Abstract
Current systemic dosages of chemotherapeutic drugs such as gemcitabine, 5-FU, cisplatin, doxorubicin are administered every 7 days over 4 cycles due to systemic toxicity. An increase in potency of the drugs will result in dosage reduction with more frequent administration and efficacy increase. Hence, we investigated how the drugs potency can be increased by combining with bromelain and N-acetylcysteine. Tumour cells (5,000/well) were seeded into a 96 well plate and treated 24 hrs later with either single agents or in combinations at various concentrations. Cell survival was assessed by the sulforhodamine B assay after 72 hours of exposure. LD 50 was determined for each treatment and the Combination Index (CI) was assessed to determine synergy using Tallarida’s method. CI indicated that synergy was dependent on the concentration of the agents used and was cell line specific. For bromelain and N-acetylcysteine, certain ratio of the two agents gave very good synergy that was prevalent in almost all cell lines. Gemcitabine and 5-FU and doxorubicin reacted favourably with most concentrations of bromelain and NAC investigated. Cisplatin and oxaliplatin were not very compatible with NAC. A value of CI <0.5 indicated that the current clinical chemotherapeutic dosage can be dramatically reduced. Bromelain with NAC showed synergy in all tumour cell lines and acting synergistically with chemotherapeutic drugs. Synergistic combinations resulting in considerable dosage reduction of chemotherapeutic agents may enable more frequent treatment with higher efficacy.
Keywords: Bromelain, N-acetylcysteine, synergy, gemcitabine, doxorubicin, 5-FU, cisplatin
Introduction
Bromelain is a complex mixture of enzymes (proteases, phosphatases, glucosidases, peroxidases, cellulases etc.) that is extracted from the fruit and stem of pineapple plant (Ananas Cosmosus) [1]. Bromelain has antiedematous, antithrombotic, anti-inflammatory, immuno-stimulatory, antimicrobial and anticancer properties [2,3]. Although its anticancer properties have been recognised decades ago through anecdotal studies in cancer patients [4], it has not been fully evaluated. We have shown that bromelain by itself or in combination with N-acetyl cysteine (NAC) is capable of inducing tumour regression [5,6]. Further we have also demonstrated that bromelain and NAC in combination acts as an efficient mucolytic agent for the solubilisation of mucin secreted in a rare cancer known as pseudomyxoma peritonei [7]. The mucin solubilising properties of bromelain has been mainly attributed to the hydrolysis of glycosidic bonds interlinking the mucin polymer [8].
Further, many of the cellular survival proteins are glycoproteins with glycosidic linkages [9], it is conceivable that bromelain with its glycosidic activity may disrupt the vital properties of these proteins. Further, the anti-tumour effect of bromelain has been shown to be mediated mainly by down regulation of growth promoting cellular proteins with cell death mainly by apoptosis [10]. Bromelain also inhibits metastasis through inhibition of cell surface proteins, NF-kB that are vital for cell adhesions, migration and inflammation [11]. Also, matrix metalloproteinase (MMP-9) expression is inhibited [12] through suppression of activator protein-1 (AP-1) and NF-kB signalling pathways.
NAC is an antioxidant that is clinically used for the treatment of acetaminophen overdose [13]. However, it also possesses anti-tumour properties [14,15]. It has also been used as a mucolytic in patient with chronic obstructive pulmonary disease and cystic fibrosis [16]. As a mucolytic it disrupts the disulfide bridges in the mucin polymers and hence depolymerise and solubilises the mucinous mass for ciliary clearance in cystic fibrosis [17]. The reductive action on disulfide bonds may also affect tumour cell membrane thus affecting the permeability and human nuclear transport proteins that facilitate the entry of certain therapeutic drugs [18].
Since NAC and bromelain have both anti-tumour and permeability enhancing properties, the two compounds when administered together at certain concentrations has shown synergistic anti-tumour action [19]. However, their action in combination with common chemotherapeutic agents such as cisplatin, oxaliplatin, gemcitabine, doxorubicin, and 5-FU has been less well investigated, particularly in pancreatic and liver tumour cells. Therefore, the current study would investigate the potential chemotherapeutic effect of these common cytotoxic drugs in combination with bromelain and NAC. By determining the IC50 values and subsequent combinations of suitable concentrations of two or more agents, the combination index (CI) was determined by Tallarida’s method [20]. Since most of these chemotherapeutic agents at the current clinical dosage carry inherent side effects such as cardiomyopathy, myelopathy, nephrotoxicity and other deleterious effects, the reduction of dosage of these agents by synergistic combination with bromelain and NAC would essentially ameliorate some of the debilitating side effects with enhancement of patient well being. Further the use of two or three agents in synergy would further enhance the anticancer effect with possible improvement in treatment efficacy.
Materials and methods
Materials
Pharmaceutical grade NAC was purchased from Sigma Aldrich, Sydney, NSW, Australia. Bromelain was supplied by Challenge Bioproducts Co. Ltd, Taiwan, China. All other laboratory reagents used were of pharmaceutical grade from Sigma Aldrich, Australia. Human pancreatic cancer cell lines CFPAC, ASPC1, HEP3B and HEPG2 were obtained from (American Type Culture Collection, Rockville, MD, USA).
Plating of 96 well plates with cell lines
All different tumour cell lines were first grown in a medium flask in RPMI 1640 or DMEM according to the manufacturers protocol supplemented with 10% heat inactivated (v/v) foetal bovine serum, 2 mM1-1 glutamine and 1% antibiotic- antimycotic solution (Gibco/Invitrogen) at 37°C, in a humidifier with 0.2% CO2 for three passages. They were then trypsinized and each well was coated with 5,000 cells in suitable cell culture media following standard procedure and kept overnight in a humidified environment at 37°C for subsequent treatment.
Treatment of cells with bromelain and NAC
All required dilutions of NAC and bromelain was carried out in RPMI or DMEM and then filtered using 20-micron filters. The micro-wells containing the cell culture were then treated with 200 ul of the reagents and then placed in humidified incubators at 37°C for 72 hours, after which they were fixed using 10% ice cold Trichloroacetic acid. They were then subjected to sulforhodamine B (SRB) assay following standard protocol [21].
For subsequent work involving the combination of bromelain and NAC, suitable combinations of the agents were carried out based on their previous IC50 values. Dilutions of the agents that were slightly above the IC50 were used.
Treatment of cells with drugs and their combination with bromelain and N-acetylcysteine
Cells were grown in a 96 well plate as described previously. They were then treated to various concentrations of drugs to determine their individual IC50 values. Subsequently, concentrations of drugs slightly above their individual IC50 values were used for combination with bromelain or NAC; in the triple combination studies a similar principal was employed. As before after 72 hours of incubation, they were fixed, stained and the cell density was read as before using SRB assay.
Plotting of graphs and determination of combination index
All graphs were plotted using Prism 7.0 software. The IC50 value was calculated from the graphs and is equal to drug concentration at which 50% inhibition of cell proliferation occurs. To determine the type of interaction between the drugs, the median-drug effect analysis was carried out using CalcuSyn software (http://www.biosoft.com/w/calcusyn.htm, version 2.0, Biosoft, Ferguson, MO, USA). CalcuSyn software allows automated data evaluation using sophisticated algorithms for median-effect analysis. CalcuSyn calculates the CI values which are quantitative representations of the pharmacological interactivity between drugs (synergy, additive and sub-additive/antagonism relationships) as determined by Tallarida [20]. CI values determine synergism (CI<1); additivity (CI = 1) or sub-additivity/antagonism (CI>1).
The combination index for synergy gives an indication on the percentage enhancement of efficacy by the combination of two drugs at specific doses. Hence, this information can be used to determine the required doses for both animal work and clinical application. When compared to the current dosage of the chemotherapeutic agents in clinical use, the percentage reduction of dosage can be calculated. The conversion of in vitro dose to in vivo (human equivalent dose) was calculated as in reference [22].
Results
Cytotoxic response (cell death) of two types of pancreatic cancer cells (CFPAC & ASPC-1) and two types of hepatic cancer cells (HEP3B & HEPG2) as analysed using Tallarida’s method to determine CI. These results are displayed both in tabular and graphical forms for each cell type. The raw data that were used for the analysis is attached in the appendix section.
CFPAC (pancreatic cancer cells) treatment with various agents in combination (Table 1; Figure 1)
Table 1.
Combination Index (CI) for CFPAC (Pancreatic cancer cells) treated with combination of different agents
| Agent 1 | Conc. mg/ml | Agent 2 | Conc. µg/ml | CI | ||||||
|
| ||||||||||
| NAC | 0.816 | Bromelain | 15 | - | ||||||
| 20 | 0.64 | |||||||||
| 25 | 0.70 | |||||||||
| 1.632 | 15 | 0.55 | ||||||||
| 20 | 0.43 | |||||||||
| 25 | 0.53 | |||||||||
|
| ||||||||||
| Agent 1 | Conc. µg/ml | Agent 2 | Conc. mg/ml | CI | ||||||
|
| ||||||||||
| BR | 6 | NAC | 8.159 | 0.774 | ||||||
| 12 | 4.079 | 0.845 | ||||||||
| 8.159 | 0.359 | |||||||||
|
| ||||||||||
| Agent 1 | Conc. µg/ml | Agent 2 | Conc. mg/ml | CI | Agent 3 | Conc. µg/ml | CI | Combo (3 agents) | CI | |
|
| ||||||||||
| GEM | 0.003 | NAC | 2.45 | 0.81 | BR | 12 | - | GEM + NAC + BR | - | |
| 0.013 | 0.80 | - | - | |||||||
| 0.026 | 0.634 | - | 0.821 | |||||||
| 0.131 | 0.383 | - | 0.444 | |||||||
| 0.263 | 0.238 | 0.741 | 0.374 | |||||||
| 1.315 | 0.238 | 0.667 | 0.251 | |||||||
| 2.631 | 0.286 | 0.843 | 0.205 | |||||||
| 5-FU | 0.065 | NAC | 2.448 | 0.774 | BR | 12.0 | - | 5-FU + NAC + BR | 0.86 | |
| 0.130 | 0.574 | - | 0.57 | |||||||
| 0.325 | 0.287 | 0.86 | 0.41 | |||||||
| 0.650 | 0.276 | 0.91 | 0.38 | |||||||
| 1.30 | 0.361 | - | 0.46 | |||||||
| 3.2 | 0.573 | - | 0.65 | |||||||
| 6.5 | 0.841 | - | 0.71 | |||||||
| OXALI | 0.397 | NAC | 2.45 | 0.998 | BR | 15 | - | OXALI + NAC + BR | 0.73 | |
| 0.993 | - | - | 0.72 | |||||||
| 1.98 | - | - | 0.75 | |||||||
| 3.97 | - | - | 0.73 | |||||||
| 5.96 | - | - | 0.78 | |||||||
| 9.93 | - | - | 0.8 | |||||||
| 19.86 | - | 0.89 | 0.78 | |||||||
|
| ||||||||||
| Agent 1 | Conc. ng/ml | Agent 2 | Conc. mg/ml | CI | Agent 3 | Conc. µg/ml | CI | Combo (3 agents) | CI | |
|
| ||||||||||
| DOX | 0.54 | NAC | 2.45 | 0.793 | BR | 15 | - | DOX + NAC + BR | - | |
| 5.4 | 0.722 | - | - | |||||||
| 27.2 | 0.686 | - | - | |||||||
| 54.4 | 0.8 | - | - | |||||||
| 271.8 | 0.633 | - | - | |||||||
| 543.5 | 0.96 | - | - | |||||||
| 1359 | 0.88 | - | - | |||||||
CI values with 1.0 or >1 is not shown in the table. CI values <0.5 are highlighted to denote good synergy.
Figure 1.
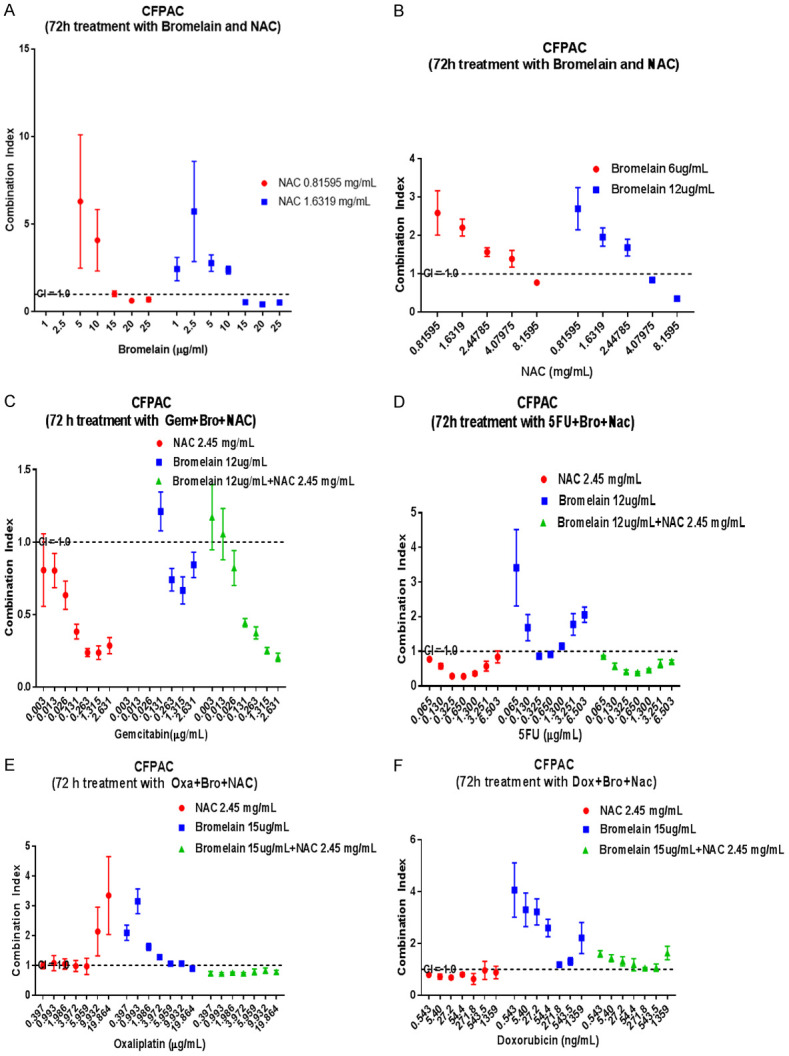
Graphical display of combination index (CI) when pancreatic cancer cells (CFPAC) are treated with concentration of bromelain (Br), N-acetylcysteine (NAC) and in combination with various chemotherapeutic agents. Br and NAC at higher concentration shows synergy (A and B) whilst gemcitabine also synergises with Br, NAC and their combinations (C). 5FU at mid range concentrations also synergises with NAC or NAC + Br (D). Oxaliplatin only synergises with Br (E) whilst Doxorubicin synergises only with NAC (F). The dotted lines show a CI = 1.0 (additivity), CI<1.0 = synergy, CI>1.0 = sub-additivity.
N-acetyl cysteine (NAC) + bromelain (BR)
Treatment with a combination of NAC and BR showed different responses, as indicated by CI values. Table 1 shows all those combinations that were synergistic (CI≤1.0). Notably, those below a value of 0.5 are highlighted to indicate that synergy was good as exemplified by NAC 1.632 mg/ml + 20 µg/ml bromelain (CI = 0.43) and bromelain 12 µg/ml + NAC 8.159 mg/ml (CI = 0.36).
Gemcitabine (GEM) + NAC; GEM + BR; GEM + NAC + BR
GEM ranging from (0.013-2.631 µg/ml) + 2.45 mg/ml NAC showed synergy and those highlighted show synergy with vales below 0.5. Although the combination of GEM with Br showed synergy the CI values did not fall below 5.0. In triple combination, GEM (0.131-2.631 µg/ml) + 2.45 mg/ml NAC + 12 µg/ml BR showed very good synergy (CI value 0.20-0.44).
5-fluorouracil (5-FU) + NAC; 5-FU + BR; 5-FU + NAC + BR
5-FU concentrations ranging from (0.065-6.5 µg/ml) + 2.45 mg/ml NAC showed varying synergy, whilst good synergy (CI<0.5) was shown when 5-FU (0.325-3.2 µg/ml) is combined with 2.45 mg/ml NAC. Triple combinations of 5-FU (0.65-3.2 µg/ml) + 2.45 mg/ml NAC + 12 µg/ml BR also showed good synergy.
Oxaliplatin (OX) + NAC; OX + BR; OX + NAC + BR
OX in combination with NAC or BR showed very little synergy, however the triple combinations showed synergy but none of the triple combinations had CI values below 0.5 (Table 1), the CI values ranged from 0.72-0.8.
Doxorubicin (DOX) + NAC; DOX + BR, DOX + NAC + BR
DOX (0.54-1359 ng/ml) in combination with 2.45 mg/ml NAC showed synergy with CI values ranging from 0.63-0.88. There was no synergy with bromelain (15 µg/ml) or with the triple combinations.
ASPC-1 (pancreatic cancer cells) treatment with various agents in combination (Table 2; Figure 2)
Table 2.
Combination Index (CI) for ASPC-1 (Pancreatic cancer cells) treated with combination of different agents
| Agent 1 | Conc. mg/ml | Agent 2 | Conc. µg/ml | CI | Agent 1 | Conc. µg/ml | Agent 2 | Conc. mg/ml | CI | |
|
| ||||||||||
| NAC | 0.082 | BR | 15 | 0.75 | BR | 7 | NAC | 0.163 | 0.65 | |
| 25 | 0.73 | 0.408 | 0.53 | |||||||
| 50 | 0.54 | 0.816 | 0.26 | |||||||
| 100 | 0.68 | 1.632 | 0.34 | |||||||
| 0.409 | 5.0 | 0.81 | 2.447 | 0.45 | ||||||
| 10 | 0.64 | 17 | 0.163 | 0.79 | ||||||
| 15 | 0.37 | 0.408 | 0.33 | |||||||
| 25 | 0.63 | 0.816 | 0.21 | |||||||
| 50 | 0.55 | 1.632 | 0.15 | |||||||
| 100 | 0.84 | 2.447 | 0.14 | |||||||
|
| ||||||||||
| Agent 1 | Conc. µg/ml | Agent 2 | Conc. mg/ml | CI | Agent 3 | Conc. µg/ml | CI | Combo (3 agents) | CI | |
|
| ||||||||||
| GEM | 0.026 | NAC | 0.407 | 0.84 | BR | 10 | - | GEM + NAC + BR | - | |
| 0.0132 | 0.51 | - | - | |||||||
| 0.0263 | 0.43 | - | 0.75 | |||||||
| 0.1315 | - | 0.23 | 0.71 | |||||||
| 0.2632 | 0.65 | 0.25 | 0.60 | |||||||
| 5-FU | 0.130 | NAC | 0.408 | - | BR | 10 | 0.56 | 5-FU + NAC + BR | - | |
| 0.650 | - | 0.92 | - | |||||||
| 1.30 | - | 0.77 | - | |||||||
| 2.08 | - | 0.76 | - | |||||||
| 3.25 | - | - | - | |||||||
| 6.50 | - | 0.95 | - | |||||||
| 13.0 | - | 0.72 | - | |||||||
| CIS | 0.15 | NAC | 0.407 | - | BR | 10 | - | |||
| 0.3 | - | - | ||||||||
| 1.5 | - | - | ||||||||
| 3.0 | - | 0.37 | ||||||||
| 6.0 | - | 0.74 | ||||||||
| 12.0 | - | - | ||||||||
| OXA | 0.397 | NAC | 0.407 | - | BR | 20 | - | OXA + NAC + BR | - | |
| 0.993 | - | - | - | |||||||
| 1.986 | - | - | - | |||||||
| 3.972 | - | - | - | |||||||
| 5.959 | - | - | - | |||||||
| 9.932 | - | - | - | |||||||
| 19.86 | - | 0.93 | - | |||||||
|
| ||||||||||
| Agent 1 | Conc. ng/ml | Agent 2 | Conc. mg/ml | CI | Agent 3 | Conc. µg/ml | CI | Combo (3 agents) | CI | |
|
| ||||||||||
| DOX | 27.18 | NAC | 0.407 | - | BR | 10 | - | DOX + BR + NAC | 0.93 | |
| 54.35 | - | - | 0.59 | |||||||
| 271 | - | - | 0.64 | |||||||
| 543 | - | - | 0.51 | |||||||
| 1359 | 0.42 | 0.02 | 0.46 | |||||||
CI values with 1.0 or >1 is not shown in the table. CI values <0.5 are highlighted to denote good synergy.
Figure 2.
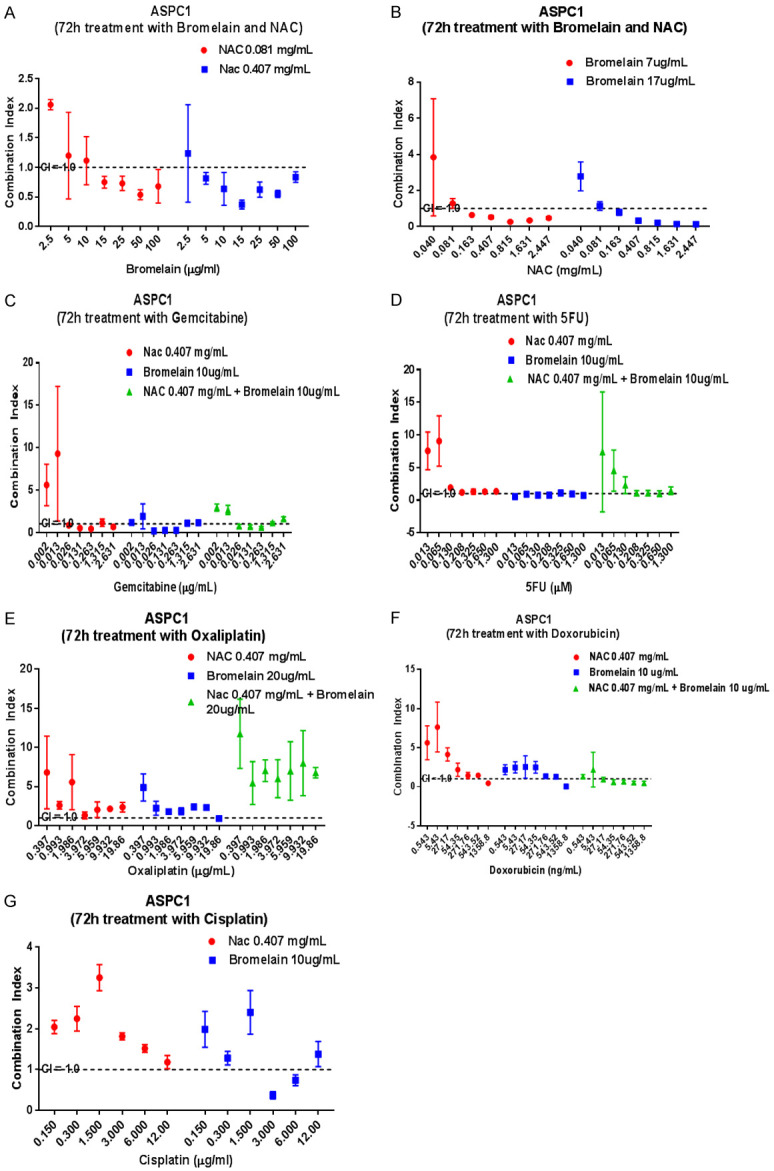
Graphical display of combination index (CI) when pancreatic cancer cells (ASPC1) are treated with varying concentrations of bromelain (Br), N-acetylcysteine (NAC) and in combinations with various chemotherapeutic agents. Synergy is shown when Br and NAC are combined at certain concentrations (A, B). Figure 1 (C) shows synergistic combinations of gemcitabine with NAC, Br and NAC + Br. (D-F) indicates almost an absence of synergy when gemcitabine, 5-FU, Oxaliplatin and doxorubicin are combined with Br, NAC or Br + NAC (G). Cisplatin at 3.0 & 6.0 ug/ml also synergises with only Br (G). The dotted lines show a CI = 1.0 (additivity), CI<1.0 = synergy, CI>1.0 = sub-additivity.
N-acetyl cysteine (NAC) + bromelain (BR)
Treatment of cells with NAC (0.082 mg/ml) in combination with BR (15-100 µg/ml) showed synergy with CI values ranging from 0.54-0.75. On the other hand, treatment with a higher concentration of NAC (0.409 mg/ml) in combination with bromelain ranging from 5.0-100 µg/ml also displayed a varied range of CI (0.37-0.84). Notably, with 15 µg/ml bromelain addition to 0.409 mg/ml NAC had a low (0.37) CI value, indicating a rather high level of synergy.
When BR (7.0 µg/ml) was combined with (0.816-2.447 mg/ml) NAC, synergy was high with CI value below 0.5. Similarly, a combination of BR 17.0 µg/ml with NAC (0.403-2.447 mg/ml) also showed very good synergy (CI<0.5). Notably, 17.0 µg/ml BR combined with 2.447 mg/ml NAC showed a CI value of 0.14.
GEM + NAC; GEM + BR; GEM + NAC + BR
GEM (0.026-0.2632 µg/ml) in combination with NAC (0.407 mg/ml) showed synergy whilst the combination of BR (10 µg/ml) with 0.1315 or 0.2632 µg/ml showed very good synergy with CI values in the region of 0.25.
5-FU + NAC; 5-FU + BR; 5-FU + NAC + BR
5-FU (0.130-13.0 µg/ml) in combination with NAC showed no synergy but there was synergy (CI range from 0.56-0.95) when combined with bromelain (10.0 µg/ml). Triple combination also showed no synergy.
Cisplatin CIS + NAC; CIS + BR; CIS + NAC + BR
CIS (0.15-12.0 µg/ml) with NAC (0.407 mg/ml) showed no synergy, however cisplatin at 3.0 & 6.0 µg/ml with bromelain (10.0 µg/ml) showed synergy (CI range 0.37-0.74).
OXA + NAC; OXA + BR; OXA + NAC + BR
OXA (0.397-19.86) µg/ml with NAC (0.407 mg/ml) showed no synergy whilst with bromelain (20.0 µg/ml) showed synergy (CI = 0.93) only with OXA (19.86 µg/ml). Triple addition showed no synergy.
DOX + NAC; DOX + BR; DOX + NAC + BR
DOX at 1359 ng/ml in combination with NAC at 0.408 mg/ml or in combination with BR (10 µg/ml showed good synergy with the latter having a CI value of 0.02. Triple combinations showed varying synergy.
HEP3B (liver cancer cells) treatment with various agents in combination (Table 3; Figure 3)
Table 3.
Combination Index (CI) for HEP3B (liver cancer cells) treated with combination of different agents
| Agent 1 | Conc. µg/ml | Agent 2 | Conc. mg/ml | CI | |||||
|
| |||||||||
| BR | 15 | NAC | 2.45 | 0.36 | |||||
| 25 | 0.39 | ||||||||
|
| |||||||||
| Agent 1 | Conc. µg/ml | Agent 2 | Conc. mg/ml | CI | Agent 3 | Conc. µg/ml | CI | Combo (3 agents) | CI |
|
| |||||||||
| GEM | 0.026 | NAC | 2.45 | - | BR | 7.0 | 0.86 | GEM + NAC + BR | 0.23 |
| 0.131 | - | - | 0.21 | ||||||
| 0.263 | - | 0.88 | 0.18 | ||||||
| 0.065 | - | - | 0.16 | ||||||
| 1.315 | 0.59 | 0.45 | 0.19 | ||||||
| 2.63 | 0.32 | 0.52 | 0.23 | ||||||
| 6.58 | 0.6 | 0.85 | 0.32 | ||||||
| 5-FU | 0.0650 | NAC | 2.45 | - | BR | 7 | 0.8 | 5-FU + NAC + BR | 0.43 |
| 0.130 | - | 0.68 | 0.18 | ||||||
| 0.325 | - | 0.68 | 0.22 | ||||||
| 0.650 | 0.59 | 0.72 | 0.17 | ||||||
| 1.3 | 0.32 | 0.88 | 0.26 | ||||||
| 2.0 | 0.6 | - | 0.17 | ||||||
| 2.6 | - | - | 0.24 | ||||||
| OXALI | 3.97 | NAC | 2.45 | - | BR | 7.0 | 0.99 | OXALI + NAC + BR | - |
| 5.96 | - | 0.54 | - | ||||||
| 9.93 | - | 0.69 | - | ||||||
| CIS | 1.5 | 1.0 | - | - | |||||
| 3.0 | 0.61 | 0.52 | 0.66 | ||||||
| 6.0 | - | 0.77 | - | ||||||
|
| |||||||||
| Agent 1 | Conc. ng/ml | Agent 2 | Conc. mg/ml | CI | Agent 3 | Conc. µg/ml | CI | Combo (3 agents) | CI |
|
| |||||||||
| DOX | 0.54 | NAC | 2.45 | - | BR | 10 | 0.68 | DOX + NAC + BR | 0.85 |
| 5.43 | - | 0.79 | 0.81 | ||||||
| 27.18 | 0.83 | 0.63 | 0.83 | ||||||
| 54.35 | 0.66 | 0.6 | - | ||||||
| 272 | 0.55 | 0.65 | 0.97 | ||||||
| 544 | 0.34 | 0.48 | 0.69 | ||||||
| 1359 | 0.74 | 0.88 | - | ||||||
CI values with 1.0 or >1.0 is not shown in the table. CI values <0.5 are highlighted to denote good synergy.
Figure 3.
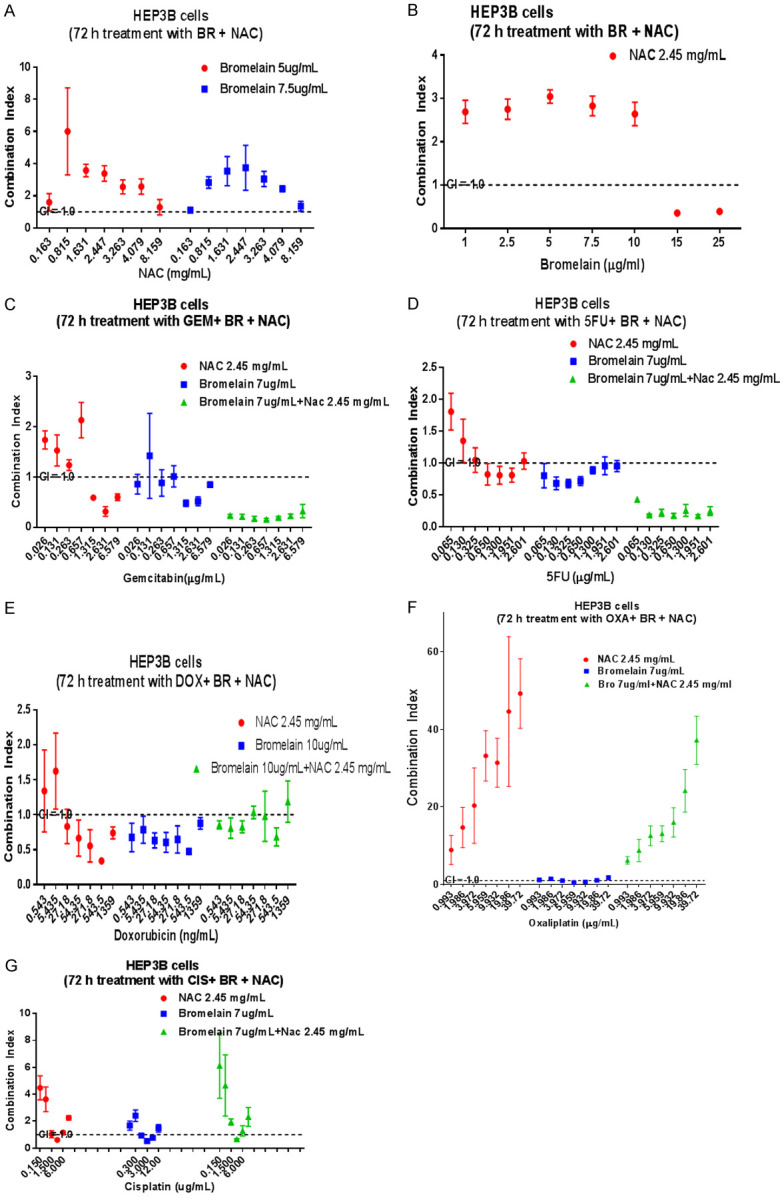
Graphical display of combination index (CI) when liver cancer cells (HEP3B) are treated with varying concentrations of bromelain (Br), N-acetylcysteine (NAC), and in combination with different chemotherapeutic agents. (A) Shows no synergy at all whilst (B) shows good synergy when 15 or 25 ug/ml bromelain is combined with 2.45 mg/ml NAC. (C) Shows synergy when gemcitabine at higher concentrations are combined with bromelain, NAC or bromelain + NAC. A similar scenario exists for 5-FU (D) whilst doxorubicin at various concentrations can be successfully combined with bromelain, NAC or bromelain + NAC to exert good synergy (E). Absence of synergy is shown when oxaliplatin is combined with Br, NAC or their combinations (F). Cisplatin at certain concentrations shows some synergy when combined with bromelain, NAC or Br + NAC (G). The dotted lines show a CI = 1.0 (additivity), CI<1.0 = synergy, CI>1.0 = sub-additivity.
BR + NAC
BR (15 or 25 µg/ml) in combination with NAC (2.45 mg/ml) showed good synergy (CI value of 0.36-0.39).
GEM + NAC; GEM + BR; GEM + NAC + BR
GEM (1.32-6.58 µg/ml) in combination with NAC (2.45 mg/ml) showed synergy, the best synergy being with 2.63 µg/ml GEM (CI = 0.32). GEM (0.026-6.58 µg/ml) in combination with BR (7.0 µg/ml) showed varying synergy, the best being at GEM, 1.315 µg/ml + 7.0 µg/ml BR (CI = 0.45). Triple combinations with varying conc. of GEM (0.026-6.58 µg/ml) in combinations with NAC (2.45 mg/ml) and BR (7.0 µg/ml) showed very good synergy (CI values ranging from 0.16-0.32).
5-FU + NAC; 5-FU + BR; 5-FU + NAC + BR
5-FU (0.650-2.0 µg/ml) in combination with NAC (2.45 mg/ml) showed synergy (CI value = 0.8). 5-FU (0.065-1.3 µg/ml) in combination with BR (7.0 µg/ml) also showed synergy (CI values ranging from 0.7-0.9). Triple combinations with 5-FU (0.065-2.6 µg/ml) + NAC (2.45 µg/ml) + BR (7.0 µg/ml) showed very good synergy, CI values ranging from 0.17-0.43.
DOX + NAC; DOX + BR; DOX + NAC + BR
DOX (27.2-1359 ng/ml) in combination with 2.45 mg/ml NAC showed varying synergy with the best CI value of 0.34 at a combination of 544 ng/ml DOX + 2.45 mg/ml NAC. DOX (0.54-1359 ng/ml) in combination with BR (10 µg/ml) showed synergy with varying CI values ranging from 0.48-0.8. Triple combinations of DOX (0.54-27.18 ng/ml) in combination with 2.45 mg/ml NAC and 10.0 µg/ml BR showed synergy (CI = 0.85). Likewise DOX (272-544 ng/ml) in combination with NAC and BR showed synergy.
OXA + NAC; OXA + BR; OXA + NAC + BR
OXA (3.97-9.93 µg/ml) in combination with BR (7.0 µg/ml) showed synergy (CI range from 0.54-0.99). With NAC 2.45 mg/ml there was no synergy.
CIS + NAC; CIS + BR; CIS + NAC + BR
CIS (3.0 µg/ml) in combination with 2.45 mg/ml NAC showed synergy (CI = 0.6). CIS (3.0-6.0 µg/ml) in combination with BR (7.0 µg/ml) also showed synergy (CI = 0.5-0.7). Triple combinations of NAC + BR showed synergy when combined with 3.0 µg/ml CIS (CI = 0.66).
HEPG2 (liver cancer cells) treatment with various agents in combination (Table 4; Figure 4)
Table 4.
Combination Index (CI) for HEPG2 (liver cancer cells) treated with combination of different agents
| Agent 1 | Conc. mg/ml | Agent 2 | Conc. µg/ml | CI | Agent 2 | Conc. µg/ml | CI | Agent 2 | Conc. µg/ml | CI |
|
| ||||||||||
| NAC | 0.163 | BR | 10 | 0.4 | BR | 20 | 0.44 | BR | 40 | 0.45 |
| 0.407 | 0.3 | 0.25 | 0.20 | |||||||
| 0.815 | 0.34 | 0.23 | 0.3 | |||||||
| 1.63 | 0.4 | 0.35 | 0.53 | |||||||
| 2.45 | 0.79 | 0.34 | 0.44 | |||||||
| 4.1 | 0.31 | 0.25 | 0.17 | |||||||
| 8.16 | 0.2 | 0.20 | 0.15 | |||||||
|
| ||||||||||
| Agent 1 | Conc. µg/ml | Agent 2 | Conc. mg/ml | CI | Agent 2 | Conc. mg/ml | CI | Agent 2 | Conc. mg/ml | CI |
|
| ||||||||||
| BR | 2.5 | NAC | 0.162 | 0.07 | NAC | 0.408 | 0.09 | NAC | 1.63 | 0.06 |
| 5.0 | 0.16 | 0.19 | 0.14 | |||||||
| 10.0 | 0.32 | 0.33 | 0.23 | |||||||
| 15.0 | 0.36 | 0.22 | 0.11 | |||||||
| 25.0 | 0.37 | 0.41 | 0.2 | |||||||
| 50 | 0.52 | 0.64 | 0.25 | |||||||
| 100 | 0.86 | - | 0.3 | |||||||
|
| ||||||||||
| Agent 1 | Conc. µg/ml | Agent 2 | Conc. mg/ml | CI | Agent 3 | Conc. µg/ml | CI | Combo (3 agents) | CI | |
|
| ||||||||||
| GEM | 0.0026 | NAC | 0.408 | - | BR | 15 | 0.13 | GEM + NAC + BR | 0.08 | |
| 0.0132 | - | 0.09 | 0.09 | |||||||
| 0.0263 | - | 0.1 | 0.1 | |||||||
| 0.132 | 0.32 | 0.11 | 0.11 | |||||||
| 0.263 | 0.45 | 0.14 | 0.11 | |||||||
| 1.316 | - | 0.25 | 0.21 | |||||||
| 2.632 | - | 0.41 | 0.45 | |||||||
| 5-FU | 0.065 | NAC | 0.408 | 0.24 | BR | 15 | 0.78 | 5-FU + NAC + BR | 0.38 | |
| 0.130 | 0.05 | 0.39 | 0.08 | |||||||
| 0.325 | 0.26 | 0.07 | 0.07 | |||||||
| 0.650 | 0.12 | 0.09 | 0.06 | |||||||
| 1.30 | 0.22 | 0.11 | 0.08 | |||||||
| 3.25 | 0.28 | 0.09 | 0.09 | |||||||
| 6.50 | 0.39 | 0.2 | 0.14 | |||||||
CI values of 1.0 or >1.0 is not shown in the table. CI values <0.5 are highlighted to denote good synergy.
Figure 4.
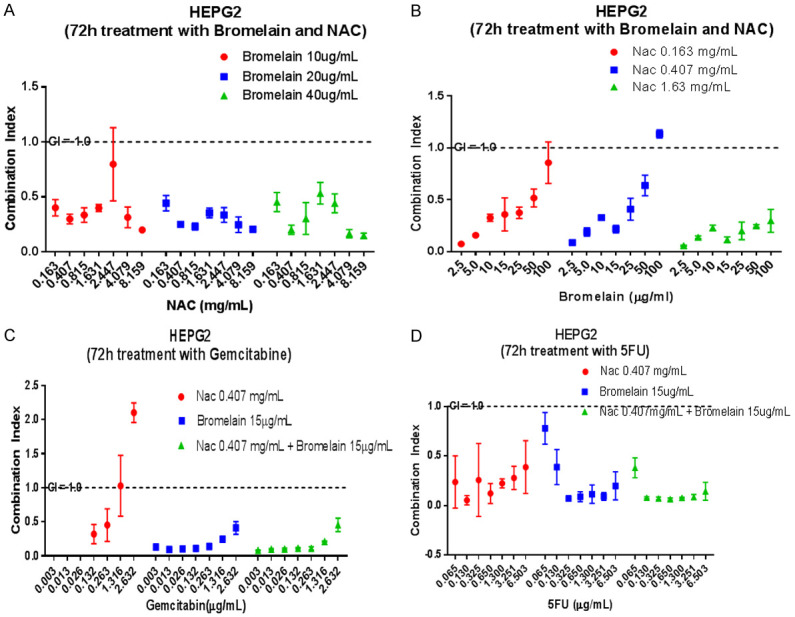
Graphical display of combination index (CI) in liver cancer cells (HEPG2) when treated with varying concentrations of bromelain, NAC, gemcitabine, 5-FU and their combinations. Bromelain + NAC at varying concentrations shows very good synergy. Very low concentrations of bromelain with NAC shows tremendous synergy (A, B). Gemcitabine at very low concentrations acts synergistically with NAC, bromelain and bromelain + NAC (C) whilst a similar scenario exists with 5-FU (D). The dotted lines show a CI = 1.0 (additivity), CI<1.0 = synergy, CI>1.0 = sub-additivity.
NAC + BR
NAC (0.163-8.16 mg/ml) in combinations with 10 µg/ml BR, 20 µg/ml BR or 40 µg/ml BR showed very good synergy. Similarly, BR 2.5-100 µg/ml in combination with NAC 0.162, 0.408 or 1.63 mg/ml showed very good synergy. The triple combinations had very good synergy based on the CI value which ranged from 0.11-0.44. The cells seem to be very responsive to the triple combinations which suggest that NAC and BR at suitable combinations may be used for treating these types of tumour cells.
GEM + NAC; GEM + BR; GEM + BR + NAC
GEM (0.132-0.263 µg/ml) in combination with NAC (0.408 mg/ml) showed very good synergy (CI value ranged from 0.32-0.45). When GEM (0.0026-2.632 µg/ml) is combined with BR (15 µg/ml), synergy was very good (CI values ranged from 0.1-0.41). Triple combinations of GEM with NAC and BR also showed very good synergy, on the whole.
5-FU + NAC; 5-FU + BR; 5-FU + NAC + BR
5-FU (0.065-6.50 µg/ml) in combinations with NAC (0.41 mg/ml) showed very good synergy (CI values range from 0.05-0.39). Similarly, in combinations of 5-FU (0.130 µg/ml) with 15.0 µg/ml BR showed very good synergy (CI values ranging from 0.07-0.39). Finally, the triple combinations were also very effective with good synergy (CI values ranging from 0.07-0.38). On the whole these liver cancer cells seem to respond very well with treatment using either NAC + BR, GEM in combination with BR or NAC or the triple combinations.
Discussion
The present study indicates that BR in combination with NAC exerts synergistic cytotoxicity on four different tumour cell lines. However, their synergistic effect seems to depend on the relative concentrations of the two agents used and is also cell line specific. BR and NAC are agents with different chemical reactivity. NAC is a reducing agent whilst BR is a proteolytic agent. NAC is a very much smaller molecule compared to BR (164 vs. 28000 Daltons) with a weight ratio of 1:117. Furthermore, BR exists as a single polypeptide chain containing 211 or 212 residues [23] and a single molecule is capable of cleaving hundreds of glycosidic bonds since it can regenerate itself, a property intrinsic with most enzymes [24]. On the other hand, a molecule of NAC reduces a disulphide linkage only once [17]. Hence, most of the synergistic ratio of NAC: BR, in terms of weight or molecular ratio, indicates that NAC is present in much larger quantity compared to BR. Since NAC is a disulfide reducing agent, the quantity of NAC required may also have a direct relationship to the number of disulfide groups present within the cellular glycoproteins. This is exemplified in CFPAC tumour cells where a combination of BR (12 µg/ml) + (4.079 mg/ml) NAC registered a CI value of 0.85 as compared to BR (12 µg/ml) + (8.159 mg/ml) NAC with a CI value that was 0.36, indicating that increasing the NAC concentration twofold reduced the CI value by half. Similarly, in ASPC-1 tumour cells, treatment with a combination of BR (17 µg/ml) + (2.447 mg/ml) NAC gave a CI value of 0.14 in comparison to treatment with BR (17 µg/ml) + (0.163 mg/ml) NAC that gave a CI value of 0.8. In addition, there is indication that once the ratio of NAC: BR is exceeded, then, the CI values rises indicating a lesser synergy. This is exemplified in the ASPC-1 cells, adding variable amount of BR (5-100 µg/ml) to a fixed quantity of NAC (0.409 mg/ml) indicated that at 5.0 µg/ml bromelain, the CI was 0.8 that gradually declined to 0.37 with the addition of 15 µg/ml bromelain (3 fold) after which further addition of 25 µg/ml bromelain increased the CI value to 0.63. Hence, there is a ration of the two agents that is crucial in maintaining the maximum synergy. A similar scenario is exemplified in all the cell lines as shown in Table 5.
Table 5.
Shows the relative ratio of the two agents used to derive a low CI that is indicative of very good synergy (CI<0.5) in pancreatic cancer cells (CPFAC & ASPC1) and hepatic cancer cells (HEP3B & HEPG2)
| Cell line | NAC (µg/ml) | BR (µg/ml) | CI | Wt. ratio NAC:BR | Molecular ratio NAC:BR |
|---|---|---|---|---|---|
| CPFAC | 1632 | 20.0 | 0.43 | 82:1 | 0.5:3.5 × 10-5 |
| 8159 | 12.0 | 0.36 | 680:1 | 4.2:3.5 × 10-5 | |
| ASPC-1 | 2447 | 17.0 | 0.14 | 144:1 | 0.88:3.5 × 10-5 |
| 408 | 17.0 | 0.33 | 24:1 | 0.15:3.5 × 10-5 | |
| HEP3B | 2450 | 15.0 | 0.36 | 163:1 | 1.0:3.5 × 10-5 |
| 2450 | 25.0 | 0.39 | 98:1 | 0.6:3.5 × 10-5 | |
| HEPG2 | 815 | 10.0 | 0.34 | 81.5:1 | 0.5:3.5 × 10-5 |
| 8160 | 10.0 | 0.2 | 816:1 | 5.0:3.5 × 10-5 | |
| 1630 | 2.5 | 0.06 | 652:1 | 4.0:3.5 × 10-5 | |
| 1630 | 10.0 | 0.23 | 163:1 | 1.0:3.5 × 10-5 |
NAC = N-acetylcysteine; BR = bromelain; CI = Combination index. The highlighted figures indicate the best synergism of the combinations used.
Pancreatic cancer cells (CFPAC and ASPC-1) are mucin expressing cells that provide both a barrier and oncogenic (survival and reproductive) function to these cells [25]. MUC5B and MUC 16 have been shown to provide survival and migration advantage in ASPC-1 cells [26] whilst MUC 1 has been found in abundance in CFPAC cells that are responsible for regulating the COX2 gene [27]. Hepatocellular tumour cells HEP3B are also mucin expressing [28] whilst HEPG2 cells does not express MUC1 but has other glycoproteins [29]. Hence, the removal of mucin and subsequent treatment with a second agent that targets other survival proteins would enable a better tumour cell kill and thereby increase the efficacy of cancer treatment.
In the present work a number of different cytotoxic agents such as gemcitabine (GEM), 5-FU, doxorubicin (DOX), cisplatin (CIS) and oxaliplatin (OXA) have been tested with the addition of BR and NAC on four different tumour cell lines to determine if synergy exists. The mechanistic action of cytotoxic agents varies although most of them are nucleosides. GEM is (2’-2’ difluoro-2’-deoxyxytidine; dFdC) is a pro-drug that is converted into a pharmacologically active triphosphate-dFdC within the cell by deoxycytidine kinases and blocks de nova synthesis of DNA [30]. Both the pancreatic tumour cells showed synergy with GEM and NAC, although CFPAC showed a higher synergy. With bromelain and GEM, again both the cell line showed synergy, with ASPC-1 exhibiting a much better synergy (CI, 0.67 vs. 0.23) however, with the triple combination (combo), CFPAC showed much better response. This differential response may be due to heterogeneity in mucin and other molecular parameters. In the case of Hepatic Cancer Carcinoma (HCC) cells, HEPG2 showed much greater synergy compared to HEP3B with either the addition of NAC or bromelain. However, the triple combination was almost equally effective with good synergy in both the cell lines. Overall, the HCC seem to react better (greater synergy) and this means that they are more sensitive to particularly the combo or triple regime. Hence, further studies to decipher the molecular features that are particularly sensitive to triple combination are necessary so that it can be applied to clinical practise.
5-FU is a pro-drug that must be converted into its active metabolites, 5-fluorodeoxyuridine monophosphate (5-FdUMP), 5-fluorodeoxyuridine triphosphate (5-FdUTP) or 5-fluorouridine triphosphate (5-FUTP). 5-FdUMP is an inhibitor of thymidylate synthase (TYMS) and depletion of dTMP results in deoxynucleotide pool imbalance affecting DNA synthesis and repair. Further when 5-FUTP is incorporated into RNA, its function is impaired [31] whilst incorporation of 5-FUTP into DNA inhibits DNA synthesis leading to single and double strand breaks. The combination of 5-FU with NAC only showed good synergy at certain concentrations of the two agents in CFPAC and noticeably absent in ASPC1 cells. With the addition of BR to 5-FU, both the cell lines showed some synergy, however the triple addition showed much better synergy in CFPAC and totally absent in ASPC1. This differential property needs further investigation, but their peculiarity may be partly be assigned to their heterogenous mucinous and other cellular parameters.
Cisplatin (cisplatinum, cis-diamminedichloroplatinum (II) or CDDP) and oxaliplatin (oxalate (trans-t-1,2-diaminocyclohexane)) platinum differs from each other in their molecular weight, the former with 301 vs. 397.3 for the latter. The leaving groups within the two compounds also differ, the former having chloride whilst the latter with oxalate, hence their reactivity varies slightly [32]. The reactive radicals generated by these platinum compounds enter the nucleus of the cell with a specific tropism for the guanine-cytosine (GC) rich sites and bind to nitrogen atom (N7) of guanine forming mono-adducts and then bi-adducts [33]. Intra-strand cross linking seems to be the main mechanism of action of DNA lesions and subsequent lethality of the cells. Since, the generation of reactive species seems to be the main mechanistic pathway; the presence of antioxidants may interfere or quench the reactive pathway. Both pancreatic cell lines reacted negatively when NAC was added to OXA indicating the antioxidant effect. Synergy was almost absent with the addition of BR, as well. With triple combination, CFPAC showed some synergistic response. The addition of NAC to CIS in ASPC1 also showed negative effect (antioxidant effect) whilst BR showed good synergy (CI = 0.37). In the HCC, the addition of NAC to OXA showed a negative effect in HEP3B whilst the addition of BR showed some synergy. When CIS was added to NAC synergy was shown at a concentration of 3.0 µg/ml CIS (CI = 0.61), similarly the triple combination also showed synergy. The molecular ratio of CIS to NAC may be a decisive parameter for synergy and it needs further investigation.
Two mechanisms by which DOX acts on cancer cells have been proposed i.e. intercalation with DNA with disruption of topoisomerase II mediated DNA repair and generation of free radicals that results in damage to cellular membranes, DNA and other cellular proteins. Although, these pathways seem plausible, none of these mechanisms are achievable at the clinical drug concentrations [34]. Recently, it has been suggested that it acts through proteolytic activation of a transcription factor cAMP response element binding protein 3-like 1 (CREB3L 1) [35] and cells expressing this factor are more responsive to DOX. DOX with NAC showed synergy in CFPAC cells but it was absent with BR or in the triple combination. However, in ASPC1 cells, the addition of 0.407 mg/ml NAC to 1359 ng/ml DOX showed good synergy (CI = 0.42) whilst with 20 µg/ml bromelain, it showed extremely high synergy (CI = 0.02). The triple combination also showed good synergy. In the HCC cell HEP3B, synergy existed with the addition of NAC or BR and also in the triple combination. Although we did not investigate DOX with HEPG2, based on its reactivity with NAC and BR, synergy is most likely present with NAC, BR or the triple combinations.
In CFPAC cells treatment with NAC and BR indicates that synergy was fairly good at different concentrations of both the agents, however translational to clinical practise may only be possible with loco regional or intra-tumoral delivery. Owing to the high dosage required for NAC, systemic delivery may lead to toxicity [36]. Similarly, although very good synergy was observed with GEM and NAC or combo, only loco regional delivery is safe. On the other hand, with the addition of BR to GEM, BR is only present at 12 µg/ml that translates to 840 mg for a 70 kg patient. The safety of bromelain has only been investigated in humans using oral delivery, animal studies by iv or ip routes have shown a median lethal dosage of 20-35 mg/kg and 36.7-85.2 mg/kg, respectively [37] and hence locoregional delivery is probably the safest route of administration. A similar scenario was seen with 5-FU. DOX also synergises with NAC, however only loco regional delivery is safe. A similar trend seems to exist with ASPC-1 cells. In the case of HCC, although, they responded very well with most of the agents in combination, owing to the high concentrations of NAC used, loco regional delivery of the therapeutic agents is probably the safest route. However, chemotherapeutic drugs in combination with BR may be used systemically. In the case of HEPG2 cells treatment of chemo agents (GEM, 5-FU) with bromelain showed tremendous synergistic efficacy indicating that the dosage of chemo agents can be reduced drastically when compared to the current dosages as shown in Table 6.
Table 6.
Shows synergy in HEPG2 cells with a comparison of dose (synergistic) to current in vivo clinical dose
| Chemo agent (in vitro) | In vivo dose (Synergy) | Current clinical dose | % reduction in dose | Bromelain dose (Synergy) | CAP |
|---|---|---|---|---|---|
| GEM 0.132 µg/ml | 132 µg/kg; 4.88 mg/M2 | 25 mg/kg; 1000 mg/M2 | 99.5 | 15.0 µg/ml (in vitro) | SYS |
| 15 mg/kg; 555 mg/M2 (in vivo) | |||||
| 5-FU 0.325 µg/ml | 325 µg/kg; 12.0 mg/M2 | 71 mg/kg; 2627 mg/M2 | 99.54 | SYS |
CAP = Clinical application; SYS = systemic. It indicates that using the principal of synergy, when combined with suitable dose of bromelain, the requirement for chemotherapeutic agents such as gemcitabine and 5-FU can be dramatically reduced.
Hence, the current study gives an indication that the combination of some of the common chemotherapeutic drugs such as GEM, 5-FU, DOX, CIS & OXAL with NAC and BR may enable a considerable reduction of the current clinical dose used with further enabling more frequent treatment. This may result in a more efficient treatment since cancer cells exposed to short cycles are prone to a higher response compared to 28-day cycles whereby resistant cells are able to recover and repopulate during the 7-day time interval between treatment regimens [38,39]. Frequent treatment is possible since very low therapeutic drug levels can be used, i.e. cutting the dosage to an absolute minimum and hence averting adverse side effects dramatically. In addition, this investigation also indicates that the combination of NAC and BR by itself can also be used effectively for cancer treatment with suitable combinations of the two agents. Although, the current findings are very promising for the development of a more effective cancer therapy, further studies that examine the efficacy of these combinations needs to be investigated in vivo models.
Disclosure of conflict of interest
None.
References
- 1.Maurer HR. Bromelain: biochemistry, pharmacology and medical use. Cell Mol Life Sci. 2001;58:1234–1245. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillai K, Akhter J, Chua TC, Morris DL. Anticancer property of bromelain with therapeutic potential in malignant peritoneal mesothelioma. Cancer Invest. 2013;31:241–250. doi: 10.3109/07357907.2013.784777. [DOI] [PubMed] [Google Scholar]
- 3.Singer AJ, Taira BR, Anderson R, McClain SA, Rosenberg L. The effects of rapid enzymatic debridement of deep partial-thickness burns with Debrase® on wound reepithelialization in swine. J Burn Care Res. 2010;31:795–802. doi: 10.1097/BCR.0b013e3181eed48e. [DOI] [PubMed] [Google Scholar]
- 4.Chobotova K, Vernallis AB, Majid FA. Bromelain’s activity and potential as an anti-cancer agent: current evidence and perspectives. Cancer Lett. 2010;290:148–156. doi: 10.1016/j.canlet.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Amini A, Ehteda A, Masoumi Moghaddam S, Akhter J, Pillai K, Morris DL. Cytotoxic effects of bromelain in human gastrointestinal carcinoma cell lines (MKN45, KATO-III, HT29-5F12, and HT29-5M21) Onco Targets Ther. 2013;6:403–409. doi: 10.2147/OTT.S43072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillai K, Ehteda A, Akhter J, Chua TC, Morris DL. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anticancer Drugs. 2014;25:150–160. doi: 10.1097/CAD.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 7.Pillai K, Akhter J, Chua TC, Morris DL. A formulation for in situ lysis of mucin secreted in pseudomyxoma peritonei. Int J Cancer. 2014;134:478–486. doi: 10.1002/ijc.28380. [DOI] [PubMed] [Google Scholar]
- 8.Wang SL, Lin HT, Liang TW, Chen YJ, Yen YH, Guo SP. Reclamation of chitinous materials by bromelain for the preparation of antitumor and antifungal materials. Bioresour Technol. 2008;99:4386–4393. doi: 10.1016/j.biortech.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Kailemia MJ, Park D, Lebrilla CB. Glycans and glycoproteins as specific biomarkers for cancer. Anal Bioanal Chem. 2017;409:395–410. doi: 10.1007/s00216-016-9880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillai K, Akhter J, Ehteda A, Badar S, Chua T, Morris D. Anti-tumour and chemosensitising effect of a combination of bromelain + N-Acetyl cysteine with cisplatin or 5-Fu on malignant peritoneal mesothelioma cells. J Glycobiol S. 2013;1:2. [Google Scholar]
- 11.Amini A, Masoumi-Moghaddam S, Ehteda A, Morris DL. Bromelain and N-acetylcysteine inhibit proliferation and survival of gastrointestinal cancer cells in vitro: significance of combination therapy. J Exp Clin Cancer Res. 2014;33:92. doi: 10.1186/s13046-014-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalra N, Bhui K, Roy P, Srivastava S, George J, Prasad S, Shukla Y. Regulation of p53, nuclear factor kappaB and cyclooxygenase-2 expression by bromelain through targeting mitogen-activated protein kinase pathway in mouse skin. Toxicol Appl Pharmacol. 2008;226:30–37. doi: 10.1016/j.taap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–292. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delneste Y, Jeannin P, Potier L, Romero P, Bonnefoy JY. N-acetyl-L-cysteine exhibits antitumoral activity by increasing tumor necrosis factor alpha-dependent T-cell cytotoxicity. Blood. 1997;90:1124–1132. [PubMed] [Google Scholar]
- 15.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadowska AM. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2012;6:127–35. doi: 10.1177/1753465812437563. [DOI] [PubMed] [Google Scholar]
- 17.Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, Sergio F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 2018;52:751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 18.Hogg PJ. Disulfide bonds as switches for protein function. Trends Biochem Sci. 2003;28:210–214. doi: 10.1016/S0968-0004(03)00057-4. [DOI] [PubMed] [Google Scholar]
- 19.Amini A, Masoumi-Moghaddam S, Ehteda A, Liauw W, Morris DL. Potentiation of chemotherapeutics by bromelain and N-acetylcysteine: sequential and combination therapy of gastrointestinal cancer cells. Am J Cancer Res. 2016;6:350–369. [PMC free article] [PubMed] [Google Scholar]
- 20.Tallarida RJ. Quantitative methods for assessing drug synergism. Genes Cancer. 2011;2:1003–1008. doi: 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orellana EA, Kasinski AL. Sulforhodamine B (SRB) assay in cell culture to investigate cell proliferation. Bio Protoc. 2016;6 doi: 10.21769/BioProtoc.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritonja A, Rowan AD, Buttle DJ, Rawlings ND, Turk V, Barrett AJ. Stem bromelain: amino acid sequence and implications for weak binding of cystatin. FEBS Lett. 1989;247:419–424. doi: 10.1016/0014-5793(89)81383-3. [DOI] [PubMed] [Google Scholar]
- 24.Cleland WW. Enzyme kinetics revisited: a commentaryon “The kinetics of enzyme-catalyzed reactions with two or more substrates or products” by WW Cleland Biochim. Biophys. Acta 67 (1963) 104 ff. with Biochim. Biophys. Acta 67 (1963) 173, 188 (summaries) Biochim Biophys Acta Gen Subj. 1989;1000:209–221. doi: 10.1016/s0006-3002(89)80019-8. [DOI] [PubMed] [Google Scholar]
- 25.Suh H, Pillai K, Morris DL. Mucins in pancreatic cancer: biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am J Cancer Res. 2017;7:1372–1383. [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Lee J, Yun JH, Jeong DG, Kim JH. DUSP28 links regulation of Mucin 5B and Mucin 16 to migration and survival of AsPC-1 human pancreatic cancer cells. Tumor Biology. 2016;37:12193–12202. doi: 10.1007/s13277-016-5079-x. [DOI] [PubMed] [Google Scholar]
- 27.Nath S, Roy LD, Grover P, Rao S, Mukherjee P. Mucin 1 regulates Cox-2 gene in pancreatic cancer. Pancreas. 2015;44:909–17. doi: 10.1097/MPA.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Liu G, Shao D, Wang J, Yuan H, Chen T, Zhai R, Ni W, Tai G. Mucin1 mediates autocrine transforming growth factor beta signaling through activating the c-Jun N-terminal kinase/activator protein 1 pathway in human hepatocellular carcinoma cells. Int J Biochem Cell Biol. 2015;59:116–25. doi: 10.1016/j.biocel.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Yu C, Hu Y, Duan J, Yuan W, Wang C, Xu H, Yang XD. Novel aptamer-nanoparticle bioconjugates enhances delivery of anticancer drug to MUC1-positive cancer cells in vitro. PLoS One. 2011;6:e24077. doi: 10.1371/journal.pone.0024077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veltkamp SA, Pluim D, van Eijndhoven MA, Bolijn MJ, Ong FH, Govindarajan R, Unadkat JD, Beijnen JH, Schellens JH. New insights into the pharmacology and cytotoxicity of gemcitabine and 2’, 2’-difluorodeoxyuridine. Mol Cancer Ther. 2008;7:2415–25. doi: 10.1158/1535-7163.MCT-08-0137. [DOI] [PubMed] [Google Scholar]
- 31.Sommer J, Mahli A, Freese K, Schiergens TS, Kuecuekoktay FS, Teufel A, Thasler WE, Müller M, Bosserhoff AK, Hellerbrand C. Analysis of molecular mechanisms of 5-fluorouracil-induced steatosis and inflammation in vitro and in mice. Oncotarget. 2017;8:13059–13072. doi: 10.18632/oncotarget.14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alcindor T, Beauger N. Oxaliplatin: a review in the era of molecularly targeted therapy. Curr Oncol. 2011;18:18–25. doi: 10.3747/co.v18i1.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–41. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 35.Denard B, Lee C, Ye J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. Elife. 2012;1:e00090. doi: 10.7554/eLife.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dósa E, Heltai K, Radovits T, Molnár G, Kapocsi J, Merkely B, Fu R, Doolittle ND, Tóth GB, Urdang Z. Dose escalation study of intravenous and intra-arterial N-acetylcysteine for the prevention of oto-and nephrotoxicity of cisplatin with a contrast-induced nephropathy model in patients with renal insufficiency. Fluids Barriers CNS. 2017;14:26. doi: 10.1186/s12987-017-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lotz-Winter H. On the pharmacology of bromelain: an update with special regard to animal studies on dose-dependent effects. Planta Med. 1990;56:249–253. doi: 10.1055/s-2006-960949. [DOI] [PubMed] [Google Scholar]
- 38.Maiti R. Metronomic chemotherapy. J Pharmacol Pharmacother. 2014;5:186–92. doi: 10.4103/0976-500X.136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bishnoi R, Shah C, Bejjanki H, Bennett JA, Reisman DN. An alternative approach with a low dose and prolonged chemotherapy for palliative treatment of locally advanced, metastatic or recurrent squamous cell head and neck cancer. Applied Cancer Research. 2017;37:43. [Google Scholar]


