Abstract
The objective of this study was to estimate the comparative effectiveness of bisphosphonate therapy on bone mineral density (BMD) in patients with corticosteroid-treated Duchenne muscular dystrophy (DMD). A retrospective, comparative effectiveness study evaluating changes in BMD and fragility fractures in patients with DMD presenting to British Columbia Children’s Hospital from 1989 to 2017 was conducted. Marginal structural generalized estimating equation models weighted by stabilized inverse-probability of treatment weights were used to estimate the comparative effectiveness of therapy on BMD. Of those treated with bisphosphonates (N = 38), 7 (18.4%), 17 (44.7%), and 14 (36.8%) cases were treated with pamidronate, zoledronic acid, or a combination of both, respectively, while 36 cases of DMD were untreated. Mean age of bisphosphonate initiation was 9.2 (SD 2.7) years. Mean fragility fractures declined from 3.5 to 1.0 following bisphosphonate therapy. Compared to the treated group, the untreated group had an additional 0.63-SD decrease (95% confidence interval [CI]: −1.18, −0.08, P = .026) in total BMD and an additional 1.04-SD decrease (95% CI: −1.74, −0.34; P = .004) in the left hip BMD, but the change in lumbar spine BMD (0.15, 95% CI: −0.36, 0.66; P = .57) was not significant. Bisphosphonate therapy may slow the decline in BMD in boys with corticosteroid-treated DMD compared to untreated counterparts. Total number of fragility fractures decreased following bisphosphonate therapy.
Keywords: Bisphosphonate, Duchenne muscular dystrophy, children, fragility fracture
Introduction
Duchenne muscular dystrophy (DMD) is an inherited, X-linked recessive condition characterized by progressive weakness and loss of skeletal and cardiac muscle.1,2 DMD is caused by mutations in the DMD gene, which codes for the muscle protein dystrophin and functions to stabilize muscle fibers.
DMD causes progressive muscle weakness and degeneration, resulting in death in the late second to third decade of life, secondary to respiratory or cardiac failure.2 Recent advances in clinical DMD care—including use of corticosteroids, improved orthopedic intervention, respiratory, and nutritional care—have resulted in improved quality of life and life expectancy, with some individuals living into their fourth decade.3 To slow muscle disease progression in DMD, corticosteroids, including prednisone and deflazacort, have become standard of care, with the latter being the standard of care in many centers because of emerging evidence suggesting that deflazacort-treated patients experience slower rates of decline in motor outcome scores and fewer negative side effects compared to prednisone.4-8
One well-described complication of DMD is low bone mineral density (BMD), likely secondary to a constellation of low bone mass and deterioration of bone microarchitecture, exacerbated by corticosteroid treatment and reduced weight bearing associated with this condition.4,9-11 In DMD, even in those patients who are ambulatory, BMD decreases significantly over time.12-15 This low BMD results in a high incidence of fragility fractures, which greatly impairs function and quality of life in this population.16,17
Notably, a recent study by Joseph and colleagues demonstrated that boys with DMD treated with daily deflazacort had 16-fold increased risk for first fracture.18
Bisphosphonates are widely used in the treatment of childhood osteoporosis and fragility fractures.19 Several studies have demonstrated reduced frequency of fragility fractures and improved BMD after bisphosphonate therapy in pediatric patients with osteogenesis imperfecta and osteoporosis.20-24 However, only small-size studies exist that report on bisphosphonate therapy3,25 in pediatric patients with DMD. Two studies describe oral bisphosphonate therapy in patients with DMD.25,26 In these studies of 16 and 52 patients with DMD treated with oral alendronate and oral risedronate therapy, respectively, total-body BMD remained stable.25,26 In a more recent retrospective study in 7 pediatric DMD patients, intravenous (IV) bisphosphonate therapy with either zoledronic acid or pamidronate was associated with stabilization of vertebral fractures and improvement in vertebral height ratios of previously fractured vertebrae.3
In spite of the common use of these medications in this population, there is limited data on the impact of IV bisphosphonate therapy on BMD and fragility fractures in boys with DMD. Therefore, the primary objective of this study was to estimate the comparative effectiveness of bisphosphonate therapy on BMD in glucocorticoid-treated DMD patients. The study also aimed to describe fragility fractures and ambulatory status over time in these 2 groups of patients (treated and not-treated with bisphosphonates).
Method
Setting
All children in BC and the Yukon, Canada with DMD are followed through the multidisciplinary Neuromuscular Clinic at British Columbia’s Children’s Hospital (BCCH). The standard of care for DMD management is in accordance with recent guidelines and includes treatment with corticosteroids, usually in the form of deflazacort, from the age of 4 years.27
At BCCH, care for bone health in children with DMD involves many disciplines, including pediatric neurology, endocrinology, orthopedics, and radiology. All care of boys with DMD at BCCH is provided by a single pediatric neurologist and medical director of the neuromuscular diseases program. As such, steroid dosing and frequency of investigations is consistent among all subjects. Deflazacort is dosed at 0.9 mg/kg/day to a maximum dose of 36 mg and prednisone 0.7 mg/kg/day. Routine bone health screening includes measuring serum 25-hydroxyvitamin D twice yearly, lateral thoracolumbar spine radiographs every 6-12 months, and BMD assessment with dual-energy X-ray absorptiometry (DXA) annually during corticosteroid treatment. In those patients who are non-ambulatory, knee height is used and converted to estimated height.
IV bisphosphonate therapy is routinely used in the endocrinology program.28 Standardized dosing for pamidronate is 1 mg/kg per infusion for 3 days every 4 months, and for zoledronic acid is 0.05 mg/kg once every 6 months. More recently, the standard of practice has shifted from using pamidronate to using zoledronic acid exclusively. For the purposes of this retrospective review at BCCH, the decision to refer to endocrinology was at the discretion of the treating neurologist; similarly, following referral, the decision to initiate bisphosphonate therapy was at the discretion of the treating endocrinologist for each study subject.
BMD measurements were completed at BCCH using a Hologic Discovery A dual-energy X-ray absorptiometry (DXA) machine with software version 13.4.2. BMD is converted to Z-scores (adjusted for age, height and sex) at the lumbar, hip and total body measurements. Over the time period of this study, BMD scans were used at regular, consistent intervals as all children with DMD are cared for by a single pediatric neurologist. Furthermore, all BMDs in DMD patients were reviewed by a single nuclear medicine radiologist (HN).
Study design
This study was approved by the University of British Columbia Clinical Research Ethics Board (H17-03002). This study is a retrospective cohort study conducted at BCCH of boys with DMD followed by the multidisciplinary neuromuscular clinic from 1989 to 2017.
Treated cases were identified as those with DMD who received at least one bisphosphonate infusion (pamidronate or zoledronic acid) during the study period. An untreated cohort of DMD subjects was identified from the BCCH neuromuscular clinic database of patients followed for DMD. Those included in the analysis were bisphosphonate-treated patients who had at least 1 BMD by DXA measured no more than 4 weeks before and after bisphosphonate treatment, and untreated patients who had at least 2 BMDs measurements at BCCH. All DXA reported results were completed head out (not including skull). Vertebrae with vertebral fractures were excluded from the BMD analysis. All DXA scan data at our center is corrected for size using the height adjusted Z-score to determine height adjusted for age.
Eligible patients’ electronic and paper records were reviewed to collect data on subject demographics, past medical history, DMD diagnosis and management, physical examination and laboratory investigations during treatment, bisphosphonate therapy, fragility fractures history prior to and following bisphosphonate treatment, and results of BMD by DXA scans. These parameters were collected from the first visit to the most recent visit at BCCH. In those treated with bisphosphonates, data were also collected at the visit prior to the first infusion and the visit after the most recent infusion.
All fracture data were collected from the patient imaging reports and patient records and included all non-traumatic fractures reported in the patient clinic visits or emergency room visits. Each fracture identified in the clinic chart was subsequently confirmed in the X-ray report by a pediatric radiologist at our center. Fragility fractures were defined as all non-traumatic fractures (including vertebrae and long bones). The cumulative number of fragility fractures in bisphosphonate-treated subjects was collected from clinic documentation and radiology reports prior to receiving bisphosphonate therapy and again from the most recent clinic visit. Post-infusion fractures are reported as total fractures since initiation of bisphosphonate therapy. In the untreated cohort, the number of total fractures was collected prior to first DXA scan and at most recent clinic visit. In those subjects where the same fracture (for example, a specific vertebral fracture) was seen prior to bisphosphonate therapy and again afterwards, it was only counted towards fragility fractures prior to therapy start.
The subject’s height and weight were used to calculate body-mass index (BMI), which was converted to a Z-score to standardize the measurement for age and gender.29
Statistical Analysis
Descriptive statistics (mean and SD for the continuous variables, or number and percentage for the categorical variables) are presented for the full cohort, and by their treatment status. As recommended in the STROBE guidelines, hypothesis testing was not done to compare the intervention groups, and hence no P-values are reported.30
Change in BMD was defined as the difference between the first and the last BMD measurement in the untreated group, and the difference between last BMD measurement (post-bisphosphonate) and the BMD measurement immediately prior to initiation of bisphosphonate therapy in the treated group. Due to the non-randomized nature of the study, we attempted to address treatment indication bias within the framework of marginal structural models.31 More specifically, we estimated the comparative effectiveness of bisphosphonate therapy by applying stabilized inverse probability of treatment weights (IPTW) in the generalized estimating equations (GEE) models. We used robust standard errors to accommodate repeated measures of BMD. Propensity score was calculated by fitting logistic regression models using an a priori list of variables including year of DMD diagnosis, and baseline weight, height, calcium supplement, fracture, and ambulation status, as the predictors of treatment allocation. The balance of the propensity score was visually checked using histograms. The numerator for the stabilization of the IPTW was the marginal probability of treatment allocation, estimated by fitting intercept-only logistic regression, for the treated group. The complement of this probability was the numerator for the untreated group. Similarly, the propensity score was the denominator for the treated group, while the complement of the propensity score was the denominator for the untreated group.32 The stabilized IPTW-weighted GEE models were further adjusted for baseline values of respective BMD Z-scores to address the mathematical phenomenon of regression towards the mean.33 Time between the first and last BMD measurement was additionally adjusted in the sensitivity analyses. We plotted the estimated changes in BMD Z-scores in the treated and untreated groups, computed at the baseline mean BMD Z-scores in the untreated group, to graphically present the predicted trajectory of children with and without bisphosphonate therapy. SAS software version 9.4 was used for the statistical analysis.34 All the tests were two-sided with a significance level of 0.05.
Results
During the study period, 38 boys with DMD were treated with bisphosphonates, and 36 boys were not treated. Six bisphosphonate-treated subjects were excluded from DXA analysis because they did not have DXA scans completed after starting treatment. The baseline characteristics of the study population are presented in Table 1. Mean (SD) age of DMD diagnosis in the entire study population was 4.2 (2.3) years, with a slightly lower mean age for those who initiated bisphosphonate therapy. All bisphosphonate-treated subjects were treated with daily deflazacort, except for 1 patient who was treated with daily prednisone for the duration of the study period. Concomitant medications are presented in Table 1 and there were no patients treated with either growth hormone or metformin. Mean follow-up between the first and the last BMD DXA evaluation was 4.4 (2.2) and 4.7 (2.6) years in treated and untreated subjects, respectively. Mean (SD) duration of bisphosphonate treatment was 4.2 (2.7) years and range was 12 months—7.5 years. In those treated with bisphosphonates, 7 (18.4%) were treated with pamidronate, 17 (44.7%) were treated with zoledronic acid, and 14 (36.8%) were treated initially with pamidronate and then switched to zoledronic acid (combination). Mean age of initiation of bisphosphonate treatment was 12.8 (2.2) years. The most common documented adverse events related to bisphosphonate treatment were chills (68.8%), aches (68.8%), numbness/tingling (65.6%), and muscle cramps/spasms (59.4%).
Table 1.
Baseline characteristics of the study population in the study on the effectiveness of bisphosphonate therapy on bone mineral density in boys with Duchenne muscular dystrophy.a
| Treated (n = 32) | Untreated (n = 36) | |
|---|---|---|
| Age at diagnosis (years) | 3.6 (1.9) | 4.8 (2.4) |
| Age at steroid start (years) | 4.2 (1.89) | 4.9 (1.78) |
| Age at bisphosphonate start (years) | 12.7 (2.17) | NA |
| Total bisphosphonate infusions | 9.9 (7.3) | NA |
| Calcium supplementation; n (%) | 15 (46.9) | 26 (72.2) |
| Vitamin D supplementation; n (%) | 32 (100.0) | 36 (100.0) |
| Deflazacort treatment; n (%) | 32 (100) | 35 (97.2) |
| Duration of bisphosphonate therapy; (years) | 4.2 (2.7) | NA |
| Time between first and last BMD measurement; (years) | 4.4 (2.2) | 4.7 (2.6) |
| Other medications | ||
| Beta blockersb; n (%) | 8 (25.0) | 8 (22.2) |
| ACE inhibitorsc; n (%) | 21 (65.6) | 11 (30.6) |
| Antidepressantsd; n (%) | 11 (34.4) | 4 (11.1) |
| Stimulantse; n (%) | 4 (12.5) | 4 (11.1) |
| Coenzyme Q; n (%) | 0 (0) | 4 (11.1) |
| Digoxin; n (%) | 3 (9.4) | 3 (8.3) |
| Losartan; n (%) | 1 (3.1) | 0 (0) |
| Testosterone; n (%) | 2 (6.3) | 0 (0) |
| Ranitidine/omeprazole; n (%) | 1 (3.1) | 0 (0) |
| Ataluren; n (%) | 1 (3.1) | 1 (2.8) |
Abbreviations: ACE, angiotensin converting enzyme; NA, not applicable; SD, standard deviation.
Data presented as mean (SD) unless otherwise specified.
Beta blockers include carvedilol, bisoprolol, and metoprolol.
ACE inhibitors include ramipril and enalapril.
Antidepresants include sertraline, citalopram, trazodone, fluoxetine, and escitalopram.
Stimulants include atomoxetine and methylphenidate.
Average number of fragility fractures dropped from 3.5 pre-bisphosphonate to 1.0 (over mean time period 4.2 years) after bisphosphonate therapy (4.4 to 0, 3.0 to 0.9, and 3.5 to 1.7, in those treated with pamidronate, zoledronic acid, and a combination, respectively) (Table 2). This trend was similar when the follow-up time was taken into account to calculate the number of fractures per year. The average number of fractures per year dropped from 2.4 (2.2) to 0.7 (1.2) overall, and 3.6 to 0, 1.6 to 0.5, and 2.4 to 1.2 in the pamidronate, zoledronic acid, and combination group, respectively.
Table 2.
Changes in anthropometric, laboratory, fracture and radiographic measurements in boys managed with and without bisphosphonate therapy.a
| Treated (n = 32) |
Untreated (n = 36) |
|||
|---|---|---|---|---|
| First | Recent | First | Recent | |
| Physical examinationb | ||||
| Height ⩽3rd centilec; n (%) | 5 (21.7) | 22 (88.0) | 10 (41.7) | 21 (61.8) |
| Weight ⩽3rd centilec; n (%) | 4 (16.0) | 8 (32.0) | 5 (14.3) | 7 (21.2) |
| BMI | 22.3 (5.4) | 26.4 (8.2) | 18.1 (4.7) | 23.7 (5.3) |
| BMI ⩾90th centilec; n (%) | 7 (21.9) | 11 (34.4) | 5 (13.9) | 18 (50.0) |
| BMI ⩾95th centilec; n (%) | 5 (15.6) | 9 (28.1) | 5 (13.9) | 17 (47.2) |
| Tanner staging | ||||
| Performed; n (%) | 13 (40.6) | 11 (34.4) | ||
| 1; n (%) | 13 (100.0) | 5 (45.5) | ||
| 2; n (%) | 0 (0) | 3 (27.3) | ||
| 3-5; n (%) | 0 (0) | 3 (27.3) | ||
| Unknown; n (%) | 19 (59.4) | 21 (65.6) | ||
| Laboratory values | ||||
| Ionized calcium (mmol/L) | 1.2 (0.0) | 1.14 (0.04) | 1.2 (0.1) | 1.12 (0.07) |
| 25-hydroxyvitamin D (nmol/L) | 90.8 (37.8) | 102.0 (32.14) | 75.0 (58.9) | 94.4 (50.27) |
| Total calcium (mmol/L) | 2.3 (0.1) | 2.29 (0.14) | 2.4 (0.1) | 2.35 (0.08) |
| Alkaline phosphatase (U/L) | 67.1 (23.0) | 54.8 (17.11) | 82.5 (27.1) | 78.6 (26.00) |
| Creatinine (µmol/L) | 25.2 (8.6) | 23.1 (8.32) | 24.9 (11.9) | 22.2 (5.56) |
| Urine Ca/Cr ratio | 1.70 (1.7) | 1.23 (0.93) | 1.9 (1.8) | 1.67 (1.11) |
| Total fractures | ||||
| Pamidronate (n = 7) | 4.4 (2.8) | 0 (0.0) | ||
| Zoledronic acid (n = 12) | 3.0 (2.7) | 0.9 (1.5) | ||
| Combinationd (n = 13) | 3.5 (3.3) | 1.7 (2.3) | ||
| Cumulative fractures (n) | 112 | 33 | 33 | 73 |
| Fractures per subject | 3.5 (2.9) | 1.0 (1.8) | 0.9 (1.1) | 2.0 (2.1) |
| Non-ambulatory; n (%) | 17 (53.1) | 23 (71.9) | 22 (61.1) | 30 (83.3) |
| Ambulatory; n (%) | 15 (46.9) | 9 (28.1) | 14 (38.9) | 6 (16.7) |
| BMD Z-scores | ||||
| Left hip | −4.6 (1.3) | −4.3 (1.0) | −2.9 (1.4) | −4.1 (1.0) |
| L1-L4 | −2.3 (0.9) | −2.6 (1.0) | −1.3 (1.2) | −1.9 (0.9) |
First = first clinic visit to the Endocrinology Clinic; Recent = most recent clinic visit at the time of data analysis.
Abbreviations: AAP, American Academy of Pediatrics; BMD, bone mineral density; BMI, body mass index; SD, standard deviation.
Data presented as mean (SD) unless otherwise specified.
Unknown values were omitted from percent calculations.
Height, weight, and BMI for age centiles were categorized as per the Centers for Disease Control29.
Combination includes pamidronate followed by zoledronic acid.
Physical examination parameters, laboratory investigations and new fractures for treated and untreated subjects are shown in Table 2. Baseline BMI was higher (22.3 vs 18.1 kg/m2) among those treated with bisphosphonate therapy compared to those who were untreated. Baseline 25-hydroxy vitamin-D levels were higher among the treated (90.8 vs 75.0 nmol/L). All subjects were Tanner stage 1 at their first visit (mean age 12.8 years). In those subjects with Tanner staging available, all were below the average Tanner stage for age at the time of bisphosphonate initiation. Tanner staging was not performed in the untreated group.
The estimates from the stabilized IPTW-weighted GEE models additionally adjusting for the respective baseline BMD Z-scores are shown in Table 3. The results show that the mean decline in BMD measurement was significantly greater in the untreated group compared to the treated group. Compared to the treated group, the untreated group had an additional 0.63-SD decrease (95% confidence interval [CI]: −1.18, −0.08, P = .026) in total BMD and an additional 1.04-SD decrease (95% CI: −1.74, −0.34; P = .004) in the left hip BMD, but the change in lumbar spine BMD (0.15, 95% CI: −0.36, +0.66; P = .57) was not statistically significant. These estimates were unchanged when further adjusted for the time between first and last BMD measurement (Table 3).
Table 3.
Change in the mean BMD Z-score (total body, left hip, and lumbar) among boys with DMD estimated from the generalized estimating equations models weighted by the stabilized inverse probability of treatment weights.
| Characteristics | BMD total |
BMD left hip |
BMD lumbar spine |
|||
|---|---|---|---|---|---|---|
| Mean (95% CI) | P value | Mean (95% CI) | P value | Mean (95% CI) | P value | |
| BMD Z-score change in the untreated compared to the treated* | −0.63 (–1.18, –0.08) | .026 | −1.04 (–1.74, –0.34) | .004 | 0.15 (–0.36, 0.66) | .570 |
| BMD Z-score change in the untreated compared to the treated** | −0.62 (–1.18, –0.07) | .028 | −1.04 (–1.75, –0.34) | .004 | 0.15 (–0.37, 0.66) | .577 |
Abbreviations: BMD, bone mineral density; CI, confidence interval; DMD, Duchenne muscular dystrophy.
Estimates from the generalized estimating equations model using stabilized inverse probability of treatment weights, and further adjusting for the respective baseline BMD Z-scores.
Previous models but additionally adjusting for time between the first and the last BMD measurements.
Figure 1 depicts the change in BMD between the first and last measurement, computed using the baseline mean Z-scores. In this analysis, a steeper decline in BMD was found in the untreated group compared to the treated group.
Figure 1.
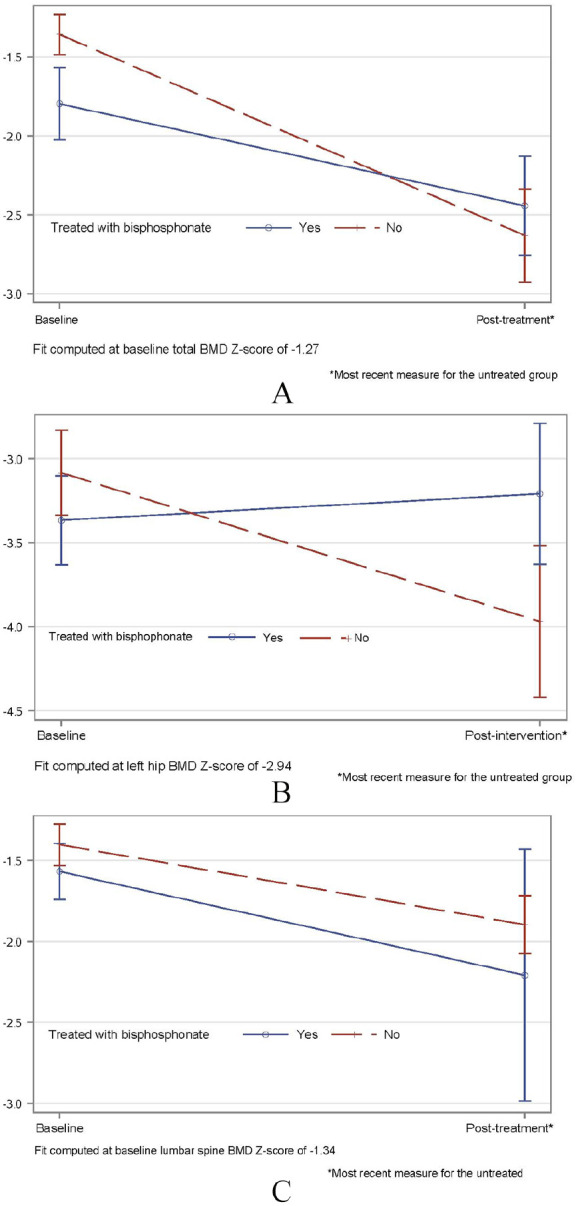
Comparative effectiveness of bisphosphonate treatment on bone mineral density in children with Duchenne muscular dystrophy estimated from the generalized estimating equations weighted by the stabilized inverse probability of treatment weights and adjusting for the respective baseline BMD Z-scores.
A: Total BMD Z-score; B: Left hip BMD Z-score; C: Lumbar spine BMD Z-score BMD, bone mineral density.
Discussion
This is the first study to describe changes in BMD in boys with corticosteroid-treated DMD treated with bisphosphonates, compared to not treated natural controls. Our data demonstrate a greater deterioration in BMD assessed by DXA based on 2 measurements in those who did not receive bisphosphonates compared to those who did. This suggests that bisphosphonates may help prevent reduction of BMD over time in corticosteroid-treated DMD.
In a similar study, Houston and colleagues compared patients with DMD who received glucocorticoids to those who did not, and their results demonstrated a significant decrease in BMD in those who received glucocorticoids.35 In a sub-analysis of this population, oral alendronate was associated with stabilization of hip BMD. Currently, a placebo-controlled study is underway evaluating IV zolendronate in children with DMD, although these results are not yet available (unpublished data; ClinicalTrials.gov Identifier: NCT00799266).
In our study, many of the subjects were prepubertal, and therefore their BMD Z-scores are likely reading lower than their healthy age peers who are in puberty. Unfortunately, we were not able to compare this data to healthy controls to evaluate this further. Pubertal status is important in the interpretation of BMD. It is likely that, similar to our center, many neurologists specializing in the care of DMD patients feel it is outside of their competence to perform these pubertal exams. These data highlight the need for systematic pubertal evaluation in boys with DMD, and the importance of collaborative care between neurology and endocrinology regardless of whether bisphosphonate treatment will be used.
Our data also show a reduction in the number of fragility fractures following treatment with both pamidronate and zoledronic acid. It is possible that fragility fractures reduction is, in part, related to subjects’ reduction in ambulation. Unfortunately, we were not able to assess the relationship between reduction in fragility fracture and change in ambulatory status. In a study in 7 boys with DMD, Sbrocchi and colleagues found an improvement in both vertebral height and pain following 2 years of treatment with either pamidronate or zoledronic acid, suggesting a reduction in vertebral compression fractures.3 As these fractures are often associated with significant pain, treatment resulting in fracture reduction is an important contributor to improving patient quality of life. In fact, we noted as part of this retrospective review that 100% of the bisphosphonate-treated subjects’ physicians commented in their medical records on “reduction in pain” following initiation of bisphosphonate treatment.
Limitations and strengths
The main limitation of this study is its retrospective nature, and thus it is restricted to the data available in the patient record. For example, Tanner staging was not available in untreated subjects because these patients were not assessed by endocrinology. In addition, the decision to start bisphosphonate treatment was at the discretion of the treating endocrinologist, and therefore varied by study subject. Furthermore, there were several bisphosphonate-treated subjects who did not have a calcium supplement recorded in the patient record despite endocrine clinic recommendations for use, which may have led to under reporting of this supplement in this study. Another limitation of this study is that it was not feasible to re-review spine radiographs with a single radiologist completed over the course of the last 20 years. Spine fracture data was collected from the radiology report, all of which were generated by a pediatric radiologist at the time of fracture diagnosis. There may have been some variation in the interpretation of these films over the years, and because of the retrospective nature of the study, the radiologist at the time may not have been blinded to the treatment status. As well, since the average number of fractures before and after BMD therapy are likely affected by variable follow-up time, we further compared the number of fractures per year, and the primary findings remained unaffected. However, owing to the retrospective nature of the data, it was challenging to precisely estimate the follow-up time, especially for the control group, because patients were not followed up with a pre-specified schedule. Furthermore, we were not able to objectively quantify changes in pain or quality of life, as no objective pain rating scale was used consistently. Nevertheless, physicians documented a subjective improvement in pain in 100% of treated subjects’ medical records. Furthermore, a strength of this study is that all boys with DMD are cared for by a single neurologist and thus glucocorticoid dosing is consistent. Another potential limitation includes the non-randomized nature of the data which are subject to treatment indication bias and residual confounding. However, we attempted to address the issue of treatment indication bias by applying a sophisticated analytic strategy, stabilized IPTW-weighted GEE models, additionally adjusting for the baseline outcome values to address the mathematical phenomenon of regression towards the mean. In our analysis, the estimates were robust and are further adjusted for time between the first and last BMD measurement.
Despite its limitations, this is the largest study to date describing BMD assessed by DXA in boys with corticosteroid-treated DMD. Furthermore, this is the first study to compare boys with DMD treated with bisphosphonates to those who were not treated using robust methodological and analytic strategies.
Conclusion
Bisphosphonate therapy may be associated with a slower decline in BMD in boys with corticosteroid-treated DMD. Fragility fractures were substantially reduced following bisphosphonate therapy in this cohort. Prospective longitudinal studies systematically evaluating BMD and fragility fractures, as well as objective measures of pain and quality of life, following bisphosphonate therapy are needed. Furthermore, studies with the newer dissociative corticosteroids such as vamorolone or other glucocorticoid-sparing treatments for DMD are needed to protect against the rapid BMD decline in this vulnerable population.
Acknowledgments
We would like to thank Ms. Brianna Cameron (University of British Columbia) for her assistance with data collection.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by a Childhood Diseases Applied Health Grant from the BC Children’s Hospital Research Institute (CP and RR). MK was funded by the BC Children’s Hospital Research Institute Summer Studentship.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Ronsley conceptualized and designed the study, designed the data collection instruments, completed data collection and initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. Islam completed statistical analyses and provided critical review and revision to the manuscript. Kang contributed to data collection, created tables and reviewed and revised the manuscript. Nadel, Reilly and Metzger contributed to interpretation of data and critically reviewed the manuscript for important intellectual content and contributed to revisions. Selby contributed to study concept and design and reviewed and revised the manuscript. Panagiotopoulos contributed to study concept and design, oversaw data collection and analyses and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Statement of Ethics: This research complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. It was approved by the University of British Columbia Research Ethics Boards.
References
- 1. Hoffman EP. The muscular dystrophies. In: Rosenberg RN, ed. The Molecular and Genetic Basis of Neurologic Disease. 2nd ed. Butterworth Heinemann; 1997: 877-912. [Google Scholar]
- 2. Dubowitz V. Muscle Disorders in Childhood. 2nd ed. WB Saunders; 1995: 34-133. [Google Scholar]
- 3. Sbrocchi AM, Rauch F, Jacob P, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne’s muscular dystrophy. Osteoporos Int. 2012; 23:2703-2711. [DOI] [PubMed] [Google Scholar]
- 4. Yilmaz O, Karaduman A, Topaloglu H. Prednisolone therapy in Duchenne muscular dystrophy prolongs ambulation and prevents scoliosis. Eur J Neurol. 2004;11:541-544. [DOI] [PubMed] [Google Scholar]
- 5. Biggar WD, Gingras M, Fehlings DL, Harris VA, Steele CA. Deflazacort treatment of Duchenne muscular dystrophy. J Pediatr. 2001;138:45-50. [DOI] [PubMed] [Google Scholar]
- 6. Tian C, Wong BL, Hornung L, et al. Bone health measures in glucocorticoid-treated ambulatory boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26:760-767. [DOI] [PubMed] [Google Scholar]
- 7. Griggs RC, Miller JP, Greenberg CR, et al. Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy. Neurology. 2016;87:2123-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shieh PB, McIntosh J, Jin F, et al. Deflazacort versus prednisone/prednisolone for maintaining motor function and delaying loss of ambulation: a post hoc analysis from the act DMD trial. Muscle Nerve. 2018;58:639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soderpalm AC, Magnusson P, Ahlander AC, et al. Low bone mineral density and decreased bone turnover in Duchenne muscular dystrophy. Neuromuscul Disord. 2007;17:919-928. [DOI] [PubMed] [Google Scholar]
- 10. Bell JM, Shields MD, Watters J, et al. Interventions to prevent and treat corticosteroid-induced osteoporosis and prevent osteoporotic fractures in Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2017;1: CD010899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Misof BM, Roschger P, McMillan HJ, et al. Histomorphometry and bone matrix mineralization before and after bisphosphonate treatment in boys with Duchenne muscular dystrophy: a paired transiliac biopsy study. J Bone Miner Res. 2016;31:1060-1069. [DOI] [PubMed] [Google Scholar]
- 12. Hsu JD. Skeletal changes in children with neuromuscular disorders. In: Dixon AD, Sarnat BG, eds. Factors and Mechanisms Influencing Bone Growth. Alan Liss; 1982: 553-557. [PubMed] [Google Scholar]
- 13. Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Paediatr Orthop. 2000;20:71-74. [PubMed] [Google Scholar]
- 14. Aparicio LF, Jurkovic M, DeLullo J. Decreased bone density in ambulatory patients with Duchenne muscular dystrophy. J Paediatr Orthop. 2002;22:179-181. [PubMed] [Google Scholar]
- 15. Hawker GA, Ridout R, Harris VA, Chase CC, Fiedling LJ, Biggar WD. Aledronate in the treatment of low bone mass in steroid-treated boys with Duchenne’s Muscular Dystrophy. Arch Phys Med Rehabil. 2005;86: 284-288. [DOI] [PubMed] [Google Scholar]
- 16. Ma J, McMillan HJ, Karaguzel G, et al. The time to and determinants of first fractures in boys with Duchenne’s muscular dystrophy. Osteoporos Int. 2017;28:597-608. [DOI] [PubMed] [Google Scholar]
- 17. Singh A, Schaeffer EK, Reilly CW. Vertebral fractures in Duchenne muscular dystrophy patients managed with Deflazacort. J Pediatr Orthop. 2018;38:320-324. [DOI] [PubMed] [Google Scholar]
- 18. Joseph S, Wang C, Bushby K, et al. Fractures and linear growth in a nationwide cohort of boys with Duchenne muscular dystrophy with and without glucocorticoid treatment. JAMA Neurol. 2019;76:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ward LM, Petryk A, Gordon C. Use of bisphosphonates in the treatment of pediatric osteoporosis. Int J Clin Rheumatol. 2009;4:657-672. [Google Scholar]
- 20. Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998; 339:947-952. [DOI] [PubMed] [Google Scholar]
- 21. Sumnik Z, Land C, Rieger-Wettengl G, Korber F, Stabrey A, Schoenau E. Effect of pamidronate treatment on vertebral deformity in children with primary osteoporosis. A pilot study using radiographic morphometry. Horm Res. 2004;61:137-142. [DOI] [PubMed] [Google Scholar]
- 22. Brumsen C, Hamdy NA, Papapoulos SE. Long-term effects of bisphosphonates on the growing skeleton. Studies of young patients with severe osteoporosis. Medicine. 1997;76:266-283. [DOI] [PubMed] [Google Scholar]
- 23. Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Bone Miner Res. 2009;24:1282-1289. [DOI] [PubMed] [Google Scholar]
- 24. Bishop N, Harrison R, Ahmed F, et al. A randomized, controlled dose-ranging study of risedronate in children with moderate and severe osteogenesis imperfecta. J Bone Miner Res. 2010;25:32-40. [DOI] [PubMed] [Google Scholar]
- 25. Hawker GA, Ridout R, Harris VA, Chase CC, Fielding LJ, Biggar WD. Alendronate in the treatment of low bone mass in steroid-treated boys with Duchennes muscular dystrophy. Arch Phys Med Rehabil. 2005;86:284-288. [DOI] [PubMed] [Google Scholar]
- 26. Srinivasan R, Rawlings D, Wood CL, et al. Prophylactic oral bisphosphonate therapy in Duchenne muscular dystrophy. Muscle Nerve. 2016;54:79-85. [DOI] [PubMed] [Google Scholar]
- 27. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17:251-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ward LM, Hadjiyannakis S, McMillan HJ, Noritz G, Weber DR. Bone health and osteoporosis management of the patient with Duchenne muscular dystrophy. Pediatrics. 2018;142:S34-S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuczmanski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;314:1-27. [PubMed] [Google Scholar]
- 30. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robins YY, Hernan JM, Brumback B. Marginal structural models and causal inference in Epidemiology. Epidemiology. 2000;11:550-560. [DOI] [PubMed] [Google Scholar]
- 32. Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215-220. [DOI] [PubMed] [Google Scholar]
- 34. StataCorp. Stata Statistical Software: Release 14. StataCorp LP; 2015. [Google Scholar]
- 35. Houston C, Mathews K, Shibli-Rahhal A. Bone density and alendronate effects in Duchenne Muscular Dystrophy patients. Muscle Nerve. 2014;49:506-511. [DOI] [PubMed] [Google Scholar]


