Abstract
Splenic abscesses are a rare infection that usually requires seeding from another primary source; however, direct contact of bacteria can occur with microperforation secondary to colon cancer leading to abscess formation. This occurrence is rare, and through literature review only 12 previous cases have been reported with associated bacteremia. Our patient is a 62-year-old female who presented with left upper quadrant pain with a history of tobacco and alcohol abuse that was febrile and hypoxic. Blood cultures were obtained that eventually grew Fusobacterium mortiferum. Computed tomography of the abdomen and the pelvis revealed 2 splenic abscesses that were cultured to grow Escherichia coli and β-hemolytic Streptococcus group C. Colonoscopy was performed, which identified 2 masses that were biopsied, and histopathology confirmed well-differentiated adenocarcinoma with possible muscular invasion. The patient had no other identifiable risk factors for bacterial seeding from another primary source. We present the first reported case report of splenic abscess secondary to colonic adenocarcinoma suspected microperforation associated with Fusobacterium mortiferum bacteremia.
Keywords: splenic abscess, colon cancer, adenocarcinoma, bacteremia
Case Presentation
A 62-year-old female presented with sharp left upper quadrant pain that worsened with inspiration. The patient has a history of tobacco abuse (35-pack-year equivalent) and alcohol abuse; however, she denied any illicit drug use. She was febrile with temperature 103 °F and hypoxic saturating 90% on room air. Physical examination demonstrated left upper quadrant pain to both light and deep palpation. Laboratory results were significant for normocytic anemia and absence of leukocytosis. Blood cultures were collected on admission with gram stain growing gram-negative rods resembling enterics and an anaerobic culture eventually growing Fusobacterium mortiferum.
An initial chest X-ray revealed a left lower lobe consolidation with a pleural effusion. Computed tomography (CT) of the abdomen with contrast revealed 2 perisplenic fluid collections, one at the lower lateral periphery measuring 4.1 × 2.1 cm in size, and the second at the inferior pole measuring 3.8 × 2.2 cm (Figure 1). The patient was then started on intravenous fluids, acetaminophen/oxycodone for pain management, and intravenous piperacillin/tazobactam 4.5 g every 6 hours.
Figure 1.
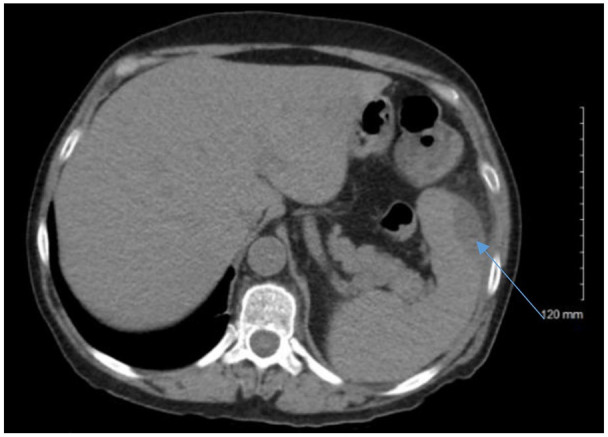
Computed tomography of abdomen with contrast demonstrating splenic abscess (blue arrow).
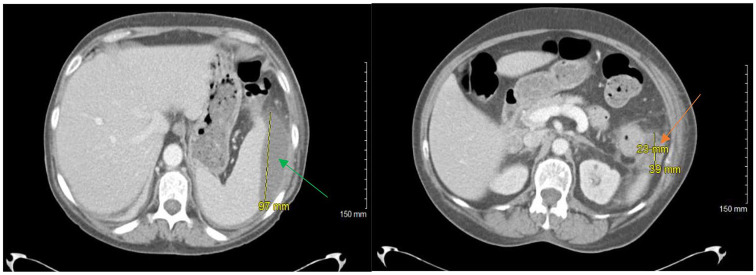
Repeat CT abdomen with contrast scan on hospital day 3 demonstrated enlargement of the 2 splenic subcapsular fluid collections, the lesion at the superior pole of the spleen now measured 10.8 cm × 7.4 cm × 9.7 cm and the second lesion at the inferior pole adjacent with the splenic flexure now measured 2.3 cm × 3.9 × 3.6 cm (Figure 2). A CT-guided aspiration and drainage of the superior perisplenic collection (100 mL removed) and of the inferior perisplenic collection (15 mL removed) was obtained. The superior aspirate culture did not grow any bacteria or fungus; however, the inferior aspirate culture grew Escherichia coli and β-hemolytic Streptococcus group C.
Figure 2.
Repeat computed tomography of abdomen with contrast demonstrating 2 perisplenic abscesses, one in the superior spleen (green arrow) and one in the inferior spleen (orange arrow).
A transesophageal echocardiogram and oral X-rays showed no vegetations or lesions. Colonoscopy revealed 2 masses in the transverse and descending colon as well as a rectal polyp. The smaller mass in the transverse colon measured 2.4 cm, and the larger tumor in the descending colon measured 8.4 cm. Bother lesions were biopsied and the smaller lesion showed well-differentiated adenocarcinoma with suspicion for muscular invasion. The larger descending colonic mass showed moderately differentiated invasive adenocarcinoma. Assessment from oncology determined the staging to be Stage IIB (pT4aN0M0).
The patient underwent colorectal surgery for left hemicolectomy with ileocolonic anastomosis. During surgery, the colon was found to be adherent to the superior pole of the spleen. The surgical pathology report revealed adenocarcinoma in the transverse colon tumor that invaded through the muscularis interna, and adenocarcinoma in the larger tumor with 15% mucinous carcinoma component that invaded through the muscularis propria and perforated the peritoneum and into the pericolic fat. The patient discharged in stable condition and was instructed to follow with oncology for adjuvant chemotherapy as an outpatient.
Discussion
This case describes the rare occurrence of a splenic abscess forming by direct spread rather than distant seeding. Splenic abscesses are a rare medical occurrence usually caused by hematogenous spread from a distant infection.1-3 Splenic abscess should be suspected in a patient with left upper quadrant pain, splenomegaly, left costovertebral tenderness, left pleural effusion on X-ray, and leukocytosis with a left shift. Associated risk factors for developing a splenic abscess include infective endocarditis, diabetes mellitus, trauma, intravenous drug abuse, or hemoglobinopathies,1,4-8 of which our patient had none. Rarely, colon cancer can perforate the bowels and communicate into the spleen allowing for direct spread of bacteria and eventual abscess formation. Through literature review, 12 cases of this phenomenon have been reported4,9-19 (Table 1), all of which had an associated bacteremia. The most common bacteria cultured in splenic abscess are Escherichia coli, Proteus mirabilis, Streptococcus group D, Klebsiella pneumonia, and Bacteroides fragilis.
Table 1.
Literature Review.
| Reference # | Author | Year | Journal |
|---|---|---|---|
| 9 | Cartanese et al | 2012 | Annali Italiani di chirugia |
| 10 | Kawamoto et al | 1993 | Jpn J Clin Oncol |
| 11 | Michowits et al | 1982 | J Surg Oncol |
| 12 | Leibovitz et al | 2002 | Harefuah |
| 13 | Nakao et al | 1999 | Anticancer Res |
| 14 | Belinkie et al | 1983 | Dis Colon Rectum |
| 15 | Parmelle et al | 2001 | J Radiol |
| 16 | Pisanu et al | 2007 | World J Gastroenterol |
| 17 | Gervaise et al | 2010 | J Radiol |
| 18 | Awotar et al | 2016 | Medicine |
| 19 | Rishitha et al | 2017 | Gastroenterology |
| 4 | Johnson et al | 1985 | Rev Infect Dis |
In the absence of endocarditis, patients presenting with a splenic abscess usually have a concomitant immunodeficiency disorder, trauma, splenic infarct, diabetes, or malignancy.1,4-8 With the absence of typical risk factors, combined with the clinical and surgical findings, we highly suspect that our patient developed a splenic abscess by direct contact via microperforation secondary to the adenocarcinoma of the distal transverse colon rather than by seeding of a distant source.
Bacteremia is another common finding that is associated with a splenic abscess.1-3 Bacteremia associated with splenic abscesses are most often gram positive due to the seeding from infective endocarditis.1-3 However, this patient’s gram-negative bacteremia grew F mortiforeum, which is typically associated with infections of the oral cavity, female genital tract, gastrointestinal tract, and often plays a role in septic shock and abscess formation.20-22 The 2 most common species of Fusobacterium widely discussed in the literature are F necrophorum and F nucleatum. F mortiferum is less commonly an isolated specimen in clinical cases related to gastrointestinal neoplasms.20-22 There is a well-established connection between Fusobacterium and colorectal cancers, with the most reported species in the literature being F nucleatum.20-22 It is possible that F mortiferum was not able to be cultured from the splenic abscess in our patient as she had received 3 days of intravenous antibiotics prior to having the CT-guided biopsy. We were unable find any documented cases of F mortiforeum bacteremia in the setting of colorectal cancer.
Although there are no specific diagnostic criteria for splenic abscess, CT-guided imaging appears to be a sufficient technique to confirm splenic abscess if there is strong clinical suspicion.4 The treatment recommendation for splenic abscess varies in the literature.4-9 Though splenectomy was once considered the gold standard of treatment for a splenic abscess, drainage and broad-spectrum antibiotics are now the preferred route over a splenectomy, but this must be weighed against the risks of bleeding.5,8 The number of splenectomies performed due to a splenic abscess is not well documented in the literature. Definitive management for a splenic abscess is typically a combination of antibiotic therapy and splenectomy.1-3 However, as in this case, antibiotic therapy and drainage may have a favorable outcome without the need for a splenectomy.
Conclusion
In our case report, we describe an unusual suspected etiology of a splenic abscess formation by direct spread, an unusual presentation of colon cancer, and a previously undocumented splenic abscess with associated F mortiferum bacteremia. Patients presenting with the clinical symptoms and associated risk factors for splenic abscess should undergo the appropriate diagnostic workup, including identifying the source of bacterial exposure to the spleen.
Footnotes
Authors’ Note: An oral presentation of this case report was presented at the 2020 Western Medical Research Conference in Carmel, California, on January 24, 2020.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Ethical approval to report this case was obtained from our institutional review board (IRB #19029).
Informed Consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Tushar Bajaj  https://orcid.org/0000-0002-8863-9399
https://orcid.org/0000-0002-8863-9399
References
- 1. Robinson SL, Saxe JM, Lucas CE, Arbulu A, Ledgerwood AM, Lucas WF. Splenic abscess associated with endocarditis. Surgery. 1992;112:781-786. [PubMed] [Google Scholar]
- 2. Brook I, Frazier EH. Microbiology of the liver and spleen abscesses. J Med Microbiol. 1998;47:1075-1080. [DOI] [PubMed] [Google Scholar]
- 3. Lee CH, Leu HS, Hu TH, Liu JW. Splenic abscess in southern Taiwan. J Microbiol Immunol Infect. 2004;37:39-44. [PubMed] [Google Scholar]
- 4. Johnson JD, Raff MJ, Drasin GF, Daffner RH. Radiology in the diagnosis of splenic abscess. Rev Infect Dis. 1985;7:10-20. [DOI] [PubMed] [Google Scholar]
- 5. Waheed A, Mathew G, Zemaitis MR. Splenic Abscess. StatPearls; 2019. [PubMed] [Google Scholar]
- 6. Divyashree S, Gupta N. Splenic abscess in immunocompetent patients managed primarily without splenectomy: a series of 7 cases. Perm J. 2017;21:16-139. doi: 10.7812/TPP/16-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ting W, Silverman NA, Arzouman DA, Levitsky S. Splenic septic emboli in endocarditis. Circulation. 1990;82(5 suppl):IV105-IV109. [PubMed] [Google Scholar]
- 8. Westh H, Reines E, Skibsted L. Splenic abscess: a review of 20 cases. Scand J Infect Dis. 1990;22:569-573. [DOI] [PubMed] [Google Scholar]
- 9. Cartanese C, Carbotta G, Candia Di, et al. A rare case of colon cancer with splenic abscess [in Italian]. Ann Ital Chir. 2013;84:201-204. [PubMed] [Google Scholar]
- 10. Kawamoto K, Teramoto T, Watanabe M, et al. Splenic abscess associated with colon cancer: a case report. Jpn J Clin Oncol. 1993;23:384-388. [PubMed] [Google Scholar]
- 11. Michowits M, Avnieli D, Lazarovici I, Solowiejczyk M. Perforation complicating carcinoma of the colon. J Surg Oncol. 1982;19:18-21. [DOI] [PubMed] [Google Scholar]
- 12. Leibovitz I, Polychuck I, Reitblat TD, Gheorghiu D, Zamir D. Splenic abscess as an unusual manifestation of colonic carcinoma [in Hebrew]. Harefuah. 2002;141:231-238, 315, 316. [PubMed] [Google Scholar]
- 13. Nakao A, Iwagaki H, Isozaki H, et al. Portal venous gas associated with splenic abscess secondary to colon cancer. Anticancer Res. 1999;19:5641-5644. [PubMed] [Google Scholar]
- 14. Belinkie SA, Narayanan NC, Russell JC, et al. Splenic abscess associated with Streptococcus bovis septicemia and neoplastic lesions of the colon. Dis Colon Rectum. 1983;26:823-824. [DOI] [PubMed] [Google Scholar]
- 15. Paramelle PJ, Ferretti G, Desroches E, Coulomb M. Quid? Cancer of the left colonic angle with colonic-splenic fistula, thrombosis of the splenic vein and left portal branch [in French]. J Radiol. 2001;82:511-513. [PubMed] [Google Scholar]
- 16. Pisanu A, Ravarino A, Nieddu R, Uccheddu A. Synchronous isolated splenic metastasis from colon carcinoma and concomitant splenic abscess: a case report and review of the literature. World J Gastroenterol. 2007;13:5516-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gervaise A, De Saint Roman C, Sockeel P, et al. Splenic abscess secondary to a colosplenic fistula as the presenting manifestation of colon cancer [in French]. J Radiol. 2010;91(12 pt 1):1259-1262. [DOI] [PubMed] [Google Scholar]
- 18. Awotar GK, Luo F, Zhao Z, et al. Splenic abscess owing to cancer at the splenic flexure a case report and comprehensive review. Medicine (Baltimore). 2016;95:e4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yelisetti R, Khurshid M, Awad A, Kaji A. Splenic abscess: an unusual complication of colon cancer. Gastroenterology Res. 2017;10:247-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prout J, Glymph R. Anaerobic septicemia secondary to Fusobacterium mortiferum. J Natl Med Assoc. 1986;78: 334,337. [PMC free article] [PubMed] [Google Scholar]
- 21. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10:71-81. doi: 10.4251/wjgo.v10.i3.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou Z, Chen J, Yao H, Hu H. Fusobacterium and colorectal cancer. Front Oncol. 2018;8:371. doi: 10.3389/fonc.2018.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]