Abstract
Objectives
To assess serum 25-hydroxycholecalciferol (25-OH vitamin D) levels in Jordanian children with bronchial asthma, and to examine correlations between 25-OH vitamin D levels and asthma severity and control.
Methods
A cross-sectional study was conducted at the Paediatric Chest Clinic, Al-Karak Governmental Hospital, Southern Jordan, between May 2015 and February 2016. Serum 25-hydroxyvitamin D level was determined in children aged 1–14 years diagnosed with bronchial asthma (6–14 years) or recurrent wheezing episodes (<6 years). Asthma severity was determined based on the Global Initiative for Asthma assessment, the Asthma Control Test, and the Childhood Asthma Control Test. Demographic and clinical characteristics were compared between patients with low and normal 25-OH vitamin D levels, and correlations between asthma severity and 25-OH vitamin D level were assessed.
Results
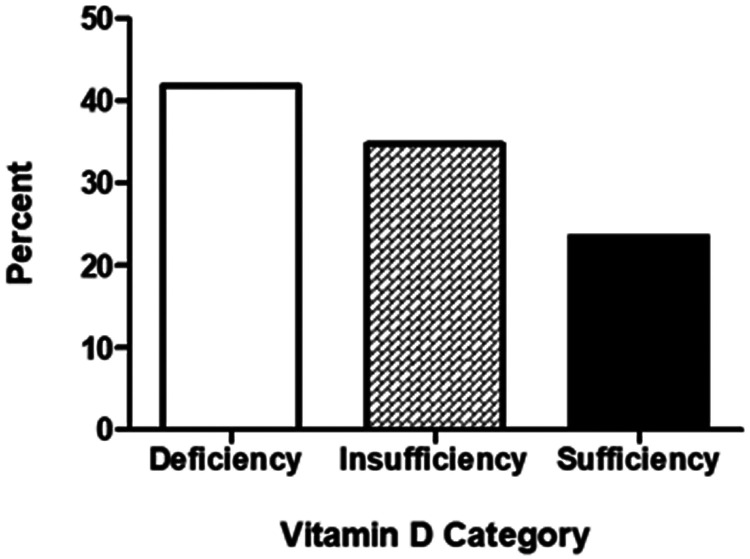
Out of 98 included children, 25-OH vitamin D levels were deficient and insufficient in 41 (41.8%) and 34 (34.7%) children, respectively. Only 23 (23.5%) had sufficient 25-OH vitamin D levels. A significant correlation was found between severity of asthma symptoms and 25-OH vitamin D deficiency.
Conclusion
25-OH vitamin D deficiency is highly prevalent in Jordanian children with bronchial asthma and correlates significantly with asthma severity.
Keywords: Asthma, Deficiency, Paediatrics, Jordan, 25-OH vitamin D
Introduction
Asthma is one of the most common chronic diseases worldwide and has increased in prevalence over the last few decades.1 In Jordan, bronchial asthma constitutes a great health burden, affecting approximately 10% of the population, according to official reports from the Ministry of Health.2 The aetiology of asthma is complex and likely involves multiple interactions between genetic and environmental factors. Environmental factors such as atmospheric pollution, dietary changes, allergen-sensitization, and lifestyle modifications may be responsible for the increasing prevalence of asthma.3 Additionally, impaired immunogenic tolerance and interplay between cells and inflammatory mediators may further promote airway obstruction.4,5
The effects of vitamin D as a hormone have gained increasing attention. Besides its numerous classical functions (calcium absorption, bone mineralization, and neuromuscular function regulation) and non-classical actions (cellular differentiation, insulin secretion, and blood pressure regulation), vitamin D is also believed to be a potent immune-system regulator, having a potential role in various allergic diseases. Vitamin D seems to have regulatory effects on every part of the immune system, whereas vitamin D deficiency has been linked to an array of immunologically-based diseases, such as asthma.4 Unfortunately, vitamin D deficiency is widespread in many middle eastern countries with abundant sunshine, such as Jordan, due to avoidance of sunlight exposure and concealed clothing style.6However, the existence of low vitamin D status has been reported even with adequate sun exposure.7
The global increase in asthma and allergic diseases may be linked to reduced serum vitamin D levels.8 Several studies have indicated an association between vitamin D deficiency and increased incidence and severity of childhood asthma,9–11 and vitamin D reportedly has protective effects against viral infections and exacerbations of asthma in children.12,13 Furthermore, vitamin D has been reported to enhance lung development in infants when taken during pregnancy, and to have prophylactic effects on wheezing and asthma, which may occur later.13,14 However, conflicting results have been reported, demonstrating either no effects or adverse effects for vitamin D on asthma incidence or severity, making the association between vitamin D and asthma unclear.15,16
The epidemic increase in asthma and related allergic disease is a major public health problem worldwide.1 Thus, identifying new risk factors for asthma development, such as vitamin D deficiency, is important to improve asthma control, reduce asthma-related morbidity, and, more importantly, help prevent the disease in early life. Therefore, the aim of the present cross-sectional study was to assess serum 25-OH vitamin D levels in children with bronchial asthma in Al-Karak, a city in south Jordan. An additional aim was to compare the severity and control of asthma between patients with different levels of 25-OH vitamin D.
Patients and methods
Study population, design and setting
This cross-sectional study included consecutive paediatric patients, aged 1–14 years, with asthma or wheezing symptoms, and was conducted at the Paediatric Chest Clinic, Al-Karak Governmental Hospital, South Jordan, between May 2015 and February 2016. The population of Al-Karak Governorate is estimated to be 317 500 (according to the Jordan Department of Statistics 2015 data [http://dosweb.dos.gov.jo/; accessed 13 November 2020]), and the required study population sample size was calculated to be 100 participants.
This study was approved by the Mu'tah University Faculty of Medicine Ethics committee (April 2015; reference No. 20153), and written informed consent was obtained from the parents or legal proxies of all participants. Patients' details were de-identified upon publication. All procedures were followed in accordance with the Helsinki Declaration of 1975.
For inclusion into the study, patients were diagnosed with asthma and/or recurrent wheezing episodes (early asthma), according to the Global Initiative for Asthma (GINA) criteria: (1) a physician’s diagnosis of asthma; (2) symptoms of recurrent (i.e., more than two) episodes of wheezing, cough, shortness of breath, or a combination of these; (3) documented reversibility with bronchodilators; and/or (4) use of medication for asthma in the previous 6 months.17
Patients who were taking vitamin D supplements were excluded from the study. For each patient, detailed personal medical histories were recorded, and a thorough physical examination was performed. Study participants were also screened for the presence of allergic conditions other than asthma, including allergic rhinitis, atopic dermatitis/eczema, allergic conjunctivitis, and food allergies. History of breastfeeding during the first six months of life was also screened.
Asthma severity and control of symptoms were determined by the following: the GINA classification,17 and a validated Asthma Control Test (ACT) questionnaire in children aged ≥ 12 years, or Childhood Asthma Control Test (C-ACT) in children aged between 4 and 11 years;18 use of systemic steroids; and admission to hospital due to asthma within the last 12 months. Patients were categorized into either intermittent, mild persistent, moderate persistent, or severe persistent asthma according to the GINA classification.17 An ACT/C-ACT score ≤19 and ACT/C-ACT score >19 were considered to represent poor and good control, respectively.20
Vitamin D measurement
Vitamin D levels were assessed by measuring serum 25-hydroxycholecalciferol (25-OH vitamin D), a vitamin D metabolite considered to be a reliable measure of serum vitamin D level. A venous blood sample (10 ml) was obtained; the blood was allowed to clot for 60 min, then serum was separated by centrifugation at 2 000 rpm for 20 min at 4°C and stored at –70°C until analysis. Serum 25-OH vitamin D was measured using a Liaison® 25-OH Vitamin D Total assay kit with the Liaison® rapid automated assay system (DiaSorin, Stillwater, MN, USA), which utilizes a chemiluminescence immunoassay to detect both forms of 25-OH vitamin D (D2 and D3). Based on published guidelines and recommendations from previous studies, 25-OH vitamin D levels were categorized as deficient (<20 ng/ml), insufficient (20–29 ng/ml), or sufficient (>29 ng/ml).19,20
Skin prick testing
To assess the association between atopy and vitamin D deficiency in children with asthma included in the study, skin prick testing for common inhaled allergens was performed. The test was performed using 11 standardized allergen extracts from a commercial test kit (Stallergenes Greer, London, UK), in accordance with published guidelines.21
The 11 tested inhaled allergens were cat pelt, Salsola kali, two strains of house dust mites (Dermatophagoides pteronyssinus and D. farina), cereal mix, olive pollen, grass mix, mould (Alternaria), dog fur, Compositae, and wall-pellitory. The test was reported to be positive if the prick site swelled to form a wheel with a diameter >4 mm. A positive control (histamine) and negative control (normal saline) were used to avoid false-positive (dermatographism) or false-negative results. Parents and caregivers of the tested children were requested to stop systemic antihistamines or leukotriene modifiers 4–5 days prior to testing.
Statistical analyses
The study population sample size was calculated using OpenEpi software, version 3.0 (www.OpenEpi.com, updated 6 April 2013). The required sample size was calculated to be 100 patients (confidence level of 95%, Z 0.95 = 1.96).
Data are presented as mean ± SD for continuous variables and frequencies and percentages for categorical variables. 25-OH vitamin D deficiency in children with bronchial asthma is presented as frequency distributions. Patients grouped according to deficient, insufficient or sufficient 25-OH vitamin D level were compared in terms of relevant demographic and clinical characteristics using analysis of variance (ANOVA) or Pearson’s χ2-test. The correlation between 25-OH vitamin D level and age was assessed using Pearson’s correlation coefficient. Multiple logistic regression was used to investigate possible predictors of low 25-OH vitamin D levels in the study population. The relationships between asthma severity or asthma control and 25-OH vitamin D levels were analysed in patients grouped according to asthma severity or control using ANOVA or independent samples t-test. Statistical analyses were performed using SPSS software, version 21 (IBM; Armonk, NY, USA), and a P value ≤ 0.05 was considered to be statistically significant.
Results
Although 100 participants were initially enrolled, two patients dropped out of the study, thus, a total of 98 patients referred to Al-Karak chest clinic and diagnosed with wheezing (aged ≤5 years) or asthma (aged 6–14 years) were included in the final analyses. Demographic and clinical characteristics, in terms of associated allergic conditions, use of inhaled and/or systemic steroids, exclusive breastfeeding during the first 6 months, and classification of asthma according to GINA criteria, are summarised in Table 1. Most of the children in this study population were male (66.3%). A total of 92 children were categorized by asthma severity according to GINA criteria. Of these, 61 patients (66.3%) had intermittent or mild persistent asthma, and 31 patients (33.7%) had moderate or severe persistent asthma.
Table 1.
Demographic and clinical characteristics among 98 paediatric patients with wheezing (aged ≤ 5 years) or asthma (aged 6–14 years).
| Characteristic | Study population |
|---|---|
| Age, years | 7.67 ± 3.83 |
| Sex, male/female | 65 (66.3)/33 (33.7) |
| Associated allergic conditions: | |
| Allergic rhinitis | 71 (76.3%) |
| Conjunctivitis | 24 (26.4%) |
| Food allergy | 2 (2.2%) |
| Eczema/atopic dermatitis | 23 (25%) |
| Use of inhaled corticosteroids | 67 (72%) |
| Exclusive breastfeeding during first 6 months | 17 (17.3%) |
| GINA classification, n = 92: | |
| Intermittent | 38 (41.3%) |
| Mild persistent | 23 (25%) |
| Moderate persistent | 23 (25%) |
| Severe persistent | 8 (8.7%) |
Data presented as mean ± SD or n (%) prevalence.
GINA, Global Initiative for Asthma.
The proportions of patients with deficient, insufficient or sufficient levels of 25-OH vitamin D are shown in Figure 1. 25-OH vitamin D was found to be deficient or insufficient in 75 children (76.5%) and sufficient in only 23 (23.5%). Age distribution varied among three categories of 25-OH vitamin D level with a mean age of 8.46 years (±3.71) for children with deficient 25-OH vitamin D level, 6.50 years (±3.90) for the 25-OH vitamin D insufficient group, and 6.09 years (±3.81) for children with sufficient 25-OH vitamin D level (P = 0.25; Table 2).
Figure 1.
Distribution of children with asthma (n = 98) categorized according to serum 25-OH vitamin D levels. Deficiency, <20 ng/ml; insufficiency, 20–29 ng/ml; sufficiency, >29 ng/ml.
Table 2.
Comparison of frequencies and age among 98 paediatric patients with asthma or wheezing categorized according to serum 25-OH vitamin D levels.
| Parameter | Serum 25-OH vitamin D level |
||
|---|---|---|---|
| Deficient (<20 ng/ml) | Insufficient (20–29 ng/ml) | Sufficient (>29 ng/ml) | |
| Frequency | 41 (41.8) | 34 (34.7) | 23 (23.5) |
| Age, years | 8.46 ± 3.71 | 6.50 ± 3.90 | 6.09 ± 3.81 |
Data presented as n (%) prevalence or mean ± SD.
P = 0.025, analysis of variance.
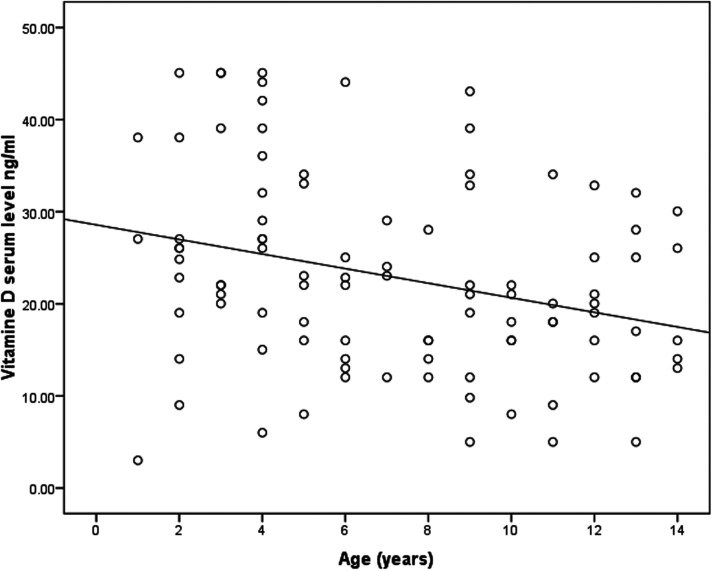
Correlations between patients’ serum 25-OH vitamin D levels and selected demographic and health characteristics were examined using multiple logistic regression. Variables, such as age, sex, and the presence of other allergic conditions, were evaluated (Table 3). Levels of 25-OH vitamin D were shown to be inversely correlated with age (P < 0.05, R2 = 0.105; Figure 2), and younger children tended to have more sufficient serum 25-OH vitamin D levels than older children. Among associated allergic conditions other than asthma (allergic rhinitis, allergic conjunctivitis, and eczema), allergic rhinitis was the only condition found to be significantly correlated with 25-OH vitamin D levels (P = 0.036; Table 3).
Table 3.
Multiple logistic regression analysis of the association between serum 25-OH vitamin D level and different clinical and demographic variables in paediatric patients with asthma.
| Independent variable | Statistical significance | AOR | 95% CI for AOR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Age | P = 0.021 | 0.866 | 0.767 | 0.979 |
| Sex | NS | 1.094 | 0.041 | 29.086 |
| Associated allergic diseases: | ||||
| Allergic rhinitis | P = 0.036 | 3.170 | 1.077 | 9.325 |
| Allergic conjunctivitis | NS | 1.869 | 0.165 | 21.191 |
| Eczema | NS | 0.852 | 0.070 | 10.372 |
AOR, adjusted odds ratio; CI, confidence interval.
NS, no statistically significant association between variable and vitamin D level (P > 0.05; Pearson’s χ2-test).
Figure 2.
Scatter plot showing correlation between serum 25-OH vitamin D levels (ng/ml) and age (years) in paediatric patients with asthma (P = 0.002; Pearson’s correlation coefficient).
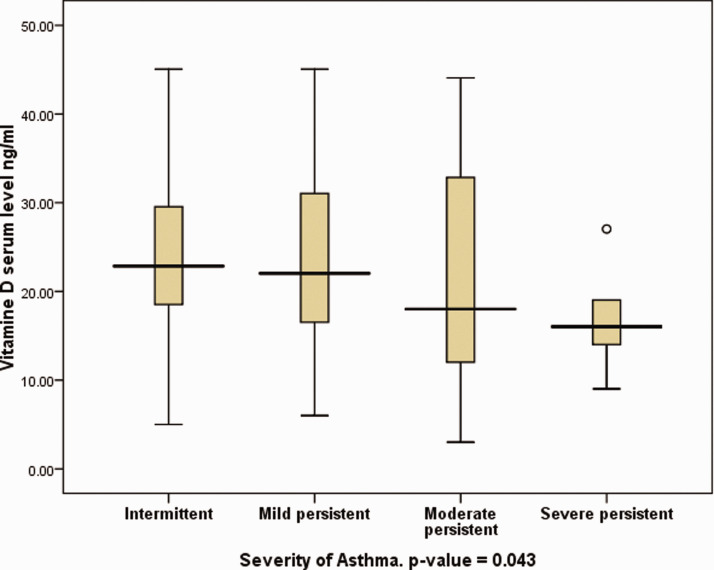
Children categorized according to asthma severity using the GINA classification, were compared in terms of 25-OH vitamin D levels. A significant correlation was found between asthma severity and 25-OH vitamin D levels, in that patients categorized with severe persistent asthma were found to have significantly lower vitamin D levels than those with intermittent asthma (Figure 3; P = 0.043; ANOVA). Six children (14.3%) with insufficient 25-OH vitamin D levels and two children (6.7%) with deficient levels had severe persistent asthma, whereas none of the children with sufficient levels were reported to have such asthma severity.
Figure 3.
Box-whisker plot showing serum 25-OH vitamin D level in 98 paediatric patients (aged ≤14 years) categorized according to asthma severity graded using the Global Initiative for Asthma (GINA) classification (P = 0.043, severe persistent versus intermittent asthma; analysis of variance). Data presented as median (central black horizontal line); 25th and 75th percentiles (extremities of the box); and minimum and maximum outliers (error bars).
The proportion of patients with 25-OH vitamin D deficiency or insufficiency who used systemic corticosteroids to treat asthma exacerbation within the last year (48.8% and 52.9%, respectively) was significantly higher than those who had sufficient 25-OH vitamin D levels (21.7%; P = 0.047, Pearson’s χ2-test; Table 4).
Table 4.
Association between 25-OH vitamin D level and different asthma assessment measures in paediatric patients with asthma.
| Measure | 25-OH vitamin D level |
Statistical significance | |||
|---|---|---|---|---|---|
| Deficientn = 41 | Insufficientn = 34 | Sufficientn = 23 | |||
| Systemic steroid use within last 12 months | 20 (48.8) | 18 (52.9) | 5 (21.7) | P = 0.047 | |
| Hospital admission within last 12 months | 29 (70.7) | 24 (70.6) | 13 (56.5) | NS | |
| Skin prick test, n = 76 | –ve | 10 (27.0) | 7 (30.4) | 3 (18.8) | NS |
| +ve | 27 (73.0) | 16 (69.6) | 13 (81.2) | ||
Data presented as n (%) prevalence.
–ve, negative response; +ve, positive response.
P ≤ 0.05, statistically significant between-group differences (Pearson’s χ2-test).
NS, no statistically significant between-group differences (P > 0.05).
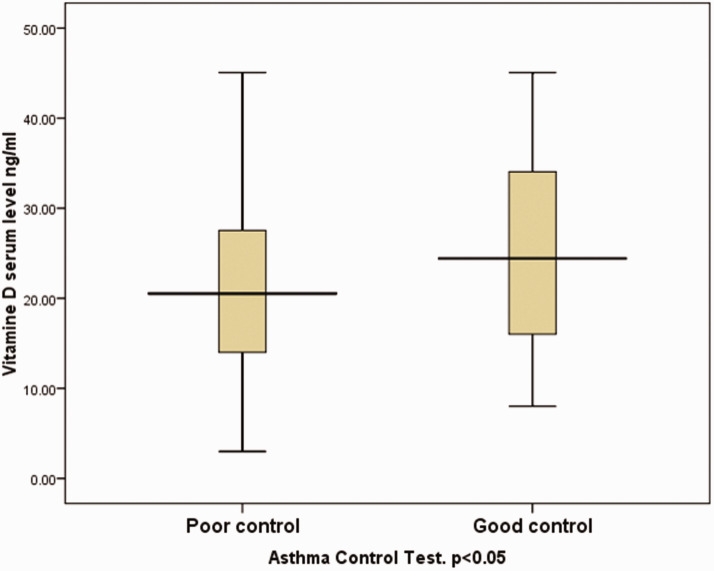
In patients grouped according to asthma control, based on ACT and C-ACT questionnaire scores, patients with poor control were found to have lower 25-OH vitamin D levels than patients with good control (P = 0.05, independent samples t-test; Figure 4). There were 52 patients with good control and 46 patients with poor control.
Figure 4.
Box-whisker plot showing serum 25-OH vitamin D level in 98 paediatric patients (aged ≤14 years) with either poor or good asthma control, according to Asthma Control Test (ACT) and Childhood (C)-ACT scores. Serum 25-OH vitamin D levels were significantly lower in patients with poor versus good control (P < 0.05, independent samples t-test). Data presented as median (central black horizontal line); 25th and 75th percentiles (extremities of the box); and minimum and maximum outliers (error bars).
Seventy-six children were tested for atopy using skin prick testing for 11 common inhaled allergens. No statistically significant differences were found between the three 25-OH vitamin D groups regarding allergen reactivity (P = 0.711; Table 4).
Discussion
The current cross-sectional study assessed serum 25-OH vitamin D levels in children with bronchial asthma or wheezing episodes in Al-Karak city of South Jordan. To the best of the authors’ knowledge, this is the first study in Jordan to address the prevalence of vitamin D deficiency in children with asthma and recurrent episodic wheeze. A high prevalence of 25-OH vitamin D deficiency (<20 ng/ml) and insufficiency (20–29 ng/ml) was observed in children with asthma or recurrent wheezing (40.1% and 34.7%, respectively). Accordingly, sufficient 25-OH vitamin D levels (≥30 ng/ml) only represented 23.5% of these patients. This finding is consistent with previous studies showing that vitamin D deficiency is prevalent in children with asthma in many regions of the world, and particularly in the Middle East.6 In a study conducted in Qatar, 52.9% of children with bronchial asthma had insufficient 25-OH vitamin D levels.22 A similar study in Turkey showed that 67% of children with asthma had deficient 25-OH vitamin D levels, whereas 20% were 25-OH vitamin D insufficient.23 Other reports published globally have also confirmed the high prevalence of 25-OH vitamin D deficiency in children with asthma.24,25 Reverse causality may account partly for the fact that children with bronchial asthma tend to avoid outdoor activities and thus have less sun exposure than those without bronchial asthma, and subsequently have lower 25-OH vitamin D levels. However, previous studies have shown that even high sunlight exposure levels do not ensure adequate 25-OH vitamin D levels.7
Although 25-OH vitamin D levels were not correlated to seasonality in the present study, seasonable variability in 25-OH vitamin D levels has previously been shown to exist, with the lowest levels reported in late summer and winter seasons.6,26
An aim of the present study was to determine whether a correlation could be established between 25-OH vitamin D levels and different demographic and clinical characteristics in the study population. Only age was significantly correlated with 25-OH vitamin D levels, and younger children were shown to have higher serum 25-OH vitamin D levels. This is consistent with findings in other countries, such as the USA, the UK, and New Zealand, where the prevalence of low vitamin D status increases with age, with adolescents being the most affected age group.27 Among the co-existing allergic conditions, only allergic rhinitis showed a significant correlation with 25-OH vitamin D levels, similar to a previous study conducted in south Jordan.28These results further support the important role of vitamin D in modulating the immunological response in different respiratory or allergy-related conditions.5
In terms of the correlation between vitamin D deficiency and asthma severity, the present study shows a significant association between the severity of asthma determined by the GINA classification and 25-OH vitamin D deficiency status. Children with severe and moderate persistent asthma were more likely to have 25-OH vitamin D deficiency than children with intermittent or mild asthma. This association was also significant in other measures of the severity of asthma; children with lower 25-OH vitamin D levels were more likely to use systemic steroids to treat asthma exacerbations, and children with poor asthma control (evaluated using ACT/C-ACT scores) were shown to have lower vitamin D levels. There was a numerically higher rate of hospital admissions and use of systemic corticosteroids in patients with low versus sufficient vitamin D levels, however, the difference did not reach statistical significance. This may have been due to the relatively small sample size in the present study population. A large Childhood Asthma Management Program (CAMP) study, investigating the relationship between serum 25-OH vitamin D levels and subsequent severe asthma exacerbations or hospitalizations, showed that 25-OH vitamin D deficiency is associated with a higher rate of severe exacerbations in children with asthma.10 This result is also supported by several other reports.29,30
The association between vitamin D levels and asthma can be explained in several aspects. Vitamin D has an important role as an immune-system modulator,5,31 and is also believed to correlate with paediatric asthma. For example, a significant inverse correlation between serum levels of 25-OH vitamin D3 and immunoglobulin E was found in paediatric patients with asthma.32 In addition, a strong association between vitamin D deficiency and respiratory tract infections, a leading trigger for asthma exacerbations, has been reported.33 In addition, several clinical and epidemiological studies have suggested that there may be a synergistic effect of vitamin D and corticosteroids in asthma outcomes.34,35 This suggests that vitamin D deficiency may impede the response of patients with asthma to steroid therapy.
Contrary to the present findings, some studies have reported inverse associations, or no associations, between asthma or recurrent wheezing and vitamin D level,36,37 which may reflect the heterogeneity of asthma, differences between populations and research methodologies, or diagnostic tools. Previous clinical trials of vitamin D supplementation revealed mixed outcomes. In some studies, prenatal vitamin D supplementation, or supplementation during infancy, resulted in better lung function and asthma control in childhood.38–40 In contrast, other studies showed an increase in the prevalence of asthma, allergic rhinitis, and atopy.41 Vitamin D supplementation might be beneficial for certain groups of children with asthma and at a certain age and dose. This may necessitate conducting randomized clinical trials on the benefits of vitamin D supplementation on a large scale.
The present results signify the importance of studying 25-OH vitamin D levels in children with bronchial asthma in south Jordan. However, a high prevalence of vitamin D deficiency in the study population may also reflect the fact that vitamin D deficiency is prevalent in the Jordanian population. Studies of vitamin D deficiency in Jordanian adults over 18 years-of-age have been conducted, where the overall prevalence of low vitamin D status (< 30 ng/ml) was 29.4%.42 Another study of vitamin D levels in newborn infants in Jordan showed extremely high rates of vitamin D deficiency at approximately 94%.43 A national study of 1 077 Jordanian children of preschool age (12–59 months) showed 19.8% deficiency (<12 ng/ml) and 56.5% insufficiency (<20 ng/ml).44 Also, vitamin D supplementation, combined with other asthma medications, has been shown to improve children's inflammatory response to asthma.45 However, to the present authors’ knowledge, this has not been assessed in Jordan.
The present results did not show a strong association between atopy (positive skin prick test to inhaled allergens) and vitamin D deficiency. However, previous studies have shown that low vitamin D levels were associated with an increased risk of atopy and asthma.46 The lack of association could be due to multiple mechanisms by which vitamin D can affect asthma other than atopy.
The results of the present study may be limited be several factors. There was no control group to assess 25-OH vitamin D level in children without bronchial asthma, and the relatively small sample size hindered the ability to detect associations of high statistical power. Furthermore, the study was conducted in a single-centre which may not be representative of children with asthma in other parts of Jordan. Finally, the study did not evaluate factors associated with lower vitamin D levels such as diet, sun exposure, and clothing.
Conclusion
The present results showed that vitamin D deficiency was highly prevalent in paediatric patients with asthma, as almost half of the tested patients had low 25-OH vitamin D levels, and vitamin D deficiency was shown to correlate with asthma severity in children. This was reflected by lower levels of vitamin D in children with higher GINA scores, and more frequent hospital admissions and use of systemic steroids to treat asthma exacerbations in children with low 25-OH vitamin D levels versus those with normal 25-OH vitamin D levels. These results show the important role vitamin D plays in the pathogenesis and severity of asthma and possibly other allergic conditions. Therefore, assessing serum 25-OH vitamin D levels in patients with bronchial asthma is important, and may be beneficial for assessment, follow-up, and treatment. The present authors recommend that all children diagnosed with bronchial asthma receive a test for serum 25-OH vitamin D levels. The current study is observational; hence further research is needed to understand the role of low vitamin D level in asthma in the Jordanian population. Future research should assess the effects of vitamin D supplementation on asthma severity and symptom control.
Footnotes
Availability of data and materials: The datasets used and/or analysed during the current study are available from the corresponding author on request.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was funded by the deanship of scientific research at the University of Mu'tah.
ORCID iD: Enas Al-Zayadneh https://orcid.org/0000-0002-5059-560X
References
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention, 2016, www.ginasthma.org (2016, accessed 20 December 2016).
- 2.Ministry of Health HKoJ, https://moh.gov.jo/ (2018, accessed 18 March 2018) [In Arabic].
- 3.Van Der Werff SD, Junco Diaz R, Reyneveld R, et al. Prediction of asthma by common risk factors: a follow-up study in Cuban schoolchildren. J Investig Allergol Clin Immunol 2013; 23: 415–420. [PubMed] [Google Scholar]
- 4.Lange NE, Litonjua A, Hawrylowicz CM, et al. Vitamin D, the immune system and asthma. Expert Rev Clin Immunol 2009; 5: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt PG andStrickland DH.. Interactions between innate and adaptive immunity in asthma pathogenesis: new perspectives from studies on acute exacerbations. J Allergy Clin Immunol 2010; 125: 963–972. [DOI] [PubMed] [Google Scholar]
- 6.Bassil D, Rahme M, Hoteit M, et al. Hypovitaminosis D in the Middle East and North Africa: prevalence, risk factors, and impact on outcomes. Dermatoendocrinol 2013; 5: 274–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binkley N, Novotny R, Krueger D, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007; 92: 2130–2135. [DOI] [PubMed] [Google Scholar]
- 8.Bener A, Ehlayel MS, Bener HZ, et al. The impact of vitamin D deficiency on asthma, allergic rhinitis and wheezing in children: an emerging public health problem. J Family Community Med 2014; 21: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brehm JM, Celedon JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009; 179: 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brehm JM, Schuemann B, Fuhlbrigge AL, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 2010; 126: 52–58.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinellato I, Piazza M, Sandri M, et al. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr 2011; 158: 437–441. [DOI] [PubMed] [Google Scholar]
- 12.Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med 2017; 5: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales E, Romieu I, Guerra S, et al. Maternal vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology 2012; 23: 64–71. [DOI] [PubMed] [Google Scholar]
- 14.Camargo CA, Jr, Ingham T, Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011; 127: e180–e187. [DOI] [PubMed] [Google Scholar]
- 15.Thuesen BH, Heede NG, Tang L, et al. No association between vitamin D and atopy, asthma, lung function or atopic dermatitis: a prospective study in adults. Allergy 2015; 70: 1501–1504. [DOI] [PubMed] [Google Scholar]
- 16.Gale CR, Robinson SM, Harvey NC, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 2008; 62: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008; 31: 143–178. [DOI] [PubMed] [Google Scholar]
- 18.Lababidi H Hijaoui A andZarzour M.. Validation of the Arabic version of the asthma control test. Ann Thorac Med 2008; 3: 44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930. [DOI] [PubMed] [Google Scholar]
- 20.Barake M, Daher RT, Salti I, et al. 25-hydroxyvitamin D assay variations and impact on clinical decision making. J Clin Endocrinol Metab 2012; 97: 835–843. [DOI] [PubMed] [Google Scholar]
- 21.Heinzerling L, Mari A, Bergmann KC, et al. The skin prick test - European standards. Clin Transl Allergy 2013; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bener A, Ehlayel MS, Tulic MK, et al. Vitamin D deficiency as a strong predictor of asthma in children. Int Arch Allergy Immunol 2012; 157: 168–175. [DOI] [PubMed] [Google Scholar]
- 23.Uysalol M, Mutlu LC, Saracoglu GV, et al. Childhood asthma and vitamin D deficiency in Turkey: is there cause and effect relationship between them? Ital J Pediatr 2013; 39: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dogru M, Kirmizibekmez H, Mutlu RGY, et al. Clinical effects of vitamin D in children with asthma. Int Arch Allergy Immunol 2014; 164: 319–325. [DOI] [PubMed] [Google Scholar]
- 25.Kolokotroni O, Middleton N, Kouta C, et al. Association of serum vitamin D with asthma and atopy in childhood: review of epidemiological observational studies. Mini Rev Med Chem 2015; 15: 881–899. [DOI] [PubMed] [Google Scholar]
- 26.Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009; 20: 1807–1820. [DOI] [PubMed] [Google Scholar]
- 27.Spiro A andButtriss JL.. Vitamin D: an overview of vitamin D status and intake in Europe. Nutr Bull 2014; 39: 322–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Shagahin HM, Albataineh EM, Alnawaiseh NA, et al. Vitamin D status among Jordanian patients with allergic rhinitis. Eur J Sci Res 2014; 119: 430–437. [Google Scholar]
- 29.Peçanha MB, Freitas RDB, Moreira TR, et al. Prevalence of vitamin D deficiency and its relationship with factors associated with recurrent wheezing. J Bras Pneumol 2019; 45: e20170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balakrishnan M, Gaki N, Vikas D, et al. Evaluation of vitamin D in bronchial asthma and the effect of vitamin D supplementation on asthma severity and control: a randomised control trial. Eur Respir J 2014; 44: 4049. [Google Scholar]
- 31.Goldsmith JR. Vitamin D as an immunomodulator: risks with deficiencies and benefits of supplementation. Healthcare (Basel) 2015; 3: 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammadzadeh I, Darvish S, Qujeq D, et al. Association of serum 25-OH vitamin D3 with serum IgE and the Pediatric Asthma Severity Score in patients with pediatric asthma. Allergy Asthma Proc 2020; 41: 126–133. [DOI] [PubMed] [Google Scholar]
- 33.Esposito S andLelii M.. Vitamin D and respiratory tract infections in childhood. BMC Infect Dis 2015; 15: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litonjua AA. Vitamin D and corticosteroids in asthma: synergy, interaction and potential therapeutic effects. Expert Rev Respir Med 2013; 7: 101–104. [DOI] [PubMed] [Google Scholar]
- 35.Wu AC, Tantisira K, Li L, et al. Effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med 2012; 186: 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali Qutub MM. Serum vitamin D and asthma control still a controversial link: a cross sectional study and literature review. AMJ 2019; 12: 110–118. [Google Scholar]
- 37.Menon J Maranda L andNwosu BU.. Serum 25-hydroxyvitamin D levels do not correlate with asthma severity in a case-controlled study of children and adolescents. J Pediatr Endocrinol Metab 2012; 25: 673–679. [DOI] [PubMed] [Google Scholar]
- 38.Wolsk HM, Chawes BL, Litonjua AA, et al. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: a combined analysis of two randomized controlled trials. PLoS One 2017; 12: e0186657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornsby E, Pfeffer PE, Laranjo N, et al. Vitamin D supplementation during pregnancy: effect on the neonatal immune system in a randomized controlled trial. J Allergy Clin Immunol 2018; 141: 269–278.e1. [DOI] [PubMed] [Google Scholar]
- 40.Forno E, Bacharier LB, Phipatanakul W, et al. Effect of vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low vitamin D levels: the VDKA randomized clinical trial. JAMA 2020; 324: 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyppönen E, Sovio U, Wjst M, et al. Infant vitamin D supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci 2004; 1037: 84–95. [DOI] [PubMed] [Google Scholar]
- 42.Batieha A, Khader Y, Jaddou H, et al. Vitamin D status in Jordan: dress style and gender discrepancies. Ann Nutr Metab 2011; 58: 10–18. [DOI] [PubMed] [Google Scholar]
- 43.Khuri-Bulos N, Lang RD, Blevins M, et al. Vitamin D deficiency among newborns in Amman, Jordan. Glob J Health Sci 2013; 6: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nichols EK, Khatib IMD, Aburto NJ, et al. Vitamin D status and associated factors of deficiency among Jordanian children of preschool age. Eur J Clin Nutr 2015; 69: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang Q, Zhang X, Liu S, et al. Correlation between the vitamin D levels and asthma attacks in children: evaluation of the effects of combination therapy of atomization inhalation of budesonide, albuterol and vitamin D supplementation on asthmatic patients. Exp Ther Med 2018; 15: 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollams EM, Hart PH, Holt BJ, et al. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J 2011; 38: 1320–1327. [DOI] [PubMed] [Google Scholar]