Highlights
-
•
Brainstem to thalamus connectivity had the largest increase during recovery from traumatic coma.
-
•
Thalamus to temporal lobe tracts are disrupted in patients who do not recover from traumatic coma.
-
•
Larger sample sizes are needed to confirm these preliminary findings.
Keywords: Coma, Traumatic brain injury, MRI, Consciousness, Brainstem
Abstract
Background
It is not currently possible to predict which patients will develop chronic disorders of consciousness (DoC) after severe traumatic brain injury (TBI). Although the ascending arousal network (AAN) supports human consciousness, it is unknown which AAN pathways must be preserved for patients to recover consciousness.
Methods
Sixteen patients with acute traumatic coma and 16 matched healthy controls were scanned with high angular resolution diffusion imaging (HARDI). All patients recovered consciousness (Recovery Cohort). Nine were scanned longitudinally: first in the ICU (Acute), then at ≥5 months post-injury (Follow-up). Six separate patients with post-traumatic DoC were scanned ≥5 months post-injury (Chronic DoC Cohort). For each AAN pathway, we computed the median relative change in Acute-to-Follow-up Connectivity Probability (CP) in the Recovery Cohort. We then used Wilcoxon tests with Bonferroni correction to compare CP in each AAN pathway in the Recovery Cohort at Follow-up versus the Chronic DoC Cohort. In an exploratory analysis, we used principal component analysis (PCA) to determine whether linear combinations of AAN CP values could separate the Chronic DoC Cohort from the Recovery Cohort and the healthy controls.
Results
In the Recovery Cohort, the largest relative AAN CP changes were in the brainstem-to-thalamus (median [IQR] = 0.7 [0.09, 0.9]) and forebrain-to-occipital lobe (−0.8 [−0.9, −0.8]) pathways. The AAN connections that differed in the cross-sectional analysis between the Recovery Cohort at Follow-up and the Chronic DoC Cohort included brainstem-to-hypothalamus (W = 53, PBonf = 0.02), brainstem-to-temporal lobe (W = 52, PBonf = 0.04), and thalamus-to-temporal lobe (W = 54, PBonf = 0.009). Plotting the first two principal components of AAN connectivity resulted in a linear separation of Chronic DoC patients from other study groups.
Conclusions
We provide evidence for a longitudinal increase in brainstem-thalamic connectivity during recovery of consciousness after traumatic coma. Cross-sectional analyses revealed that brainstem-hypothalamus, brainstem-temporal lobe, and thalamus-temporal lobe connectivity differed between patients who recovered consciousness and those with a chronic DoC. These observations provide the basis for further investigation into AAN connectivity as a biomarker for recovery of consciousness after traumatic coma.
1. Introduction
Among patients who survive a severe traumatic brain injury (TBI), 7–8% remain in a vegetative state (VS), and 37% remain in a minimally conscious state (MCS) or completely dependent at six months (Wilkins et al., 2019). Alternatively, 17% have a good or near-complete recovery (Wilkins et al., 2019). The lack of accurate prognostic tools in the intensive care unit (ICU) creates uncertainty and increases the risk of premature withdrawal of life-sustaining therapy (WLST) in patients with recovery potential (Turgeon et al., 2011, Izzy et al., 2013).
One imaging biomarker with potential prognostic relevance is structural connectivity within the ascending arousal network (AAN) (Snider et al., 2019). The AAN is a collection of arousal-promoting brainstem (mesopontine tegmentum) and subcortical (hypothalamus, basal forebrain, thalamus) nuclei synaptically connected with each other and the cerebral cortex (Lindsley et al., 1949, Parvizi and Damasio, 2001). Using probabilistic tractography, we recently demonstrated that relative to healthy control subjects, patients with traumatic coma have an approximately 20% reduction of AAN tracts connecting the brainstem tegmentum to the thalamus, basal forebrain, and hypothalamus (Snider et al., 2019). However, it remains unknown whether AAN connectivity changes during recovery of consciousness. Furthermore, the specific AAN connections essential for recovery of consciousness have not been identified.
We conducted a longitudinal analysis of AAN connectivity in patients who recovered from traumatic coma, and a cross-sectional analysis of AAN connectivity in patients with and without a chronic disorder of consciousness (DoC). We used high angular resolution diffusion imaging (HARDI) and probabilistic tractography to answer the following questions: (1) Where are the peak changes in AAN connectivity during recovery from traumatic coma? (2) Where are the largest differences in AAN connectivity between patients who recover consciousness and patients with chronic post-traumatic DoC?
2. Materials and methods
2.1. Patients
We prospectively enrolled 18 patients who presented with acute traumatic coma (Glasgow Coma Scale [GCS] ≤ 6 and no eye opening for 24 h) to an academic medical center as described previously (Snider et al., 2019, Edlow et al., 2017). Etiologies included motor vehicle accident (MVA), fall, and assault (Table 1). Two patients died from WLST in the ICU and were excluded from the analysis. Level of consciousness was assessed with GCS prior to ICU admission and with the Coma Recovery Scale-Revised (CRS-R) (Giacino et al., 2004) at the time of each MRI scan. Acute MRI scans were performed as soon as was deemed safe by treating ICU clinicians. All 16 surviving patients fully recovered consciousness by six months (Table 1, Recovery Cohort). Nine of these patients returned for a follow-up MRI approximately six months after the initial injury (Table 1, Recovery Cohort, Longitudinal).
Table 1.
Patient Characteristics.
| Recovery (N = 16) |
Chronic DoC (N = 6) | ||
|---|---|---|---|
| Acute Only (N = 7) | Longitudinal (N = 9) | ||
| Age (median [IQR]) | 32 [25, 36] | 26 [21, 28] | 24 [22, 26] |
| Sex (M) | 6 (86%) | 6 (67%) | 3 (50%) |
| Mechanism | MVA 3 (43%), Fall 3 (43%), Assault 1 (14%) | MVA 6 (67%), Fall 3 (33%) | MVA 6 (100%) |
| Time from injury to Acute MRI (days) | 12 [10,14] | 7 [3,14] | – |
| Time from injury to Follow-Up MRI (days) | – | 206 [190, 370] | 172 [151, 925] |
| Level of consciousness at Follow-Up | – | Conscious (100%) | VS 3 (50%), MCS 3 (50%) |
| CRS-R total score at Follow-Up | – | 23 [23, 23] | 6.5 [5, 8.75] |
We prospectively identified and enrolled a separate, convenience sample of six patients with chronic DoC, as defined by a behavioral diagnosis of VS or MCS on the CRS-R (Giacino et al., 2018), at least five months post-TBI (Table 1, Chronic DoC Cohort). The burden of structural injury in these patients as well as in the Recovery Cohort at Follow-up is reported in Supplementary Figure 1.
2.2. Imaging
MRI for all patients and healthy subjects was performed on the same 3 Tesla Skyra scanner (Siemens Medical Solutions) using a 32-channel head coil. HARDI sequence parameters have been described previously (Snider et al., 2019) and are notable for 2 mm isotropic resolution, 60 diffusion-encoding directions, and a contrast of b = 2,000 sec/mm2. Anatomical T1-weighted multi-echo MPRAGE (MEMPRAGE) volumes were also acquired as previously described (Edlow et al., 2017). All HARDI data are available at https://openneuro.org/datasets/ds003367/versions/1.0.0.
2.3. Image processing
Pre-processing of the HARDI data was performed in FSL (FMRIB, Oxford, UK), including brain extraction (bet2, f parameter 0.2) and eddy current and inter/intra volume head motion correction (EDDY 6.0.1 (Andersson and Sotiropoulos, 2016)). We ran EDDY with slice-wise outlier replacement (signal > 4 standard deviations below mean signal intensity) and slice-to-volume alignment to correct for intra-volume movement (estimated with 20-order model and 5 iterations). Average absolute movement for the entire study, and by group, is reported in Supplementary Figure 2. Diffusion parameters were estimated with BEDPOSTX (6.0.1), using default parameter values. Image registration into MNI space was performed using Advanced Normalization Tools (ANTs, http://stnava.github.io/ANTs/) as described previously (Snider et al., 2019). In one case (Chronic DoC), the subject’s fractional anisotropy (FA) image was aligned first to the T1 MEMPRAGE image and then to the MNI template to improve anatomical alignment. The accuracy of the individual registrations is illustrated in Supplementary Figures 3-4.
2.4. Probabilistic tractography
We used previously-described bilateral AAN ROIs (Snider et al., 2019) and MNI atlas segmentations of cortical lobes (Kötter et al., 2001), transformed into subject diffusion space and visually screened for misalignments. The insular and temporal lobe ROIs were combined given their proximity. Tracts connecting pairs of ROIs were generated with probabilistic tractography (FSL, PROBTRACKX2 5.0.7) using default parameters. Because cortico-cortical association fibers were not considered here, a callosal exclusion mask was used to remove anatomically implausible tracts that crossed the midline. In 7 subjects (6 Chronic DoC, 1 Recovery Follow-up), hypothalamic, thalamic or basal forebrain masks were manually edited (FSLeyes) in diffusion space according to the Allen Brain Atlas (Ding et al., 2016) to correct misalignments. A representative example is shown in Supplementary Figure 5.
A connectivity probability (CP) between each pair of ROIs (Snider et al., 2019) was defined as , where = tracts from seed reaching target, = tracts from target reaching seed, = total tracts launched from seed (5000 × seed voxels), and = total tracts launched from target (5000 × target voxels). This formula models CP as a binomial distribution with each pi = ki/ni derived from ki tracts reaching the relative target from ni trials (5000 × seed voxels). With two samples (seed-to-target and target-to-seed), the resulting probability (CP) is a weighted average of the two pi, each weighted by ni trials. Because ROIs were bilateral, CP additionally reflects the average of left- and right-sided connectivity, also weighted by the left and right hemispheric size of each ROI.
We analyzed subcortical (connecting brainstem tegmentum, hypothalamus, basal forebrain, and thalamus) and projection AAN tracts (connecting subcortical structures and a cortical lobe). For visualization, we transformed into MNI space the tracts connecting every pair of subcortical structures and normalized each voxel by the number of tracts launched (total voxel size of all ROIs × 5000).
2.5. Statistics
Among the Recovery Cohort patients, we defined the relative change in CP (rel CP) as (CPFollow-up − CPacute) / CPacute. To identify AAN connections with the greatest differences between the Chronic DoC (N = 6) and Recovery Cohort at Follow-up (N = 9), we ran Wilcoxon rank sum tests for each pair of ROIs, using a Bonferroni P-value correction. As a benchmark for comparison, we analyzed CP values in an age- and sex-matched healthy control cohort (N = 16), as well as in all Recovery Cohort Acute patients (N = 16). To ensure that any difference in CP between the Chronic DoC and Recovery cohorts was not driven by excess motion in the Chronic DoC group, we repeated the Wilcoxon analysis for any significant results, after excluding subjects who were motion outliers (i.e. >1 standard deviation from the group mean average absolute motion). To identify the axes explaining the largest proportion of variance between the Chronic DoC and Recovery Cohort at Follow-up, we performed principal component analysis (PCA) on the set of centered and scaled AAN CP values. We then projected the Control and Recovery Cohorts’ (Acute) AAN CP values onto these same axes to assess for linear separability among the groups. Analyses were performed in RStudio (Version 1.1.442).
3. Results
3.1. Longitudinal recovery: Acute to Follow-up (N = 9)
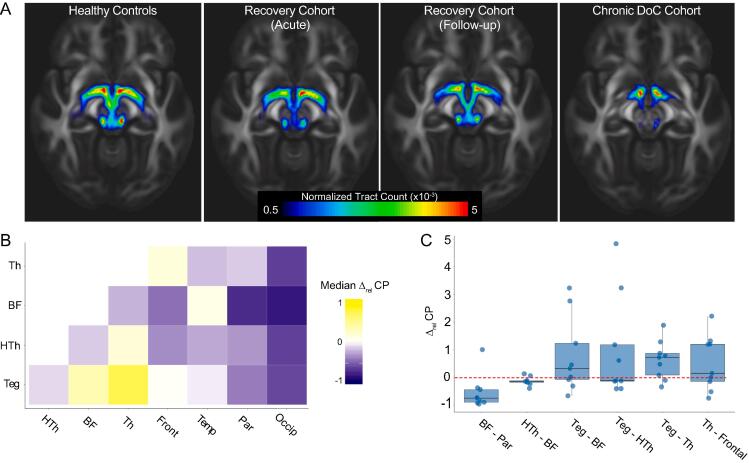
Normalized tract plots illustrate differences in subcortical AAN connectivity between Controls, Recovery (Acute and Follow-up), and Chronic DoC patients (Fig. 1A). Among the Recovery patients, the largest proportional connectivity increase (rel CP) from the Acute to Follow-up scans occurred between the brainstem tegmentum and thalamus (median 0.7, IQR [0.09, 0.9], Fig. 1B) and the largest decrease occurred in the projection fibers connecting the basal forebrain to the occipital lobe (−0.8 [−0.9, −0.8], Fig. 1B). However, even among the six connections with the lowest coefficient of variation (1st quartile, Fig. 1C), individual subject results were heterogeneous.
Fig. 1.
Peak changes in AAN connectivity during recovery from traumatic coma. (A) Group-median values for streamlines between all subcortical AAN structures (normalized by total number of streamlines launched) plotted in MNI T1 1 mm space. (B) Each value in the matrix represents the group median relative change in CP (rel CP) for each pair of ROIs in Recovery patients between the Acute and Follow-up time points. (C) Individual subject rel CP values. The six AAN connections with coefficient of variation less than 1st quartile are displayed, illustrating heterogeneity between subjects, even among connections experiencing the most uniform trends. Abbreviations: Th = Thalamus, BF = Basal Forebrain, HTh = Hypothalamus, Teg = Brainstem Tegmentum, Front = Frontal Lobe, Temp = Temporal Lobe, Par = Parietal Lobe, Occip = Occipital Lobe.
3.2. Cross-sectional: chronic DoC (N = 6) versus recovery follow-up (N = 9)
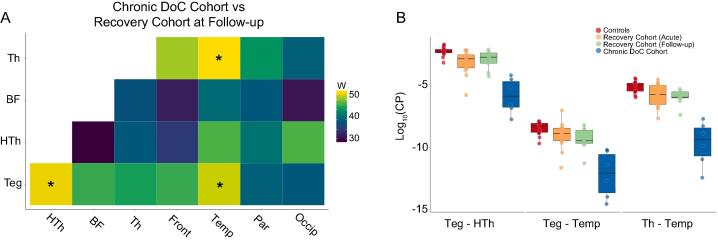
We next investigated maximal connectivity differences between patients who did or did not recover consciousness. We identified three AAN connections with significant CP reduction in Chronic DoC compared with Recovery Follow-up (Fig. 2A): brainstem tegmentum - hypothalamus (W = 53, PBonf = 0.02), tegmentum - temporal lobe (W = 52, PBonf = 0.04), and thalamus - temporal lobe (W = 54, PBonf = 0.009). The largest thalamus - temporal lobe CP value in the Chronic DoC Cohort was smaller than the smallest CP value in all other study groups (Fig. 2B). This lack of between-group overlap was only observed for this particular connection. All three connections remained significantly different between groups after excluding patients who were motion outliers (Supplementary Figure 6) or who were imaged after long delays (>2 years) post injury. All three connections trended in the same direction and two of three (tegmentum - hypothalamus (P = 0.048) and thalamus - temporal (P = 0.024)) remained significantly different after excluding three subjects from the Recovery Cohort at Follow-up who had a non-MVA mechanism of injury.
Fig. 2.
Structural connectivity is reduced in multiple AAN pathways in patients with chronic DoC after TBI. (A) Areas of maximal difference in connectivity probability (CP) between patients with a chronic DoC and those who recovered consciousness (Recovery Cohort at Follow-up). Wilcoxon rank sum values are plotted for each connection. * = Bonferroni adjusted p < 0.05. (B) Individual subjects’ log-transformed CP values are displayed for the three significant connections from (A) across all study groups. For Teg-HTh, Recovery Cohort Follow-up (green) subjects’ (untransformed) group median CP was 60% that of Controls (red), while Chronic DOC (blue) subjects’ group median CP was 4% that of Controls. For Teg-Temp, Recovery Follow-up was 30% vs. Chronic DoC 3%, and for Th-Temp, Recovery Follow-up was 40% vs. Chronic DoC 1%. Abbreviations: Th = Thalamus, BF = Basal Forebrain, HTh = Hypothalamus, Teg = Brainstem Tegmentum, Front = Frontal Lobe, Temp = Temporal Lobe, Par = Parietal Lobe, Occip = Occipital Lobe. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
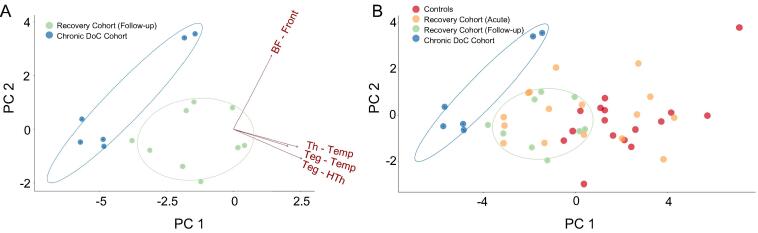
Finally, we performed PCA on all AAN CP values in an exploratory analysis to assess the separability of Chronic DoC patients from those who recovered consciousness. The first two principal components (loaded most heavily with tegmentum-hypothalamus and forebrain-frontal CP values, respectively) explained a cumulative 71% of the variance between the Chronic DoC Cohort and Recovery Cohort at Follow-up (Fig. 3A). Projecting all Controls (N = 16) and Recovery Cohort Acute (N = 16) subjects onto the same axes demonstrated linear separability of the Chronic DoC cohort from all other study participants (Fig. 3B).
Fig. 3.
Principal component analysis (PCA) separates Chronic DoC from conscious patients. (A) Individual subjects are plotted on the first two principal component axes from a PCA to identify the largest sources of variance among the AAN CP values of Chronic DoC and Recovery Cohort Follow-up patients. Confidence intervals for the plot area occupied by each group are shown as ellipses. Arrows represent the loading of each variable (AAN connection) onto each principal component axis. (B) Controls and Recovery Cohort Acute patients are plotted on these same axes, illustrating consistent linear separability between the Chronic DoC Cohort and other study groups. Abbreviations: PC 1 = 1st Principal Component, PC 2 = 2nd Principal Component, Th = Thalamus, BF = Basal Forebrain, HTh = Hypothalamus, Teg = Brainstem Tegmentum, Front = Frontal Lobe, Temp = Temporal Lobe.
4. Discussion
We provide the first report on longitudinal changes in AAN connectivity during post-traumatic recovery of consciousness and identify AAN connections whose integrity differed between patients who did or did not recover consciousness. In traumatic coma patients who recovered consciousness, the largest AAN connectivity increases occurred subcortically, between the brainstem tegmentum and thalamus, and the largest decreases occurred in the cortical projections.
Consistent with prior work in structural connectivity after severe TBI (Wang et al., 2011), longitudinal AAN connectivity changes varied between patients who recovered consciousness. Just as there are different patterns of injury that can produce unconsciousness, there may be multiple potential pathways to the restoration of consciousness, supported by different combinations of subcortical connections. Substantial heterogeneity in patterns of injury and recovery have implications for prognostic and therapeutic studies, which typically rely on sample averages to derive conclusions. In this context, larger samples amenable to a precision medicine approach (Edlow et al., 2020) are needed to identify the range of potential mechanisms of injury and recovery.
While the brainstem’s connectivity with the hypothalamus and thalamus was reduced in chronic DoC patients, only thalamus-temporal lobe had no CP values that overlapped with other study groups, warranting further investigation into the pathophysiologic relevance of this pathway in human consciousness. The brainstem and thalamus have long been known to be critical for arousal and awareness, but networks within the temporal lobe have more recently been identified as important for consciousness. Temporal lobe functional connectivity within the auditory network has been shown to differ significantly between DoC patients with versus without awareness (Boly et al., 2004, Demertzi et al., 2015, Martínez et al., 2020). Furthermore, the temporal lobe’s connections with the medial thalamus and rostral brainstem are thought to be responsible for loss of consciousness in temporal lobe seizures (Blumenfeld and Taylor, 2003).
In our previous study (Snider et al., 2019); tegmentum - hypothalamus and tegmentum - thalamus tracts showed the largest degree of disruption in acute traumatic coma as compared with control subjects. Connections between the thalamus and temporal lobe were not investigated in our prior work, which exclusively focused on brainstem pathways. Even so, connections that can produce coma when damaged may differ from those essential for recovery of consciousness. While multiple tegmental pathways may produce a coma when disrupted, it is possible that a persistent DoC occurs only when thalamo-temporal connections are damaged.
The burden of structural injury accompanying subcortical disconnections was not independently accounted for here. Apart from sample size constraints, such an analysis presents a fundamental challenge. In the chronic, post-injury setting, cortical volumes in regions connected by a white matter fiber tract are closely correlated with diffusion MRI parameter measurements within the tract itself (Warner et al., 2010). Structural injury is therefore fundamentally intertwined with measured connectivity.
Using input from all AAN connections, PCA identified the linear combination of connectivity values that explained the most variance between patients with chronic DoC from those who recovered. Whether this technique will enable more robust connectivity-based separation of chronic DoC from recovered patients will need to be tested in larger datasets. Furthermore, it remains to be determined whether individual tract disruptions are evident on the acute scans of TBI patients who will develop a chronic DoC, or whether they become apparent only after time has elapsed. Future work will also be needed to determine if connectivity measurements obtained acutely can independently predict the degree of cognitive and functional recovery, as measured by standardized scales like the CRS-R and Disability Rating Scale.
The goal of structural connectivity analyses is to use tractography to reconstruct diffusion MRI data and quantify the number of intact axonal connections between two structures. In animal models of TBI, changes in diffusion MRI parameters correspond to focal edema and/or axonal injury (Mac Donald et al., 2007), but these observations have not yet been histologically validated in humans. The CP metric used here seeks to quantify structural connectivity, while mitigating some of the biases (e.g. accounting for seed and target size) and limitations (e.g. using a bidirectional diffusion measure because there is no information about the direction of electrical signaling) inherent to tractography. Axonal injury or focal edema within axon bundles should reduce the measured CP between two structures, but this remains unproven.
A fundamental limitation of this study is its small sample size. In studies performing brain-wide correlations between a neuroimaging feature and behavior, estimates of effect sizes inversely correlate with sample size, and small samples tend to produce noisy, unstable estimates (Lorca-Puls et al., 2018, Button et al., 2013, Marek et al., 2020). To mitigate the potential for obtaining non-reproducible results, we restricted our testing to the AAN and used the most conservative statistical method to correct for multiple comparisons (Bonferroni correction). However, there is no absolute way to avoid false negatives and spurious associations without a larger sample size. For this reason, the findings reported here should be considered preliminary until validated in a larger, independent dataset.
A second limitation of this study is the differential amount of head motion between groups. While we processed the data with a sophisticated motion-correction algorithm and demonstrated that our results were robust to the exclusion of motion outliers, fully accounting for the confounding effects of motion is difficult. In larger datasets, estimates of head motion should be included as nuisance regressors (Yendiki et al., 2014) when modeling the effect of DTI parameters on patient characteristics.
Additional limitations include the differential time to imaging post-injury, the well-established problems with the neuroanatomic sensitivity and specificity of tractography (Maier-Hein et al., 2017) and our use of sub-maximal angular resolution (Prčkovska et al., 2013). Furthermore, as subcortical ROI-based tractography requires transformation of atlas-based masks onto 2 mm isotropic diffusion sequences, small errors in mask alignment would go undetected. Additionally, given our use of whole-thalamus ROIs, thalamocortical projections analyzed here would have also included fibers between thalamic nuclei and cortical structures not believed to be involved in arousal or awareness. Finally, the structural connectivity quantification used here (CP) has not been histologically validated.
In summary, we provide initial characterization of longitudinal connectivity changes within the human AAN following traumatic coma. We show that specific AAN connections may discriminate between patients with or without a chronic DoC. These findings warrant further investigation into AAN connectivity as a potential biomarker for recovery of consciousness after traumatic coma.
5. Study funding
This study was supported by the NIH National Institute of Neurological Disorders and Stroke (K23NS094538, R21NS109627, RF1NS115268), NIH Director’s Office (DP2HD101400), NIH National Institute of Biomedical Imaging and Bioengineering (K01EB019474), James S. McDonnell Foundation, Rappaport Foundation, Tiny Blue Dot Foundation, and National Institute on Disability, Independent Living and Rehabilitation Research (NIDILRR), Administration for Community Living (90DP0039, Spaulding-Harvard TBI Model System).
CRediT authorship contribution statement
Samuel B. Snider: Conceptualization, Data curation, Formal analysis, Methodology, Writing - original draft. Yelena G. Bodien: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Writing - original draft. Aina Frau-Pascual: Conceptualization, Methodology. Marta Bianciardi: Methodology, Formal analysis. Andrea S. Foulkes: Methodology. Brian L. Edlow: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank the nursing staffs of the Massachusetts General Hospital Neurosciences ICU, Multidisciplinary ICU, and Surgical ICU. We also thank the Massachusetts General Hospital MRI technologists for assistance with data acquisition. We are grateful to the patients and families in this study for their participation and support.
Author disclosures
Drs. Snider, Bodien, Frau-Pascual, Bianciardi, Foulkes, and Edlow report no relevant disclosures.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102503.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T.E., Beers S.R., Borrasso A.J. Favorable Functional Recovery in Severe Traumatic Brain Injury Survivors beyond Six Months. J. Neurotrauma. 2019;36(22):3158–3163. doi: 10.1089/neu.2018.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon A.F., Lauzier F., Simard J.-F. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. Can. Med. Assoc. J. 2011;183(14):1581–1588. doi: 10.1503/cmaj.101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzy S., Compton R., Carandang R. Self-Fulfilling Prophecies Through Withdrawal of Care: Do They Exist in Traumatic Brain Injury, Too? Neurocrit Care. 2013;19(3):347–363. doi: 10.1007/s12028-013-9925-z. [DOI] [PubMed] [Google Scholar]
- Snider S.B., Bodien Y.G., Bianciardi M. Disruption of the ascending arousal network in acute traumatic disorders of consciousness. Neurology. 2019;93(13):e1281–e1287. doi: 10.1212/WNL.0000000000008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D.B., Bowden J.W., Magoun H.W. Effect upon the EEG of acute injury to the brain stem activating system. Electroencephalogr. Clin. Neurophysiol. 1949;1(1-4):475–486. [PubMed] [Google Scholar]
- Parvizi J., Damasio A. Consciousness and the brainstem. Cognition. 2001;79:135–160. doi: 10.1016/s0010-0277(00)00127-x. [DOI] [PubMed] [Google Scholar]
- Edlow B.L., Chatelle C., Spencer C.A. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140:2399–2414. doi: 10.1093/brain/awx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino J.T., Kalmar K., Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Katz D.I., Schiff N.D. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91(10):450–460. doi: 10.1212/WNL.0000000000005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötter R., Mazziotta J., Toga A. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Phil. Trans. R. Soc. Lond. B. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S.L., Royall J.J., Sunkin S.M. Comprehensive cellular-resolution atlas of the adult human brain. J. Comp. Neurol. 2016;524:3127–3481. doi: 10.1002/cne.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.Y., Bakhadirov K., Abdi H. Longitudinal changes of structural connectivity in traumatic axonal injury. Neurology. 2011;77(9):818–826. doi: 10.1212/WNL.0b013e31822c61d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow B.L., Barra M.E., Zhou D.W. Personalized Connectome Mapping to Guide Targeted Therapy and Promote Recovery of Consciousness in the Intensive Care Unit. Neurocritical Care. 2020;33:364–375. doi: 10.1007/s12028-020-01062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M., Faymonville M.-E., Peigneux P. Auditory Processing in Severely Brain Injured Patients: Differences Between the Minimally Conscious State and the Persistent Vegetative State. Arch Neurol. 2004;61(2):233. doi: 10.1001/archneur.61.2.233. [DOI] [PubMed] [Google Scholar]
- Demertzi A., Antonopoulos G., Heine L. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain. 2015;138(9):2619–2631. doi: 10.1093/brain/awv169. [DOI] [PubMed] [Google Scholar]
- Martínez D.E., Rudas J., Demertzi A. Reconfiguration of large‐scale functional connectivity in patients with disorders of consciousness. Brain Behav. 2020;10(1) doi: 10.1002/brb3.v10.110.1002/brb3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H., Taylor J. Why do Seizures Cause Loss of Consciousness? Neuroscientist. 2003;9(5):301–310. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- Warner M.A., de la Plata C.M., Spence J. Assessing Spatial Relationships between Axonal Integrity, Regional Brain Volumes, and Neuropsychological Outcomes after Traumatic Axonal Injury. J. Neurotrauma. 2010;27(12):2121–2130. doi: 10.1089/neu.2010.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Dikranian K., Bayly P. Diffusion Tensor Imaging Reliably Detects Experimental Traumatic Axonal Injury and Indicates Approximate Time of Injury. J. Neurosci. 2007;27(44):11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca-Puls D.L., Gajardo-Vidal A., White J. The impact of sample size on the reproducibility of voxel-based lesion-deficit mappings. Neuropsychologia. 2018;115:101–111. doi: 10.1016/j.neuropsychologia.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P.A., Mokrysz C. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Marek S., Tervo-Clemmens B., Calabro F.J. Towards Reproducible Brain-Wide Association Studies. bioRxiv. 2020 2020:2020.2008.2021.257758. [Google Scholar]
- Yendiki A., Koldewyn K., Kakunoori S. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage. 2014;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein K.H., Neher P.F., Houde J.C. The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun. 2017;8:1349. doi: 10.1038/s41467-017-01285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prčkovska V., Achterberg H.C., Bastiani M. Optimal Short-Time Acquisition Schemes in High Angular Resolution Diffusion-Weighted Imaging. Int. J. Biomed. Imaging. 2013;2013:1–17. doi: 10.1155/2013/658583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.