Abstract
Objective
MicroRNA (miR)-22 plays crucial roles in malignant tumors and is involved in regulation of chemosensitivity. Additionally, altered expression of circulating miR-22 has been reported in various cancers. This study was designed to investigate plasma miR-22 expression in patients with osteosarcoma (OS) and determine its diagnostic, prognostic, and chemosensitivity prediction value.
Methods
Plasma miR-22 levels in 120 patients with OS and 120 healthy controls were detected by real-time quantitative reverse transcription PCR. Associations of plasma miR-22 expression with the patients’ clinicopathological features and prognosis were then assessed.
Results
Plasma miR-22 levels in patients with OS were significantly lower than those in healthy controls. Low plasma miR-22 levels were correlated with large tumor size, advanced clinical stages, positive distant metastasis, and poor tumor response to preoperative chemotherapy. Plasma miR-22 could discriminate OS patients from controls and distinguish patients with a good response to therapy from those with a poor response to therapy. Multivariate analysis revealed that low plasma miR-22 expression was a significant independent predictor of unfavorable prognosis.
Conclusions
Altered plasma levels of miR-22 might serve as a novel, noninvasive biomarker for OS diagnosis, prognosis, and chemosensitivity prediction.
Keywords: microRNA-22, osteosarcoma, biomarkers, diagnosis, prognosis, chemosensitivity
Introduction
Osteosarcoma (OS) accounts for approximately 60% of bone tumors presenting in the first two decades of life; it occurs mainly in the metaphyses of the long bones, especially in the distal and proximal femur and the proximal humerus, with a bimodal age distribution and a propensity to develop predominantly in adolescents and young adults.1 It is characterized by rapid progression, high metastatic potential (especially to the lung), and poor clinical prognosis.2 Although advanced strategies such as surgery and adjuvant chemotherapy are used in the clinic, the long-term survival of patients with OS remains unsatisfactory.3 Chemotherapy combined with surgery leads to a 5-year survival rate of 60% to 70% for non-metastatic patients; the clinical outcome of metastatic or relapsed patients is progressively worse, with 20% survival. Moreover, morbidity is rising by 1.4% per year, mainly because of delayed diagnosis, distant metastasis, and chemoresistance. Chemoresistance has become a major obstacle during management of OS. The mutational landscape of OS is highly heterogeneous across different histotypes, making it very difficult to understand its molecular pathogenesis and identify specific therapeutic molecules. For these reasons, there is an urgent need to better understand the molecular mechanisms underlining OS and to identify novel noninvasive biomarkers for the diagnosis, prognosis, and chemosensitivity prediction of OS. This would greatly facilitate the development of effective biological-based therapies and optimize treatment strategies for OS patients.
MicroRNAs (miRNAs) are endogenous noncoding small RNAs that negatively control gene expression after transcription by binding to the 3′-untranslated region (3′-UTR) of their targets.4 Emerging evidence shows that miRNAs are essential physiological regulators, and dysregulation of miRNA expression contributes to the pathogenesis of many human diseases, including cancer.5 Accumulating evidence has demonstrated that miRNAs are significantly associated with the development and progression of OS.6–8 Rehei et al.9 exhibited that miR-214 could significantly promote invasion and migration of OS cells by targeting TNF receptor associated factor 3 (TRAF3). Zhuang et al.10 found that miR-524 promoted cell proliferation of OS by targeting phosphatase and tensin homolog (PTEN). Wang and colleagues discovered that miR-141-3p was a key negative regulator of the epidermal growth factor receptor (EGFR) pathway and may provide a new theoretical basis for the treatment of OS.11 More importantly, recent studies have demonstrated that miRNAs can be detected in the blood and may be used as noninvasive biomarkers for diseases.12,13 For example, plasma miR-145, miR-20a, and miR-223 could be potential novel biomarkers for early-stage non-small-cell lung cancer (NSCLC) screening.14 Serum miR-21 expression is associated with poor overall survival in breast cancer (BC),15 colorectal cancer (CRC),16 and NSCLC.17 High serum miR-200c expression was significantly associated with advanced clinical stage and a poor tumor response to platinum-based chemotherapy in patients with esophageal squamous cell carcinoma (ESCC).18 In addition, many studies have been conducted to identify circulating miRNAs as biomarkers for cancers, such as OS.19–21 For example, Cong et al.22 reported that serum miR-124 might be a satisfactory diagnostic and prognostic biomarker for OS.
miR-22, an evolutionarily conserved gene, is located on chromosome 17p13 and aberrantly expressed in various cancers, showing different effects.23 Wang et al. showed that miR-22 was downregulated in OS tissue and its expression was correlated with a variety of clinical and pathological parameters. miR-22 may also serve as a promising biomarker to predict prognosis of OS.24 In vitro functional experiments confirmed that blockage of autophagy by miR-22 inhibited OS cell proliferation, migration, and invasion.25 Those studies indicated that miR-22 is a critical regulator of tumorigenesis and development in OS. More importantly, recent studies have suggested that miR-22 is aberrantly expressed in the serum or plasma of patients with intrahepatic cholangiocarcinoma (ICC),26 hepatocellular carcinoma (HCC),27 ESCC,28 pancreatic cancer,29 prostatic cancer,30 and NSCLC,31 confirming its utility as a promising biomarker. To our knowledge, there are no data in the literature regarding the clinical significance of circulating miR-22 in patients with OS.
In view of the elusive roles of miR-22 in tumorigenesis and tumor progression of OS, we detected plasma levels of miR-22 in 120 patients with OS and 120 healthy controls using real-time quantitative reverse transcription PCR (qRT-PCR). Then, we analyzed the relationship between plasma miR-22 levels and clinicopathologic factors or survival of OS patients. The aim of the present study was to determine the clinicopathological significance and diagnostic and prognostic value of plasma miR-22 in human OS.
Materials and methods
Ethical approval
This study was approved by the Ethical Committee of Daqing Oilfield General Hospital (Approval Number: 20-029). All participants gave written informed consent.
Study population
Primary OS patients treated in Daqing Oilfield General Hospital (Daqing, China) from January 2014 to December 2015 were retrospectively enrolled in this study. Exclusion criteria were previous malignant tumors in another organ or system; patients with no clinical and pathological data; hematological disorders; patients with end-stage cancer not qualified for chemotherapy; and any patients unable or unwilling to participate. Histopathological analysis of the surgically excised tissues was performed to confirm the diagnosis of OS, and tumor stage was determined on the basis of the surgery findings. All patients received neoadjuvant chemotherapy, with a combination of doxorubicin and methotrexate, and underwent surgical resection. The demographic and clinical characteristics of the study subjects were obtained from their medical records, and follow-up was performed by a combination of outpatient visits, telephone calls, and letters. The patients had follow-up visits with physical and radiography examinations every 3 months, as well as computed tomography (CT) or magnetic resonance imaging (MRI) when necessary. Patients were followed for overall survival after therapy, which was defined as the interval from surgery to death or to the date of the last follow-up. An equal number of age- and sex-matched healthy individuals were recruited as the control group. Controls were selected from family members of patients or visitors and were free of cancers.
For healthy individuals, blood samples (4–8 mL) were collected into K2-EDTA-coated tubes (Becton, Dickinson Co., Franklin Lakes, NJ, USA). Blood samples were obtained from patients before any treatment was administered. The samples were stored for up to 24 hours at 4°C and prepared in accordance with the following protocol: centrifuged at 1500 × g at 4°C for 15 minutes, and then at 14,000 ×g at 4°C for another 15 minutes. After centrifugation, the supernatant fraction (plasma) was aliquoted into RNase/DNase-free tubes and stored at −80°C for subsequent experiments. To minimize the effects of freeze–thaw on circulating miRNAs, we only used plasma samples that had not been previously thawed. The chemotherapeutic response was assessed according to the Huvos grading system.32 More than 90% tumor necrosis in response to neoadjuvant chemotherapy was classified as a good pathologic response, whereas less than 90% tumor necrosis was defined as a poor pathologic response.
Isolation of circulating RNA from plasma
Total RNA was extracted from frozen plasma using TRIzol LS Reagent (Invitrogen, Carlsbad, CA, USA). Briefly, 250 μL of plasma was mixed with 750 μL of TRIzol LS and incubated for 15 minutes at room temperature to permit the complete dissociation of nucleoprotein complexes. Then, 200 μL of chloroform was added and the mixture was vigorously vortexed for 15 s. Extraction was performed for 15 minutes at room temperature and the mixture centrifuged at 12,000 × g at 4°C for 15 minutes. The upper aqueous phase was transferred to a clean tube, an equal volume of cold isopropanol was added to the tube, and the mixture was stored overnight at −20°C. The samples were centrifuged at 12,000 × g for 15 minutes at 4°C, the supernatant was carefully removed, and the RNA pellet was washed with 1 mL of 75% ethanol. For normalization, each sample was supplemented with 25 pM Caenorhabditis elegans miR-39 (cel-miR-39) after the TRIzol LS was added.33 RNA was dissolved in 30 μL of RNase-free water. The total RNA purity and concentration were evaluated by their absorbance ratio at 260/280 nm.
miRNA analysis
A total of 100 ng of total RNA was used for reverse transcription with a TaqMan microRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) in a 5-μL reaction system. A 5-μL reaction system comprising 0.5 μL of 10× reverse transcription buffer, 0.33 μL of Multiscribe reverse transcriptase (50 U/μL), 0.063 μL of RNase inhibitor (20 U/μL), 0.05 μL of 100 mM dNTPs, 0.5 μL of primers, the RNA sample, and RNase-free water was incubated for 10 minutes at 30°C, followed by incubation for 30 minutes at 50°C, 5 minutes at 95°C, and then held at 4°C. Real-time quantitative PCR (qPCR) was performed using a TaqMan microRNA Assay Kit on a 7500 Sequence Detection System (Applied Biosystems). The reaction system was 20 μL, containing 10 μL of 2× Universal PCR Master Mix, 1 μL of the TaqMan microRNA Assay, 1 μL of reverse transcriptase product, and 8 μL of nuclease-free water. Each sample was examined in triplicate, U6 RNA was used as the normalization control, and relative miR-22 expression was calculated by the 2−ΔΔCt cycle threshold method.34
Statistical analysis
Statistical analysis was carried out using SPSS version 20.0 (IBM Corp., Armonk, NY, USA), and the level of significance was set at P < 0.05. The Mann–Whitney U test was used to compare plasma miR-22 levels between OS patients and healthy controls. The correlations between plasma miR-22 expression and clinicopathological parameters were explored by Student’s t test or chi-square test. Variables with significant effects in the Student’s t test analyses were further entered into a linear regression model to investigate the effects of these variables. Receiver operating characteristic (ROC) curve analysis was applied to determine the diagnostic utility of plasma miR-22. The cumulative overall survival rates were calculated using the Kaplan–Meier method, and the independence of risk factors was tested using a Cox proportional hazards regression model.
Results
Downregulation of plasma miR-22 and its diagnostic value
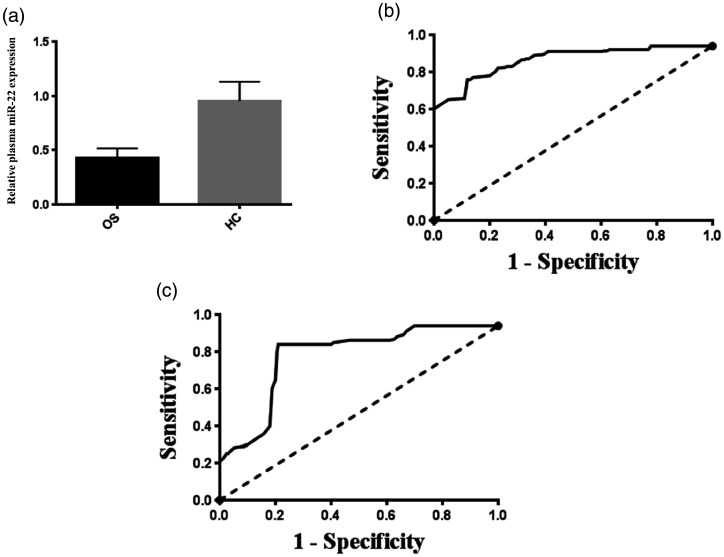
In total, 120 primary OS patients were included in the present study, and 87 of them were under 20 years of age (Table 1). This is in agreement with previous reports showing that the majority of OS patients are under the age of 25 years and that most develop OS in the second decade of life (<20 years).35 The clinicopathological data of all OS patients are presented in Table 1. Plasma miR-22 expression was detected in all subjects by TaqMan qPCR. As shown in Figure 1A, plasma miR-22 expression in patients with OS was significantly lower than that in healthy controls (P < 0.01). ROC analyses were conducted to evaluate the sensitivity and specificity for plasma miR-22 to discriminate OS patients from healthy controls. As shown in Figure 1B, the AUC was 0.91 (95% CI, 0.86–0.95) for miR-22 to differentiate patients with OS from controls with a sensitivity of 84.1% and a specificity of 80.2% (cut-off value = 0.48).
Table 1.
Association of plasma microRNA-22 (miR-22) expression with clinicopathological features of osteosarcoma.
| Clinicopathological features | Number of cases | Plasma miR-22 expression |
P-valuea | Plasma miR-22 expressionb | P-valuec | Standard regression coefficientd | |
|---|---|---|---|---|---|---|---|
| Low, n (%) | High, n (%) | ||||||
| Age | |||||||
| <20 years | 87 | 43 (49.4) | 44 (50.6) | NS | 0.385 ± 0.129 | NS | — |
| ≥20 years | 33 | 17 (51.5) | 16 (48.5) | 0.367 ± 0.145 | |||
| Sex | |||||||
| Male | 80 | 38 (47.5) | 42 (52.5) | NS | 0.395 ± 0.146 | NS | — |
| Female | 40 | 22 (55.0) | 18 (45.0) | 0.351 ± 0.098 | |||
| Tumor size | |||||||
| >8 cm | 36 | 26 (72.2) | 10 (27.8) | 0.003 | 0.341 ± 0.154 | 0.057 | 0.052 |
| ≤8 cm | 84 | 34 (40.5) | 50 (59.5) | 0.397 ± 0.121 | |||
| Tumor location | |||||||
| Tibia/femur | 85 | 45 (52.9) | 40 (47.1) | NS | 0.382 ± 0.139 | NS | — |
| Elsewhere | 35 | 15 (42.9) | 20 (57.1) | 0.376 ± 0.120 | |||
| Clinical stage | |||||||
| IIA | 65 | 24 (36.9) | 41 (63.1) | 0.003 | 0.430 ± 0.139 | <0.001 | 0.060 |
| IIB/III | 55 | 36 (65.5) | 19 (34.5) | 0.322 ± 0.099 | |||
| Distant metastasis | |||||||
| Absent | 68 | 25 (36.8) | 43 (63.2) | 0.002 | 0.423 ± 0.142 | <0.001 | 0.123 |
| Present | 52 | 35 (67.3) | 17 (32.7) | 0.325 ± 0.097 | |||
| Response to chemotherapy | |||||||
| Good | 66 | 19 (28.8) | 47 (71.2) | <0.001 | 0.448 ± 0.124 | <0.001 | 0.140 |
| Poor | 54 | 41 (75.9) | 13 (24.1) | 0.298 ± 0.092 | |||
aChi-square test.
bData presented as mean ± standard deviation.
cStudent’s t-test.
dLinear regression analysis.
NS = not significant.
Figure 1.
Plasma microRNA-22 (miR-22) expression in osteosarcoma (OS) patients and its potential value in diagnosis and the prediction of tumor chemosensitivity. (a) Plasma miR-22 expression in patients with OS was significantly lower than that of healthy controls (HC) (P < 0.01). (b) Receiver operating characteristic (ROC) curve analysis demonstrated that plasma miR-22 was a reliable biomarker for distinguishing patients with OS from healthy controls. (c) ROC curve analysis illustrated that plasma miR-22 could distinguish patients with a good response to therapy from those with a poor response to therapy.
Plasma miR-22 expression, clinicopathological features, and prediction of chemosensitivity and prognosis
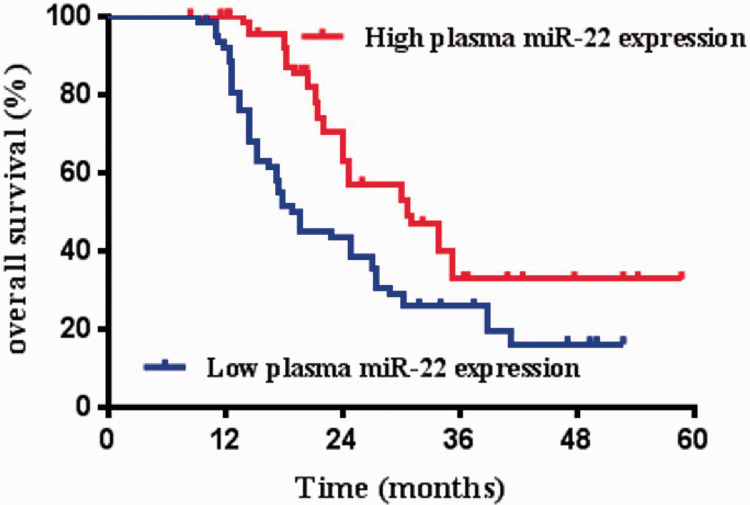
Correlations between plasma miR-22 level and clinical data were analyzed in all OS patients. We divided the OS patients into a high-miR-22 expression group and low-miR-22 expression group on the basis of the median value of plasma miR-22 expression levels as a cut-off point. As summarized in Table 1, low plasma miR-22 level was significantly associated with large tumor size (P = 0.003), advanced clinical stage (P = 0.003), distant metastasis (P = 0.002), and poor response to chemotherapy (P < 0.001). We also analyzed the association between plasma miR-22 expression and clinicopathological features using Student’s t test and linear regression analysis. As shown in Table 1, plasma miR-22 was significantly downregulated in patients with advanced clinical stage (IIB-III vs. IIA), distant metastasis (vs. no distant metastasis), and poor response to chemotherapy (P < 0.001 for all comparisons). The standard regression coefficient of response to chemotherapy was the largest, followed by the distant metastasis status and then by the clinical stage, indicating that response to chemotherapy was more strongly correlated with plasma miR-22 expression. At the optimal cut-off point (cut-off value = 0.28), the AUC was 0.86 (95% CI, 0.77–0.94) for plasma miR-22 to distinguish a good from a poor tumor response with a sensitivity of 85.0% and a specificity of 85.4% (Figure 1C). On the basis of the Kaplan–Meier analysis and Log-Rank test results, OS patients with low plasma miR-22 expression had shorter overall survival than OS patients with high plasma miR-22 expression (P = 0.002) (Figure 2). The results of the univariate analysis indicated that tumor size, clinical stage, distant metastasis, and response to chemotherapy were also risk factors affecting the overall survival in OS patients (all P < 0.05) (Table 2). Multivariate analysis confirmed plasma miR-22 expression as an independent prognostic factor for OS patients (P = 0.006) (Table 2).
Figure 2.
Prognostic value of plasma microRNA-22 (miR-22) for patients with osteosarcoma. Patients with low plasma miR-22 expression had shorter overall survival than those with high plasma miR-22 expression (P = 0.002).
Table 2.
Univariate and multivariate survival analyses of overall survival in 120 patients with osteosarcoma.
| Clinicopathological features | Number of cases | 4-year survival, n (%) | 4-year death, n (%) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|---|
| P-value | HR | P-value | HR | ||||
| Age | NS | 0.83 | — | — | |||
| <20 years | 87 | 26 (29.9) | 61 (70.1) | ||||
| ≥20 years | 33 | 10 (30.3) | 23 (69.7) | ||||
| Sex | NS | 1.07 | — | — | |||
| Male | 80 | 23 (28.8) | 57 (71.2) | ||||
| Female | 40 | 13 (32.5) | 27 (67.5) | ||||
| Tumor size | 0.031 | 2.24 | 0.035 | 2.08 | |||
| >8 cm | 36 | 6 (16.7) | 30 (83.3) | ||||
| ≤8 cm | 84 | 30 (35.7) | 54 (64.3) | ||||
| Tumor location | NS | 0.98 | — | — | |||
| Tibia/femur | 85 | 27 (31.8) | 58 (68.2) | ||||
| Elsewhere | 35 | 9 (25.7) | 26 (74.3) | ||||
| Clinical stage | 0.001 | 4.52 | 0.004 | 4.38 | |||
| IIA | 65 | 29 (44.6) | 36 (55.4) | ||||
| IIB/III | 55 | 7 (12.7) | 48 (87.3) | ||||
| Distant metastasis | 0.003 | 3.83 | 0.002 | 3.96 | |||
| Absent | 68 | 30 (44.1) | 38 (55.9) | ||||
| Present | 52 | 6 (11.5) | 46 (88.5) | ||||
| Response to chemotherapy | 0.014 | 2.94 | 0.024 | 2.67 | |||
| Good | 66 | 28 (42.4) | 38 (57.6) | ||||
| Poor | 54 | 8 (14.8) | 46 (85.2) | ||||
| Plasma miR-22 expression | 0.004 | 3.72 | 0.006 | 3.31 | |||
| Low | 60 | 12 (20.0) | 48 (80.0) | ||||
| High | 60 | 24 (40.0) | 36 (60.0) | ||||
HR, hazard ratio; miR-22, microRNA-22; NS = not significant.
Discussion
Accumulating knowledge of the underlying molecular pathogenesis of malignant tumors has provided new insights for disease characterization and therapeutic applications; identification of reliable biomarkers with high sensitivity and specificity is of great clinical significance for early detection and prediction of therapeutic outcome for OS. Numerous studies have indicated that circulating miRNAs may serve as promising biomarkers in different human malignancies, including OS.12 For example, Liu et al.36 concluded that reduced expression of serum miR-375 in OS patients might serve as a biomarker for OS diagnosis. Monterde et al.19 reported circulating miR-215-5p and miR-642a-5p to be potential diagnostic biomarkers for OS in a Mexican population. Li and colleagues showed that the expression levels of miR-542-3p in peripheral blood could serve as a new type of noninvasive biomarker for tumor monitoring and outcome prediction in OS patients.37
In the present study, we found decreased plasma miR-22 levels in patients with OS and observed that the decreased expression of plasma miR-22 was correlated with tumor size, clinical stage, distant metastasis, and response to neoadjuvant chemotherapy. Metastasis status at initial presentation is a patient-related factor that can predict the survival of OS patients.38 We found that plasma expression levels of miR-22 were significantly decreased in patients with metastatic disease compared with that in patients without metastases. This is further supported by previous studies, demonstrating that miR-22 may regulate the migration of cancer cells.25 Taken together, our results showed that dysregulated circulating miR-22 may also be associated with some clinical and pathological parameters of OS patients, which needs to be further investigated in studies with larger cohorts. A low plasma miR-22 level was an independent unfavorable prognostic factor for patients with OS. The ROC analyses revealed that plasma miR-22 could serve as a biomarker for OS diagnosis and prediction of tumor chemosensitivity. As far as we know, this is the first study to examine plasma miR-22 expression and its clinical significance in OS patients. The expression trends of plasma miR-22 in patients with OS were consistent with those in tissues or OS cell lines as shown previously.
Previous research has investigated the tumor-suppressive function of miR-22 in several human malignancies, including OS. Guo et al.25 reported that miR-22 inhibited the proliferation, migration, and invasion of OS cells. Gai et al.39 showed that overexpressing miR-22 could diminish the proliferation and increase apoptosis of MG63 cells. Aberrant circulating miR-22 expression has been reported to be a potential biomarker in several human cancers. The low expression of serum miR-22 in patients with ICC and HCC may be an independent promising biomarker for early diagnosis of these cancers.26,27 In contrast, high expression of serum miR-22 in patients with ESCC, pancreatic cancer, and metastatic prostatic cancer suggests that miR-22 may be a reliable, noninvasive biomarker with the desired sensitivity and specificity for cancer diagnosis.28–30 Alterations in the expression of circulating miR-22 may also directly mirror therapeutic effects for cancer patients. The elevated expression of serum miR-22 in NSCLC patients is significantly associated with tumor aggression and unresponsiveness to chemotherapeutic drugs,31 implying its potential role in monitoring chemotherapeutic effects in NSCLC. Taken together, detecting alterations in miR-22 expression in cancer tissues or body fluids may be of great potential significance in cancer diagnosis, particularly in early diagnosis, as well as in assessing therapeutic effects and predicting prognosis. These findings may help to evaluate the diagnostic and prognostic value of circulating miR-22 in OS patients. The expression and clinical significance of circulating miR-22 in other cancers is worthy of further study.
Despite the rapid development and clinical use of multi-agent chemotherapy regimens, resistance to chemotherapy remains a substantial therapeutic problem in the management of OS. Therefore, identification of reliable and predictive biomarkers of chemoresistance remains a crucial challenge for appropriate and timely treatments. miRNAs can also predict a response to chemotherapeutic agents in OS. For example, in the study by Luo et al.,40 the authors found that downregulation of circulating miR-125b might predict poor response to cisplatin-based chemotherapy and unfavorable prognosis. Previous studies have suggested that miR-22 is involved in the regulation of chemosensitivity in several types of cancers. p53-wild-type CRC cells display chemoresistance to paclitaxel partly by enhanced miR-22 binding to and activation of phosphatase and tensin homolog (PTEN), which then counteracts a cascade of PI3K/Akt events and stimulates apoptosis.41,42 There are other possible mechanisms with respect to miR-22 in enhancing chemosensitivity, including the autophagy and apoptosis pathways. By separately targeting the B-cell translocation gene 1 (BTG1) and high-mobility group box 1 (HMGB1), miR-22 could induce apoptosis and inhibit autophagy, contributing to the reverse chemoresistance in CRC and OS.25,43 Moreover, miR-22 may profoundly enhance the efficacy of chemotherapeutic agents, especially anticancer drugs, by intimately interacting with other miRNAs. The PI3K/Akt/mTOR pathway is predominantly repressed by the combined and simultaneous effects of miR-100 directly targeting fibroblast growth factor receptor 3 (FGFR3) and mTOR and by miR-22 targeting EVI1, resulting in increased chemosensitivity to everolimus in clear cell ovarian cancer.44 Taken together, these data suggest that miR-22 may markedly enhance chemosensitivity or reverse chemoresistance of cancer to corresponding anticancer drugs through a variety of molecular mechanisms, and that multiple drugs with mainly therapeutic effects may further maximize their advantages or minimize side effects by regulating miR-22 expression to inhibit proliferation, migration, and invasion of cancer cells. Hence, to obtain the best treatment effects for miR-22 as a therapeutic intervention, understanding the potential mechanisms of miR-22 in regulating chemosensitivity or chemoresistance will be greatly beneficial in the short term. In this study, we observed a significant relationship between plasma levels of miR-22 and tumor response to neoadjuvant chemotherapy. ROC curve analysis confirmed plasma miR-22 as a potential biomarker to distinguish good from poor pathologic response. Therefore, circulating miR-22 might act as an biomarker to predict the chemosensitivity of human malignancies.
Although our results are encouraging, some limitations need to be acknowledged. First, all subjects were drawn from a single center in a restricted geographic area, and it is not clear whether these results apply to the whole population. Second, the sample size investigated in this study was relatively small, and data from more participants are needed. Third, our conclusions were derived from clinical data; laboratory-based evidence and the mechanism by which miR-22 regulates chemosensitivity of OS have not been investigated. Mechanisms of resistance to chemotherapy are complicated, including drug inactivation, decreased intracellular drug accumulation, autophagy-related chemoresistance, perturbations of signal transduction pathways, and miRNA dysregulation.45,46 Considering that exosomes have been reported to participate in cell-to-cell communication,47 we assume potential relationships between miR-22 and exosomes. Illustrating the exact mechanism involved in miR-22 regulation of OS response to chemotherapy warrants further investigation. Fourth, we only detected plasma levels of miR-22 in the present study. Other circulating miRNAs, such as miR-199a and miR-148a, are also abnormally expressed in the peripheral blood of patients with OS.48,49 Genome-wide microarray or next-generation sequencing analysis might be an ideal tool to identify more circulating miRNAs as diagnostic, prognostic, and predictive biomarkers with improved sensitivity and specificity.
In summary, this study provides the first evidence that an altered plasma miR-22 level in patients with OS is a potential noninvasive biomarker for the diagnosis, prognosis, and chemosensitivity prediction of IS. Further studies with a larger sample size are needed to confirm our results and to explore the underlying mechanisms of dysregulated plasma miR-22 in OS.
Footnotes
Authors’ contributions: Zhen-bin Diao and Tian-xiao Sun contributed equally to this work. Yuan-sheng Xia designed the experiments and edited the manuscript; Zhen-bin Diao and Tian-xiao Sun conducted the experiments and wrote the manuscript; Yi Zong and Bo-chuan Lin performed experiments and analyzed data. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work. The data sets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yuan-sheng Xia https://orcid.org/0000-0002-4127-7036
References
- 1.Bielack S, Carrle D, Casali PG, et al. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009; 20: 137–139. [DOI] [PubMed] [Google Scholar]
- 2.Shi ZW, Wang JL, Zhao N, et al. Single nucleotide polymorphism of hsa-miR-124a affects risk and prognosis of osteosarcoma. Cancer Biomark 2016; 17: 249–257. [DOI] [PubMed] [Google Scholar]
- 3.Han K, Chen X, Bian N, et al. MicroRNA profiling identifies MiR-195 suppresses osteosarcoma cell metastasis by targeting CCND1. Oncotarget 2015; 6: 8875–8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee RC Feinbaum RL andAmbros V.. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75: 843–854. [DOI] [PubMed] [Google Scholar]
- 5.Wang S Wu W andClaret FX.. Mutual regulation of microRNAs and DNA methylation in human cancers. Epigenetics 2017; 12: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng XM Xu CW andWang F.. MiR-33b inhibits osteosarcoma cell proliferation through suppression of glycolysis by targeting lactate dehydrogenase A (LDHA). Cell Mol Biol (Noisy-le-grand) 2018; 64: 31–35. [PubMed] [Google Scholar]
- 7.Wang JW, Wu XF, Gu XJ, et al. Exosomal miR-1228 from cancer-associated fibroblasts promotes cell migration and invasion of osteosarcoma by directly targeting SCAI. Oncol Res 2019; 27: 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Lu XQ, Zhou XQ, et al. NEAT1 induces osteosarcoma development by modulating the miR-339-5p/TGF-β1 pathway. J Cell Physiol 2019; 234: 5097–5105. [DOI] [PubMed] [Google Scholar]
- 9.Rehei AL, Zhang L, Fu YX, et al. MicroRNA-214 functions as an oncogene in human osteosarcoma by targeting TRAF3. Eur Rev Med Pharmacol Sci 2018; 22: 5156–5164. [DOI] [PubMed] [Google Scholar]
- 10.Zhuang M, Qiu X, Cheng D, et al. MicroRNA-524 promotes cell proliferation by down-regulating PTEN expression in osteosarcoma. Cancer Cell Int 2018; 18: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Wang G, Li B, et al. miR-141-3p is a key negative regulator of the EGFR pathway in osteosarcoma. Onco Targets Ther 2018; 11: 4461–4478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105: 10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Song Y, Yao L, et al. Circulating microRNAs: promising biomarkers involved in several cancers and other diseases. DNA Cell Biol 2017; 36: 77–94. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Mao F, Shen T, et al. Plasma miR-145, miR-20a, miR-21 and miR-223 as novel biomarkers for screening early-stage non-small cell lung cancer. Oncol Lett 2017; 13: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav P, Mirza M, Nandi K, et al. Serum microRNA-21 expression as a prognostic and therapeutic biomarker for breast cancer patients. Tumour Biol 2016; 37: 15275–15282. [DOI] [PubMed] [Google Scholar]
- 16.Menéndez P, Padilla D, Villarejo P, et al. Prognostic implications of serum microRNA-21 in colorectal cancer. J Surg Oncol 2013; 108: 369–373. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZX, Bian HB, Wang JR, et al. Prognostic significance of serum miRNA-21 expression in human non-small cell lung cancer. J Surg Oncol 2011; 104: 847–851. [DOI] [PubMed] [Google Scholar]
- 18.Yu H, Duan B, Jiang L, et al. Serum miR-200c and clinical outcome of patients with advanced esophageal squamous cancer receiving platinum-based chemotherapy. Am J Transl Res 2013; 6: 71–77. [PMC free article] [PubMed] [Google Scholar]
- 19.Monterde-Cruz L, Ramírez-Salazar EG, Rico-Martínez G, et al. Circulating miR-215-5p and miR-642a-5p as potential biomarker for diagnosis of osteosarcoma in Mexican population. Hum Cell 2018; 31: 292–299. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Xu T, Hu X, et al. Elevated circulating miR-182 acts as a diagnostic biomarker for early colorectal cancer. Cancer Manag Res 2018; 10: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Wen Q. miR-3120-5p acts as a diagnostic biomarker in non-small cell lung cancer and promotes cancer cell proliferation and invasion by targeting KLF4. Mol Med Rep 2018; 18: 4621–4628. [DOI] [PubMed] [Google Scholar]
- 22.Cong C, Wang W, Tian J, et al. Identification of serum miR-124 as a biomarker for diagnosis and prognosis in osteosarcoma. Cancer Biomark 2018; 21: 449–454. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Li Y, Ding M, et al. Molecular mechanisms and clinical applications of miR-22 in regulating malignant progression in human cancer. Int J Oncol 2017; 50: 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G, Shen N, Cheng L, et al. Downregulation of miR-22 acts as an unfavorable prognostic biomarker in osteosarcoma. Tumour Biol 2015; 36: 7891–7895. [DOI] [PubMed] [Google Scholar]
- 25.Guo S, Bai R, Liu W, et al. miR-22 inhibits osteosarcoma cell proliferation and migration by targeting HMGB1 and inhibiting HMGB1-mediated autophagy. Tumour Biol 2014; 35: 7025–7034. [DOI] [PubMed] [Google Scholar]
- 26.Kawahigashi Y, Mishima T, Mizuguchi Y, et al. MicroRNA profiling of human intrahepatic cholangiocarcinoma cell lines reveals biliary epithelial cell-specific microRNAs. J Nippon Med Sch 2009; 76: 188–197. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Yang Y, Yang T, et al. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br J Cancer 2010; 103: 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, Wang C, Chen X, et al. Expression profile of microRNAs in serum: A fingerprint for esophageal squamous cell carcinoma. Clin Chem 2010; 56: 1871–1879. [DOI] [PubMed] [Google Scholar]
- 29.Ganepola GA, Rutledge JR, Suman P, et al. Novel blood-based microRNA biomarker panel for early diagnosis of pancreatic cancer. World J Gastrointest Oncol 2014; 6: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knyazev EN, Samatov TR, Fomicheva KA, et al. MicroRNA hsa-miR-4674 in hemolysis-free blood plasma is associated with distant metastases of prostatic cancer. Bull Exp Biol Med 2016; 161: 112–115. [DOI] [PubMed] [Google Scholar]
- 31.Franchina T, Amodeo V, Bronte G, et al. Circulating miR-22, miR-24 and miR-34a as novel predictive biomarkers to pemetrexed-based chemotherapy in advanced non-small cell lung cancer. J Cell Physiol 2014; 229: 97–99. [DOI] [PubMed] [Google Scholar]
- 32.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer 1982; 49: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 33.Huang S, Chen M, Li L, et al. Circulating microRNAs and the occurrence of acute myocardial infarction in Chinese populations. Circ Cardiovasc Genet 2014; 7: 189–198. [DOI] [PubMed] [Google Scholar]
- 34.Wu C, Li M, Hu C, et al. Clinical significance of serum miR-223, miR-25 and miR-375 in patients with esophageal squamous cell carcinoma. Mol Biol Rep 2014; 41: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 35.Mirabello L Troisi RJ andSavage SA.. International osteosarcoma incidence patterns in children and adolescents, middle ages, and elderly persons. Int J Cancer 2009; 125: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W, Zhao X, Zhang YJ, et al. MicroRNA-375 as a potential serum biomarker for the diagnosis, prognosis, and chemosensitivity prediction of osteosarcoma. J Int Med Res 2018; 46: 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Song S, Ni G, et al. Serum miR-542-3p as a prognostic biomarker in osteosarcoma. Cancer Biomark 2018; 21: 521–526. [DOI] [PubMed] [Google Scholar]
- 38.Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 2006; 106: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 39.Gai P, Sun H, Wang G, et al. miR-22 promotes apoptosis of osteosarcoma cells via inducing cell cycle arrest. Oncol Lett 2017; 13: 2354–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo Z, Liu M, Zhang H, et al. Association of circulating miR-125b and survival in patients with osteosarcoma-A single center experience. J Bone Oncol 2016; 5: 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Zhang Y, Zhao J, et al. Overexpression of miR‑22 reverses paclitaxel-induced chemoresistance through activation of PTEN signaling in p53-mutated colon cancer cells. Mol Cell Biochem 2011; 357: 31–38. [DOI] [PubMed] [Google Scholar]
- 42.Tsuchiya N, Izumiya M, Ogata-Kawata H, et al. Tumor suppressor miR-22 determines p53-dependent cellular fate through post-transcriptional regulation of p21. Cancer Res 2011; 71: 4628–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Tang J, Li C, et al. miR-22 regulates 5-FU sensitivity by inhibiting autophagy and promoting apoptosis in colorectal cancer cells. Cancer Lett 2015; 356: 781–790. [DOI] [PubMed] [Google Scholar]
- 44.Nagaraja AK, Creighton CJ, Yu Z, et al. A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol Endocrinol 2010; 24: 447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou AJ andGorlick R.. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther 2006; 6: 1075–1085. [DOI] [PubMed] [Google Scholar]
- 46.He H Ni J andHuang J.. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett 2014; 7: 1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang YN, Zhang R, Du JW, et al. Predictive role of UCA1-containing exosomes in cetuximab-resistant colorectal cancer. Cancer Cell Int 2018; 18: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma W, Zhang X, Chai J, et al. Circulating miR-148a is a significant diagnostic and prognostic biomarker for patients with osteosarcoma. Tumour Biol 2014; 35: 12467–12472. [DOI] [PubMed] [Google Scholar]
- 49.Zhou G, Lu M, Chen J, et al. Identification of miR-199a-5p in serum as noninvasive biomarkers for detecting and monitoring osteosarcoma. Tumour Biol 2015; 36: 8845–8852. [DOI] [PubMed] [Google Scholar]