Abstract
Objective: To explore the effects of miR-137 on cognitive dysfunction in rats induced by propofol (PRO). Methods: Male SD rats and SK-N-SH cells were purchased, and control and PRO groups were set up in the rats, and the same groups were set up in the cells. On the basis of the PRO group, miR-137 and PTN were up-regulated or down-regulated, and cognitive dysfunction and cell biological functions in each group were detected. Results: The cognitive function of rats induced by PRO might be affected. We observed that the escape latency of PRO group was significantly prolonged, with significantly lower percentage of time for target platform exploration and times of crossing the platform, while over-expression of miR-137 or knock down of PTN could change the above results. Under PRO intervention, the expression of miR-137 in SK-N-SH cells decreased in a dose-dependent manner, while the expression and protein level of PTN in SK-N-SH cells increased in a dose-dependent manner. Cytotoxicity test yielded a 30 μM concentration of PRO as the optimal experimental concentration. When miR-137 and PTN were up-regulated or down-regulated, PRO-induced cell apoptosis, proliferation and PTN/PTPRZ pathway protein phosphorylation level were effectively reversed. Dual luciferase reporter confirmed that miR-137 and PTN have targeted relationship. Conclusion: Up-regulation of miR-137 can at least partially regulate PTN/PTPRZ pathway through the inhibition of PTN in a targeted manner, effectively inhibit cell apoptosis, and protect cognitive dysfunction caused by PRO.
Keywords: Propofol, miR-137, PTN, Alzheimer’s disease
Introduction
Alzheimer’s disease (AD), as a progressive neurodegenerative disease closely related to age, is one of the main causes of cognitive dysfunction in the elderly [1,2]. According to statistics, AD is a major cause of death in humans, with an estimated 700,000 deaths over the age of 65, and it affects 36.5 million people worldwide [3,4]. The identification of AD depends on neuropathological examination and measurement of widely used biological indicators (e.g., β-amyloid protein), but there is no AD treatment that can effectively halt the disease process [5,6]. Aging, aluminum and general anesthesia are all potential risk factors that may cause body dysfunction and further induce AD. Propofol (PRO), as a commonly used general anesthetic, can destroy lysosomal homeostasis by mediating activation of Ca2+ inositol triphosphate receptor or ryanodine receptor, further increasing body neurotoxicity and cognitive dysfunction [7,8]. We will apply PRO as an inducing medium for cognitive dysfunction to explore potential regulatory mechanisms in vivo and in vitro, which is of great significance for reversing the AD process and reducing AD-related mortality.
miRNAs are small non-coding RNA molecules that target physiological and pathological processes in the body. Its regulation lies in post-translation inhibition, degradation and silence caused by the combination of complementary sequences. Its application scope even involves the diagnosis of neurodegenerative diseases including AD [9,10]. In the research of Müller et al. [11], miR-29a can be used as a biological diagnostic index for AD in acellular cerebrospinal fluid. The report of Salta et al. [12] also suggests that miR-132 shows significant dysregulation in AD, and targeting or alternative treatment of miR-132 may become a novel treatment strategy for AD. Currently, a number of miRNAs have been found to be closely associated with anesthesia-related cognitive dysfunction. For example, miR-24 alleviates isoflurane-induced neurotoxicity in rat hippocampus via mediating oxidative stress mechanisms, and knockdown of miR-124 attenuates hippocampal neurodegeneration induced by ketamine anesthesia [13,14].
The protagonist of this study, miR-137, has been reported to have a potential role in the regulation of neurotoxicity under anesthesia with sevoflurane and PRO, but the mechanism of the potential neuroprotective effects of miR-137 under PRO has not been elucidated, so this study will focus on the relevant exploration of miR-137 [15,16]. As a member of miRNAs family involved in neurodevelopment, miR-137 shows abnormal down-regulation in the pathogenesis of AD. It can inhibit NF-κB pathway by targeting TNFAIP1 of Neuro2a cells, thus alleviating β-amyloid induced neurotoxicity [17,18]. In addition, it is also reported that miR-137 can inhibit inflammation, oxidative stress, and neuronal damage in a mouse model of ischemic stroke via blocking the MAPK signaling pathway, thus improving cognitive impairment in mice [19]. All of the above studies suggest that miR-137 may have certain neuroprotective effects, and increasing its expression level may have certain therapeutic efficacy. On the other hand, PTN is a neurotrophic factor, and its abnormal over-expression often occurs in drug abuse, brain degeneration and other situations, which has certain neuroprotective effect on neurodegenerative diseases [20]. PTN/PTPRZ pathway is involved in the regulation of neuroinflammation, which regulates the formation of developmental myelin sheath and stress behavior after injury, and neuroinflammation is one of the pathogenesis of AD, suggesting that PTN/PTPRZ pathway may participate in AD pathological regulation process [21].
We found that miR-137 has a target site with PTN, and there may be potential targeting relationship between the two. We suspected that the two could mediate the potential neuroprotective effect of PTN/PTPRZ pathway on PRO-induced cognitive dysfunction, which was verified as follows.
Material and methods
Animals and groups
Fifty Sprague Dawley male rats aged 7 weeks were purchased from Tianqin Biotechnology Co., Ltd. (Changsha, China). They were kept in light and darkness for 12 hours each, acclimated for 7 days, provided with food and water, and kept at the Experimental Animal Center of Dalian Medical University, China. Then, they were stochasticly divided into a control group and a PRO group. Rats in the control group (n = 10) were inhaled with air regularly for 4 hours, and rats in the PRO group (n = 40) were injected intravenously with PRO at 0.9 mg/kg/min and 19 mg/kg [22] (Mengya Biotechnology Co., Ltd., Shanghai, China, D24475-5 mg) for 4 hours. Then they were transfected and evenly divided into a PRO alone group, a PRO + vehicle group, a PRO + miR-137 mimics (mimics) group, and a PRO + si-PTN group. Animal transfection method [23]: Within 10 min after PRO administration, transfection mimics, si-PTN and solvent control (equivalent) were put into the left ventricle through pre-drilled cranial holes. Rats were given a recovery time of seven days, during which time they were normally fed. The construction of miR-137 over-expressed plasmid (mimics) and RNA interference plasmid of PTN (si-PTN) was completed by Shanghai Meixuan Biological Science and Technology Co., LTD. At the end of the experiment, rats were anesthetized using 10% chloral hydrate (0.4 g/kg) (Yulong Algae Co., Ltd., Qingdao, China, H37022673) and sacrificed by cervical dislocation. This animal experimental programme has been authorized by the animal experimental ethics committee of The First Affiliated Hospital of Dalian Medical University.
Evaluation of cognitive function in rats
Morris water maze (MWM) [24] test was applied for relevant assessments. The rats were tested by MWM before injection to determine whether there was any physical disorder. After 7 days of injection, the rats were evaluated for 4 days of spatial learning ability and tested once a day in different quadrants. The rats were placed in the water facing the pool wall. If no platform was found by them within 2 min, they would be led to the platform to rest for 0.5 min, and the test was finished. If the rats found the platform within 2 min, they would be given a rest on the platform for 0.5 min as well. The training interval in each quadrant was 1 min, and the escape latency from entering water to finding the platform, average swimming speed, percentage of time spent in exploring the target platform, and times of crossing the platform of rats were recorded.
Terminal dexynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) analysis and haematoxylin and eosin (HE) staining
We applied the TUNEL detection kit (Qiming Biotechnology Co., Ltd., Shanghai, China, OX02752) for analysis. First, hippocampus of rats was fixed at room temperature for 10 minutes with 4% paraformaldehyde. Tissue sections that had been paraffin-embedded, dewaxed, and washed with ethanol at a stepped concentration were harvested and incubated with 20 µg/mL protease K at indoor temperature for 30 minutes, and then rinsed with PBS for 10 minutes. TUNEL reaction mixture was added to the tissue for 2-hour incubation at room temperature. The tissues rinsed with PBS were dyed with propidium iodide (PI, 25 µg/mL) for 30 minutes at indoor temperature. Finally, the samples were observed using a confocal microscope (Leica Microscopy Trading Co., Ltd., Shanghai, China, Leica TCS SP8 CARS).
Tissue sections were stained with HE and analyzed with HE staining kit (Hengfei Biotechnology Co., Ltd., Shanghai, China, AR1180). First, the tissues were first dehydrated and washed with different concentrations of ethanol and xylenol, and then the nuclei were stained with 5% hematoxylin solution, rinsed with distilled water after 10 minutes, and incubated in 0.1% HCl-ethanol for 30 seconds. Then, the eosin solution was used to re-stain the tissues for 2 minutes. After being washed and dehydrated again, they were observed under an optical microscope.
Cell culture and transfection
Human neuron SK-N-SH (Zishi Biotechnology Co., Ltd., Shanghai, China, WD100310) neuroblastoma cells were cultured at 37°C with 5% CO2 in a DMEM (Luyuan Bode Biotechnology Co., Ltd., Beijing, China, PM150220B) containing 10% PBS and 100 U/mL penicillin (Yihui Biological Technology Co., Ltd., Shanghai, China, 07500). SK-N-SH cells were pretreated with PRO at a concentration of 30 μM (the optimal concentration determined by cytotoxicity test) for 30 min. Cell transfection was carried out, and mimics (over-expressed sequence of miR-137), inhibitor (inhibited sequence of miR-137), miR negative control (miR-NC), targeted PTN RNA (si-PTN), targeted over-expression PTN RNA (sh-PTN), and negative control RNA (si-NC) were transfected with Lipofectamine™ 2000 kit (BioMag Scientific Inc., Wuxi, China, 11668019), and the procedures were conducted strictly in the light of kit instruction.
qRT-PCR detection
Extraction of total RNA adopted TRIzol kit (Mingjing Biology Co., Ltd., Shanghai, China, 5003050). cDNA was synthesized using Bio-RADSSO FAST EVA Green Supermixkit (Yihui Biological Technology Co., Ltd., Shanghai, China, 1725202) and Life 7500 real-time PCR system (Anmaige Trading Co., Ltd., Beijing, China). Reaction system: m-MLV 1 μl, Olig (d T) 1 μl, RNAase inhibitor 0.5 μl, d NTPs 1 μl, and RNAse free water was added to replenish to 15 μl. Design and synthesis of all primers were completed by Shanghai Xinfan Biotechnology Co., Ltd. The primer sequence was as follows: miR-137 sense oligodeoxynucleotides (SODN): 5’-TTATTGCTTAAGAATACGCG-3’, miR-137 antisense oligodeoxynucleotides (ASODN): 5’-TCGTATCCAGTGCAGGGTC-3’. U6 SODN: 5’-GCTCGCTTCGGCAGCACAT-3’, U6 ASODN: 5’-AAAATATGGAACGCTTCACG-3’. PTN SODN: 5’-AGAAGCAATTTGGCGCGGA-3’, PTN ASODN: 5’-TGCACCACCAACTGCTTAGC-3’. β-Actin SODN: 5’-GTATCCTGACCCTGAAGTACC-3’, β-Actin ASODN: 5’-TGAAGGTCTCAAACATGATCT-3’. PCR operation required 35 cycles, namely 94°C, 55°C, 72°C for 1 min each, and then extended at 72°C for 5 min. miRNA and mRNA used U6 and β-Actin as internal references, respectively, and data analysis adopted 2-ΔΔct.
Western blot analysis
RIPA lysate (Kanglang Biological Technology Co., Ltd., Shanghai, China, KL-X135) was added to each group of cultured cells for total protein extraction. The protein concentration was determined using BCA assay (Chreagen Biotechnology Co., Ltd., Beijing, China, 120982), which was then adjusted to 4 μg/μL, isolated by 12% SDS-PAGE (LMAI Bio Co., Ltd., Shanghai, China) electrophoresis, and transferred to PVDF membrane. The membrane was blocked with 5% skimmed milk powder (Yiyan Biological Technology Co., Ltd., Shanghai, China, SL1330-100 ml) for 2 h, and PTN, Bax, Caspase-3, Caspase-9, and β-Actin primary antibody at the diluted ratio of 1:500 were added to block overnight at 4°C. All the antibodies were purchased from Hengfei Biotechnology Co., Ltd., Shanghai. The primary antibody was removed through washing the membrane, and added with horseradish peroxidase labeled goat anti rabbit secondary antibody (1:1000) for one-hour incubation at 37°C, with 5 min each. Excess liquid was absorbed from the membrane with filter paper. ECL was used to illuminate and develop in a dark room (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China, R30199, R21313). Protein band scan and grayscale value analysis were carried out with Quantity One software.
Detection of cell viability
Cells after 24 hours of transfection were collected to adjust their density to 5×103 cells/well, transferred to 96-well plates, and incubated for 24 h, 48 h and 72 h at 37°C. MTT solution (20 μL, 5 μmg/mL) was put into the plate at each time point for 4 hours culture at 37°C. Dimethyl sulfoxide (200 μL) was put into each well, and measurement of OD value was conducted at 490 nm wavelength by spectrophotometer. When the optimal concentration of PRO was determined in the pre-experiment, three concentrations of 15 μM, 30 μM and 45 μM [25] and the control group were set up to measure the cell viability after 48 hours of intervention.
Detection of apoptosis ability
After being digested with 0.25% trypsin (Yuanye Bio-Technology Co., Ltd., Shanghai, China, R20109), transfected cells underwent PBS wash and mixture with 100 μL binding buffer. After being configured into suspension (1*106/mL) and the successive addition of annexinv-FITC and PI, cells underwent 5-minute incubation at indoor temperature away from light. Flow cytometry (FCM, Delika Biotechnology Co., Ltd., Beijing, China, DLK0002051) was utilized for determination.
Detection of dual luciferase activity
miR-137 downstream target genes were predicted using Targetscan7.2. PTN-Wt, PTN-Mut, miR-137-mimics and miR-NC were transferred into SK-N-SH cells using Lipofectamine™ 2000 kit, and dual luciferase reporter kit (Baiao Laibo Technology Co., Ltd., Beijing, China, KFS303-LBV) was utilized to determine the luciferase activity.
Statistical analysis
GraphPad 6 was applied for data analysis and image rendering. Comparison between two groups adopted independent sample t test. One-way analysis of variance (ANOVA) and LSD-t test were applied for comparison among multiple groups and pair-wise comparison afterwards, respectively. Repeated measurement ANOVA and Bonferroni were utilized for expression at multiple time points and back testing, respectivey. When P < 0.05, statistical difference was indicated.
Results
Pathological effects of PRO on hippocampus in rats
To analyze the pathological impacts of PRO on hippocampus in rats and to determine the successful construction of the cognitive impairment model, we performed neuronal apoptosis analysis as well as HE staining analysis on hippocampus of rats. The results showed that the level of neuronal apoptosis in hippocampal tissues of rats in the PRO group was notably increased under the PRO intervention compared to the Control group. In contrast, the abnormally high level of neuronal apoptosis caused by PRO decreased significantly under the effect of mimics or si-PTN, suggesting that mimics or si-PTN may have a neuroprotective effect against neuroapoptosis. Then, in the HE analysis, we found that the PRO group showed notably increased pyramidal nucleus in the hippocampus, significantly decreased cytoplasmic staining, nuclear membrane wrinkling, and significantly increased glial cells when compared with the Control group. The above pathological conditions were significantly improved under the intervention of mimics or si-PTN, indicating that mimics or si-PTN has different degrees of neuroprotective effects. As shown in Figure 1.
Figure 1.
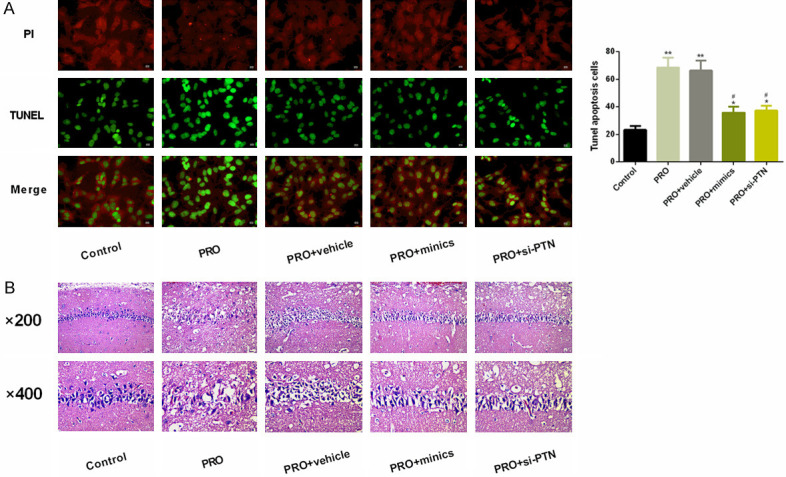
Pathological effects of PRO on hippocampus in rats. A. TUNEL analysis detected the effects of PRO on neuron apoptosis in rat hippocampus. It was found that PRO significantly increased the level of neuron apoptosis in rat, and this abnormally up-regulated level of neuron apoptosis could be significantly inhibited under mimics or si-PTN intervention. B. HE staining analyzed the effects of PRO on morphology of rat hippocampus. It was found that PRO significantly damaged morphology of rat hippocampus, but such damage was improved under the impacts of mimics or si-PTN. Notes: Compared with the Control group, *denotes P < 0.05. Compared with the PRO group, #denotes P < 0.05.
Effects of PRO on cognitive function in SK-N-SH cells in rats
No considerable difference could be found in the swimming speed among rats in the four groups (P > 0.05). The escape latency in the PRO group on the second and third days was significantly longer, the percentage of time spent exploring the target platform, and the number of times of crossing the platform were notably lower when compared with the control group, with statistically significant differences (P < 0.05). Compared with PRO group, the escape latency of PRO + mimics and PRO + si-PTN group was evidently shorter on the second and third days, and the percentage of time spent exploring the target platform, and the number of times of crossing the platform were evidently higher, with statistically significant difference (P < 0.05). Compared with the control group, no remarkable difference could be seen in escape latency, the percentage of time spent exploring the target platform, and number of times of crossing the platform between PRO + mimics and PRO + si-PTN groups (P > 0.05). As shown in Figure 2.
Figure 2.
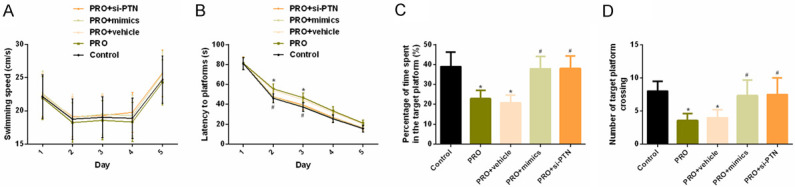
Effects of PRO on spatial learning and memory in rats. A. Effects of PRO on swimming speed of rats. B. Effects of PRO on escape latency of rats. C. Effect of PRO on the percentage of time spent exploring the target platform of rats. D. Effects of PRO on the times of rats crossing the platform. Notes: Compared with the mice test on the first day or the control group, *P < 0.05. Compared with the PRO group, #P < 0.05.
Effects of PRO on miR-137 and PTN in SK-N-SH cells
Under PRO intervention, miR-137 level in SK-N-SH cells decreased in a dose-dependent manner, while PTN expression and protein level in SK-N-SH cells elevated in a dose-dependent manner, with statistically significant differences (P < 0.05). Through cytotoxicity test, we found that the cell viability of the cells interfered with 45 μM PRO was significantly inhibited, so we chose 30 μM PRO for further experimental intervention. As shown in Figure 3.
Figure 3.
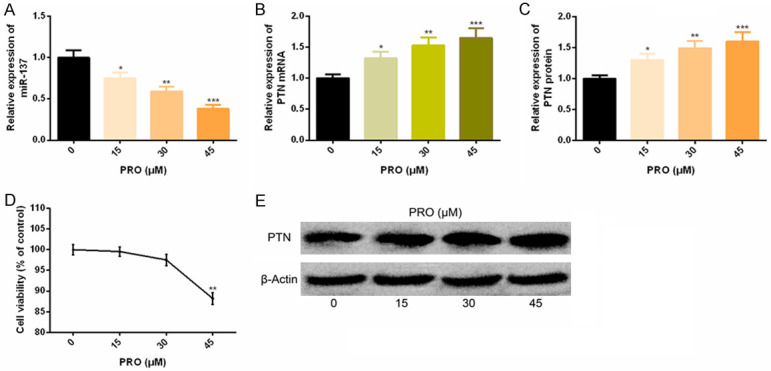
PRO down-regulates miR-137 and up-regulates PTN in in vitro experiments. A-C. With the increase of PRO concentration, miR-137 expression in SK-N-SH cells decreased, while PTN expression and protein level increased. D. Cytotoxicity test of PRO. E. Corresponding protein figure. Note: Compared with 0 μM PRO, *P < 0.05, **P < 0.01, ***P < 0.001.
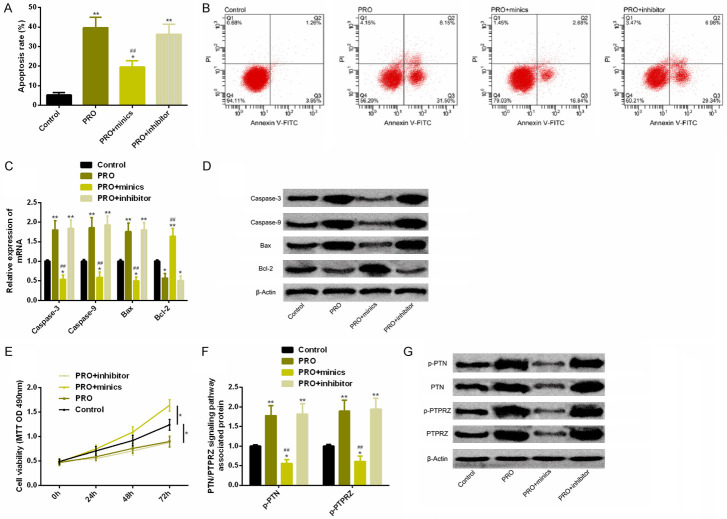
Up-regulation of miR-137 in SK-N-SH cells has neuroprotective effects
Comparison of the control group revealed that the apoptosis rate in PRO group was notably increased, Caspase-3, Caspase-9 and Bax expression were notably increased, and Bcl-2 expression was significantly decreased. The cell proliferation ability was significantly reduced, and p-PTN and p-PTPRZ protein levels were notably elevated. The PRO + mimics group with up-regulated miR-137 could reverse the above results of cell apoptosis, proliferation and protein phosphorylation of PTN/PTPRZ pathway, with statistically significant differences (P < 0.05). As shown in Figure 4.
Figure 4.
Up-regulation of miR-137 has neuroprotective effect in in vitro experiments. A. Up-regulation of miR-137 could significantly reverse the notably increased apoptosis rate under PRO intervention. B. Flow cytometry. C. Up-regulation of miR-137 could significantly reverse the notably changed expression levels of apoptosis-related factors under PRO intervention. D. Protein figure of apoptosis-related factors. E. Up-regulation of miR-137 could significantly reverse the notably reduced cell activity under PRO intervention. F. Up-regulation of miR-137 could significantly reverse the phosphorylation level of PTN/PTPRZ pathway-related proteins under PRO intervention. G. Protein figure of PTN/PTPRZ pathway related proteins. Notes: Compared with the control group or between the two groups, *P < 0.05, **P < 0.01. Compared with PRO group, ##P < 0.01.
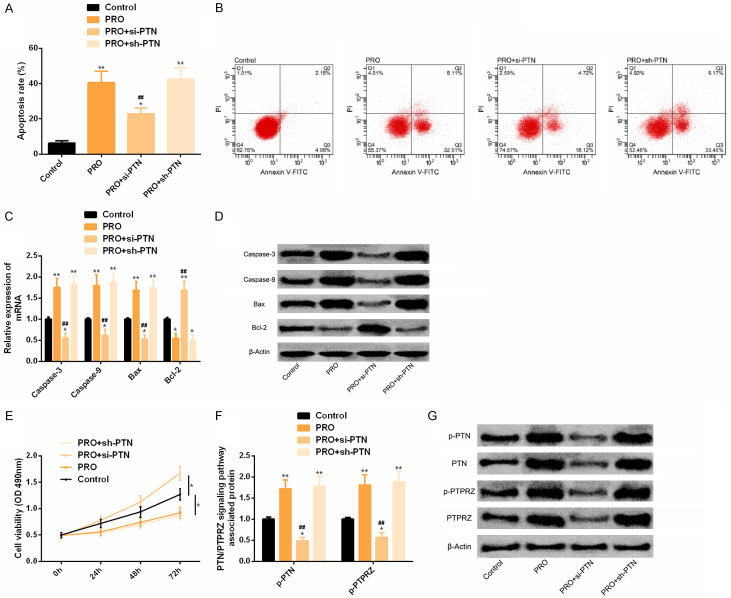
Down-regulation of PTN in SK-N-SH cells has neuroprotective effects
Comparison of the control group indicated that the apoptosis rate in PRO group was notably increased. Caspase-3, Caspase-9 and Bax expression were notably elevated, while Bcl-2 expression was significantly decreased. The cell proliferation ability was significantly reduced, and p-PTN and p-PTPRZ protein levels were significantly increased. The PRO + si-PTN group with PTN reduction could reverse the above results of cell apoptosis, proliferation and protein phosphorylation of PTN/PTPRZ pathway, with statistically significant differences (P < 0.05). As shown in Figure 5.
Figure 5.
Down-regulation of PTN has neuroprotective effect in in vitro experiments. A. Down-regulation of PTN could significantly reverse the notably increased apoptosis rate under PRO intervention. B. Flow cytometry. C. Downregulation of PTN could significantly reverse the notably changed expression level of apoptosis-related factors under PRO intervention. D. Protein figure of apoptosis-related factors. E. Down-regulation of PTN could significantly reverse the notably reduced cell activity under PRO intervention. F. Downregulation of PTN could significantly reverse the phosphorylation level of PTN/PTPRZ pathway-related proteins under PRO intervention. G. Protein figure of PTN/PTPRZ pathway related proteins. Notes: Compared with the control group or between the two groups, *P < 0.05, **P < 0.01. Compared with PRO group, ##P < 0.01.
Identification of miR-137 target genes in SK-N-SH cells
There were targeted binding sites between PTN and miR-137 via Targetscan7.2. And dual luciferase reporter revealed that PTN 3’UTR-Wt luciferase activity decreased remarkably after up-regulation of miR-137 (P < 0.05), but it had no influence on PTN 3’UTR-Mut luciferase activity (P > 0.05). WB assay showed that PTN protein expression was notably decreased after transfection of miR-137-mimics (P < 0.05). As shown in Figure 6.
Figure 6.
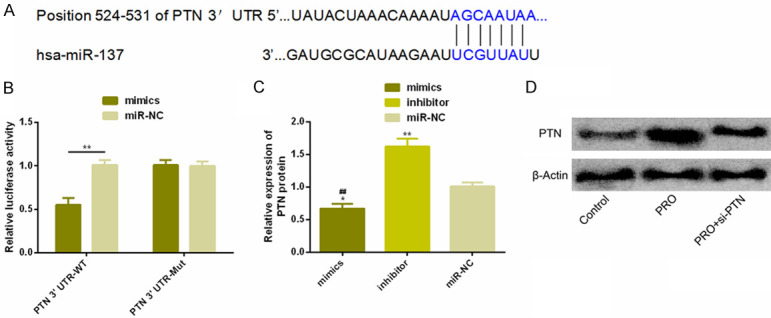
Detection of dual luciferase reporter. A. Targeting binding sites existed between miR-137 and PTN. B. Relative luciferase activity-dual luciferase reporter. C. Expression of PTN protein in transfected HCC827 and NCI-H524 cells. D. Protein figure. Notes: **P < 0.01.
Discussion
More and more researchers pay attention to the regulation mechanism of miR-137 in neuroprotection, and have carried out related studies. For example, in reports of Shi [26], miR-137 can target Notch1 to cut off Notch1 signaling pathway, thus exerting the protective effect of neurons. Another example is the research of Olde et al. [27] which suggests that miR-137 is a key regulator of the chain cognitive dysfunction induced by glutamate dysfunction. Regulating miR-137 is beneficial to restore synaptic efficacy and synaptic plasticity. In this study, after PRO intervention was performed on rats, the escape latency of PRO group was prolonged in MWM test, and the percentage of time spent exploring the target platform and the number of times to cross the platform was remarkably lower. However, after miR-137 was up-regulated or PTN was down-regulated and the above results of rats were remarkably improved, suggesting that miR-137 and PTN might participate in the pathological mechanism of cognitive dysfunction induced by PRO, and reverse treatment of expression of miR-137 and PTN was helpful to play a neuroprotective role. To this end, we conducted in-depth exploration to understand the potential regulatory mechanisms of the two. In vitro, we intervened SK-N-SH cells with PRO. The expression of miR-137 and PTN decreased or increased in a PRO dose-dependent manner, suggesting that miR-137 and PTN expression might be closely related to PRO. Then, we conducted a cytotoxicity test and determined PRO with a concentration of 30 μM was the best experimental concentration. We analyzed cell apoptosis, proliferation, PTN/PTRZ signal transduction pathway and so on. The results revealed that the PRO group had a significantly higher cell apoptosis rate. Besides Bcl-2, the other apoptosis-related factors of Caspase-3, Caspase-9, and Bax, showed a higher level, the cell viability was notably inhibited, and the protein levels of p-PTN, P-PTRZ in PTN/PTRZ pathway were significantly increased. In the study of Su et al. [28], Caspase-3, Caspase-9 and Bax showed higher levels in anesthesia induction group as apoptosis-promoting factors, while Bcl-2 showed opposite results as anti-apoptosis factor. After treatment and intervention, the above results were reversed, which was similar to our research results. However, when we over-expressed miR-137 and knocked down PTN, PRO-induced apoptosis, proliferation and protein phosphorylation of PTN/PTPRZ pathway were remarkably reversed. Dual luciferase reporter confirmed that miR-137 has a targeted relationship with PTN. If miR-137 expression was increased, PTN protein level would be significantly inhibited. The above studies showed that up-regulation of miR-137 could regulate PTN/PTPRZ pathway through inhibition of PTN in a target manner, which had neuroprotective effect on PRO-induced cognitive dysfunction.
At present, there is little research on the specific protective mechanism of PTN/PTPRZ signal pathway in cognitive dysfunction. It is understood that PTN is also a multi-effect growth factor, which can induce the orientated growth of neurites and has mitogenic effect on a variety of cells such as fibroblasts. PTRZ is a receptor protein tyrosine phosphatase active in the nervous system and participates in physiological processes such as cell adhesion and signal transduction [29,30]. PTN/PTRZ signaling pathway has been reported in previous studies, which can stimulate differentiation of oligodendrocyte precursor cells by mediating tyrosine phosphorylation of AFAP1L2, thus initiating PI3K/AKT pathway. PI3K/AKT pathway also participates in the pathological mechanism of AD, suggesting that PTN/PTRZ signaling pathway may be the initial activation pathway of AD pathology [31,32].
Conclusion
To sum up, up-regulation of miR-137 can inhibit PTN in a target manner to regulate PTN/PTPRZ pathway to prevent cognitive dysfunction caused by PRO. However, there is still room for improvement in this study. First of all, we can supplement the research on whether miR-137 and PTN have inhibitory effects on inflammatory factors and explore their potential protective effects on neuroinflammation. In addition, we can also increase their regulation of oxidative stress indicators to explore whether they participate in the protective mechanism of oxidative stress injury.
Disclosure of conflict of interest
None.
References
- 1.Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement (N Y) 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2018;6:CD001190. doi: 10.1002/14651858.CD001190.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Robinson M, Lee BY, Hane FT. Recent progress in Alzheimer’s disease research, part 2: genetics and epidemiology. J Alzheimers Dis. 2017;57:317–330. doi: 10.3233/JAD-161149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R Contributors. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folch J, Petrov D, Ettcheto M, Abad S, Sanchez-Lopez E, Garcia ML, Olloquequi J, Beas-Zarate C, Auladell C, Camins A. Current research therapeutic strategies for Alzheimer’s disease treatment. Neural Plast. 2016;2016:8501693. doi: 10.1155/2016/8501693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Exley C. Aluminum should now be considered a primary etiological factor in Alzheimer’s disease. J Alzheimers Dis Rep. 2017;1:23–25. doi: 10.3233/ADR-170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang M, Wang Y, Liang G, Xu Z, Chu CT, Wei H. Alzheimer’s disease presenilin-1 mutation sensitizes neurons to impaired autophagy flux and propofol neurotoxicity: role of calcium dysregulation. J Alzheimers Dis. 2019;67:137–147. doi: 10.3233/JAD-180858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganju A, Khan S, Hafeez BB, Behrman SW, Yallapu MM, Chauhan SC, Jaggi M. miRNA nanotherapeutics for cancer. Drug Discov Today. 2017;22:424–432. doi: 10.1016/j.drudis.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Giau V, An SS. Emergence of exosomal miRNAs as a diagnostic biomarker for Alzheimer’s disease. J Neurol Sci. 2016;360:141–152. doi: 10.1016/j.jns.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Muller M, Jakel L, Bruinsma IB, Claassen JA, Kuiperij HB, Verbeek MM. MicroRNA-29a is a candidate biomarker for Alzheimer’s disease in cell-free cerebrospinal fluid. Mol Neurobiol. 2016;53:2894–2899. doi: 10.1007/s12035-015-9156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salta E, De Strooper B. microRNA-132: a key noncoding RNA operating in the cellular phase of Alzheimer’s disease. FASEB J. 2017;31:424–433. doi: 10.1096/fj.201601308. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Yue L, Wang J, Wan Z, Bu W. MicroRNA-24 alleviates isoflurane-induced neurotoxicity in rat hippocampus via attenuation of oxidative stress. Biochem Cell Biol. 2020;98:208–218. doi: 10.1139/bcb-2019-0188. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Zhang J, Zhou W, Feng Y, Teng S, Song X. The role of miR-124 in modulating hippocampal neurotoxicity induced by ketamine Anesthesia. Int J Neurosci. 2015;125:213–220. doi: 10.3109/00207454.2014.919915. [DOI] [PubMed] [Google Scholar]
- 15.Ye J, Zhang Z, Wang Y, Chen C, Xu X, Yu H, Peng M. Altered hippocampal microRNA expression profiles in neonatal rats caused by sevoflurane anesthesia: microRNA profiling and bioinformatics target analysis. Exp Ther Med. 2016;12:1299–1310. doi: 10.3892/etm.2016.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan J, Zhou Q, Qin Z, Tao T. Effect of propofol on microRNA expression in rat primary embryonic neural stem cells. BMC Anesthesiol. 2016;16:95. doi: 10.1186/s12871-016-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoudi E, Cairns MJ. MiR-137: an important player in neural development and neoplastic transformation. Mol Psychiatry. 2017;22:44–55. doi: 10.1038/mp.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He D, Tan J, Zhang J. miR-137 attenuates Abeta-induced neurotoxicity through inactivation of NF-kappaB pathway by targeting TNFAIP1 in Neuro2a cells. Biochem Biophys Res Commun. 2017;490:941–947. doi: 10.1016/j.bbrc.2017.06.144. [DOI] [PubMed] [Google Scholar]
- 19.Tian R, Wu B, Fu C, Guo K. miR-137 prevents inflammatory response, oxidative stress, neuronal injury and cognitive impairment via blockade of Src-mediated MAPK signaling pathway in ischemic stroke. Aging (Albany NY) 2020;12:10873–10895. doi: 10.18632/aging.103301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herradon G, Perez-Garcia C. Targeting midkine and pleiotrophin signalling pathways in addiction and neurodegenerative disorders: recent progress and perspectives. Br J Pharmacol. 2014;171:837–848. doi: 10.1111/bph.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herradon G, Ramos-Alvarez MP, Gramage E. Connecting metainflammation and neuroinflammation through the PTN-MK-RPTPbeta/zeta axis: relevance in therapeutic development. Front Pharmacol. 2019;10:377. doi: 10.3389/fphar.2019.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang N, Li L, Li Z, Ni C, Cao Y, Liu T, Tian M, Chui D, Guo X. Protective effect of dapsone on cognitive impairment induced by propofol involves hippocampal autophagy. Neurosci Lett. 2017;649:85–92. doi: 10.1016/j.neulet.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Lu X, Lv S, Mi Y, Wang L, Wang G. Neuroprotective effect of miR-665 against sevoflurane anesthesia-induced cognitive dysfunction in rats through PI3K/Akt signaling pathway by targeting insulin-like growth factor 2. Am J Transl Res. 2017;9:1344–1356. [PMC free article] [PubMed] [Google Scholar]
- 24.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang CY, Du J, Zhang R, Jin J, Qiao LY. Erythropoietin attenuates propofol-induced hippocampal neuronal cell injury in developing rats by inhibiting toll-like receptor 4 expression. Neurosci Lett. 2020;716:134647. doi: 10.1016/j.neulet.2019.134647. [DOI] [PubMed] [Google Scholar]
- 26.Shi F, Dong Z, Li H, Liu X, Liu H, Dong R. MicroRNA-137 protects neurons against ischemia/reperfusion injury through regulation of the Notch signaling pathway. Exp Cell Res. 2017;352:1–8. doi: 10.1016/j.yexcr.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Olde Loohuis NF, Ba W, Stoerchel PH, Kos A, Jager A, Schratt G, Martens GJ, van Bokhoven H, Nadif Kasri N, Aschrafi A. MicroRNA-137 controls AMPA-receptor-mediated transmission and mGluR-dependent LTD. Cell Rep. 2015;11:1876–1884. doi: 10.1016/j.celrep.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 28.Su R, Sun P, Zhang D, Xiao W, Feng C, Zhong L. Neuroprotective effect of miR-410-3p against sevoflurane anesthesia-induced cognitive dysfunction in rats through PI3K/Akt signaling pathway via targeting C-X-C motif chemokine receptor 5. Genes Genomics. 2019;41:1223–1231. doi: 10.1007/s13258-019-00851-5. [DOI] [PubMed] [Google Scholar]
- 29.Stoica GE, Kuo A, Aigner A, Sunitha I, Souttou B, Malerczyk C, Caughey DJ, Wen D, Karavanov A, Riegel AT, Wellstein A. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276:16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- 30.Bouyain S, Watkins DJ. The protein tyrosine phosphatases PTPRZ and PTPRG bind to distinct members of the contactin family of neural recognition molecules. Proc Natl Acad Sci U S A. 2010;107:2443–2448. doi: 10.1073/pnas.0911235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanga N, Kuboyama K, Kishimoto A, Kiyonari H, Shiraishi A, Suzuki R, Watanabe T, Fujikawa A, Noda M. The PTN-PTPRZ signal activates the AFAP1L2-dependent PI3K-AKT pathway for oligodendrocyte differentiation: targeted inactivation of PTPRZ activity in mice. Glia. 2019;67:967–984. doi: 10.1002/glia.23583. [DOI] [PubMed] [Google Scholar]
- 32.Guo J, Cheng J, North BJ, Wei W. Functional analyses of major cancer-related signaling pathways in Alzheimer’s disease etiology. Biochim Biophys Acta Rev Cancer. 2017;1868:341–358. doi: 10.1016/j.bbcan.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]