Abstract
Background: Myeloid-derived suppressor cells (MDSCs) play key roles in sepsis, but whether the bone marrow is considered the only source remains unclear. The current knowledge about the mechanism of MDSCs leading to myocardial injury in sepsis is poor. Methods: In sepsis patients with cardiac dysfunction, the circulating percentage of CD14-CD11b+ and serum concentrations of IL-6 and IL-1β were measured. A mouse sepsis model was established through caecum ligation and puncture (CLP). Animals were divided into four groups: control, sham, CLP and CLP+splenectomy (CLPS). Serum concentrations of IL-6, IL-1β, TnI and NT-proBNP were measured. CD11b+Gr-1+ cells were detected by immunofluorescence staining and RT-PCR. Myocardial injury was detected by HE, Masson and TUNEL staining. The expression of mTOR, P53 and caspase-3 was measured by Western blot. Results: In sepsis patients, circulating MDSCs were increased, and the serum concentrations of IL-6 and IL-1β were elevated. The serum concentrations of IL-6 and IL-1β were correlated with the ratio of circulating MDSCs. In the mouse sepsis model, the spleen was the major source of CD11b+Gr-1+ cells that migrated into circulation and the heart in sepsis. The serum concentrations of IL-6 and IL-1β were also elevated. Echocardiography and serum biomarkers showed that cardiomyocyte damage and cardiac hypofunction in sepsis-induced myocardial injury. The expression of CD11b, Gr-1 and pro-inflammatory cytokines in the heart was significantly higher in sepsis patients than that in controls. Pathological staining and TUNEL staining showed obvious myocardial damage and cell apoptosis. The Western blot analysis indicated that in the heart, the activation of mTOR was inhibited and that the expression of P53 and caspase-3 was elevated in sepsis-induced myocardial injury. Conclusion: In sepsis-induced myocardial injury, splenic reservoir CD11b+Gr-1+ cells rapidly migrated into circulation and the heart, further impairing heart function via the high expression of P53 through the inhibition of mTOR.
Keywords: Sepsis, myocardial injury, MDSCs, inflammation, mTOR
Introduction
Sepsis is a severe and complicated syndrome that has a high mortality. Myocardial injury is a common complication of sepsis in clinical practice. A growing amount of evidence suggests that myeloid cells are a major component in the role of promoting inflammation [1-3]. Recent studies have suggested that myeloid-derived suppressor cells (MDSCs) include immature granulocytes, macrophages and dendritic cells at different stages of differentiation [4]. Both in sepsis and trauma, MDSCs participate in the progression of inflammation [5,6]. In sepsis, the activation of MDSCs is related to pro-inflammatory cytokine production. Furthermore, MD-SCs can regulate T cell responses through the production of large amounts of nitric oxide (NO) and reactive oxygen species (ROS) [7,8]. However, the role of MDSCs needs to be fur-ther discussed.
The major known functions of the spleen are the removal of aging erythrocytes and the recycling of iron, elicitation of immunity, and supply of erythrocytes after hemorrhagic shock [9]. The presence of numerous monocytes in the spleen contributes to the progress of inflammation. A previous study showed that the spleen has numerous MDSCs that express Gr-1+CD11b+. In a mouse myocardial infarction model, the increased circulating MDSCs did not originate in the bone marrow. However, a great number of splenic MDSCs were recruited into the blood [10]. Although it has been suggested by other research that in an animal sepsis model, CD11b+Gr-1+ MDSCs gradually increase [6], the behavior and origin of the MDSCs remain unclear in sepsis-induced myocardial injury.
Accordingly, this study was designed to confirm whether the number of circulating MDSCs increase and are further recruited into the myocardial tissue, leading to sepsis-induced myocardial injury. Further, we aimed to determine the origin of circulating MDSCs. Moreover, the preliminary mechanism of MDSC recruitment and the myocardial injury was discussed.
Methods
Sepsis patients
Peripheral venous blood samples were collected from patients with sepsis and cardiac injury/dysfunction (n=10) in the Emergency Department Intensive Care Unit of Yi Ji Shan Hospital affiliated with Wan Nan Medical College (n=2), Department of Critical Care Medicine of Wuhu No. 1 Peoples’ Hospital (n=2), Department of Critical Care Medicine of Wuhu No. 2 Peoples’ Hospital (n=5) and Department of Critical Care Medicine of Wuhu Traditional Chinese Medicine Hospital (n=1). Human blood samples were collected contributes to the medical ethics committees of Yi Ji Shan Hospital, Wuhu No. 1 Peoples’ Hospital, Wuhu No. 2 Peoples’ Hospital and Wuhu Traditional Chinese Medicine Hospital and performed according to the Declaration of Helsinki. This study was approved by the Yi Ji Shan Hospital Ethics Committee and has informed consent from all patients and healthy control. Samples from 6 age- and sex-matched healthy volunteers were collected as controls. Peripheral venous blood samples were collected into EDTA-coated tubes and a procoagulant tube. The serum was then separated from the procoagulant tube, collected into EP tubes and stored at -40°C. Serum concentrations of IL-6 and IL-1β weremeasured using a Human IL-6 ELISA kit and aHuman IL-1β ELISA Kit (Boster, China), respec-tively. Mononuclear cells were isolated on hy-droxypropyl methylcellulose (HaoYang, China) by centrifugation at 500 g for 20 min. Cellswere fixed in 1% BSA in PBS. The cells werethen incubated with PE-conjugated anti-Hu-man CD14 (BD Biosciences, USA) and FITC-conjugated anti-Human CD11b (BD Biosciences, USA) for 1 h on ice. After washing threetimes with 1% BSA in PBS, cells were analyzedon a FACScan flow cytometer (Becton Dickinson, USA) to detect CD14-CD11b+ cells.
Animal sepsis model
A sepsis model was generated in male C57BL/6J mice (8-10 weeks old, weight: 20-25 g) via caecum ligation and puncture (CLP). The C57BL/6J mice were purchased from Qing Long Shan Animal Breeding Farm, Nanjing, China. All the mice were fed in the SPF animalHouse. This animal study was approved by the Yi Ji Shan Hospital Animal Care Committee. Themice were handled according to the guidelines of the Local Ethics Committee for Animal Experimentation. Written informed consent to use the animals was obtained from Yi Ji Shan Hospital Animal Care Committee. The mice were randomly divided into four groups: controlnon-operated (control, n=5), control operated (sham, n=5), CLP (n=10) and CLP+splenectomy (CLPS, n=10). Sepsis was induced by CLP based on previous descriptions [11,12]. Briefly, mice were anesthetized intraperitoneally with 1% pentobarbital (Sigma, 35 mg/kg). The caecum was ligated with a 5.0 silk suture at itsbase and then punctured one single time with a 26-gauge needle. After the caecum was pl-aced back in the abdominal cavity, the peritneal wall and skin incision were sutured. Sham group mice underwent a similar surgery with-out ligated or punctured. CLPS mice underwent the same procedure of CLP after splenectomy. All mice received 1 ml of sterile saline subcutaneously after surgery. No antibiotics or anti-inflammatories were administered. After 24 h of the sepsis model, all mice were sacrificed under deep anesthesia with overdose pentobarbital (Sigma, 130 mg/kg intraperitoneal injection).
Echocardiography
After 24 h of the sepsis model established, 2-dimensional echocardiography was perform-ed on the mice using a transthoracic echocar-diogram (Visual Sonic, Canada) as previouslydescribed [13]. The ejection fraction (EF) was measured and the shortening fraction (FS) was calculated.
Determination of serum concentrations of IL-6, IL-1β, TnI and NT-proBNP in the animal model
After echocardiography was performed, micewere sacrificed. Mouse blood was collectedinto an EDTA-coated tube. After centrifugationat 500 g for 20 min, serum was collected. Serum concentrations of IL-6, IL-1β, TnI and NT-proBNP were analyzed using a Mouse IL-6 ELISA Kit (Beyotime, China), Mouse IL-1β ELISA Kit (Beyotime, China), Mouse TnI ELISA Kit (Enzyme-linked Biotechnology, China) and Mouse NT-proBNP ELISA Kit (Elabscience, China), respectively.
Cells preparation for FACS analysis
Bone marrow-derived cells (BMDCs) from the femurs of euthanized mice were flushed with ice-cold PBS containing 1% BSA and depletedof red blood cells (RBCs) using RBC lysing buffer (BD Biosciences). The spleen was cut intosmall pieces, grinded into a cell suspensionand depleted of red blood cells (RBCs) using RBC lysing buffer (BD Biosciences) (except for CLPS). Total nucleated cells in the peripheral blood were isolated after erythrocyte lysis. Forthe FACS analysis, cell suspensions were stained with PE-conjugated anti-mouse CD11b antibodies and FITC-conjugated anti-mouse Gr-1 for 1 h on ice, and then they were washed with 1% BSA in PBS. The percentage of CD11b+Gr-1+ cells was evaluated by multi-color flow cytometry using a flow cytometer (EPICS® ALTRA, Beckman Coulter).
mRNA expression of CD11b, Gr-1, IL-6 and IL-1β in the myocardium of mice
In mouse hearts, mRNA levels of various fac-tors, including CD11b, Gr-1, IL-6 and IL-1β, were detected by RT-PCR. The mouse hearts werestored in Trizol at -80°C. Total RNA was extract-ed as previously described and cDNA was synthesized using a PrimeScriptTM RT reagent kitwith gDNA Eraser (TAKARA, Japan). RT-PCR was performed using CD11b primers (Forward: CCATGACCTTCCAAGAGAATGC, Reverse: ACCGGCTTGTGCTGTAGTC), Gr-1 primers (Forward: TCATCCTTCTTGTGGTCCTA, Reverse: AAGGGGCAGGTAGTTGTG), IL-6 (Forward: TAGTCCTTCCTACCCCAATTTCC, Reverse: TTGGTCCTTAGCCACTCCTTC), IL-1β (Forward: GCAACTGTTCCTGAACTCAACT, Reverse: ATCTTTTGGGGTCCGTCAACT) and GAPDH (Forward: GCCTCAAGATCATCAGCAAT, Reverse: GGACTGTGGTCATGAGTCCT). After RT (50°C, 30 min), hot start (94°C, 15 min), and 40-42 cycles of PCR (94°C, 1 min; 52.5°C, 1 min; 72°C, 1 min), CD11b, Gr-1, IL-6 and IL-1β mRNA expression was normalized to GAPDH and calculated as 2-ΔΔCt.
Cardiac histopathology
The myocardial tissue was fixed in 10% formaldehyde, and then embedded in paraffin. Tissue sections (2 μm thick) were stained with hematoxylin and eosin (HE) and Masson staining using an HE Staining Kit (Beyotime, China) and Masson Staining Kit (Nanjing KeyGen Biotech, China), respectively, according to the manufacturer’s protocol. Morphological changes of myocardial tissue were assessed using an optical microscope (Olympus, Tokyo, Japan). The morphological evaluations were performed in a blinded manner by 2 independent investigators.
Immunofluorescence staining
A frozen section of the heart was prepared. The myocardial tissue section (2 μm thick) was stained with PE-conjugated anti-mouse CD11b antibodies and FITC-conjugated anti-mouse Gr-1 for 1 h at room temperature to measure the expression of CD11b and Gr-1. The tissue was further stained with 4,6-diamidino-2-phenylindole (DAPI, Roche). Fluorescence microscopy (Olympus, Tokyo, Japan) was used to detect the fluorescence.
TUNEL immunohistochemistry (IHC) staining
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was performed to detect apoptotic nuclei by TUNEL Staining Kit (Roche, Indianapolis, IN) according to the manufacturer’s protocol. The number of apoptotic cells with TUNEL-positive nuclei was counted by 2 independent observers blinded to the treatment group and expressed as a percentage of the total myocyte population.
Western blot analysis
For the Western blot analysis, the protein was extracted from mouse myocardial tissue using a Tissue Protein Extraction Kit (Beyotime, China) according to the protocol provided by the manufacturer. Protein concentrations were measured using the BCA Assay Kit (Pierce, USA). Western blots of the protein samples were performed to detect the mammalian target of rapamycin (mTOR) and phosphorylated Ser2448-mTOR (P-Ser2448 mTOR) using rabbit monoclonal anti-mouse mTOR antibodies and the rabbit monoclonal anti-mouse phosphorylated Ser2448-mTOR antibody (Cell Signaling Technology, USA). P53 and caspase-3 expression were detected by rabbit monoclonal anti-mouse P53 (Cell Signaling Technology, USA) and rabbit anti-mouse caspase-3 (Abcam, UK). GAPDH was used as a loading control using GAPDH Antibody (Cell Signaling Technology, USA). After primary antibody incubation, the blots were incubated with the appropriate secondary horseradish peroxidase-conjugated mouse monoclonal anti-rabbit antibody (Boster, China). Each membrane was washed and then developed using the Super Signal chemiluminescent substrate (Pierce, USA).
Statistical analyses
For all experiments, the data were analyzed using either a one way ANOVA, Student’s t-test or Bonferroni’s test. The correlation was analyzed by Pearson correlation. Values are expressed as the means ± SEM. All statistical analyses were performed using SPSS software (SAS Institute Inc., USA); p values <0.05 were considered to indicate significance.
Results
Increased CD14-CD11b+ cells and serum concentration of inflammatory factors in peripheral circulation of sepsis patients with myocardial injury
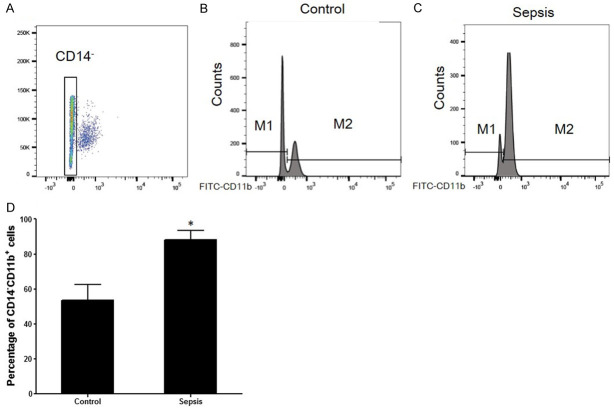
No differences in age or sex were found between the sepsis with the myocardial injury group (n=10) and the control group (n=6). The percentage of CD14-CD11b+ MDSCs was higher in the sepsis group than in the control group (88.1±5.3% vs 53.4±9.1%, P<0.05, Figure 1). Furthermore, the serum concentrations of IL-6 and IL-1β in sepsis patients were significantly higher than in controls (24.0±1.4 pg/mL vs 16.7±1.0 pg/mL and 11.4±1.4 pg/mL vs 7.4±0.5 pg/mL, respectively, P<0.05, Figure 2A and 2B). Furthermore, the serum concentrations of IL-6 (r=0.728, P<0.001) and IL-1β (r=0.884, P<0.001) were statistically related to the percentage of CD14-/CD11b+ cells in the blood (Figure 2C, 2D).
Figure 1.
The percentage of CD14-CD11b+ cells in peripheral blood of sepsis patients with myocardial injury. The percentage of CD11b+Gr-1+ cells in blood, spleen and bone marrow. A: The gating of CD14 negative cells. CD14- cells were gated for further analysed. B: Representative flow cytometry analyses of CD14-CD11b+ cells expression in control (n=6). C: Representative flow cytometry analyses of CD14-CD11b+ cells expression in sepsis with myocardial injury (n=10). D: Histogram showed the percentage of CD14-CD11b+ cells in sepsis patients was higher than control (*P<0.05 vs control).
Figure 2.

Serum concentration of IL-6 and IL-1β in control (n=6) and sepsis with myocardial injury group (n=10). A: Histogram showed the serum concentration of IL-6 was higher in sepsis than control. B: Histogram showed the serum concentration of IL-1β was higher in sepsis than control. C: The relationship between serum IL-6 concentration and percentage of CD14-CD11b+ cells. Pearson correlation analysis showed that percentage of circulating CD14-CD11b+ cells has correlated with serum concentration of IL-6 (r=0.728, P<0.001). D: The relationship between serum IL-1β concentration and percentage of CD14-CD11b+ cells. Pearson correlation analysis showed that percentage of circulating CD14-CD11b+ cells has correlated with serum concentration of IL-1β (r=0.884, P<0.001) (*P<0.05 vs control).
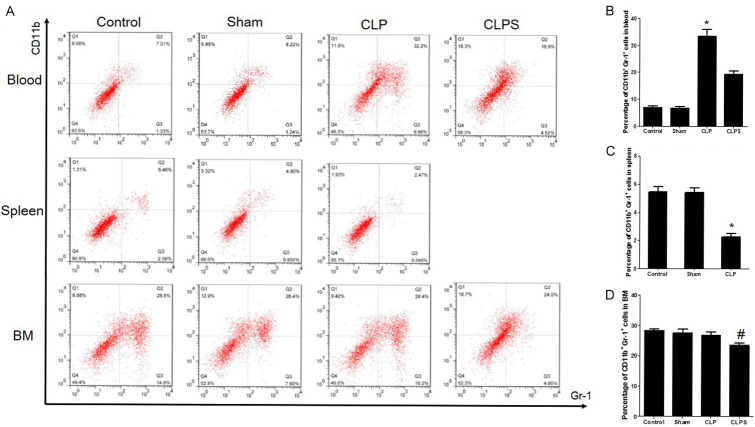
The percentage of CD11b+Gr-1+ cells in blood, spleen and bone marrow of mouse sepsis models
As shown in Figure 3, compared with the control and sham groups, the percentage of CD11b+Gr-1+ cells in the blood was significantly higher in the CLP group (mean: 33.6±2.4% vs 6.9±1.0% and 6.8±0.8%, respectively, P<0.001). Furthermore, in the CLPS group, the percentage of CD11b+Gr-1+ cells in the blood was lower than that in the CLP group (mean: 19.3±1.3% vs 33.6±2.4%, P<0.001). However, the percentage of CD11b+Gr-1+ cells in the spleen was significantly lower in the CLP group than in the control and sham groups (mean: 2.5±0.3% vs 5.46±0.3% and 4.80±0.3%, respectively, P<0.001). In the bone marrow, the percentage of CD11b+Gr-1+ cells in the CLP group was similar to the CLPS, control and sham groups (mean: 28.4±1.2% vs 24.0±0.7%, 26.4±0.5% and 28.8±1.3%, respectively, P>0.05).
Figure 3.
Flow cytometry analyses of CD11b+Gr-1+ cells expression in blood, spleen and bone marrow among four groups. Percentage of circulating CD11b+Gr-1+ cells in sepsis was higher than control, sham and CLPS. However, percentage of splenic CD11b+Gr-1+ cells in sepsis was lower than control and sham. In bone marrow, percentage of CD11b+Gr-1+ cells in four groups was similar. A: Representative scatter plot showed the CD11b+Gr-1+ cells expression in blood, spleen and bone marrow. B: Histogram showed the percentage of CD11b+Gr-1+ in blood. C: Histogram showed the percentage of CD11b+Gr-1+ in spleen. D: Histogram showed the percentage of CD11b+Gr-1+ in bone marrow (n=5 for control and sham, n=10 for CLP and CLPS, *P<0.001 vs control, sham and CLPS, #P>0.05 vs control, sham and CLP).
Cardiac function of mouse sepsis models
In the sepsis group, the EF% and FS% were significantly lower than in the control and sham groups (EF%: 56.0±3.0% vs 76.1±3.0% and 73.0±3.4%, respectively, P<0.001; FS%: 27.4±1.0 vs 45.4±1.4% and 42.0±1.7%, respectively, P<0.001). In the CLPS group, the EF% and FS% were significantly higher than in the CLP group (EF%: 64.2±4.6% vs 56.0±3.0%, P<0.001; FS%: 35.3±2.2% vs 27.4±1.0%, P<0.001, Figure 4).
Figure 4.
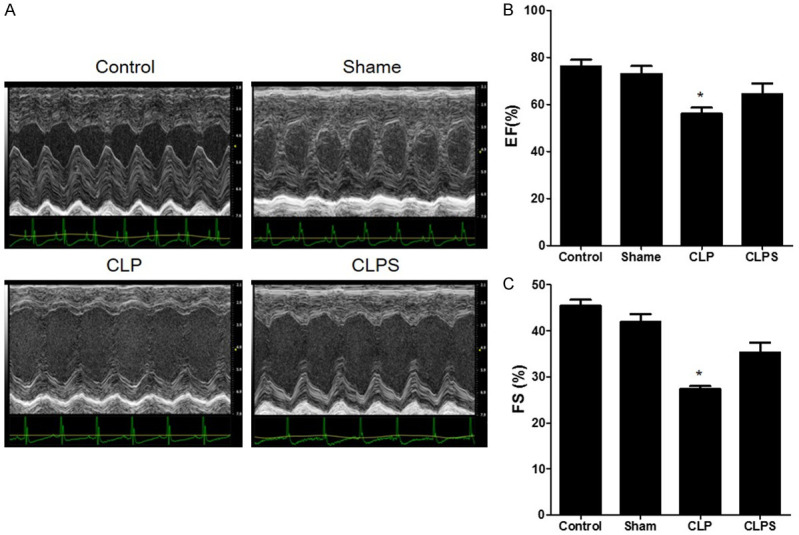
The cardiac function in four groups. A: The representative echocardiography image of four groups. B: Histogram showed the EF% among four groups. EF% in sepsis group was lower than control, sham and CLPS. C: Histogram showed the FS% among four groups. FS% in sepsis group was lower than control, sham and CLPS (n=5 for control and sham, n=10 for CLP and CLPS, *P<0.001 vs control, sham and CLPS).
The serum concentration of troponin I (TnI), NT-proBNP and the expression of inflammatory factors in mouse sepsis models
The serum concentration of TnI was significantly higher in the CLP group than that in the control, sham and CLPS groups (6.23±0.24 ng/mL vs 0.03±0.01 ng/mL, 0.04±0.01 ng/mL and 3.68±0.17 ng/mL, respectively, P<0.05). The serum NT-proBNP concentration was significantly higher in the CLP group than that in the control, sham and CLPS groups (2799±536 pg/mL vs 93±8.0 pg/mL, 100±4.0 pg/mL and 1011±148 pg/mL, respectively, P<0.05, Figure 5A and 5B).
Figure 5.

Serum concentration of TnI, NT-proBNP, IL-6 and IL-1β in animal models. A: Histogram showed the serum concentration of TnI in four groups. Serum concentration of TnI in sepsis was higher than other three groups. B: Histogram showed the serum concentration of NT-proBNP in four groups. Serum concentration of NT-proBNP in sepsis was higher than other three groups. C: Histogram showed the serum concentration of IL-6 in four groups. Serum concentration of IL-6 was higher than other three groups. D: Histogram showed the serum concentration of IL-1β in four groups. Serum concentration of IL-1β was higher than other three groups (n=5 for control and sham, n=10 for CLP and CLPS, *P<0.05 vs control, sham and CLPS).
The serum concentration of IL-6 was also higher in the CLP group than that in the control, sham and CLPS groups (19.5±0.3 pg/mL vs 9.8±0.7 pg/mL, 4.2±1.0 pg/mL and 4.1±0.3 pg/mL, respectively, P<0.05). Furthermore, the serum concentration of IL-1β in the CLP group was higher than that in the control, sham and CLPS groups (27.5±1.1 pg/mL vs 8.6±0.1 pg/mL, 8.3±0.2 pg/mL and 15.4±0.8 pg/mL, respectively, P<0.05, Figure 5C and 5D).
RT-PCR showed that the expression of IL-6 and IL-1β was significantly higher in the CLP group than that in the control and sham groups. In the CLPS group, the expression of IL-6 and IL-1β was downregulated more than in the CLP group (Figure 6A and 6B).
Figure 6.
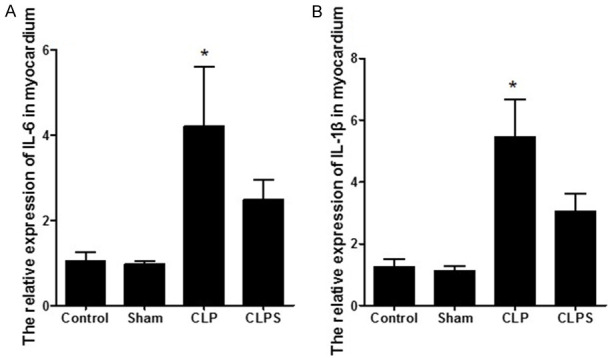
The relative expression of IL-6 and IL-1β in myocardial tissue. A: Histogram showed the relative expression of IL-6 in four groups. Relative expression of IL-6 in myocardial tissue was higher than other three groups. B: Histogram showed the relative expression of IL-1β in four groups. Relative expression of IL-1β in myocardial tissue was higher than other three groups (n=5 for control and sham, n=10 for CLP and CLPS, *P<0.05 vs control, sham and CLPS).
Expression of CD11b and Gr-1 in myocardium tissue and cell apoptosis analysis
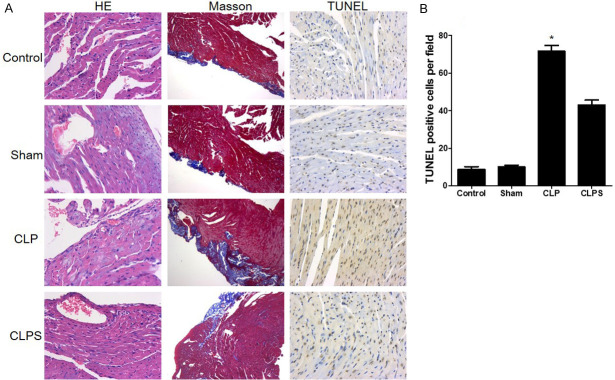
HE staining showed the number of inflammatory cells that gathered in the myocardium tissue in the CLP group compared with the control and sham groups. In the CLPS group, the number of inflammatory cells was decreased more than in the CLP group (Figure 7). Masson staining showed that collagen fibers were detected instead of muscle fibers in sepsis (Figure 7). TUNEL staining showed that apoptotic cardiomyocytes had no obvious change between the control and sham groups. In the CLP group, apoptotic cardiomyocytes increased markedly in the myocardium tissue. However, apoptotic cardiomyocytes decreased in the CLPS group compared with the CLP group (Figure 7). Immunofluorescence indicated that CD11b+Gr-1+ cells in the myocardium tissue tended to be higher in the CLP group than in the control or sham groups. In the CLPS group, the number of CD11b+Gr-1+ cells was lower than in the CLP group (Figure 8A and 8B). The histogram showed the CD11b+Gr-1+ cells number in myocardial tissue (Figure 8B). RT-PCR further showed that the expression of CD11b and Gr-1 was significantly higher in the CLP group than in the control and sham groups. In the CLPS group, the expression of CD11b and Gr-1 was lower than in the CLP group (Figure 8C and 8D).
Figure 7.
Monocyte mobilized to myocardial tissue in sepsis and led to myocardial damage. A: The first column: representative HE staining showed that monocyte accumulated to myocardial tissue in CLP group. No obviously inflammatory cells occurred in control, sham and CLPS. Myocardial dissolving and inflammatory cell infiltration was observed in sepsis group (400*). The second column: representative Masson staining photograph. Collagen fiber was detected in sepsis instead of muscle fibers (100*). The third column: representative TUNEL staining showed that apoptotic cardiomyocytes were no obviously changed between control and sham (400*). Markedly increased apoptotic cardiomyocytes in the CLP group. In CLPS, the number of apoptotic cardiomyocytes is lower than CLP. B: Histogram showed the number of apoptotic cardiomyocytes among the four groups (n=5 for control and sham, n=10 for CLP and CLPS, *P<0.05 vs control, sham and CLPS).
Figure 8.
CD11b+Gr-1+ cells mobilized to myocardial tissue in sepsis. A: Representative immunofluorescence staining showed that in CLP group, both CD11b and Gr-1 expressed in myocardial tissue. In control and sham, CD11b and Gr-1 negatively expressed in myocardial tissue. In CLPS, the expression of CD11b and Gr-1 was lower than CLP. B: Histogram showed the CD11b+Gr-1+ cells number in myocardial tissue. The number of CD11b+Gr-1+ cells was significantly higher in myocardial tissue in CLP. C: Histogram showed the relative expression of Gr-1 in four groups. Relative expression of Gr-1 in myocardial tissue was higher than other three groups. D: Histogram showed the relative expression of CD11b in four groups. Relative expression of CD11b in myocardial tissue was higher than other three groups (n=5 for control and sham, n=10 for CLP and CLPS, *P<0.05 vs control, sham and CLPS).
mTOR/P53 signaling pathway participated in sepsis-induced myocardial injury
In the CLP group, mTOR was dephosphorylated and the expression of P53 and caspase-3 was upregulated compared to the control and sham groups. In the CLPS group, the phosphorylation of mTOR was higher than in the CLP group. The expression of P53 and caspase-3 was downregulated in the CLPS group compared to the CLP group (Figure 9).
Figure 9.
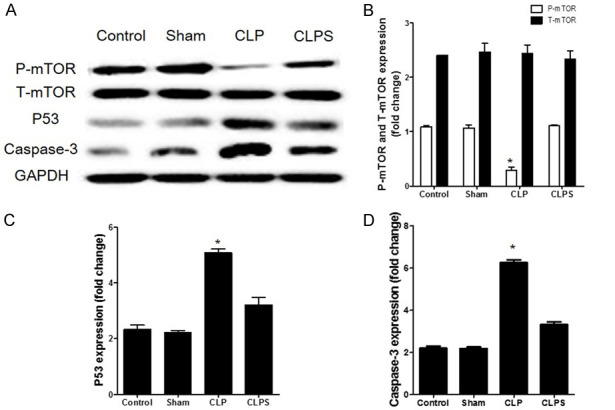
The mTOR/P53 pathway in septic myocardial tissue. A: Representative Western blots normalized to GAPDH showed that in CLP group, the phosphorylation of mTOR was inhibited compared to control and sham. However, the phosphorylation of mTOR was higher in CLPS than CLP. Further, the expression of P53 and caspase-3 was down-regulated in CLPS compared to CLP. B: Densitometry quantitation of P-mTOR and T-mTOR expression levels are shown as fold changes in histogram. C: Densitometry quantitation of P53 expression levels are shown as fold changes in histogram. D: Densitometry quantitation of caspase-3 expression levels are shown as fold changes in histogram (n=5 for control and sham, n=10 for CLP and CLPS, *P<0.05 vs control, sham and CLPS).
Discussion
In this study, we revealed that spleen-derived CD11b+Gr-1+ cells mobilize into circulation and myocardial tissue and further inhibit the phosphorylation of mTOR that leads to apoptosis of cardiac myocytes through the upregulated expression of P53 and caspase-3 in sepsis-induced myocardial injury.
Severe sepsis contributes to the main causes of mortality in ICU patients and increases the medical burden [14,15]. The inflammatory response is a key characteristic in the development of organ injury in sepsis. Myocardial dysfunction is an important characteristic in sepsis that is associated with poor prognoses such as cognitive impairment and functional disability [16]. Pro-inflammatory factors released by inflammatory cells are a major mechanism participating in the progress of sepsis-induced myocardial dysfunction [17,18]. CD11b+Gr-1+ cells are a group of mononuclear cells usually defined as myeloid-derived suppressor cells (MDSCs). In humans, MDSCs are defined as CD14-CD11b+ [8]. CD11b+Gr-1+ cells have numerous functions, including producing pro-inflammatory cytokines and immunoregulatory properties that migrate to injured or infected sites [19,20]. CD11b+Gr-1+ cells participate in the systemic inflammatory response through different pathways during early and late sepsis [6]. However, under different circumstances, CD11b+Gr-1+ cells have a paradoxical role in sepsis, trauma and ischaemic injury [5,21]. It has remained in doubt whether CD11b+Gr-1+ cells are protective or injury-promoting cells. A widely accepted viewpoint considered that in different disease phases, different groups of CD11b+Gr-1+ cells showed contrary effects [6]. In this study, we indicated that CD11b+Gr-1+ cells migrated to the circulation of sepsis patients and the myocardial tissue in animal sepsis models. The concentration of blood pro-inflammatory cytokines increased dramatically in both patients and animal models, which further influenced the signaling pathway associated with cell survival.
Bone marrow is rich in mononuclear cells containing CD11b+Gr-1+ cells. Various researches have indicated that bone marrow is the major source of CD11b+Gr-1+ cells [22]. However, the spleen contains numerous monocytes or promonocytes. In myocardial infarction, spleen-derived CD11b+Gr-1+ cells contributed maximally to circulation and myocardial tissue [10,23]. In other pathological injuries, splenic reservoir CD11b+Gr-1+ cells were also the major source of circulating inflammatory cells [24]. Our research also indicated that in sepsis-induced myocardial injury, spleen-derived CD11b+Gr-1+ cells were the major source that rapidly mobilized into circulation and the target organ to trigger an inflammatory reaction and damage of the organ through the CLPS model. The spleen and its primary cells have the potential to become a probable new therapeutic target.
mTOR is a key molecule that regulates CD11b+Gr-1+ differentiation and immunomodulation during an inflammatory reaction [25,26]. The relationship between mTOR and organ damage is a key point that attracts a number of researchers. Previous research suggested that the activation of mTOR protected against murine immunological hepatic injury by limiting the recruitment of CD11b+Gr-1+Ly6Chigh cells [27]. However, in different organs and microenvironments, the role of mTOR showed an obvious heterogeneity. The activation of mTOR associated with vasodilator and pro-inflammatory mediator formation contributes to LPS-induced hypotension and inflammation [28]. In acute kidney injury, CD11b+Gr-1+ cells are recruited to the injured kidney following mTOR inhibition, and further protect mouse kidneys against AKI in vivo [26]. The key role of mTOR may act to adjust the balance of pro-inflammatory and anti-inflammatory responses [25]. Our data indicated that in sepsis-induced myocardial injury, the expression of phosphorylated mTOR in myocardial tissue was lower than that in the control group. Nevertheless, phosphorylated mTOR was highly expressed in CLPS mice, similar to healthy controls, due to few CD11b+Gr-1+ cells that mobilized from the spleen and migrated to the myocardial tissue. These results suggest that the inflammatory reaction inhibits the activation of mTOR and further leads to myocardial tissue damage in sepsis.
The mTOR signaling pathway has been widely studied in the senescence of tumour cells and immortalized cell lines [29]. It had been proven that the mTOR signalling pathway plays a key role in cell apoptosis and senescence. Inhibition of mTOR activates the expression of P53 and further leads to cell senescence and organ dysfunction [30]. P53 is considered a key molecular that regulates the cell cycle and loss of cell function. A previous study suggested that P53 mediates the accelerated onset of senescence of endothelial progenitor cells in diabetes [31]. Nevertheless, activation of the p53 tumour suppressor can lead to cell cycle arrest [32]. However, the relationship between the mTOR pathway and cell and organ injury is still under debate. In LPS-induced rat damage models, activation of mTOR is the key mechanism involved in inflammation [28]. In sepsis, activation of the mTOR signalling pathway contributes to long-term neuronal loss [33]. Our research demonstrated that in sepsis-induced myocardial injury, expression of phosphorylated mTOR was significantly inhibited and further led to the upregulation of P53, and finally, to cell apoptosis, which resulted in impaired heart function. These data may partly explain the role of mTOR/P53 in sepsis-induced myocardial injury.
There are several apparent limitations in this study. Whether the mTOR signalling pathway is involved in the differentiation of CD11b+Gr-1+ cells and the roles of different types of MDSCs need to be further studied.
Conclusion
In a mouse sepsis-induced myocardial injury model, splenic reservoir CD11b+Gr-1+ cells rapidly migrated into circulation and the target organ, further impairing heart function via the high expression of P53 through the inhibition of mTOR.
Acknowledgements
We thank Dr. Yueping Chen, Dr. Yongli Zhao, Dr. Xiang Kong, Dr. Qing Zhai and Dr. Jinhan Chen in Department of Endocrine, Yi Ji Shan hospital for their kindly support. The abstract of this manuscript has been presented as conference abstract in ESC congress 2019. This work was supported by National Natural Science Foundation of China (81700265 to Cong Fu and 81702092 to Yuhan Cao) and Colleges and Universities Natural & Science Fund of Anhui (No. KJ2017A270 to Cong Fu, No. KJ2017A269 to Yuhan Cao).
Disclosure of conflict of interest
None.
References
- 1.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 2.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 3.Shubin NJ, Monaghan SF, Ayala A. Anti-inflammatory mechanisms of sepsis. Contrib Microbiol. 2011;17:108–124. doi: 10.1159/000324024. [DOI] [PubMed] [Google Scholar]
- 4.Ueha S, Shand FH, Matsushima K. Myeloid cell population dynamics in healthy and tumor-bearing mice. Int Immunopharmacol. 2011;11:783–788. doi: 10.1016/j.intimp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17:281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brudecki L, Ferguson DA, McCall CE, El GM. Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infect Immun. 2012;80:2026–2034. doi: 10.1128/IAI.00239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 12.Spiller F, Costa C, Souto FO, Vinchi F, Mestriner FL, Laure HJ, Alves-Filho JC, Freitas A, Rosa JC, Ferreira SH, Altruda F, Hirsch E, Greene LJ, Tolosano E, Cunha FQ. Inhibition of neutrophil migration by hemopexin leads to increased mortality due to sepsis in mice. Am J Respir Crit Care Med. 2011;183:922–931. doi: 10.1164/rccm.201002-0223OC. [DOI] [PubMed] [Google Scholar]
- 13.Sheng Z, Yao Y, Li Y, Yan F, Huang J, Ma G. Bradykinin preconditioning improves therapeutic potential of human endothelial progenitor cells in infarcted myocardium. PLoS One. 2013;8:e81505. doi: 10.1371/journal.pone.0081505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 15.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 16.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 17.Gamkrelidze M, Intskirveli N, Vardosanidze K, Goliadze L, Chikhladze K, Ratiani L. Myocardial dysfunction during septic shock (review) Georgian Med News. 2014:40–6. [PubMed] [Google Scholar]
- 18.Fan TT, Feng XY, Yang YZ, Gao F, Liu Q. Downregulation of PI3K-gamma in a mouse model of sepsis-induced myocardial dysfunction. Cytokine. 2017;96:208–216. doi: 10.1016/j.cyto.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286:23591–23599. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Lv G, Min Y, Yang L, Lin PC. Intravenous administration of Gr-1+CD11b+ myeloid cells increases neovascularization and improves cardiac function after heart infarction. Int J Cardiol. 2013;168:1702–1705. doi: 10.1016/j.ijcard.2013.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei WC, Lin SY, Lan CW, Huang YC, Lin CY, Hsiao PW, Chen YR, Yang WC, Yang NS. Inhibiting MDSC differentiation from bone marrow with phytochemical polyacetylenes drastically impairs tumor metastasis. Sci Rep. 2016;6:36663. doi: 10.1038/srep36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Li H, Zhao G, Sun A, Zong NC, Li Z, Zhu H, Zou Y, Yang X, Ge J. Hydrogen sulfide attenuates the recruitment of CD11b(+)Gr-1(+) myeloid cells and regulates Bax/Bcl-2 signaling in myocardial ischemia injury. Sci Rep. 2014;4:4774. doi: 10.1038/srep04774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao T, Lu W, Zhu J, Jin X, Ma G, Wang Y, Meng S, Zhang Y, Li Y, Shen C. Role of CD11b+Gr-1+ myeloid cells in AGEs-induced myocardial injury in a mice model of acute myocardial infarction. Int J Clin Exp Pathol. 2015;8:3238–3249. [PMC free article] [PubMed] [Google Scholar]
- 25.Cobbold SP. The mTOR pathway and integrating immune regulation. Immunology. 2013;140:391–398. doi: 10.1111/imm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C, Wang S, Li J, Zhang W, Zheng L, Yang C, Zhu T, Rong R. The mTOR signal regulates myeloid-derived suppressor cells differentiation and immunosuppressive function in acute kidney injury. Cell Death Dis. 2017;8:e2695. doi: 10.1038/cddis.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Bi Y, Yang H, Chen X, Liu H, Lu Y, Zhang Z, Liao J, Yang S, Chu Y, Yang R, Liu G. mTOR limits the recruitment of CD11b+Gr1+Ly6Chigh myeloid-derived suppressor cells in protecting against murine immunological hepatic injury. J Leukoc Biol. 2014;95:961–970. doi: 10.1189/jlb.0913473. [DOI] [PubMed] [Google Scholar]
- 28.Temiz-Resitoglu M, Kucukkavruk SP, Guden DS, Cecen P, Sari AN, Tunctan B, Gorur A, Tamer-Gumus L, Buharalioglu CK, Malik KU, Sahan-Firat S. Activation of mTOR/IkappaB-alpha/NF-kappaB pathway contributes to LPS-induced hypotension and inflammation in rats. Eur J Pharmacol. 2017;802:7–19. doi: 10.1016/j.ejphar.2017.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Dando I, Cordani M, Donadelli M. Mutant p53 and mTOR/PKM2 regulation in cancer cells. IUBMB Life. 2016;68:722–726. doi: 10.1002/iub.1534. [DOI] [PubMed] [Google Scholar]
- 30.Sung JY, Lee KY, Kim JR, Choi HC. Interaction between mTOR pathway inhibition and autophagy induction attenuates adriamycin-induced vascular smooth muscle cell senescence through decreased expressions of p53/p21/p16. Exp Gerontol. 2018;109:51–58. doi: 10.1016/j.exger.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Rosso A, Balsamo A, Gambino R, Dentelli P, Falcioni R, Cassader M, Pegoraro L, Pagano G, Brizzi MF. p53 Mediates the accelerated onset of senescence of endothelial progenitor cells in diabetes. J Biol Chem. 2006;281:4339–4347. doi: 10.1074/jbc.M509293200. [DOI] [PubMed] [Google Scholar]
- 32.Engeland K. Cell cycle arrest through indirect transcriptional repression by p53: i have a DREAM. Cell Death Differ. 2018;25:114–132. doi: 10.1038/cdd.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo JN, Tian LY, Liu WY, Mu J, Zhou D. Activation of the Akt/mTOR signaling pathway: a potential response to long-term neuronal loss in the hippocampus after sepsis. Neural Regen Res. 2017;12:1832–1842. doi: 10.4103/1673-5374.219044. [DOI] [PMC free article] [PubMed] [Google Scholar]