cause constitutive activation of lymphocyte proliferation and/or blockage of cell death, which can promote the occurrence and development of tumours [52]. Two GEP (gene expression profiling) studies have found significant enrichment of genes in the NF-κB pathway in cancer [53]. It has been proven that there is pathogenic cooperation between p53 deletion and NF-κB activity and that activation of NF-κB is related to poor survival in ABC DLBCL [54]. In one study, p52 (NF-κB subunit) nuclear staining was observed in 65.2% of ENKTCL cases as an alternative indicator to determine activation of NF-κB and correlate its status with survival. Moreover, the 2-year PFS and OS rates of 8 p52-positive patients were lower than those of 8 p52-negative patients [55]. Therefore, the NF-κB signalling pathway is an attractive therapeutic target in T- and B-cell malignancies.
Bortezomib, a dipeptidyl boric acid, is associated with tumourigenesis, cell cycle progression, apoptosis and multiple drug resistance, which can lead to stabilization of the inhibitory protein IκBα and decrease the activity of NF-κB [56]. Bortezomib has achieved remarkable results in the treatment of relapsed and refractory multiple myeloma [57]. And bortezomib can also play an anti-tumour role in leukaemia. In both laboratory and clinical studies, bortezomib has shown some progress in the treatment of chronic myeloid leukaemia, chronic lymphocytic leukaemia, acute myeloid leukaemia, acute lymphocytic leukaemia, adult T-lymphocytic leukaemia and plasma cell leukaemia. Bortezomib-based combinations targeting NF-κB have also been evaluated for ENKTCL, though data are available for only small groups of patients. In a study involving six ENKTCL patients, the ORR due to B-GIFOX (bortezomib (B), gemcitabine (G), oxaliplatin (OX) and ifosfamide (IF)) regimens was 42.8% in newly diagnosed patients, but the median PFS was relatively short at approximately 4 months [58] (Table 2). B-GIFOX is a promising regimen for ENKTCL, and a prospective phase 2 study is currently being designed to verify its efficacy in treating ENKTCL. In addition, bortezomib inhibits the repair of DNA damage induced by fludarabine, and this synergistic effect enhances the anti-tumour effect accordingly, bortezomib-fludarabine chemotherapy may be an effective remedial strategy for recurrent ENKTCL. In one case, a 40-year-old patient who relapsed after hormone therapy combined with radiotherapy and chemotherapy was treated with two courses of remedial therapy containing bortezomib and fludarabine and received autologous haematopoietic stem cell transplantation; the patient remained disease-free as of the last follow-up (March 2017) [59] (Table 2). Nevertheless, larger trials are needed to provide a more comprehensive assessment of the efficacy and safety of bortezomib, and early clinical trials of ENKTCL are underway.
JAK3 inhibitors
Janus kinase (JAK) family members include JAK1, JAK2, JAK3 and TYK2, which play roles in different signalling pathways mediated by cytokines and growth factor receptors (Figure 2). JAK3, a non-receptor tyrosine kinase involved in the JAK-STAT pathway, is mainly expressed in haematopoietic cells and helps to regulate the development of lymphocytes [60]. In one report, 5 of 71 ENKTCL patients (7.0%) harboured novel JAK3 mutations (JAK3 H583Y and JAK3 G589D), which inhibited the proliferation of Ba/F3 cells and were carcinogenic in NKTCL [60]. Pathway inhibition might be a therapeutic option for NKTCL patients with JAK3 mutations.
Tofacitinib can significantly inhibit JAK3 activity in vivo and in vitro, but its clinical application in cancer treatment is limited by pan-JAK inhibitory activity. PRN371 is a small molecule that was designed as an effective and selective JAK3 inhibitor, that specifically binds to cysteine 909 of JAK3 kinas. PRN371 has high selectivity and durability and stronger anti-tumour activity as well as fewer side effects than other JAK3 inhibitors. M.-L. Nairismägi and colleagues preclinically evaluated PRN371 for the first time and found that it significantly inhibited tumour growth in an NKTCL xenotransplantation model carrying JAK3 activating mutations, which is consistent with in vitro results [61]. The JAK3 inhibitory antineoplastic activity seen in preclinical/in vitro models demonstrates the biological basis for targeting these pathways. Although the frequency of JAK3 mutations is controversial, PRN371 is effective in most ENKTCL cells, including most cases with STAT3 mutations, and can act on other downstream pathways of JAK3, such as the EZH2 pathway. Therefore, a subset of patients stratified based on appropriate molecular markers can use PRN371 as an alternative to current chemotherapies, a strategy that can be translated into clinical trials to clarify clinical impact and effectiveness [61]. In addition, the JAK1/2 inhibitor ruxolitinib has been approved for treatment of bone marrow fibrosis. Phase 2 clinical trials evaluating JAK inhibitors in ENKTCL are being conducted in patients with recurrent ENKTCL (NCT02974647 and NCT03598959) [62].
LMP-CTLs
Tumour cells from approximately 40% of patients with Hodgkin’s or non-Hodgkin’s lymphoma express type II Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) and LMP2, inducing attack by LMP-cytotoxic T lymphocytes (CTLs). Therefore, expanding LMP-CTLs in ENKTCL to target EBV antigens can achieve anti-tumor effects [63]. In fact, this strategy has shown therapeutic efficacy in a variety of EBV-derived lymphomas and can result in a lasting complete response without significant toxicity but with high specificity [63]. Using this robust and cost-effective approach in the treatment of EBV-related lymphoproliferative diseases after transplantation also leads to continuous complete remission (CRs in 68-84% of patients) [64]. Bollard and colleagues treated 52 patients with EBV-related lymphoma, using LMP1/2-or LMP2-targeted and stimulated CTLs [63]. LMP2- or LMP1/2-specific CTLs were administered to 50 EBV-related HL or NHL patients, which proved to be safe. The 2-year EFS rate was 82% in 29 patients with high-risk or recurrent disease in the remission stage. Among the 21 patients with active disease, 11 received CTL treatment and entered into continuous CR, and the other 2 achieved PR. Five of the 11 ENKTCL patients received CTLs as consolidation therapy after initial radiotherapy or autologous stem cell transplantation and remained in CR for 2-6 years. A patient with a primary refractory disease who acquired CR after autologous stem cell transplantation and retained it for 2 years after CTL infusion may prove the effectiveness of this approach.
Although these results may be significant for high-risk relapsed/refractory patients, they do not demonstrate the role of CTLs as a maintenance therapy for local disease after first-line treatment. Cho and colleagues studied LMP1/2-directed CTL treatment in eight patients with localized disease and two advanced ENKTCL patients, and all patients reached CR in 4 years, with OS and PFS values of 100% and 90%, respectively. [65] Only one patient had an initial recurrence of IVE disease after 32 months, which suggests that CTLs can induce persistent complete remission without significant toxicity. However, as the 5-year survival rate of chemical radiation can be as high as 90%, it remains unclear whether patients with early disease truly benefit from maintaining CTL infusion (Table 2). In conclusion, T cell immunotherapy with immunity stimulated by Epstein-Barr virus (EBV) may play an important role in the targeted treatment of EBV-related cancer.
Intracellular immunotherapy in humans
CAR-T (chimeric antigen receptor T) cells are a type of antigen-specific T cell with extracellular single-chain variant fragments that recognize antigens coupled to activated intracellular domains and then bind to them. Thus far CAR-T cell immunotherapy has achieved remarkable therapeutic effects in the treatment of haematological malignancies, for example, the treatment of B cell malignancies with allogeneic CD19-CAR-T cells [66] (Table 2). Targeting CD30 with CD30 CAR T cells provides an opportunity for the rapid production of tumour-specific T cells in various lymphoma patients, reducing side effects and enhancing anti-tumour activity, regardless of EBV status. In a phase 1 dose-escalation study, CD28 co-stimulated anti-CD30 CAR-T cells were used to treat 7 patients with relapsed/refractory HL and 2 patients with ALCL [67]. Seven patients experienced disease progression during brentuximab treatment. However, two patients with HL who reached complete remission (CR) sustained it for more than 2 years, and 3 patients had transient stable disease. One ALCL patient achieved CR for more than 9 months, and no patient developed impaired virus-specific immunity. Additionally, Wang CM et al. treated 17 HL patients and 1 cutaneous ALCL patient with a 4-1BB co-stimulated anti-CD30 CAR-T cell construct, with seven achieving PR and six stable disease (Table 1). Hence, an increasing number of studies have applied CAR-T cell therapy for breast cancer, sarcoma, neuroblastoma and other solid tumours and explored its clinical value [68]. Compared with haematological malignancies, the application of CAR-T cells for solid tumours is limited by many factors, including inhibited T cell functions and T cell localization [69]. Therefore, the application of CAR-T cell immunotherapy in solid tumours remains at the safety-determining stage. Overall, CAR-T cell therapy is feasible for the treatment of NKTCL and even the relapsed or refractory NKTCL. Several clinical trials with anti-CD7 CAR-T cells and similar molecules are ongoing (NCT04004637, NCT04008394, NCT03049449, NCT02274584, NCT04033302, and NCT03910842) (Table 3).
Table 1.
Summary of available therapies for NK/T cell lymphoma using targeting markers or viral antigens
| Target | Percentage of ENKTCLs with positive expression | Prognostic relevance | Targeted agent | Research stage in ENKTCL |
|---|---|---|---|---|
| CD30 | 50-70% [11] | disputed | brentuximab vedotin | clinical |
| CD30 CAR-T cells | clinical | |||
| CD38 | 95% [29] | negative | daratumumab | clinical |
| PD-1 | NA | NA | pembrolizumab | clinical |
| nivolumab | clinical | |||
| NF-κB | NA | NA | bortezomib | clinical |
| JAK3 | NA | NA | tofacitinib | clinical |
| PRN371 | preclinical | |||
| EBV antigens | NA | NA | LMP-CTLs | clinical |
Conclusion
Overall, there has been great progress in our understanding and treatment of NK/T cell lymphoma in recent years. As immunotherapy has broader anticancer effects and fewer side effects than existing individual therapies and combination therapies, it may provide more effective treatment options. There are specific drugs for CD30, CD38, PD-1, NF-κB, JAK1/2/3 inhibitors, but EBV antigen and CAR-T cell treatment strategies need to be further researched. Nevertheless, few patients have improved outcomes, and there are almost no phase 3 clinical trials to guide treatment in this area. In brief, the effect of existing therapeutic methods is still unsatisfactory, especially for relapsed/refractory ENKTCL. A large number of new immunotherapy clinical trials need to be carried out.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 8187010857).
Disclosure of conflict of interest
None.
Abbreviations
- EBV
Epstein-Barr virus
- CR
Complete response
- PR
Partial response
- SD
Stable disease
- HL
Hodgkin’s lymphoma
- ALCL
Anaplastic large cell lymphoma
- ENKTCL
Extranodal NK/T cell lymphoma
- PTCL
Peripheral T cell lymphoma
- OS
Overall survival
- PFS
Progression-free survival
- B-ALL
B cell acute lymphoblastic leukaemia
- PD1
Programmed death 1
- LMP
Latent membrane protein
- CAR-T cell
Chimeric antigen receptor T cell
- PTCL NOS
Peripheral T cell lymphoma not otherwise specified
- DLBCL NOS
Diffuse large B cell lymphoma not otherwise specified
- PMBCL
Primary mediastinal B cell lymphoma
- T-ALL
T cell acute lymphoblastic leukaemia
- TCL
T cell lymphoma
- AML
Acute myeloid leukaemia
- NHL
Non-Hodgkin’s lymphoma
- MDR
Multidrug resistance
- CCRT
Concurrent chemoradiotherapy
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- EBER
EBV-encoded RNA
- UAT
Upper aerodigestive tract
- CD
Cluster of differentiation
- COV
Critical value
- BV
Brentuximab vedotin
- CDC
Complement-dependent cytotoxicity
- MM
Multiple myeloma
- APCs
Antigen-presenting cells
References
- 1.Haverkos BM, Pan Z, Gru AA, Freud AG, Rabinovitch R, Xu-Welliver M, Otto B, Barrionuevo C, Baiocchi RA, Rochford R. Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT): an update on epidemiology, clinical presentation, and natural history in North American and European cases. Curr Hematol Malig Rep. 2016;11:514–527. doi: 10.1007/s11899-016-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng Y, Rao H, Lei Y, Huang Y, Wang F, Zhang Y, Xi S, Wu Q, Shao J. CD30 expression in extranodal natural killer/T-cell lymphoma, nasal type among 622 cases of mature T-cell and natural killer-cell lymphoma at a single institution in South China. Chin J Cancer. 2017;36:43. doi: 10.1186/s40880-017-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams SV, Newcomb PA, Shustov AR. Racial patterns of peripheral t-cell lymphoma incidence and survival in the United States. J. Clin. Oncol. 2016;34:963–71. doi: 10.1200/JCO.2015.63.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu B, Oki Y. Novel immunotherapy options for extranodal NK/T-cell lymphoma. Front Oncol. 2018;8:139. doi: 10.3389/fonc.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki R. NK/T cell lymphoma: updates in therapy. Curr Hematol Malig Rep. 2018;13:7–12. doi: 10.1007/s11899-018-0430-5. [DOI] [PubMed] [Google Scholar]
- 6.Somasundaram N, Lim JQ, Ong CK, Lim ST. Pathogenesis and biomarkers of natural killer T cell lymphoma (NKTL) J Hematol Oncol. 2019;12:28. doi: 10.1186/s13045-019-0717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaffe ES, Chan JK, Su IJ, Frizzera G, Mori S, Feller AC, Ho FC. Report of the workshop on nasal and related extranodal angiocentric T/natural killer cell lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol. 1996;20:103–111. doi: 10.1097/00000478-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Ando M, Sugimoto K, Kitoh T, Sasaki M, Mukai K, Ando J, Egashira M, Schuster SM, Oshimi K. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol. 2005;130:860–868. doi: 10.1111/j.1365-2141.2005.05694.x. [DOI] [PubMed] [Google Scholar]
- 9.Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, Tilly H, Morschhauser F, Thieblemont C, Ysebaert L, Devidas A, Petit B, de Leval L, Gaulard P, Feuillard J, Bordessoule D, Hermine O GELA and GOELAMS Intergroup. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117:1834–1839. doi: 10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- 10.Varelas AN, Ganti A, Eggerstedt M, Tajudeen BA. Prognostic indicators of survival in sinonasal extranodal natural killer/T-cell lymphoma. Laryngoscope. 2019;129:2675–2680. doi: 10.1002/lary.27886. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Li L, Zhang L, Wang J. The landscape of new drugs in extranodal NK/T-cell lymphoma. Cancer Treat Rev. 2020;89:102065. doi: 10.1016/j.ctrv.2020.102065. [DOI] [PubMed] [Google Scholar]
- 12.Ahn HK, Kim SJ, Hwang DW, Ko YH, Tang T, Lim ST, Kim WS. Gemcitabine alone and/or containing chemotherapy is efficient in; refractory or relapsed NK/T-cell lymphoma. J Hematol Oncol. 2013;31:469–472. doi: 10.1007/s10637-012-9889-4. [DOI] [PubMed] [Google Scholar]
- 13.Jeong S. Extranodal NK/T cell lymphoma. Blood Res. 2020;55:S63–S71. doi: 10.5045/br.2020.S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu B, Oki Y. Novel immunotherapy options for extranodal NK/T-cell lymphoma. Front Oncol. 2018;8:139. doi: 10.3389/fonc.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang GN, Zhao WG, Li L, Zhang DD, Gao XZ, Zhou J, Zhang L, Fu XR, Zheng XY, Li Y, Li Z, Zhang MZ, Li WC. Prognostic significance of CD30 expression in nasal natural killer/T-cell lymphoma. Oncol Lett. 2017;13:1211–1215. doi: 10.3892/ol.2017.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang QP, Liu S, Peng J, Xiong H, Xiong ZT, Yang Y, Tan X, Gao X. An extraordinary T/NK lymphoma, nasal type, occurring primarily in the prostate gland with unusual CD30 positivity: case report and review of the literature. Diagn Pathol. 2013;8:94–94. doi: 10.1186/1746-1596-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wook Youn K, Soo Jeong N, Sehui K, Tae Min K, Dae Seog H, Chul-Woo K, Yoon Kyung J. Prognostic implications of CD30 expression in extranodal natural killer/T-cell lymphoma according to treatment modalities. Leuk Lymphoma. 2015;56:9. doi: 10.3109/10428194.2014.974048. [DOI] [PubMed] [Google Scholar]
- 18.Wang GN, Zhao WG, Li L, Zhang DD, Gao XZ, Zhou J, Zhang L, Fu XR, Zheng XY, Li Y, Li Z, Zhang MZ, Li WC. Prognostic significance of CD30 expression in nasal natural killer/T-cell lymphoma. Oncol Lett. 2017;13:1211–1215. doi: 10.3892/ol.2017.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Ramchandren R, Bartlett NL, Cheson BD, de Vos S, Forero-Torres A, Moskowitz CH, Connors JM, Engert A, Larsen EK, Kennedy DA, Sievers EL, Chen R. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller P, Martin K, Theurich S, Schreiner J, Savic S, Terszowski G, Lardinois D, Heinzelmann-Schwarz V, Schlaak M, Kvasnicka H, Spagnoli G, Dirnhofer S, Speiser D, von Bergwelt-Baildon M, Zippelius A. Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol Res. 2014;2:741–755. doi: 10.1158/2326-6066.CIR-13-0198. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett NL, Chen R, Fanale MA, Brice P, Gopal A, Smith SE, Advani R, Matous JV, Ramchandren R, Rosenblatt JD, Huebner D, Levine P, Grove L, Forero-Torres A. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111:1848–1854. doi: 10.1182/blood-2008-01-127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O’Connor OA, Siddiqi T, Kennedy DA, Oki Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123:3095–3100. doi: 10.1182/blood-2013-12-542142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartlett NL, Chen R, Fanale MA, Brice P, Gopal A, Smith SE, Advani R, Matous JV, Ramchandren R, Rosenblatt JD, Huebner D, Levine P, Grove L, Forero-Torres A. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24. doi: 10.1186/1756-8722-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Weyden CA, Pileri SA, Feldman AL, Whisstock J, Prince HM. Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood Cancer J. 2017;7:e603. doi: 10.1038/bcj.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz S, O’Connor OA, Pro B, Illidge T, Fanale M, Advani R, Bartlett NL, Christensen JH, Morschhauser F, Domingo-Domenech E, Rossi G, Kim WS, Feldman T, Lennard A, Belada D, Illés Á, Tobinai K, Tsukasaki K, Yeh SP, Shustov A, Hüttmann A, Savage KJ, Yuen S, Iyer S, Zinzani PL, Hua Z, Little M, Rao S, Woolery J, Manley T, Trümper L ECHELON-2 Study Group. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet (London, England) 2019;393:229–240. doi: 10.1016/S0140-6736(18)32984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen R, Hou J, Newman E, Kim Y, Donohue C, Liu X, Thomas SH, Forman SJ, Kane SE. CD30 downregulation, MMAE resistance, and MDR1 upregulation are all associated with resistance to brentuximab vedotin. Mol Cancer Ther. 2015;14:1376–1384. doi: 10.1158/1535-7163.MCT-15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HK, Moon SM, Moon JH, Park JE, Byeon S, Kim WS. Complete remission in CD30-positive refractory extranodal NK/T-cell lymphoma with brentuximab vedotin. Blood Res. 2015;50:254–256. doi: 10.5045/br.2015.50.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon LM, Kwong YL. Complete remission of refractory disseminated NK/T cell lymphoma with brentuximab vedotin and bendamustine. Ann Hematol. 2016;95:847–849. doi: 10.1007/s00277-016-2627-9. [DOI] [PubMed] [Google Scholar]
- 29.van de Donk NW, Janmaat ML, Mutis T, Lammerts van Bueren JJ, Ahmadi T, Sasser AK, Lokhorst HM, Parren PW. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270:95–112. doi: 10.1111/imr.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Wang H, Li P, Lu Y, Xia Z, Huang H, Zhang Y. CD38 expression predicts poor prognosis and might be a potential therapy target in extranodal NK/T cell lymphoma, nasal type. Ann Hematol. 2015;94:1381–1388. doi: 10.1007/s00277-015-2359-2. [DOI] [PubMed] [Google Scholar]
- 31.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson KC, Lokhorst HM, van de Winkel JG, Parren PW. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 32.Pick M, Vainstein V, Goldschmidt N, Lavie D, Libster D, Gural A, Grisariu S, Avni B, Ben YD, Gatt ME. Daratumumab resistance is frequent in advanced stage multiple myeloma patients irrespectively of CD38 expression, and is related to dismal prognosis. Eur J Haematol. 2018;100:494–501. doi: 10.1111/ejh.13046. [DOI] [PubMed] [Google Scholar]
- 33.Taylor RP, Lindorfer MA. Fcγ-receptor-mediated trogocytosis impacts mAb-based therapies: historical precedence and recent developments. Blood. 2015;125:762–766. doi: 10.1182/blood-2014-10-569244. [DOI] [PubMed] [Google Scholar]
- 34.Inger SN. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128:959–970. doi: 10.1182/blood-2016-03-703439. [DOI] [PubMed] [Google Scholar]
- 35.Matas-Céspedes A, Vidal-Crespo A, Rodriguez V, Villamor N, Delgado J, Giné E, Roca-Ho H, Menéndez P, Campo E, López-Guillermo A, Colomer D, Roué G, Wiestner A, Parren PW, Doshi P, van Bueren JL, Pérez-Galán P. The human CD38 monoclonal antibody daratumumab shows antitumor activity and hampers leukemia-microenvironment interactions in chronic lymphocytic leukemia. Clin Cancer Res. 2017;23:1493–1505. doi: 10.1158/1078-0432.CCR-15-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganzel C, Kharit M, Duksin C, Rowe JM. Daratumumab for relapsed/refractory Philadelphia-positive acute lymphoblastic leukemia. Haematologica. 2018;103:e489–e490. doi: 10.3324/haematol.2018.197640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hari P, Raj RV, Olteanu H. Targeting CD38 in refractory extranodal natural killer cell-T-cell lymphoma. N Engl J Med. 2016;375:1501–1502. doi: 10.1056/NEJMc1605684. [DOI] [PubMed] [Google Scholar]
- 38.Stefanie A, Christoph D, Lukas G, Felicitas H. Systemic treatment of a patient with relapsed and refractory extranodal NK/T-cell lymphoma (ENKL) and meningeosis leukemica with daratumumab. Hematol Oncol. 2018;36:713–714. doi: 10.1002/hon.2533. [DOI] [PubMed] [Google Scholar]
- 39.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng SB, Chung TH, Kato S, Nakamura S, Takahashi E, Ko YH, Khoury JD, Yin CC, Soong R, Jeyasekharan AD. Epstein-Barr virus-associated primary nodal T/NK-cell lymphoma shows a distinct molecular signature and copy number changes. Haematologica. 2018;103:278–287. doi: 10.3324/haematol.2017.180430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jo JC, Kim M, Choi Y, Kim HJ, Kim JE, Chae SW, Kim H, Cha HJ. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2017;96:25–31. doi: 10.1007/s00277-016-2818-4. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood. 2018;131:2528–2540. doi: 10.1182/blood-2017-12-791418. [DOI] [PubMed] [Google Scholar]
- 43.Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, Ueda S, Takahara M, Kumai T, Ishibashi K, Kosaka A, Aoki N, Oikawa K, Uno Y, Akiyama N, Sado M, Takei H, Celis E, Harabuchi Y, Kobayashi H. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66:877–890. doi: 10.1007/s00262-017-1987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia Y, Medeiros LJ, Young KH. Signaling pathway and dysregulation of PD1 and its ligands in lymphoid malignancies. Biochim Biophys Acta. 2016;1865:58–71. doi: 10.1016/j.bbcan.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Cheng Y, Zhang M, Yan J, Li L, Fu X, Zhang X, Chang Y, Sun Z, Yu H, Zhang L, Wang X, Wu J, Li Z, Nan F, Tian L, Li W, Young KH. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11:15. doi: 10.1186/s13045-018-0559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiss KA, Forde PM, Brahmer JR. Harnessing the power of the immune system via blockade of PD-1 and PD-L1: a promising new anticancer strategy. Immunotherapy. 2014;6:459–475. doi: 10.2217/imt.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganzel C, Kharit M, Chen D, Rowe JM. Daratumumab for relapsed/refractory Philadelphia-positive acute lymphoblastic leukemia. Haematologica. 2018;103:e489–e490. doi: 10.3324/haematol.2018.197640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ, Lebovic D, Dhodapkar M, Avigan D, Chapuy B, Ligon AH, Freeman GJ, Rodig SJ, Cattry D, Zhu L, Grosso JF, Bradley Garelik MB, Shipp MA, Borrello I, Timmerman J. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J. Clin. Oncol. 2016;34:2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan T, Li J, Loong F, Khong P, Tse E, Kwong Y. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann Hematol. 2018;97:193–196. doi: 10.1007/s00277-017-3127-2. [DOI] [PubMed] [Google Scholar]
- 50.Jaccard A, Hermine O. A major turning point in NK/T-cell lymphoma? Blood. 2017;129:2342. doi: 10.1182/blood-2017-03-769075. [DOI] [PubMed] [Google Scholar]
- 51.Kim SJ, Hyeon J, Cho I, Ko YH, Kim WS. Comparison of efficacy of pembrolizumab between epstein-barr virus-positive and -negative relapsed or refractory non-hodgkin lymphomas. Cancer Res Treat. 2019;51:611–622. doi: 10.4143/crt.2018.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–2707. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 53.Yenlin H, Aurélien DR, Laurence DL, Bouchra G, Nadine MG, Marion T, Jacques B, Josette B, Barbara P, Emilie T. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood. 2010;115:1226. doi: 10.1182/blood-2009-05-221275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pascual M, Mena-Varas M, Robles EF, Garcia-Barchino MJ, Panizo C, Hervas-Stubbs S, Alignani D, Sagardoy A, Martinez-Ferrandis JI, Bunting KL, Meier S, Sagaert X, Bagnara D, Guruceaga E, Blanco O, Celay J, Martínez-Baztan A, Casares N, Lasarte JJ, MacCarthy T, Melnick A, Martinez-Climent JA, Roa S. PD-1/PD-L1 immune checkpoint and p53 loss facilitate tumor progression in activated B cell diffuse large B-cell lymphomas. Blood. 2019;133:2401–2412. doi: 10.1182/blood.2018889931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Wang B, Ma X, Guo Y. NF-kappaB activation through the alternative pathway correlates with chemoresistance and poor survival in extranodal NK/T-cell lymphoma, nasal type. Jpn J Clin Oncol. 2009;39:418–424. doi: 10.1093/jjco/hyp037. [DOI] [PubMed] [Google Scholar]
- 56.Huynh M, Pak C, Markovina S, Callander NS, Chng KS, Wuerzberger-Davis SM, Bakshi DD, Kink JA, Hematti P, Hope C, Asimakopoulos F, Rui L, Miyamoto S. Hyaluronan and proteoglycan link protein 1 (HAPLN1) activates bortezomib-resistant NF-κB activity and increases drug resistance in multiple myeloma. J Biol Chem. 2018;293:2452–2465. doi: 10.1074/jbc.RA117.000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun CY, Li JY, Chu ZB, Zhang L, Chen L, Hu Y. Efficacy and safety of bortezomib maintenance in patients with newly diagnosed multiple myeloma: a meta-analysis. Biosci Rep. 2017;37:BSR20170304. doi: 10.1042/BSR20170304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farid M, Yau YW, Tay K, Quek R, Tao M, Koo GC, Loong S, Lim ST. A promising new regimen for the treatment of advanced extranodal NK/T cell lymphoma. Acta Oncol. 2011;50:589–590. doi: 10.3109/0284186X.2010.516272. [DOI] [PubMed] [Google Scholar]
- 59.Chen C, He H. Treatment of relapsed extranodal natural killer/T-cell lymphoma with bortezomib plus fludarabine. Mol Clin Oncol. 2017;7:525–528. doi: 10.3892/mco.2017.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sim SH, Kim S, Kim TM, Jeon YK, Nam SJ, Ahn YO, Keam B, Park HH, Kim DW, Kim CW. Novel JAK3-activating mutations in extranodal NK/T-cell lymphoma, nasal type. Am J Pathol. 2017;187:980–986. doi: 10.1016/j.ajpath.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Nairismägi ML, Gerritsen ME, Li ZM, Wijaya GC, Chia BKH, Laurensia Y, Lim JQ, Yeoh KW, Yao XS, Pang WL, Bisconte A, Hill RJ, Bradshaw JM, Huang D, Song TLL, Ng CCY, Rajasegaran V, Tang T, Tang QQ, Xia XJ, Kang TB, Teh BT, Lim ST, Ong CK, Tan J. Oncogenic activation of JAK3-STAT signaling confers clinical sensitivity to PRN371, a novel selective and potent JAK3 inhibitor, in natural killer/T-cell lymphoma. Leukemia. 2018;32:1147–1156. doi: 10.1038/s41375-017-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Mel S, Hue SS, Jeyasekharan AD, Chng WJ, Ng SB. Molecular pathogenic pathways in extranodal NK/T cell lymphoma. J Hematol Oncol. 2019;12:33. doi: 10.1186/s13045-019-0716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bollard CM, Stephen G, Vicky T, Oumar D, Stephanie K, Yasmin H, George C, Carlos R, Luis F, Shpall EJ. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heslop HE, Slobod KS, Pule MA, Hale GA, Alexandra R, Smith CA, Bollard CM, Hao L, Meng-Fen W, Rochester RJ. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ekaterina D, Banu OS, Prockop SE, Kernan NA, Sara A, Julie TF, Cyrus H, Chou JF, Glenn H, Barker JN. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119:2644–56. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang T, Cao L, Xie J, Shi N, Zhang Z, Luo Z, Yue D, Zhang Z, Wang L, Han W, Xu Z, Chen H, Zhang Y. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: a meta-analysis. Oncotarget. 2015;6:33961–33971. doi: 10.18632/oncotarget.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramos C, Ballard B, Zhang H, Dakhova O, Gee A, Mei Z, Bilgi M, Wu M, Liu H, Grilley B, Bollard C, Chang B, Rooney C, Brenner M, Heslop H, Dotti G, Savoldo B. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J Clin Invest. 2017;127:3462–3471. doi: 10.1172/JCI94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kosti P, Maher J, Arnold J. Perspectives on chimeric antigen receptor T-cell immunotherapy for solid tumors. Front Immunol. 2018;9:1104. doi: 10.3389/fimmu.2018.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holzinger A, Barden M, Abken H. The growing world of CAR T cell trials: a systematic review. Cancer Immunol Immunother. 2016;65:1433–1450. doi: 10.1007/s00262-016-1895-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan J, Niu Q, Deng B, Liu S, Tong C. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019;33:2854–2866. doi: 10.1038/s41375-019-0488-7. [DOI] [PubMed] [Google Scholar]


