Abstract
In patients with stable coronary artery disease (CAD) blood hypercoagulability figures among factors leading to thrombosis. Tissue factor (TF) exposure at ruptured plaque initiates blood coagulation and hypercoagulability is responsible for thrombus formation. Early identification of patients eligible for angiography is a challenging issue for effective prevention of ACS. This pilot study aimed to identify biomarkers of hypercoagulability that can be prospectively used in risk assessment tools for the evaluation of CAD severity. Biomarkers of hypercoagulability could be a used for the evaluation of CAD severity. Platelet-poor plasma from 66 patients who were referred to coronary angiography was assessed for thrombin generation, phospholipid-dependent clotting time (Procoag-PPL ® ) and D-Dimers, and evaluated against atherosclerotic burden. Patients with CAD, as compared to controls, showed attenuated thrombin generation lag time: 4.7 (3.8-5.4) min versus 2.5 (2.1-2.9) min; p < 0.0001, shorter Procoag-PPL® clotting time 55.0(32-66) s versus 62.8 (42-85) s; p = 0.001), and higher D-Dimer levels 0.509 (0.27-2.58) μg/ml versus 0.309 (0.23-0.39) μg/ml; p = 0.038. Multivariate logistic regression model showed excellent discriminatory value in predicting CAD severity. The ROADMAP-CAD study showed that the Procoag-PPL® clotting time and thrombin Peak are informative for the the burden of the coronary atherosclerotic disease. The clinical relevance of this observation in the development of a new clinic-biological risk assessment model for early diagnosis of severe CAD has to be examined in a prospective study.
Keywords: coronary artery disease, thrombin generation, risk assessment model, procoagulant phospholipids
Introduction
Cardiovascular disease (CVD) is the single most common cause of death in the developed world.1 In 2025, there are expected 7.8 million premature cardiovascular deaths without modification of risk factors and coronary artery disease (CAD) is responsible for about half of these cardiovascular deaths.2 Originally, CAD thought to be dominantly a lipid storage disease. However, our current understanding of the atherosclerosis pathogenesis also implicates endothelial cell activation, inflammation together with platelet and blood coagulation activation. Following endothelial injury, smooth muscle cells and monocytes/macrophages migrate into arterial intima in the pre-atherosclerotic stage and become foam cells by accumulating cholesterol.3 Altered shear stress, high oxidative stress, smoking, and insulin resistance are additional factors that may trigger endothelial injury.4 Coronary microvascular disease may represent an epiphenomenon or contribute to the pathogenesis of cardiovascular disease.5 The development of atherosclerotic disease may be an age-related asymptomatic process or lead to stable ischemic heart disease or an acute coronary syndrome (ACS). In patients with stable CAD the annual mortality rate is approximately 2% and the annual rate of myocardial infarction (MI) and stroke is 4.5%, having thrombosis as major pathogenetic mechanism.6–9 Following rupture or erosion of a vulnerable atherosclerotic plaque, thrombosis results from the association of endothelial cell injury and blood hypercoagulability together with flow alteration due to localization and geometric characteristics of the plaque. Thrombin is a key actor in progression of atherothrombosis and plaque vulnerability by inducing platelet activation and endothelial cells remodeling.10,11 Plaque rupture, resulting in the exposure of tissue factor (TF) on the vessel wall activates blood coagulation but thrombosis is driven by blood borne hypercoagulability.12,13 According to the “perfect storm” scenario, erosion or rupture of the coronary atherosclerotic plaque is typically required for an event to happen, but only when coinciding with a blood borne hypercoagulability at the site of plaque rupture or erosion it leads to a thrombotic event.14,15 Accordingly blood hypercoagulability could reflect endothelial cell suffering, platelet activation and sustained thrombin generation. Evaluation of blood borne hypercoagulability appears as an interesting strategy for prompt identification of patients with severe CAD. Among the numerous tests of blood coagulation, thrombin generation assay and the measurement of procoagulant phospholipids and D-dimer in plasma provide information on hypercoagulability of cellular and plasma origin. Nevertheless, their relationship with the atherosclerotic burden in patients with CAD has not been investigated. Aiming to identify clinically relevant biomarkers of hypercoagulability that could be used in a risk assessment tool for prompt identification of patients with high atherosclerotic burden we designed the prospective, observational study ROADMAP-CAD (pROspective risk Assessment anD bioMArkers of hypercoagulability for the identification of Patients with severe CAD). The pilot ROADMAP-CAD study enrolled patients with stable coronary artery disease who underwent coronary angiography in the setting of an angiographically-controlled population.
Methods
Study Design and Participants
The ROADMAP-CAD study is a prospective non-interventional trial performed from January 2017 to January 2018 in patients who were eligible for coronary angiography for CAD assessment. All patients underwent coronary angiography under the standard recommended procedure.16 Exclusion criteria were: age younger than 18 years, ongoing pregnancy, major psychiatric disorders, recent (<6 months) episode of VTE, recent (<3 months) surgery, current anticoagulant or antiplatelet treatment for any indication, recent hospitalization for acute medical illness other than CAD event, active malignancy, surgery in the preceding 3 months. All patients provided written informed consent.
Coronary Angiography Procedure
Patients underwent coronary angiography according to standard clinical practice and standard projectional views were used for quantitative analysis. Angiograms were analyzed with CASS QCA Software (PIE Medical Imaging, Maastricht, the Netherlands). The patient population was ranked into categories according to the severity of CAD assessed by quantitative analysis as follows: 0: no significant coronary atherosclerosis (luminal diameter stenosis <50%), 1: significant CAD (luminal diameter stenosis ≥50%) in one major epicardial vessel, 2: significant CAD in 2 major epicardial vessels, 3: significant CAD in 3 major epicardial vessels.
Molecular and Functional Blood Analysis
Before initiation of coronary angiography procedure, blood samples were obtained by atraumatic antecubital venipuncture and collected in Vacutainer® tubes (5 ml tubes, containing 0.109 mol/L trisodium citrate; 1 volume trisodium citrate to 9 volumes blood). Platelet-poor plasma (PPP) was prepared by double centrifugation of citrated whole blood at 2000 g for 20 minutes at room temperature and plasma aliquots were stored at −80°C until assayed. Plasma samples were centralized to the core laboratory at the INSERM UMRS 938 (Centre de Recherche Saint Antoine). Procoagulant phospholipid-dependent clotting time (Procoag-PPL ® ) was measured with STA®Procoag-PPL, according to the manufacturer’s instructions.17,18 The levels of D-Dimers were measured with commercially available assays according to the manufacturer’s instructions, on a STA-R® analyzer. The assays and the analyzer were purchased from Diagnostica Stago, Asnières France. Thrombin generation was assessed in samples of PPP with the PPP-Reagent® (5 pM TF and 4 μM procoagulant phospholipids) on Calibrated Automated Thrombogram® (Stago, France) according to the manufacturer instructions. The following parameters of thrombogram were analyzed: (a) lag-time that indicates the initiation phase of thrombin generation, (b) time to reach maximum concentration of thrombin (ttPeak), (c) maximum concentration of thrombin (Peak), d) mean rate index (MRI) of the propagation phase of thrombin generation calculated by the formula: Peak/(ttPeak—lag-time) and expressed in nM/min and e) endogenous thrombin potential (ETP) that shows the integral enzymatic activity of thrombin as described elsewhere.19-21 The inter- and intra-assay coefficients of variation of the assays were from 3% to 7%. Biomarkers measured in the cohort of patients were compared against values previously measured in a group of healthy individuals with similar age and sex distribution, not taking any medication for at least 1 month before blood sampling. The normal values of the studied biomarkers were defined in the control group and were compared to the corresponding normal reference range used by our laboratory. These normal ranges have been established according to the requirements for the good quality of laboratory practice by performing the tests in healthy individuals representative of the general population regarding age, sex, ethnicity and BMI.
End Points
The primary study end-point was the presence of significant CAD (luminal diameter stenosis ≥50%). The ranking levels 2 (significant CAD in one major epicardial vessel), 3 (significant CAD in 2 major epicardial vessels) and 4 (significant CAD in 3 major epicardial vessel) were grouped in one category. Patients without significant CAD (rank 1) constituted the control group.
Statistical Analysis
Statistics
Continuous variables are described as mean ± standard deviation or as means and ranges and categorical variables as frequency and percentage. The number of patients included in the study was calculated so that the model for the prediction of significant CAD had to be constructed according to the rule of thumb, the so-called events per variable (EPV) 10-1.22,23 In view of the deviation from normality (as evidenced by the Shapiro-Wilk test), the comparison of biomarker levels between patients and healthy individuals was performed using the Mann-Whitney-Wilcoxon test for independent samples. Concerning the inter-correlations between biomarkers in patients, Spearman’s rank correlation coefficients were estimated. Univariate logistic regression analysis examined the associations between biomarkers and treatment resistance. At the univariate analysis the level of statistical significance was set at 0.05. Regarding the associations between the clinical outcome and the biomarkers, the latter were converted to binary variables through Receiver Operating Characteristic (ROC) curve analysis; the selection of cut-off levels was based on the maximization of Youden’s index. Subsequently, multivariate logistic regression analysis was performed with the clinical outcome as the dependent variable; biomarker variables proven significant at the univariate logistic regression analysis were examined as possible independent variables. Using a stepwise procedure, at the final multivariate logistic model all variables with p-value less than 0.10 were retained; the area under the ROC curve (AUC) was estimated to describe the fit of the multivariate model. Calibration of the model was controlled with the Hosmer-Lemeshow test. Data were analyzed using the STATA/SE version13 statistical software (Stata Corp., College Station, TX, USA).
Results
Study Population
A total of 66 patients with indication for coronary angiography were enrolled in the study. No patients were excluded from analysis due to missing data. The CAD group comprised 61 patients with significant CAD, stratified according to the severity ranking as follows: 5 patients (8%) had no angiographically proven significant CAD (rank 0), these patients were not included in the statistical analysis. Among the others, 17 patients (26%) had 1-vessel disease (rank 2), 18 patients (27%) had 2-vessel disease (rank 3) and 26 patients (39%) had 3-vessel disease (rank 4). The demographics and clinical characteristics of the patients at the inclusion are summarized in Table 1.
Table 1.
Baseline Demographic, Clinical and Biological Characteristics of Patients.
| Significant CAD (n = 61) | |
|---|---|
| Age (years) | 64.5 ± 10.4 |
| Male n (%) | 52/85% |
| BMI (kg/m2) | 29.2 ± 5.3 |
| Diabetes n (%) | 17 (27.9%) |
| Hypercholesterolemia n (%) | 26 (42.6%) |
| Obesity (BMI > 30) n (%) | 18/56 (32.1%) |
| Arterial hypertension n (%) | 26 (42.6%) |
BMI: body mass index; CAD: coronary artery disease.
Biomarkers of Hypercoagulability and the Presence of Coronary Atherosclerosis
At inclusion, patients with significant CAD (rank level 1, 2 and 3) showed significantly shorter Procoag-PPL® clotting time 55.0 (32-66) s versus 62.8 (42-85) s, p = 0.001) and significantly attenuated thrombin generation as compared to the control group lag time: 4.7 (3.8-5.4) min versus 2.5 (2.1-2.9) min, p < 0.0001, ETP: 1345.5 (1242-1501) nMxmin versus 1520 (1299-1690) nMxmin, p = 0.018, Peak thrombin 227.4 (187.9-277.9) nM versus 284.5 (251.5-322.8) nM, p < 0.0001, ttPeak 7.9 (6.7-9.4) min versus 5.0 (4.6-6.0) min, p < 0.0001, MRI: 71.3 (49.0-100.2) nM/min versus 109 (84.5-135.5) nM/min, p < 0.0001. Patients with coronary atherosclerosis had higher D-Dimer levels compared to the controls 0.509 (0.27-2.58) μg/ml versus 0.309 (0.23-0.39) μg/ml, respectively; p = 0.038. Data are summarized in Table 2.
Table 2.
Biomarkers of Hypercoagulability in Patients Enrolled in the ROADMAP-CAD Study.
| Normal reference range | Control (n = 30) | Cases (n = 61) | |
|---|---|---|---|
| Lag-time (min) | 2.1-3.8 | 2.5 (2.1-2.9) | 4.7 (3.8-5.4) * |
| tt-Peak (min) | 4.0-6.6 | 5.0 (4.6-6.0) | 7.9 (6.7-9.4) * |
| Peak (nM) | 222-330 | 284.5 (251.5-322.8) | 227.4 (187.9-277.9) * |
| ETP (nMxmin) | 1496.8 ± 191.4 | 1520 (1299-1690) | 1345.5 (1242-1501) |
| MRI (nM/min) | 60-120 | 109 (84.5-135.5) | 71.3 (49.0-100.2) * |
| Procoag-PPL® (s) | 42-85 | 62.8 (42-85) | 55.0 (32-66) ** |
| D-Dimers (μg/ml) | <0.50 | 0.309 (0.23-0.39) | 0.509 (0.27-2.58) ** |
*p < 0.05 cases versus controls. Data are show as means and ranges of the observed values.
ETP: endogenous thrombin potential; MRI: mean rate index; Procoag-PPL: procoagulant phospholipid dependent clotting time.
Hypercoagulable State and CAD Risk
Univariate analysis showed that the Procoag-PPL® clotting time and the thrombogram parameters lag-time, ttPeak, Peak and MRI were significantly associated with the presence of CAD (Table 3). In multivariate analysis, lag time (OR: 5.967, 95%CI: 2.497-14.259, p < 0.001), PPL (OR: 0.843, 95%CI: 0.758-0.939, p = 0.002), and Peak (OR: 0.983, 95% CI: 0.967-0.999, p = 0.042) were independently associated with the presence of CAD. This multivariate logistic regression model corresponded to the following equation:
Table 3.
Univariate Analysis and Odds Ratio of the Biomarkers of Hypercoagulability for the Outcome of the Presence of Angiographically Documented CAD.
| Variable | Odds ratio | 95% CI | p |
|---|---|---|---|
| Procoag-PPL® | 0.91 | 0.87-0.96 | <0.0001 |
| ttPeak | 3.68 | 2.12-6.40 | <0.0001 |
| lagtime | 7.75 | 3.44-17.47 | <0.0001 |
| Peak | 0.98 | 0.98-0.99 | <0.0001 |
| MRI | 0.97 | 0.96-0.99 | <0.0001 |
MRI: mean rate index; Procoag-PPL: procoagulant phospholipid dependent clotting time.
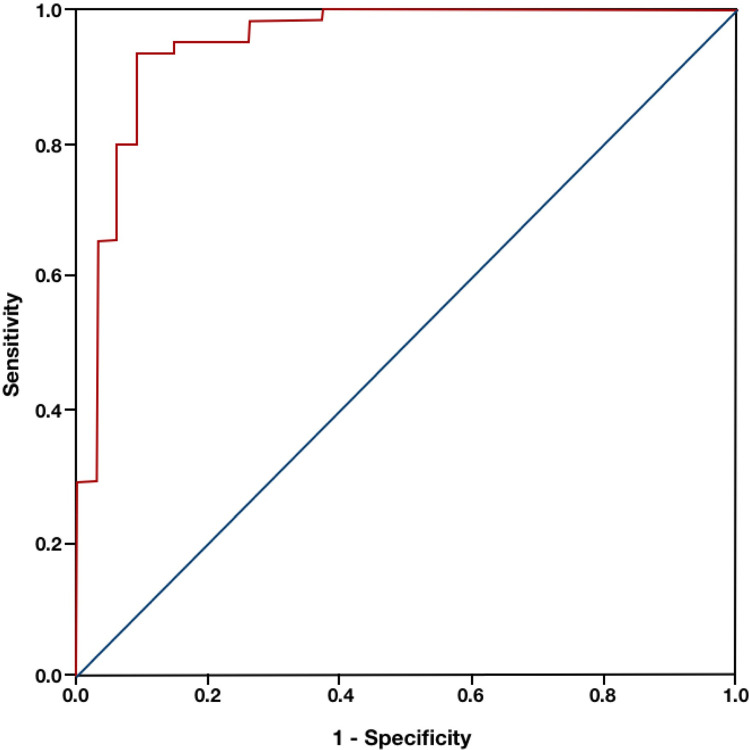
According to ROC curve analysis, a score based on the above model showed excellent discriminatory value in predicting significant CAD (AUC: 0.953, 95%CI: 0.890-0.986, p < 0.001) (Figure 1). An optimal cut-off of > 0.588 for the model-based score achieved 93.4% sensitivity and 91.4% specificity for the prediction of significant CAD. According to the Hosmer-Lemeshow test, a value of p = 0.217 showed that the model was well calibrated.
Figure 1.
ROC analysis of the experimental model for prediction of CAD severity (AUC = 0.953). CAD: coronary artery disease; AUC: area under the curve.
Biomarkers of Hypercoagulability and CAD Severity
When biomarkers of hypercoagulability were considered according to the severity of CAD, ttPeak, lag-time, were positively correlated with CAD severity (rs = 0.636, p < 0.0001; rs = 0.699, p < 0.0001), whereas there was a negative correlation observed between Procoag-PPL clotting time, ETP, Peak of thrombin, MRI and CAD severity (rs = −0.270, p = 0.009; rs = −0.228, p = 0.029; rs = −0.390, p < 0.0001; rs = −0.410, p < 0.0001). Univariate analysis showed that Procoag-PPL®, the lag-time, ttPeak, MRI, Peak) were significantly associated with the severity of CAD (Table 4).
Table 4.
Univariate Analysis and Odds Ratio of the Biomarkers of Hypercoagulability for the Outcome According to CAD Severity.
| Variable | Odds ratio | 95% CI | p |
|---|---|---|---|
| Procoag-PPL® | 0.95 | 0.92-0.98 | 0.004 |
| Lagtime | 2.83 | 1.98-4.05 | <0.0001 |
| ttPeak | 1.91 | 1.51-2.41 | <0.0001 |
| MRI | 0.98 | 0.97-0.99 | <0.0001 |
| Peak | 0.99 | 0.98-0.996 | 0.001 |
MRI: mean rate index; Procoag-PPL: procoagulant phospholipid dependent clotting time; CI: confidence interval.
In multivariate analysis, lag time (OR: 2.55, 95% CI: 1.17-5.55, p = 0.018) and PPL (OR: 0.91, 95% CI: 0.95-0.98, p = 0.004) were independently associated with the severity of CAD.
Using ROC curve analysis, optimal cut-off levels for the prediction of the presence of significant obstructive CAD were determined for several biomarkers of hypercoagulability. The optimal cut-off for Procoag-PPL levels was ≤61 s with a sensitivity of 75.4% and a specificity of 62.9% (area under the ROC Curve: 0.732, P < 0.0001). The optimal cut-off for ttPeak was > 6.3, with a sensitivity of 86.9% and a specificity of 85.7% (area under the ROC curve: 0.900, P < 0.0001). The optimal cut-off for lag-time was >3.19 min, with a sensitivity of 100% and a specificity of 85.7% (area under the ROC curve: 0.936, P < 0.0001). The optimal cut-off for ETP was ≤1623.8 nMxmin, with a sensitivity of 86.9% and a specificity of 42.9% (area under the ROC curve: 0.629, P = 0.033). The optimal cut-off for Peak was ≤238.45 nM, with a sensitivity of 55.7% and a specificity of 91.4% (area under the ROC curve: 0.745, P < 0.0001). The optimal cut-off for MRI was ≤66.1 nM/min, with a sensitivity of 49.2% and a specificity of 97.1% (area under the ROC curve: 0.760, P < 0.0001).
Discussion
The prospective observational cohort study ROADMAP-CAD in patients with CAD explored the complex coagulation profile related with atherothrombosis. The ROADMAP-CAD study identified clinically relevant biomarkers of hypercoagulability, which could identify patients with significant CAD. To the best of our knowledge the ROADMAP-CAD study provides for the first-time biological evidence linking blood borne hypercoagulability to the severity of the atherosclerotic disease evidenced by coronary angiography. The study was prospective, and the clinical features of the derivation cohort responded to the principal generalizability criteria for risk assessment tools.22,23 Multivariate analysis led to derivation of a new score which combines Procoag-PPL® clotting time and thrombin generation parameters and accurately stratifies patients into high and intermediate/low risk for significant CAD. The sensitivity and the specificity of the new score is 71.4% and 61.8%, respectively and the AUC of the ROC analysis is 0.953. These data allow to suggest that the assessment of the Procoag-PPL® clotting time and thrombin generation using the Throbmogram-Thrombinoscope® assay could be a useful tool to identify patients with high probability of severe CAD who could be further explored with imaging techniques. This concept has to be validated in a large prospective cohort trial. The present study provides some interesting information for the origin of hypercoagulability in patients with CAD. It is well established that the Procoag-PPL® clotting time is correlated with the concentration of procoagulant microparticles derived from platelets or other cells.24 Most of the patients had shorter Procoag-PPL® clotting time than the normal values of this test. Patients also showed an unexpected attenuation of thrombin generation in plasma. Indeed, lag-time and ttPeak were significantly prolonged, whereas Peak, MRI and ETP were significantly lower as compared to the group of healthy individuals. This finding may seem a paradox but is in agreement with the data published previously in patients with arterial disease as well as in patients with venous thromboembolism.25-27 Moreover our findings are further supported by the results of the population based Gutenberg Health Study which included 5000 subjects and showed that in patients with cardiovascular disease or cardiovascular risk factors the prolongation of the lag-time of thrombin generation is associated with mortality.28 Based on the analysis of the previously published studies a methodological approach is required for the interpretation of this finding. Thrombogram-Thrombinoscope® assay is performed with exogenously added PPP-reagent® which contains optimal concentrations of TF and procoagulant phospholipids (5pM and 4 μM respectively). Thus, the sensitivity of the test to variations of TF or procoagulant phospholipids concentrations in plasma is limited. In contrast, in these experimental conditions the test is sensitive to the variations of the natural coagulation inhibitors mainly the tissue factor pathway inhibitor (TFPI) and thrombomodulin (data from in vitro experiments not shown). The attenuation of thrombin generation is related to increased plasma concentration of TFPI and TM or to TFPI polymorphisms.29,30 Herein we should note that increase of TFPI or TM in patients’ plasma reflects endothelial cell activation.31-33 Following this rationale we assume that in the studied cohort of CAD patients the attenuation of thrombin generation should be interpreted as a reflection of endothelial cell activation. The increased levels of D-Dimers found in CAD patients’ plasma document an environment of sustained fibrin formation and lysis being in accordance with previous studies.34 The prospective design of the ROADMAP-CAD study led to the establishment of cut-off values for each one of the biomarkers of hypercoagulability with a predictive value for CAD severity. Procoag-PPL® clotting time shorter than 61 s had a sensitivity of 75.4% and a specificity of 62.9% for the prediction of significant obstructive CAD. Prolonged lag-time more than 3.19 min and decreased MRI (≤66.1 nM/min), Peak (≤238.45 nM), and ETP (≤1623.8 nMxmin) could accurately identify patients at high risk for significant obstructive CAD. Furthermore, the new ROADMAP-CAD score, composed by the lag-time, Peak and Procoag-PPL® clotting time showed excellent discriminatory value in predicting significant CAD (AUC: 0.953). An optimal cut-off of >0.588 for the model-based score achieved 93.4% sensitivity and 91.4% specificity for the prediction of significant CAD. The feasibility of the new ROADMAP-CAD score for the prediction of CAD severity is a challenge for the new strategy of personalized medicine in patients with CAD. Both, Procoag-PPL® and Thrombinoscope® are commercially available assays, easy to perform and do not require a specialized laboratory infrastructure. The Procoag-PPL® clotting time is a commercially available, user-friendly, fully automated, quick and reproducible technique which can be installed in any blood coagulation analyzer. The measurement of thrombogram performed in PPP with the TF 5pM PPP-Reagent® using the Calibrated Automated Thrombogram-Thrombinoscope® assay is an automated, standardized technique, available in the market worldwide. Important steps toward the standardization of the external quality control procedure have been accomplished.35,36 The new generation of the calibrated automated thrombin generation analyzer recently presented by the manufacturer (Genesia®), will render this method accessible to hematological laboratories which are not highly specialized in blood coagulation exploration. A financial analysis remains to be performed so that the benefits of the new score will not be restricted by the cost of the laboratory assessment.
The present study has some limitations. The monocentric design did not allow the evaluation of the potential influence of other therapeutic practices or procedures on the predictive capacity of the studied biomarkers. Nevertheless, we should note that biomarkers were assessed at inclusion, before any treatment administration or the application of any therapeutic intervention. Therefore, the design of the ROADMAP-CAD study did not aim to capture the impact of the antiplatelet treatment on the biomarkers of hypercoagulability. Statin treatment, although according to the statistical analysis was not found to be a confounder parameter, could have some potential impact on the attenuation of thrombin generation.37 This issue has to be explored in the ongoing prospective study organized by our group.
The size of the derivation cohort and the number of patients with significant coronary atherosclerosis provides sufficient statistical power for the derivation of the new score. However, the sample size did not allow any internal validation of the model and this is the aim of the ongoing ROADMAP-CAD project. The findings of the present study are restricted to the assessment of procoagulant phospholipids and thrombin generation in plasma using only the assays and the reagents employed. The performance of other methods which measure thrombin generation (i.e. in-house assays, other combinations of reagents or other techniques available in the market) should be assessed in suitably designed prospective studies. In addition, the longitudinal changes of thrombin generation and PPL-ct in patients with stable CAD need to be investigated in future studies.
In conclusion, the prospective ROADMAP-CAD study demonstrates the presence of pronounced blood borne hypercoagulability in patients with CAD eligible for angiography. The Procoag-PPL® clotting time and thrombin generation assay reflect cellular derived hypercoagulability and can be proposed for the screening of patients with severe CAD. This concept has to be validated in a large prospective study.
Acknowledgments
The authors like to acknowledge Mr Matthieu Grusse for his skillful technical assistance.
Authors’ Note: G.T.G. as principal investigator has made substantial contributions to conception and design of the study, analysis and interpretation of data, has been involved in drafting the manuscript, has given final approval of the version to be published, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. T.Z has made substantial contribution in patient’s recruitment and statistical analysis. I.P: has made substantial contribution in patients’ recruitment and angiography realization and evaluation. E.L. has made substantial contribution in data analysis and critical revision of the manuscript. A.C: contributed in the biological data management and validation of biomarkers. P.V.D. has made substantial contribution to the design of the study, in the organization of the assessment of the biomarkers, the interpretation of the data, the writing and editing of the paper. DK: as a co-principal investigator has made substantial contribution to the design and the implementation of the study protocol, the enrollment of patients, the organization of the biobank and the clinical databank of the study, in the organization of the assessment of the biomarkers, the interpretation of the data, the writing and editing of the paper.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Diagnostica Stago which offered the assays and reagents.
ORCID iD: Grigoris T. Gerotziafas  https://orcid.org/0000-0003-2316-6348
https://orcid.org/0000-0003-2316-6348
Jawed Fareed  https://orcid.org/0000-0003-3465-2499
https://orcid.org/0000-0003-3465-2499
References
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2013;380(9859):2095–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roth GA, Nguyen G, Forouzanfar MH, Mokdad AH, Naghavi M, Murray CJ. Estimates of global and regional premature cardiovascular mortality in 2025. Circulation. 2015;132(13):1270–1282. [DOI] [PubMed] [Google Scholar]
- 3. Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129(15):1551–1559. [DOI] [PubMed] [Google Scholar]
- 4. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368(21):2004–2013. [DOI] [PubMed] [Google Scholar]
- 5. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35(17):1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. 2014;35(42):2950–2959. [DOI] [PubMed] [Google Scholar]
- 8. Crea F, Liuzzo G. Pathogenesis of acute coronary syndromes. J Am Coll Cardiol. 2013;61(1):1–11. [DOI] [PubMed] [Google Scholar]
- 9. Libby P. Mechanisms of acute coronary syndromes. N Engl J Med. 2013;369(9):883–884. [DOI] [PubMed] [Google Scholar]
- 10. Martorell L, Martinez-Gonzalez J, Rodriguez C, Gentile M, Calvayrac O, Badimon L. Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb Haemost. 2008;99(2):305–315. [DOI] [PubMed] [Google Scholar]
- 11. DeWood MA, Spores J, Notske R. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303(16):897–902. [DOI] [PubMed] [Google Scholar]
- 12. Abbate R, Cioni G, Ricci I, Miranda M, Gori AM. Thrombosis and acute coronary syndrome. Thromb Res. 2012;129(3):235–240. [DOI] [PubMed] [Google Scholar]
- 13. Aberg M, Eriksson O, Siegbahn A. Tissue factor noncoagulant signaling: mechanisms and implications for cell migration and apoptosis. Semin Thromb Haemost. 2015;41(7):691–699. [DOI] [PubMed] [Google Scholar]
- 14. Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardio. 2015;65(8):846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012;125(9):1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katritsis DG, Siontis GC, Kastrati A, et al. Optimal timing of coronary angiography and potential intervention in non-ST-elevation acute coronary syndromes. Eur Heart J. 2011;32(1):32–40. [DOI] [PubMed] [Google Scholar]
- 17. Van Dreden P, Rousseau A, Fontaine S, Woodhams BJ, Exner T. Clinical evaluation of a new functional test for detection of plasma procoagulant phospholipids. Blood Coag Fibrinolysis. 2009;20(7):494–502. [DOI] [PubMed] [Google Scholar]
- 18. Van Dreden P, Rousseau A, Savoure A, Lenormand B, Fontaine S, Vasse M. Plasma thrombomodulin activity, tissue factor activity and high levels of circulating procoagulant phospholipid as prognostic factors for acute myocardial infarction. Blood Coagul Fibrinolysis. 2009;20(8):635–641. [DOI] [PubMed] [Google Scholar]
- 19. Dargaud Y, Wolberg AS, Luddington R, et al. Evaluation of a standardized protocol for thrombin generation measurement using the calibrated automated thrombogram: an international multicentre study. Thromb Res. 2012;130(6):929–934. [DOI] [PubMed] [Google Scholar]
- 20. Gerotziafas GT, Depasse F, Busson J, Leflem L, Elalamy I, Samama MM. Towards a standardization of thrombin generation assessment: the influence of tissue factor, platelets and phospholipids concentration on the normal values of Thrombogram-Thrombinoscope assay. Thromb J. 2005;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spronk HM, Dielis AW, De Smedt E, et al. Assessment of thrombin generation II: validation of the calibrated automated thrombogram in platelet-poor plasma in a clinical laboratory. Thromb Haemost. 2008;100(2):362–364. [PubMed] [Google Scholar]
- 22. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. [DOI] [PubMed] [Google Scholar]
- 23. Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313(13):793–799. [DOI] [PubMed] [Google Scholar]
- 24. Patil R, Ghosh K, Shetty S. A simple clot based assay for detection of procoagulant cell-derived microparticles. Clin Chem Lab Med. 2016;54(5):799–803. [DOI] [PubMed] [Google Scholar]
- 25. Liew A, Failla G, Molinari G, et al. Thrombin generation profile in patients with steady state peripheral arterial disease. Clin Appl Thromb Hemost. 2018;24(1):193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dimberg A, Alström U, Ståhle E, Christersson C. Higher preoperative plasma thrombin potential in patients undergoing surgery for aortic stenosis compared to surgery for stable coronary artery disease. Clin Appl Thromb Hemost. 2018;24(5):1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wexels F, Dahl OE, Pripp AH, Seljeflot I. Thrombin generation in patients with suspected venous thromboembolism. Clin Appl Thromb Hemost. 2017;23(5):416–421. [DOI] [PubMed] [Google Scholar]
- 28. van Paridon PCS, Panova-Noeva M, van Oerle R, et al. Thrombin generation in cardiovascular disease and mortality—results from the Gutenberg Health Study. Haematologica. 2019. [published online December 5, 2019] doi:10.3324/haematol.2019.221655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Opstad TB, Pettersen AA, Bratseth V, Arnesen H, Seljeflot I. The influence of tissue factor and tissue factor pathway inhibitor polymorphisms on thrombin generation in stable coronary artery disease. Pathophysiol Haemost Thromb. 2010;37:98–103. [DOI] [PubMed] [Google Scholar]
- 30. Syrigos K, Grapsa D, Sangare R, et al. Prospective assessment of clinical risk factors and biomarkers of hypercoagulability for the identification of patients with lung adenocarcinoma at risk for cancer-associated thrombosis: the observational ROADMAP-CAT study. Oncologist. 2018;23(11):1372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seigneur M, Constans J, Blann A, et al. Soluble adhesion molecules and endothelial cell damage in HIV infected patients. Thromb Haemost. 1997;77(4):646–649. [PubMed] [Google Scholar]
- 32. Winckers K, ten Cate H, Hackeng TM. The role of tissue factor pathway inhibitor in atherosclerosis and arterial thrombosis. Blood Rev. 2013;27(3):119–132. [DOI] [PubMed] [Google Scholar]
- 33. Ten Cate H, Hemker HC. Thrombin generation and atherothrombosis: what does the evidence indicate? J Am Heart Assoc. 2016. August 8;5(8):e003553 doi:10.1161/JAHA.116.003553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di Castelnuovo A, Agnoli C, de Curtis A, et al. Elevated levels of D-dimers increase the risk of ischaemic and haemorrhagic stroke. Findings from the EPICOR Study. Thromb Haemost. 2014;112(5):941–946. [DOI] [PubMed] [Google Scholar]
- 35. Perrin J, Depasse F, Lecompte T, French-speaking CAT Group . Large external quality assessment survey on thrombin generation with CAT: further evidence for the usefulness of normalisation with an external reference plasma. Thromb Res. 2015;136(1):125–130. [DOI] [PubMed] [Google Scholar]
- 36. Bagot CN, Leishman E. Establishing a reference range for thrombin generation using a standard plasma significantly improves assay precision. Thromb Res. 2015;136(1):139–143. [DOI] [PubMed] [Google Scholar]
- 37. Orsi FA, Biedermann JS, Kruip M, et al. Rosuvastatin use reduces thrombin generation potential in patients with venous thromboembolism: a randomized controlled trial. J Thromb Haemost. 2019;17(2):319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]