Abstract
Spontaneous internal hemorrhage from a hepatic hemangioma is rare. This case describes a 59-year-old woman who was hospitalized with recurrent cough and fever for 6 months. The doctor thought that she had pneumonia, but other infectious diseases could not be ruled out. Therefore, related tests were performed and strong antibiotics were used, but the symptom of fever was persistent and recurred. Enhanced computed tomography (CT) findings showed a right hepatic giant hemangioma with hemorrhage, while tuberculosis, liver abscess, and immune disease were excluded by the physician. Because the patient’s fever was associated with a large hepatic hemangioma, the patient was transferred to surgery. During treatment, the amount of bleeding increased, so she underwent a right hepatic hemangioma resection in the emergency department. Her postoperative fever symptoms subsequently resolved. Pathological examination confirmed hemorrhagic necrosis with infection in hepatic hemangioma. Follow-up showed that the patient was afebrile.
Keywords: Hepatic hemangioma, spontaneous internal hemorrhage, fever, hemorrhagic necrosis, benign mesenchymal tumor, infection
Introduction
Hepatic hemangioma is the most common benign mesenchymal tumor with an estimated prevalence of 0.4% to 20%, and it is usually found by chance on computed tomography (CT) scan or ultrasonography during examinations for upper abdominal organs.1,2 Most hepatic hemangomias are small in size and asymptomatic, and they are especially prevalent in women. Their growth may be affected by changes in female hormones.3–5 Lesions larger than 4 cm have been defined as giant hemangiomas, which can trigger complications such as abdominal pain, bloating, nausea, vomiting, jaundice, and spontaneous or traumatic rupture.6–9 There are few reports of spontaneous internal hemorrhage in hepatic hemangioma,10–13 and it is even rarer to have fever of unknown origin.10 In this context, we report a case of spontaneous internal hemorrhage with infection in a patient with a giant hepatic hemangioma, and we also reviewed the literature.
Case presentation
A 59-year-old woman was admitted to our hospital for treatment in July 2019 due to “recurrent cough and sputum accompanied by fever for 6 months and symptom aggravation for 3 months”. There was a small amount of white mucus sputum, but no blood in the sputum. The patient’s body temperature was intermittently elevated up to 40°C, and this was occasionally accompanied by night sweats that were independent of chills and diurnal changes. During the period of onset, the patient lost 5 kg in body weight. The patient had no history of hypertension, diabetes, cardiovascular and cerebrovascular diseases, hepatitis, tuberculosis, malaria, surgery, trauma, abdominal discomfort, dysuria, joint pain, or other diseases. She had gone through menopause at age 50 years. An abdominal examination was performed, but no mass was touched. A chest CT showed thickening of the bronchial wall in the left upper lobe and dilatation of the left upper lobe, and it also showed mass flaky shadows in the right liver lobe (Figure 1a). After admission, the laboratory assessment showed a hemoglobin (Hb) level of 104 g/L (normal range: 115–150 g/L), a platelet count (PLT) of 376,000/mm3 (normal range: 125,000–350,000/mm3), a white blood cell (WBC) count of 15.71 × 109/L (normal range: 4–10 × 109/L), an erythrocyte sedimentation rate (ESR) of 90 mm/hour (normal range: 0–26 mm/hour), and a C-reactive protein (CRP) level of 40.05 mg/L (normal range: 0–5 mg/L). Biochemical assays revealed the following: an alkaline phosphatase (ALP) level of 187.4 U/L (normal range: 50–135 U/L), a gamma-glutamyltransferase (γ-GT) level of 209.3 U/L (normal range: 7–45 U/L), and a serum albumin (ALB) level of 39.1 g/L (normal range: 40–55 g/L). Levels of alanine aminotransaminase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL) were within the normal range.
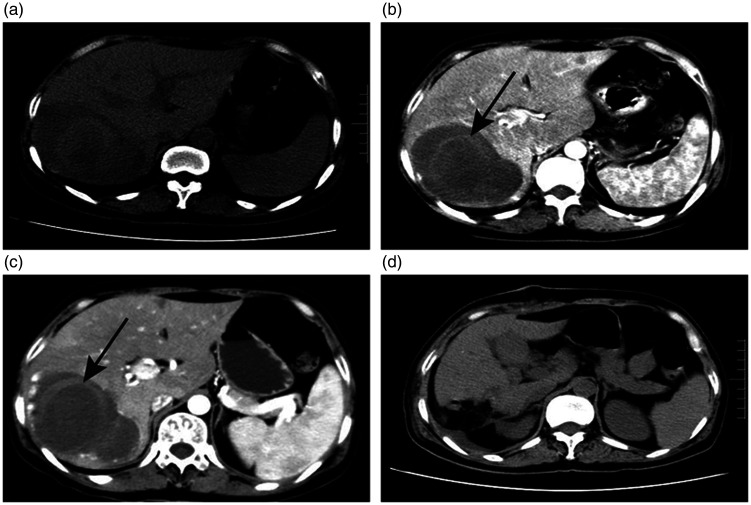
Figure 1.
a. On admission, chest CT scan revealed a mass flaky shadow of the right liver lobe. b. The first abdominal augmentation CT examination showed that the right liver lobe had a circular mixed density mass of about 10 × 9 cm, which had a clear boundary and contained a ring-shaped, strip-like slightly high-density shadow (shown by a white arrow). c. After 2 weeks, the high-density hemorrhage foci in the lesion increased compared with 2 weeks before. d. A postoperative CT scan showed a small amount of effusion.
CT, computed tomography.
The abdominal enhanced CT scan revealed a 9- × 6.6- × 10-cm round mass with significant edge enhancement, mixed density, and a relatively clear boundary, which originated from the right liver lobe. The possibility of hemangioma accompanied by bleeding was considered (Figure 1b). Physicians did not rule out causes of fever such as pulmonary infection, tuberculosis, immune diseases, liver abscesses, or liver cancer with infection. Therefore, the sputum smear, repeated examination of acid-fast bacilli, routine fecal tests, routine urine tests, lymphocyte immune typing, sputum culture, myocardial injury markers, examination before blood transfusion, blood culture, alpha-fetoprotein (AFP), and other tests were performed, but these tests showed normal results. During treatment, meropenem and other broad-spectrum antibiotics were used. Fortunately, the patient’s temperature dropped slightly (fluctuated between 37.6 and 38.6°C), but her fever did not disappear. After ruling out the above diagnosis, the doctor believed that the fever was related to internal bleeding of the hepatic hemangioma. Therefore, the patient was transferred to surgery. Re-examination of the patient’s abdominal CT (the interval was 2 weeks) and routine blood examination revealed an increase in internal bleeding in the right liver mass (Figure 1c), and the Hb level decreased to 87 g/L; however, the patient did not have gastrointestinal bleeding such as hematemesis and bloody stool. The right hepatic hemangioma was excised in the emergency department after the surgeon considered that the patient’s internal hemangioma bleeding was still increasing and might rupture. Intraoperatively, hemangioma was found to be located in the posterior lobe of the right liver; it had a diameter of about 10 cm and a prominent surface with clear boundaries from the surrounding tissues (Figure 2a). Cross-sectional examination of the mass revealed areas of necrosis within a hemangioma (Figure 2b). Histopathological reports confirmed a cavernous hemangioma of the liver. Hematoxylin–eosin (H&E) staining of the tissue showed hemorrhage and necrosis, accompanied by a large amount of inflammatory cell infiltration (Figure 3a–d). There were no postoperative complications such as bleeding, bile leakage, or liver or kidney failure. The recurrent fever that was present before surgery resolved, and the patient was discharged from the hospital on day 10 after surgery. Follow-up showed that the patient was afebrile, and there was a small amount of effusion visible on the CT scan (Figure 1d). The patient provided consent to publish the details of this case.
Figure 2.
a. A well-encapsulated right hepatic hemangioma was observed during surgery. b. Cross-sectional examination of the mass revealed areas of necrosis within the hemangioma.
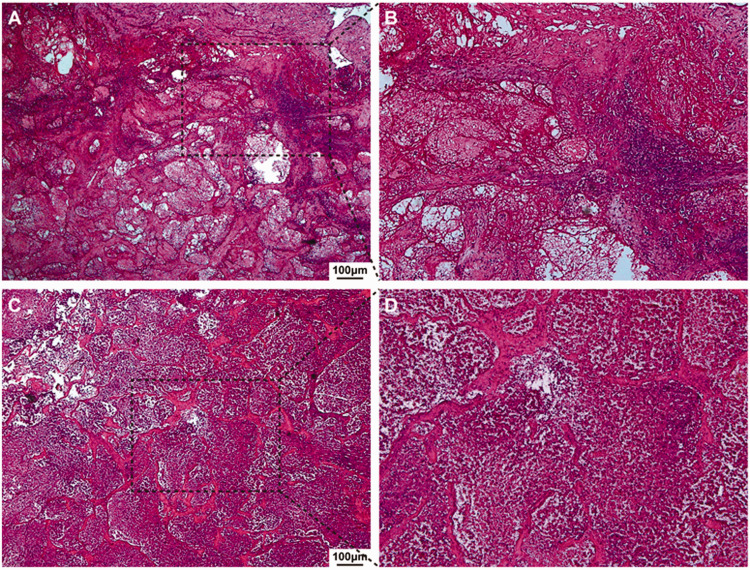
Figure 3.
a. H&E staining, ×40 and b. H&E staining, ×100. Pathology demonstrated a cavernous hemangioma of the liver with hemorrhagic necrotic areas and inflammatory cell infiltration. c. H&E staining, ×40 and d. H&E staining, ×100. Numerous inflammatory cells infiltrated the cavernous hemangioma of the liver, and the hemangioma structure was destroyed.
H&E, hematoxylin and eosin.
Discussion
Cavernous hemangiomas are the second most common cause of liver tumors after metastatic cancer, and they are the most common benign liver tumor.1,14 They are reported to have a prevalence of 7.3% on autopsy.15 In clinical practice, most cases of hepatic hemangioma rarely show symptoms, mainly because of their very slow growth patterns. However, when the hemangioma growth is quite large, some patients may have symptoms such as abdominal discomfort.16 Spontaneous rupture of a large hepatic hemangioma is not a common complication, but it is a serious event,17,18 and the surgical mortality rate for ruptured hemangioma is reported to be about 36.5%.19 Because of the occult clinical manifestations of hepatic hemangioma, its diagnosis relies more on an imaging examination. The sensitivity of ultrasound, CT, or magnetic resonance imaging (MRI) for diagnosing hepatic hemangioma is over 90%. In ultrasound examination, hemangioma usually presents as a homogeneous hyperechoic mass with relatively clear boundaries. However, when there is internal tumor bleeding, the hemangioma often present as an internal dense mass on CT imaging.11,20–22 In this case, we found a large hemangioma that was located in the posterior hepatic lobe using enhanced CT, and it contained high-density hemorrhagic foci.
Fever of unknown origin that is caused by hepatic hemangioma is an unusual presentation. We reviewed seven published reports of fever that was caused by giant hepatic hemangioma.10,23–27 Their characteristics and those of the current case report are summarized in Table 1. Most patients were female and the mass was located in the right liver lobe. Although the tumor size was not specifically described in three cases, it covered most of the left or right liver and was called a giant hemangioma. Six patients were treated via surgery, and two were treated with antipyretic drugs or prednisone. For all patients, their body temperature returned to normal and they reached the clinical standard for a cure. Fever associated with hepatic hemangioma may be due to the release of endogenous pyrogens or an acute thrombosis and necrosis within the tumor, but rarely due to internal hemorrhage. Our patient’s postoperative pathology showed hemorrhage and tumor necrosis, and a large number of inflammatory cells infiltrated into the tumor rather than around it. Therefore, we believe that the main cause of fever in this patient was post-necrotic inflammation, and bleeding and infection should also be taken into account.
Table 1.
Review of liver hemangioma characteristics with the complication of unexplained fever.
| CaseNo. | Gender | Age(years) | Size of hemangioma(diameter/measurement method) | Location | Cause of fever | Management | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 37 | 5 cm/US | Right posterior segment | Uncertain | Antipyretic | Cured | 27 |
| 2 | Male | 47 | giant/CT | Right lobe | Thrombosis and necrosis | Resection | Cured | 26 |
| 3 | Male | 44 | giant/CT | Left lobe | Thrombosis and necrosis | Resection | Cured | 26 |
| 4 | Female | 43 | giant/US | Right lobe and Left lobe | Uncertain | Prednisone | Cured | 25 |
| 5 | Male | 33 | 20 cm/US | Right lobe | Necrosis | Resection | Cured | 24 |
| 6 | Female | 52 | 15 cm/US | Right lobe | Internal hemorrhage | interventional therapy and Resection | Cured | 10 |
| 7 | Female | 49 | 15 cm/CT | Left lateral lobe | Necrosis | Resection | Cured | 23 |
| 8 | Female | 59 | 10 cm/CT | Right posterior lobe | Infected necrosis and internal hemorrhage | Resection | Cured | Present case |
US, ultrasound; CT, computed tomography.
Recognized indications for surgical treatment are mainly limited to hepatic hemangioma complications such as tumor rupture, internal hemorrhage, Kasabach–Meritt syndrome, and organ or vascular compression (e.g., limited gastric volume, Budd–Chiari syndrome).28–30 Schnelldorfer et al.31 summarized the case data from patients with hepatic hemangioma for 20 years and found that tumor size was not correlated with adverse events, health status, or quality of life between the surgery group and the observation group. They also found that even giant hepatic hemangiomas (>10 cm in diameter) were not correlated with an increased incidence of complications. Therefore, size cannot be the only indication for the surgical treatment of hepatic hemangioma. The patient’s age, sex, occupation, and clinical symptoms should be comprehensively assessed, and the patient’s safety should be the most important factor. In this case, internal hemorrhage of the hepatic hemangioma combined with infection resulted in the increased body temperature, and CT examination and blood routine examination showed increased bleeding and decreased Hb. Therefore, after obtaining the patient’s consent, we performed emergency surgery, and the infection symptoms and bleeding were subsequently resolved.
For fever with an unknown cause and a poor antibiotic treatment effect, the possibility of hepatic hemangioma with necrosis and infection was considered after excluding other common causes. Spontaneous intratumoral internal hemorrhage may be aggravated during treatment, so close attention should be paid to blood routine and imaging changes, and the patient should undergo surgery as early as possible.
Footnotes
Author contributions: All the authors participated in treating this patient. Ankang Wang, Hao Chen, and Zhiwei Huang wrote the manuscript and provided the original images. Ankang Wang, Hong Tang, Hao Shi, and Jian Wen collected the clinical, radiological, and pathologic data. Qiu Li, Yu Jiang, and Wenguang Fu reviewed the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Ankang Wang https://orcid.org/0000-0002-1349-2871
References
- 1.Choi BY andNguyen MH.. The diagnosis and management of benign hepatic tumors. J Clin Gastroenterol 2005; 39: 401–412. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez VM, Zamora AS, Morote SC, et al. Pendulous hepatic hemangioma. Surgery 2017; 161: 1735–1736. [DOI] [PubMed] [Google Scholar]
- 3.Bara T, Jr, Gurzu S, Jung I, et al. Giant cavernous hepatic hemangioma diagnosed incidentally in a perimenopausal obese female with endometrial adenocarcinoma: a case report. Anticancer Res 2016; 36: 769–772. [PubMed] [Google Scholar]
- 4.El-Hashemite N Walker V andKwiatkowski DJ.. Estrogen enhances whereas tamoxifen retards development of Tsc mouse liver hemangioma: a tumor related to renal angiomyolipoma and pulmonary lymphangioleiomyomatosis. Cancer Res 2005; 65: 2474–2481. [DOI] [PubMed] [Google Scholar]
- 5.Van Malenstein H, Maleux G, Monbaliu D, et al. Giant liver hemangioma: the role of female sex hormones and treatment. Eur J Gastroenterol Hepatol 2011; 23: 438–443. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava DN, Gandhi D, Seith A, et al. Transcatheter arterial embolization in the treatment of symptomatic cavernous hemangiomas of the liver: a prospective study. Abdom Imaging 2001; 26: 510–514. [DOI] [PubMed] [Google Scholar]
- 7.Giavroglou C Economou H andIoannidis I.. Arterial embolization of giant hepatic hemangiomas. Cardiovasc Intervent Radiol 2003; 26: 92–96. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Wei X, Wang K, et al. Enucleation versus anatomic resection for giant hepatic hemangioma: a meta-analysis. Gastrointest Tumors 2017; 3: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel Wahab M, El Nakeeb A, Ali MA, et al. Surgical management of giant hepatic hemangioma: single center’s experience with 144 patients. J Gastrointest Surg 2018; 22: 849–858. [DOI] [PubMed] [Google Scholar]
- 10.Hao F, Yang X, Tian Y, et al. Spontaneous internal hemorrhage of a giant hepatic hemangioma: a case report. Medicine (Baltimore) 2017; 96: e8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimoji K, Shiraishi R, Kuwatsuru A, et al. Spontaneous subacute intratumoral hemorrhage of hepatic cavernous hemangioma. Abdom Imaging 2004; 29: 443–445. [DOI] [PubMed] [Google Scholar]
- 12.Feldman PA andRegev A.. Atypical giant hepatic hemangiomas with intratumoral hemorrhage. Clin Gastroenterol Hepatol 2007; 5: A24. [DOI] [PubMed] [Google Scholar]
- 13.Kim JM, Chung WJ, Jang BK, et al. Hemorrhagic hemangioma in the liver: a case report. World J Gastroenterol 2015; 21: 7326–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dima-Cozma LC, Bitere OR, Pantazescu AN, et al. Cavernous liver hemangioma complicated with spontaneous intratumoral hemorrhage: a case report and literature review. Rom J Morphol Embryol 2018; 59: 557–561. [PubMed] [Google Scholar]
- 15.Ishak KG andRabin L.. Benign tumors of the liver. Med Clin North Am 1975; 59: 995–1013. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W Guo X andDong J.. Spontaneous rupture of hepatic hemangioma: a case report and literature review. Int J Clin Exp Pathol 2015; 8: 13426–13428. [PMC free article] [PubMed] [Google Scholar]
- 17.Doklestic K, Stefanovic B, Karamarkovik A, et al. Spontaneous rupture of giant liver hemangioma: case report. Srp Arh Celok Lek 2013; 141: 95–99. [DOI] [PubMed] [Google Scholar]
- 18.Cappellani A, Zanghi A, Di Vita M, et al. Spontaneous rupture of a giant hemangioma of the liver. Ann Ital Chir 2000; 71: 379–383. [PubMed] [Google Scholar]
- 19.Jain V, Ramachandran V, Garg R, et al. Spontaneous rupture of a giant hepatic hemangioma - sequential management with transcatheter arterial embolization and resection. Saudi J Gastroenterol 2010; 16: 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valette PJ Pilleul F andCrombe-Ternamian A.. MDCT of benign liver tumors and metastases. Eur Radiol 2003; 13: M31–M41. [DOI] [PubMed] [Google Scholar]
- 21.Ho HY, Wu TH, Yu MC, et al. Surgical management of giant hepatic hemangiomas: complications and review of the literature. Chang Gung Med J 2012; 35: 70–78. [DOI] [PubMed] [Google Scholar]
- 22.Toro A, Mahfouz AE, Ardiri A, et al. What is changing in indications and treatment of hepatic hemangiomas. A review. Ann Hepatol 2014; 13: 327–339. [PubMed] [Google Scholar]
- 23.Pandit N, Awale L, Chaudhary S, et al. Fever of unknown origin: a rare presentation of giant hepatic hemangioma. J Surg Case Rep 2018; 2018: rjy143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Yang Z, Tan H, et al. Fever of unknown origin caused by giant hepatic hemangioma. J Gastrointest Surg 2018; 22: 366–367. [DOI] [PubMed] [Google Scholar]
- 25.Fenster LF Freeny PC andBeebe HG.. Case reports. Cavernous hemangioma of the liver presenting with fever. Successful treatment with prednisone. West J Med 1978; 129: 138–140. [PMC free article] [PubMed] [Google Scholar]
- 26.Pateron D, Babany G, Belghiti J, et al. Giant hemangioma of the liver with pain, fever, and abnormal liver tests. Report of two cases. Dig Dis Sci 1991; 36: 524–527. [DOI] [PubMed] [Google Scholar]
- 27.Lee CW, Chung YH, Lee GC, et al. A case of giant hemangioma of the liver presenting with fever of unknown origin. J Korean Med Sci 1994; 9: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terkivatan T, Vrijland WW, Den Hoed PT, et al. Size of lesion is not a criterion for resection during management of giant liver haemangioma. Br J Surg 2002; 89: 1240–1244. [DOI] [PubMed] [Google Scholar]
- 29.Van Rosmalen BV, Bieze M, Besselink MG, et al. Long-term outcomes of resection in patients with symptomatic benign liver tumours. HPB (Oxford) 2016; 18: 908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brouwers MA, Peeters PM, De Jong KP, et al. Surgical treatment of giant haemangioma of the liver. Br J Surg 1997; 84: 314–316. [PubMed] [Google Scholar]
- 31.Schnelldorfer T, Ware AL, Smoot R, et al. Management of giant hemangioma of the liver: resection versus observation. J Am Coll Surg 2010; 211: 724–730. [DOI] [PubMed] [Google Scholar]