Abstract
Background and Aims
Despite concerns that liver transplant (LT) recipients may be at increased risk of unfavorable outcomes from COVID-19 due the high prevalence of co-morbidities, immunosuppression and ageing, a detailed analysis of their effects in large studies is lacking.
Methods
Data from adult LT recipients with laboratory confirmed SARS-CoV2 infection were collected across Europe. All consecutive patients with symptoms were included in the analysis.
Results
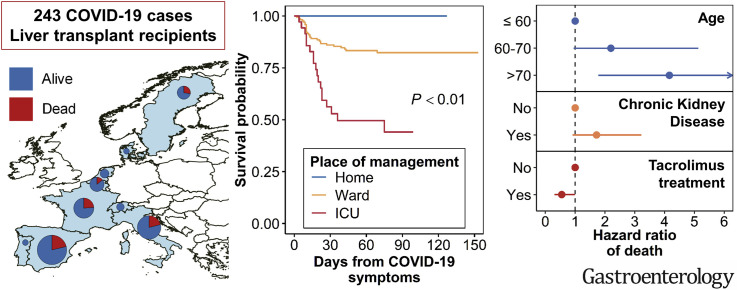
Between March 1 and June 27, 2020, data from 243 adult symptomatic cases from 36 centers and 9 countries were collected. Thirty-nine (16%) were managed as outpatients while 204 (84%) required hospitalization including admission to the ICU (39 of 204, 19.1%). Forty-nine (20.2%) patients died after a median of 13.5 (10–23) days, respiratory failure was the major cause. After multivariable Cox regression analysis, age >70 (HR, 4.16; 95% CI, 1.78–9.73) had a negative effect and tacrolimus (TAC) use (HR, 0.55; 95% CI, 0.31–0.99) had a positive independent effect on survival. The role of co-morbidities was strongly influenced by the dominant effect of age where comorbidities increased with the increasing age of the recipients. In a second model excluding age, both diabetes (HR, 1.95; 95% CI, 1.06–3.58) and chronic kidney disease (HR, 1.97; 95% CI, 1.05–3.67) emerged as associated with death
Conclusions
Twenty-five percent of patients requiring hospitalization for COVID-19 died, the risk being higher in patients older than 70 and with medical co-morbidities, such as impaired renal function and diabetes. Conversely, the use of TAC was associated with a better survival thus encouraging clinicians to keep TAC at the usual dose.
Keywords: COVID-19, Liver transplantation, Outcome, Tacrolimus
Abbreviations used in this paper: ALT, alanine aminotransferase; CI, confidence interval; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; CsA, cyclosporine A; ELITA, European Liver Transplantation Association; ELTR, European Liver Transplant Registry; ICU, intensive care unit; LT, liver transplant; MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; TAC, tacrolimus
Graphical abstract
See editorial on page 1012.
What You Need to Know.
Background and Context
Few studies have analyzed the impact of Cocid-19 in liver transplant recipients and the association of co-morbidities, immunosuppression and ageing on the mortality risk.
New Findings
Age > 70 and tacrolimus use had respectively a negative and a positive independent effect on survival. The role of co-morbidities was strongly influenced by the dominant effect of age as the number of comorbidities increased with the increasing age of the recipients.
Limitations
Although we attempted to collect data on major co-variables there remains the possibility of missing confounders.
Impact
Thees findings should encourage clinicians to keep Tacrolimus at the usual dose as it may be beneficial when treating COVID-19.
The current coronavirus disease 2019 (COVID-19) pandemic has presented unforeseen challenges to health care systems worldwide, with several issues remaining unmet. To date, firm knowledge on disease evolution, risk factors, and optimal management in specific categories of patients is lacking. All transplant recipients are potentially vulnerable to severe acute respiratory syndrome coronavirus (CoV) 2 (SARS-CoV-2) infection, with immune suppression, aging, and metabolic or cardiovascular comorbidities likely being risk factors for symptomatic disease and its severe complications.1 Liver transplant (LT) patients, in particular, represent one of the largest immunosuppressed cohorts in Europe, with 102,116 alive recipients being reported in the European Liver Transplant Registry (ELTR), 42,432 (41.6%) of whom are in their 60s and 12,669 in their 70s or older.2
At present, available data related to COVID-19 in LT patients are limited to a small number of case series,3, 4, 5 to preliminary reports from 2 international registries,6, 7, 8 and to a single international prospective cohort of 57 patients.9 All authors agreed that greater case numbers were urgently required to accurately improve our understanding of individual risk in LT recipients. Thus, a large-scale collaborative study promoted by the European Liver Transplant Association (ELITA) and European Liver Transplant Registry (ELTR) was performed, the main aim being the search for risk factors associated with mortality during the COVID-19 pandemic and with a specific focus on comorbidities and immunosuppression.
Methods
Study Population
ELITA called for a COVID-19 study, which was circulated on March 30, 2020, among 149 LT centers affiliated to ELTR and located in 30 European countries. All centers that reported at least 1 case were provided with a database and instructions on how to record structured data. Data collection was managed by ELTR. Responses were received from 114 centers (76.5%), with 56 centers (38%) having observed COVID-19 in adult LT recipients between March 1 and May 19, 2020. The study included all patients with symptoms and with SARS-CoV-2 infection confirmed by a positive result on a reverse-transcriptase polymerase chain reaction (RT-PCR) assay of a specimen collected on a nasopharyngeal swab or on bronchoalveolar lavage.
Data Collection and Definitions
Demographic and clinical data, including clinical symptoms or signs at presentation, laboratory, and radiologic results during COVID-19 management, as well as administered antiviral therapies and antithrombotic prophylaxis were retrospectively collected. All laboratory tests and radiologic assessments were performed at the discretion of the treating physician. Serum creatinine was converted to mg/dL for analysis. Information on baseline immunosuppression and on changes during COVID-19, namely reduction or discontinuation, was also obtained.
Obesity was defined as a given body mass index of >30 kg/m2. Liver injury during COVID-19 was defined as alanine aminotransferase (ALT) level >30 IU/L for male patients and 19 IU/L for female patients in those with normal ALT levels at the last outpatient visit.10 Hepatic flare was defined as ALT level ≥5 times the upper limit of normal. The time on study started at occurrence of COVID-19 symptoms.
All submitted files from each center were manually reviewed to assess for data quality, completeness, and inconsistencies. In addition, submitting clinicians were contacted and asked to provide corrections or data integration whenever needed.
Ethical and Regulatory Approval
Data were collected in accordance with General Data Protection Regulation, the European Union legislation, and the ELTR privacy policy.
Statistical Analysis
Analysis was led by the Research Centre on Public Health, University of Milan-Bicocca, Monza, Italy. A descriptive analysis of the cohort was performed on the overall population and after stratifying the population by site of management: at home, in general wards, or in intensive care units (ICUs). Categorical variables are summarized through percentages, and continuous variables through median, first quartile and third quartile. Categorical variables were compared using the χ2 or Fisher’s exact tests; continuous variables were compared using the Mann-Whitney U test or the Kruskal-Wallis test, when appropriate. All tests were 2-sided and used a significance level of 0.05.
The rates of missing data for each variable were reported. For each patient, the time between the date of COVID-19 symptoms and death or end of follow-up was computed, and the association between mortality and baseline patients’ characteristics was evaluated through univariate Cox’s proportional hazard models. All characteristic analyzed in the univariate model were included in a stepwise selection process that identified the best multivariate model. The same process was repeated after excluding age from potential predictors. Given the exploratory nature of the study and the limited sample size, a 0.1 significance level was established to retain predictors in the final multivariate models possibly favoring the tracing of borderline significant associations that could be the basis for further studies on wider samples. All statistical analyses were conducted using SAS 9.4 software (SAS Institute, Inc, Cary, NC) and R 4.0.0 software (R Core Team, Vienna, Austria). The map was drawn using QGIS 3.10 software (QGIS Development Team).
Results
Demographic and General Characteristics of Patients
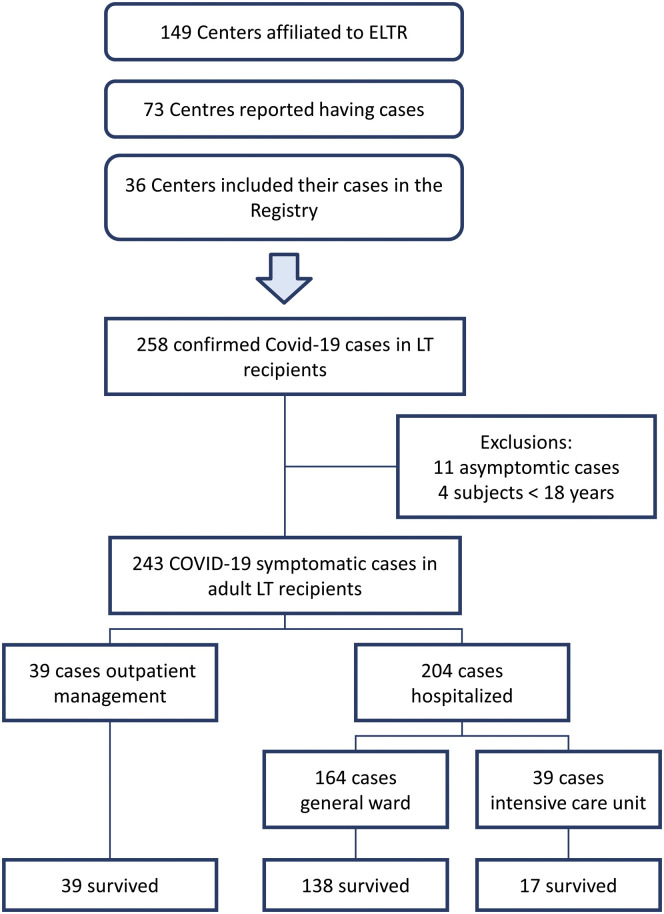
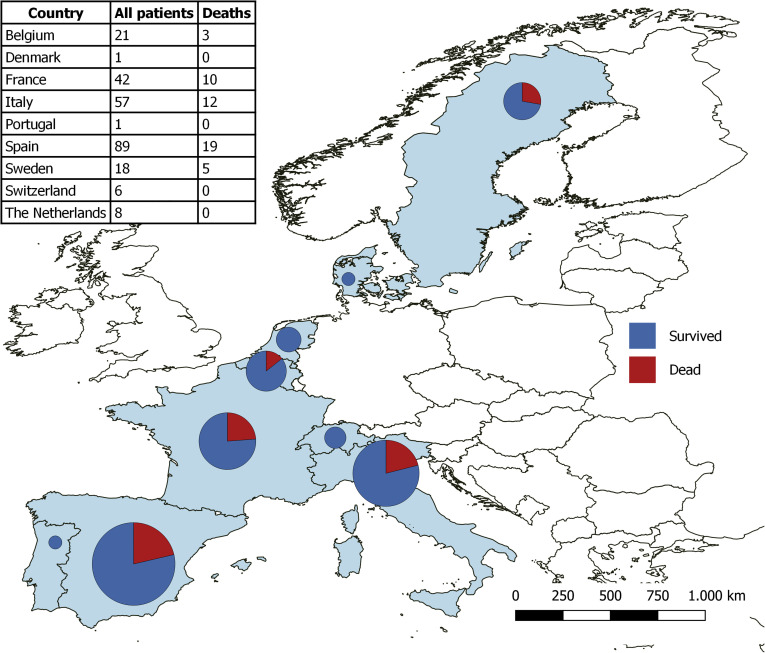
The COVID-19 pandemic was not uniformly experienced in Europe, with large areas being spared. This explains why of the 111 centers responding to the ELITA/ELTR call, only 36 centers from 9 European countries observed at least 1 patient with RT-PCR–confirmed SARS-CoV-2 infection (Figures 1 and 2 ). Of the 29,981 alive patients in regular follow-up at the participating centers, 258 (0.9%) have been consecutively reported in the registry. Excluded from the study were 11 patients (4.3%) who were asymptomatic, in whom the RT-PCR test was performed according to surveillance protocols in case of contact with a SARS-CoV-2–positive individual. Four additional patients were excluded because they were aged <18 years. The remaining 243 symptomatic patients were considered for statistical analysis, with 39 patients (16%) receiving home care, and the remaining 204 requiring hospitalization (Figure 2). Of these, 167 patients (68.7%) were treated in a general ward and 37 in ICUs. Baseline patient characteristics are reported in Table 1 . Thirty-two LT recipients with COVID-10 analyzed in this study were also included in the report from Becchetti et al.9
Figure 1.
Flowchart shows the selection of the study population.
Figure 2.
Patients with COVID-19 included in the study by country.
Table 1.
Baseline Characteristics of the Study Population
| Variables | Place of management |
Total (N = 243) | P value | ||
|---|---|---|---|---|---|
| Home (n = 39) | Ward (n = 167) | ICU (n = 37) | |||
| Male sex | 24 (61.54) | 121 (72.46) | 26 (70.27) | 171 (70.37) | .4051 |
| Age at symptoms, ya,b | 54 (37.0–61.0) | 64 (57.0–72.0) | 64 (58.0–68.0) | 63 (55.0–69.0) | <.0001 |
| Age class at symptoms, ya,b | <.0001 | ||||
| ≤50 | 16 (41.03) | 20 (11.98) | 3 (8.11) | 39 (16.05) | |
| 50–60 | 11 (28.21) | 39 (23.35) | 10 (27.03) | 60 (24.69) | |
| 60–70 | 9 (23.08) | 59 (35.33) | 20 (54.05) | 88 (36.21) | |
| >70 | 1 (2.56) | 48 (28.74) | 4 (10.81) | 53 (21.81) | |
| Location of patient at occurrence of symptomsb | .0119 | ||||
| Home | 39 (100.00) | 148 (88.62) | 30 (81.08) | 217 (89.30) | |
| Hospital | 0 (0.00) | 19 (11.38) | 7 (18.92) | 26 (10.70) | |
| Time between last LT and COVID-19 symptoms, y | 6 (2.2–10.9) | 9 (3.8–15.4) | 5 (1.5–13.3) | 8 (3.1–15.0) | .0295 |
| Time between last LT and COVID-19 symptoms | .1005 | ||||
| <1 year | 5 (12.82) | 19 (11.38) | 7 (18.92) | 31 (12.76) | |
| 1–5 years | 12 (30.77) | 32 (19.16) | 11 (29.73) | 55 (22.63) | |
| 5–10 years | 9 (23.08) | 34 (20.36) | 7 (18.92) | 50 (20.58) | |
| ≥10 years | 10 (25.64) | 81 (48.50) | 10 (27.03) | 101 (41.56) | |
| Missing | 3 (7.69) | 1 (0.60) | 2 (5.41) | 6 (2.47) | |
| Indication for LT | |||||
| Decompensated cirrhosis | 21 (53.85) | 96 (57.49) | 24 (64.86) | 141 (58.02) | .6034 |
| Hepatocellular carcinoma | 8 (20.51) | 43 (25.75) | 12 (32.43) | 63 (25.93) | .4933 |
| Otherb | 10 (25.64) | 29 (17.37) | 1 (2.70) | 40 (16.46) | .0226 |
| Etiology | |||||
| Alcohola | 3 (7.69) | 49 (29.34) | 8 (21.62) | 60 (24.69) | .0149 |
| After nonalcoholic steatohepatitis | 2 (5.13) | 10 (5.99) | 6 (16.22) | 18 (7.41) | .1262 |
| Hepatitis B virus | 5 (12.82) | 34 (20.36) | 4 (10.81) | 43 (17.70) | .2492 |
| Hepatitis C virus active or inactive | 10 (25.64) | 41 (24.55) | 11 (29.73) | 62 (25.51) | .8282 |
| Othera | 20 (51.28) | 49 (29.34) | 10 (27.03) | 79 (32.51) | .0256 |
| Missing | 0 (0.00) | 2 (1.20) | 0 (0.00) | 2 (0.82) | |
| Body mass index, kg/m2 | 25.5 (22.0–28.9) | 25.8 (23.4–29.4) | 27.9 (24.5–29.9) | 25.9 (23.4–29.4) | .1701 |
| Missing | 3 (7.69) | 18 (10.78) | 1 (2.70) | 22 (9.05) | |
| Body mass index >30 kg/m2 | 7 (17.95) | 30 (17.96) | 9 (24.32) | 46 (18.93) | .7924 |
| Comorbidities | |||||
| Nonea,b | 19 (48.72) | 35 (20.96) | 3 (8.11) | 57 (23.46) | <.0001 |
| Diabetesb | 8 (20.51) | 67 (40.12) | 19 (51.35) | 94 (38.68) | .0176 |
| Hypertensionb,c | 11 (28.21) | 71 (42.51) | 29 (78.38) | 111 (45.68) | <.0001 |
| Chronic lung disease | 3 (7.69) | 20 (11.98) | 2 (5.41) | 25 (10.29) | .5267 |
| Chronic kidney diseased | 4 (10.26) | 37 (22.16) | 8 (21.62) | 49 (20.16) | .2419 |
| Coronary artery disease | 3 (7.69) | 9 (5.39) | 5 (13.51) | 17 (7.00) | .2071 |
| Other | 4 (10.26) | 34 (20.36) | 5 (13.51) | 43 (17.70) | .2541 |
| Number of comorbiditiesa,b | .0002 | ||||
| 0 | 19 (48.72) | 35 (20.96) | 3 (8.11) | 57 (23.46) | |
| 1 | 11 (28.21) | 57 (34.13) | 11 (29.73) | 79 (32.51) | |
| ≥2 | 9 (23.08) | 75 (44.91) | 23 (62.16) | 107 (44.03) | |
| Drugs | |||||
| β-Blockers | 6 (15.38) | 34 (20.36) | 10 (27.03) | 50 (20.58) | .4515 |
| ACE inhibitors or angiotensin II receptor antagonistsa,b | 1 (2.56) | 47 (28.14) | 11 (29.73) | 59 (24.28) | .0025 |
| Smoking | .3508 | ||||
| Missing | 0 (0.00) | 1 (0.60) | 1 (2.70) | 2 (0.82) | |
| No | 35 (89.74) | 151 (90.42) | 30 (81.08) | 216 (88.89) | |
| Yes | 4 (10.26) | 15 (8.98) | 6 (16.22) | 25 (10.29) | |
| Type of immunosuppressante | |||||
| TAC | 32 (82.05) | 106 (63.47) | 24 (64.86) | 162 (66.67) | .0831 |
| MMF | 15 (38.46) | 80 (47.90) | 24 (64.86) | 119 (48.97) | .0627 |
| Steroids | 7 (17.95) | 35 (20.96) | 14 (37.84) | 56 (23.05) | .0625 |
| mTOR | 5 (12.82) | 27 (16.17) | 5 (13.51) | 37 (15.23) | .8296 |
| CsA | 1 (2.56) | 23 (13.77) | 5 (13.51) | 29 (11.93) | .1188 |
| Other | 0 (0.00) | 1 (0.60) | 0 (0.00) | 1 (0.41) | >.9999 |
| Combinations of immunosuppressant | |||||
| CsA only | 1 (2.56) | 10 (5.99) | 2 (5.41) | 13 (5.35) | .8264 |
| CsA, MMF | 0 (0.00) | 7 (4.19) | 2 (5.41) | 9 (3.70) | .3842 |
| CsA, steroids | 0 (0.00) | 3 (1.80) | 0 (0.00) | 3 (1.23) | .9999 |
| CsA, MMF, steroids | 0 (0.00) | 3 (1.80) | 1 (2.70) | 4 (1.65) | .5697 |
| TAC only | 12 (30.77) | 36 (21.56) | 6 (16.22) | 54 (22.22) | .2918 |
| TAC, MMF | 12 (30.77) | 35 (20.96) | 5 (13.51) | 52 (21.40) | .1806 |
| TAC, mTOR | 2 (5.13) | 10 (5.99) | 0 (0.00) | 12 (4.94) | .4209 |
| TAC, steroids, or other | 6 (15.38) | 16 (9.58) | 5 (13.51) | 27 (11.11) | .4473 |
| TAC, MMF, mTOR | 0 (0.00) | 0 (0.00) | 1 (2.70) | 1 (0.41) | .1523 |
| TAC, MMF, steroidsb | 0 (0.00) | 9 (5.39) | 6 (16.22) | 15 (6.17) | .011 |
| TAC, MMF, mTOR, steroids | 0 (0.00) | 0 (0.00) | 1 (2.70) | 1 (0.41) | .1523 |
| MMF only | 3 (7.69) | 17 (10.18) | 4 (10.81) | 24 (9.88) | .8966 |
| MMF, mTOR | 0 (0.00) | 7 (4.19) | 3 (8.11) | 10 (4.12) | .1712 |
| MMF, steroids | 0 (0.00) | 2 (1.20) | 1 (2.70) | 3 (1.23) | .4484 |
| mTOR only | 2 (5.13) | 9 (5.39) | 0 (0.00) | 11 (4.53) | .4577 |
| mTOR, steroids | 1 (2.56) | 1 (0.60) | 0 (0.00) | 2 (0.82) | .5286 |
| Steroids only | 0 (0.00) | 2 (1.20) | 0 (0.00) | 2 (0.82) | >.9999 |
| Most recent values before symptoms | |||||
| White blood cells, 109/L | 5.1 (4.4–6.5) | 5.2 (3.9–6.7) | 6.0 (4.3–6.7) | 5.2 (4.0–6.7) | .9274 |
| Bilirubin, mg/dL | 0.8 (0.5–1.0) | 0.6 (0.4–1.0) | 0.6 (0.5–1.0) | 0.7 (0.5–1.0) | .7569 |
| Creatinine, mg/dLa,b | 1.0 (0.9–1.1) | 1.1 (0.9–1.5) | 1.2 (1.0–1.6) | 1.1 (0.9–1.4) | .019 |
| ALT, U/L | 23.0 (17.0–32.0) | 20.0 (15.0–31.0) | 23.0 (17.0–34.0) | 20.0 (16.0–32.0) | .3607 |
NOTE. Data are presented n (%) or median (1st–3rd quartile).
ACE, angiotensin converting enzyme; mTOR, mammalian target of rapamycin inhibitors.
P value ward vs home ≤.05.
P value ICU vs home ≤.05.
P value ICU vs ward ≤.05.
Plasma creatinine >2 mg/dL.
Patients can be treated with >1 therapy; therefore, percentages do not sum to 100.
Comorbidities
A total of 111 patients (45.7%) had arterial hypertension, 94 (38.7%) had diabetes mellitus, 49 (20.2%) had chronic kidney disease with a creatinine >2 mg/dL, and 25 (10.3%) had chronic lung diseases. Concurrent comorbidities were frequent, with 107 patients (44%) having ≥2 (Table 1). The prevalence of at least 2 comorbidities increased with age being observed in 25.3%, 53.4%, and 64.2% in recipients aged <60 years, 60 to 70 years, or >70 years, respectively.
Immunosuppressive Drugs and Other Drugs
Tacrolimus (TAC) and cyclosporine A (CsA) were considered as the main immunosuppressive drugs. Because some of the patients were off a calcineurin inhibitor (CNI), the proportion of patients receiving each immunosuppressive drug or combination of drugs was also obtained. At the time of analysis, 162 patients (66.7%) were on TAC, alone or in combination, 29 (11.9%) were on CsA alone or in combination, 119 (49.0%) were on mycophenolate mofetil (MMF) alone or in combination, and 37 (15.2%) were on mammalian target of rapamycin inhibitors alone or in combination (Table 1).
Clinical Presentation and Course of Liver Transplant Recipients With COVID-19
At the time of diagnosis, the most commonly self-reported symptoms included fever in 190 patients (78.2%), cough in 143 (58.8%), dyspnea in 82 (33.7%), muscle pain or asthenia in 90 (37.0%), anosmia or dysgeusia in 21 (8.6%), and diarrhea in 55 (22.6%). Radiologic findings on computed tomography scan or on chest radiography showed typical ground-glass opacities in 145 patients (59.7%) (Table 2 ). Overall, 137 patients (56.4%) required respiratory support during hospitalization, with 26 requiring noninvasive ventilation and 25 mechanical ventilation (Table 2). Specific anti–SARS-CoV-2 treatment was administered to 149 patients: 116 (47.7%) were treated with hydroxychloroquine alone or in combination, 41 (16.9%) with lopinavir-ritonavir, 34 (14.0%) with high doses of corticosteroids, and 15 (6.2%) with tocilizumab.
Table 2.
Clinical Presentation and Course After COVID-19 Symptoms
| Variable | Place of management |
Total (N = 243) | P value | ||
|---|---|---|---|---|---|
| Home (n = 39) | Ward (n = 167) | ICU (n = 37) | |||
| Symptoms: at clinical diagnosis | |||||
| Fever >37.2°Ca | 25 (64.10) | 137 (82.04) | 28 (75.68) | 190 (78.19) | .0468 |
| Cough | 21 (53.85) | 106 (63.47) | 16 (43.24) | 143 (58.85) | .0609 |
| Polypnea or dyspneaa,b,c | 4 (10.26) | 57 (34.13) | 21 (56.76) | 82 (33.74) | .0001 |
| Diarrheaa | 3 (7.69) | 46 (27.54) | 6 (16.22) | 55 (22.63) | .0171 |
| Anosmia and dysgeusiaa | 9 (23.08) | 10 (5.99) | 2 (5.41) | 21 (8.64) | .0061 |
| Muscle paina | 13 (33.33) | 24 (14.37) | 4 (10.81) | 41 (16.87) | .0098 |
| Confusion | 0 (0.00) | 4 (2.40) | 3 (8.11) | 7 (2.88) | .0969 |
| Thoracic pain | 3 (7.69) | 11 (6.59) | 1 (2.70) | 15 (6.17) | .717 |
| Asthenia | 11 (28.21) | 34 (20.36) | 4 (10.81) | 49 (20.16) | .1669 |
| Other | 4 (10.26) | 11 (6.59) | 0 (0.00) | 15 (6.17) | .1591 |
| Time between symptoms and positive test, db | 9 (3–19) | 5 (2–9) | 3 (0–7) | 4 (2–10) | .0226 |
| Chest x-ray or thorax CT scan | |||||
| Noa,b | 16 (41.03) | 8 (4.79) | 4 (10.81) | 28 (11.52) | <.0001 |
| Yes, normalb,c | 15 (38.46) | 51 (30.54) | 0 (0.00) | 66 (27.16) | .0002 |
| Yes, ground-glass opacitiesa,b,c | 7 (17.95) | 106 (63.47) | 32 (86.49) | 145 (59.67) | <.0001 |
| Yes, lobar opacitiesc | 1 (2.56) | 6 (3.59) | 7 (18.92) | 14 (5.76) | .0044 |
| Ground-glass or lobar opacitiesa,b,c | 8 (20.51) | 108 (64.67) | 33 (89.19) | 149 (61.32) | <.0001 |
| Respiratory supportc | <.0001 | ||||
| Oxygen support | 1 (50.00) | 78 (79.59) | 7 (18.92) | 86 (62.77) | |
| Noninvasive ventilation | 1 (50.00) | 17 (17.35) | 8 (21.62) | 26 (18.98) | |
| Mechanical ventilation | 0 (0.00) | 3 (3.06) | 22 (59.46) | 25 (18.25) | |
| Added lung infection | |||||
| Noneb,c | 39 (100.00) | 154 (92.22) | 25 (67.57) | 218 (89.71) | <.0001 |
| Bacterialb | 0 (0.00) | 11 (6.59) | 7 (18.92) | 18 (7.41) | .0064 |
| Fungalc | 0 (0.00) | 1 (0.60) | 5 (13.51) | 6 (2.47) | .0011 |
| Other | 0 (0.00) | 2 (1.20) | 0 (0.00) | 2 (0.82) | >.9999 |
| Renal replacement therapyb,c | 0 (0.00) | 10 (5.99) | 11 (29.73) | 21 (8.64) | <.0001 |
| Vasoactive drugs (NA)b,c | 1 (2.56) | 1 (0.60) | 19 (51.35) | 21 (8.64) | <.0001 |
| Myocarditis | 0 (0.00) | 0 (0.00) | 1 (2.70) | 1 (0.41) | .1523 |
| Peak laboratory values | |||||
| Bilirubin, mg/dLc | 0.8 (0.5–1.1) | 0.7 (0.4–1.0) | 1.2 (0.8–2.7) | 0.8 (0.5–1.2) | .0034 |
| International normalized ratiob,c | 1.1 (1.0–1.2) | 1.1 (1.1–1.3) | 1.3 (1.1–1.7) | 1.1 (1.1–1.3) | .0039 |
| Creatinine, mg/dLb,c | 1.0 (0.9–1.6) | 1.2 (0.9–1.8) | 2.2 (1.2–4.0) | 1.3 (0.9–2.0) | .0009 |
| ALT, U/Lb,c | 28.0 (19.0–39.0) | 32.0 (19.0–51.5) | 59.5 (32.5–134.5) | 34.0 (20.0–55.0) | .0014 |
| COVID-19 therapy | |||||
| Noneab | 33 (84.62) | 46 (27.54) | 15 (40.54) | 94 (38.68) | <.0001 |
| Lopinavir/ritonavira,b | 0 (0.00) | 35 (20.96) | 6 (16.22) | 41 (16.87) | .007 |
| Hydroxychloroquinea,b,c | 4 (10.26) | 99 (59.28) | 13 (35.14) | 116 (47.74) | <.0001 |
| High-dose steroidsa,b | 0 (0.00) | 26 (15.57) | 8 (21.62) | 34 (13.99) | .0144 |
| Remdesevir | 0 (0.00) | 0 (0.00) | 1 (2.70) | 1 (0.41) | .1523 |
| Tocilizumab | 0 (0.00) | 11 (6.59) | 4 (10.81) | 15 (6.17) | .0962 |
| Azythromicina | 2 (5.13) | 57 (34.13) | 8 (21.62) | 67 (27.57) | .0009 |
| Otherb | 1 (2.56) | 15 (8.98) | 8 (21.62) | 24 (9.88) | .0215 |
| Immunosuppression changes | |||||
| Yesab | 4 (10.26) | 71 (42.51) | 22 (59.46) | 97 (39.92) | <.0001 |
| Stop CNI | 0 (0.00) | 11 (6.59) | 5 (13.51) | 16 (6.58) | .0441 |
| 25%-50% reduction in CNI | 2 (5.13) | 28 (16.77) | 8 (21.62) | 38 (15.64) | .1091 |
| Stop antimetabolitesb | 1 (2.56) | 26 (15.57) | 8 (21.62) | 35 (14.40) | .0455 |
| Stop mTOR inhibitors | 0 (0.00) | 9 (5.39) | 1 (2.70) | 10 (4.12) | .3305 |
| Other | 1 (2.56) | 5 (2.99) | 0 (0.00) | 6 (2.47) | .1479 |
| Outcomea,b,c | <.0001 | ||||
| Alive | 39 (100.00) | 138 (82.63) | 17 (45.95) | 194 (79.84) | |
| Dead | 0 (0.00) | 29 (17.37) | 20 (54.05) | 49 (20.16) | |
| Time between symptoms and last follow-up, db,c | 70 (48–88) | 66 (42–88) | 29 (17–75) | 65 (35–87) | .007 |
| Missing | 3 (7.69) | 1 (0.60) | 2 (5.41) | 6 (2.47) | |
| Cause of death | |||||
| Refractory pneumonia | 23 (79.31) | 15 (75.00) | 38 (77.55) | .7405 | |
| Liver-related death | |||||
| Without lung failure | 1 (3.45) | 0 (0.00) | 1 (2.04) | >.9999 | |
| With lung failure | 2 (6.90) | 1 (5.00) | 3 (6.12) | >.9999 | |
| Other | 3 (10.34) | 4 (20.00) | 7 (14.29) | .4221 | |
| Heparina,b | <.0001 | ||||
| Missing | 13 (33.33) | 20 (11.98) | 6 (16.22) | 39 (16.05) | |
| No | 24 (61.54) | 53 (31.74) | 10 (27.03) | 87 (35.80) | |
| Yes | 2 (5.13) | 94 (56.29) | 21 (56.76) | 117 (48.15) | |
| Average CNI level pre–COVID-19 | .0235 | ||||
| No CNI | 4 (10.26) | 5 (2.99) | 1 (2.70) | 10 (4.12) | |
| CsA ≤50 ng/L | 1 (2.56) | 6 (3.59) | 4 (10.81) | 11 (4.53) | |
| CsA 50–100 ng/L | 1 (2.56) | 2 (1.20) | 0 (0.00) | 3 (1.23) | |
| CsA >100 ng/L | 0 (0.00) | 35 (20.96) | 6 (16.22) | 41 (16.87) | |
| TAC ≤4 ng/mL | 3 (7.69) | 22 (13.17) | 6 (16.22) | 31 (12.76) | |
| TAC 4–6 ng/mL | 10 (25.64) | 25 (14.97) | 6 (16.22) | 41 (16.87) | |
| TAC >6 ng/mL | 6 (15.38) | 25 (14.97) | 6 (16.22) | 37 (15.23) | |
NOTE. Data are presented n (%) or median (1st–3rd quartile).
CT, computed tomography; mTOR, mammalian target of rapamycin; NA, noradrenaline.
P value ward vs home ≤.05
P value ICU vs home ≤.05
P value ICU vs ward ≤.05
Thromboprophylaxis, mainly with low-molecular-weight heparin, was started on COVID-19 diagnosis in 117 patients (48.2%). Thrombotic events occurred in 7 of 204 (3.4%) hospitalized patients, comprising 3 pulmonary embolisms, 2 deep vein thromboses, and 2 strokes.
An acute liver injury was observed in 56 patients with previous persistently normal ALT levels, being in the flare range in 10 patients. Acute rejection was reported in 3 patients. Notably, CNI had been withdrawn in 2 patients, and the dose of mammalian target of rapamycin had been halved in the third patient.
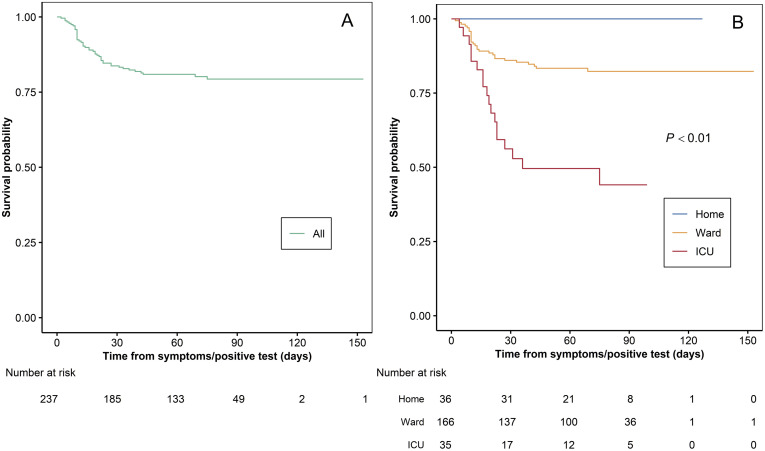
Forty-nine patients (20.2%) died after a median of 13.5 days (first–third quartile, 10–23 days) from the diagnosis of COVID-19. Causes of death were respiratory failure in 39 patients (77.6%), end-stage liver disease with respiratory failure in 2, end-stage liver disease without respiratory failure in 1, hemorrhagic shock in 2, pulmonary embolism in 1, metastatic cancer in 1 septic shock in 1, and septic complication from tracheal fistula in 1. Overall Kaplan-Meier survival from the date of COVID-19 symptoms is given in Figure 3 . Estimated a probability of survival was 88.2% (95% confidence interval [CI], 82.5%–92.1%) at 30 days and 84.4% (95% CI, 77.7%–89.2%) at 90 days.
Figure 3.
Kaplan-Meier survival curve from the date of COVID-19 symptoms (A) overall and (B) stratified by place of management.
Clinical Features and Outcomes of Liver Transplant Recipients With COVID-19 Treated at Home, in General Wards, and in Intensive Care Units
Baseline characteristics of patients with less severe symptoms who could be treated at home and those with more severe symptoms requiring hospitalization in general wards and ICUs are reported in Table 2. Patients treated at home were younger, had fewer comorbidities, and were more frequently receiving TAC as the primary immunosuppressant. Kaplan-Meier survival after stratification by place of management, at home, general ward, or ICU is provided in Figure 3. Patients managed at home survived, whereas the probability of survival at 30 days was 93.1% (95% CI, 86.7%–96.5%) and 57.0% (95% CI, 37.6%–72.4%), respectively, for patients in ward and in ICUs, and it declined to 89.8% (95% CI, 82.1%–94.3%) and 46.6% (95% CI, 26.2%–64.6%) at 90 days. Notably, 12 patients with advanced COVID-19 disease were not admitted to an ICU, 8 because they were deemed too sick for the ICU due to a combination of advanced age and severe comorbidities and 4 because ICUs were overwhelmed.
Factors Associated With Death
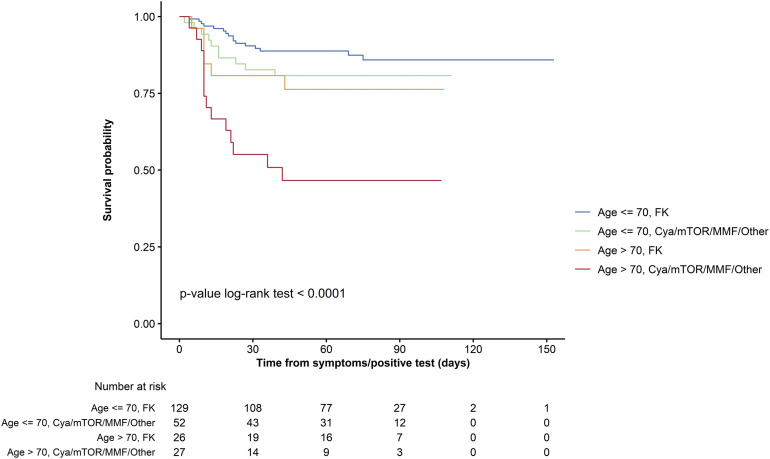
Factors by univariable analysis significantly associated with death were increased age of the recipient, time from LT, diabetes, chronic kidney disease, number of comorbidities, and use of TAC (Table 3 ). After multivariable analysis, advanced age (>70 vs <60 years) remained independently associated with an increased mortality risk (hazard ratio, 4.16; 95% CI, 1.78–9.73), whereas use of TAC was confirmed independently associated with a reduced mortality risk (hazard ratio, 0.55; 95% CI, 0.31–0.99). The Kaplan-Meier survival curves stratified by age (>70 or <70) and type of immunosuppressant (TAC vs non-TAC) may be helpful for the clinician to better understand the individual risk (Supplementary Figure 1).
Table 3.
Results From Univariate and Multivariate Analysis of Predictors of Mortality, From Cox’s Proportional Hazard Regression Models
| Variable | Univariate models |
Multivariate models |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | ||||
| Linear (1-year increase) | 1.06 (1.03–1.10) | <.0001 | ||
| 60–70 vs ≤60 years | 2.58 (1.12–5.94) | .0255 | 2.20 (0.94–5.13) | .068 |
| >70 vs ≤60 years | 5.49 (2.42–12.48) | <.0001 | 4.16 (1.78–9.73) | .001 |
| Sex (male vs female) | 1.39 (0.71–2.73) | .3438 | ||
| Indication for LT | ||||
| Decompensated cirrhosis | 1.11 (0.61–2.00) | .736 | ||
| Hepatocellular carcinoma | 1.25 (0.67–2.34) | .4846 | ||
| Other | 0.63 (0.25–1.61) | .3362 | ||
| Time between LT and COVID-19 symptoms (1-year increase) | 1.05 (1.01–1.09) | .0054 | ||
| Body mass index (1-unit increase) | 1.00 (0.94–1.07) | .9936 | ||
| Comorbidities | ||||
| Diabetes | 1.98 (1.11–3.54) | .0212 | ||
| Hypertension | 1.76 (0.98–3.17) | .0584 | ||
| Chronic lung disease | 0.55 (0.17–1.76) | .3126 | ||
| Chronic kidney diseasea | 2.20 (1.19–4.08) | .0123 | 1.72 (0.92–3.22) | .0912 |
| Coronary artery disease | 1.37 (0.49–3.81) | .5518 | ||
| Other | 1.71 (0.89–3.31) | .1095 | ||
| Comorbidities, n | ||||
| 1 vs 0 | 3.54 (1.02–12.33) | .0468 | ||
| ≥2 vs 0 | 5.63 (1.72–18.50) | .0044 | ||
| Smoking (yes vs no) | 1.62 (0.72–3.63) | .241 | ||
| Type of immunosuppressant | ||||
| CsA vs all other | 2.29 (1.13–4.60) | .0209 | ||
| TAC vs all other | 0.43 (0.24–0.77) | .0042 | 0.55 (0.31–0.99) | .0472 |
| MMF vs all other | 1.30 (0.73–2.33) | .3704 | ||
| mTOR inhibitors vs all other | 1.37 (0.66–2.84) | .3969 | ||
| Treatment with ACE inhibitors or angiotensin II receptor antagonists (yes vs no) | 1.92 (1.06–3.49) | .0328 | ||
| Country | ||||
| Spain vs Other | 1.52 (0.67–3.48) | .3178 | ||
| Italy vs Other | 1.34 (0.54–3.34) | .5253 | ||
| France vs Other | 1.48 (0.55–3.94) | .4355 | ||
| Center recruiting more than 9 patients vs other centers | 1.47 (0.82–2.65) | .1993 | ||
NOTE. Bold values are statistically significant (P < .05).
ACE, angiotensin converting enzyme; CT, computed tomography; HR, hazard ratio; mTOR, mammalian target of rapamycin.
Plasma creatinine >2 mg/dL.
Supplementary Figure 1.
Kaplan-Meier curves for survival from the date of COVID-19 diagnosis, stratified by age (2 categories) and main immunosuppressant. Cya, cyclosporin A; FK, tacrolimus; mTOR, mammalian target of rapamycin.
Because the number of comorbidities increased with the increasing age of the recipient, a second model excluding age was constructed. This allowed diabetes and chronic renal failure to emerge as predictors of mortality, their effect having been shadowed in the first model by the dominant effect of age (Supplementary Table 1).
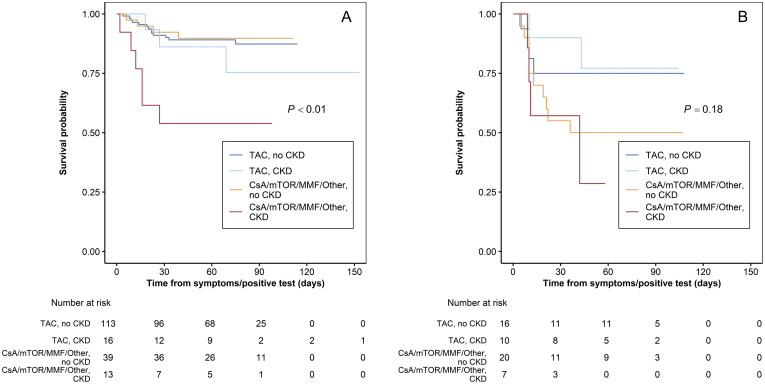
The interplay among age of the recipient, primary immunosuppressant, and chronic renal failure is summarized in Supplementary Table 2 and Supplementary Figure 2, where the negative impact of chronic kidney disease is dramatically evident in recipients not maintained on TAC. Finally, in Supplementary Table 3, patients receiving TAC-based vs non–TAC-based regimens are compared with respect to some relevant clinical variables such as age, time from transplant, chronic renal failure, concurrent exposure to angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and presence of hepatocellular carcinoma. In fact, patients receiving TAC were younger and had fewer comorbidities, these variables being potentially associated with a better outcome. Conversely patients on TAC were much less frequently treated with angiotensin-converting enzyme or angiotensin receptor blocker inhibitors, this therapy being associated with a better outcome. All these variables were included in the multivariable analysis that confirmed the independent protective role of TAC.
Supplementary Figure 2.
Kaplan-Meyer curves for survival from the date of COVID-19 diagnosis show the interplay between age of the recipient, primary immunosuppressant, and chronic renal failure (CRF). mTOR, mammalian target of rapamycin.
Discussion
As more than 200 countries worldwide are still struggling with the COVID-19 pandemic, all solid-organ transplant recipients are at risk of infection and poor outcome due to chronic immunosuppression, high rates of comorbidities, advanced age, and frequent hospitalization. We have analyzed the characteristics, management, and outcome of a large multinational European cohort of LT recipients with symptomatic SARS-CoV-2 infection.
Rates of hospitalization and death in the current study were 85% and 20.2%, confirming what we already showed in our preliminary report on the first 103 patients,7 where some patients were still experiencing their disease course. These findings concur with the 23% mortality risk reported by Webb et al,6 but compare unfavorably with the 12% mortality risk observed by Becchetti et al,9 possibly due to the lower percentage of patients requiring hospitalization in this latter study. Our study confirmed that abdominal symptoms and, more specifically, diarrhea are at least twice more frequent than in the general population9 and are possibly associated to MMF. This hypothesis is supported by the fact that almost 50% of the 26 patients maintained on MMF as the primary immunosuppressant had diarrhea as presenting symptom. Clinicians should therefore be vigilant and consider SARS-CoV-2 testing in transplant recipients presenting with diarrhea, particularly if using MMF.
However, the main finding of the present study is the significant variation in mortality risk with both age of the recipients and use of TAC as immunosuppressant. The role of advanced age confirms what has been extensively observed in the general population, with patients older than 70 having an increased 4-fold mortality risk.11, 12, 13, 14 The lower risk of death for patients maintained on TAC was unexpected and to our knowledge has not been previously reported. In particular Becchetti et al9 could not explore this association in their prospective cohort of 57 LT recipients with COVID-19 because the great majority of their patients were receiving TAC. Notably, in our analysis, the beneficial impact of TAC was robust and persisted after controlling for various confounders. The biological explanation of the potential favorable role of TAC is unknown but may be dual: inhibition of viral replication and interaction with the immune response. Some studies have shown that CoV replication, depends on active immunophilin pathways and that TAC is capable of strongly inhibiting the growth of some human CoV, notably SARS CoV-1, probably by binding the immunophilin FK506-binding proteins, although not specifically SARS-CoV-2.10 , 15 , 16
Another potential driver of the TAC protective effect could be related to the immunosuppressive property of this CNI.17 By inhibiting calcineurin and suppressing the early phase of T-cell activation, TAC reduces the production of many cytokines, notably proinflammatory cytokines, as tumor necrosis factor-α and interferon-γ, and possibly mitigates the cytokine storm that characterizes stage III COVID-19. Interestingly, this background recently prompted a group of Spanish investigators to test the effect of TAC in combination with steroids in the management of COVID-19 occurring in immunocompetent individuals (clinicaltrials.gov/ct2/show/NCT04341038). While waiting for studies on larger cohorts of transplant recipients that would allow a more precise estimate of the protective effect of TAC, reducing or withdrawing the doses of TAC during COVID-19 should be discouraged, if not indicated for other clinical reasons.
The role of comorbidities as relevant risk factors for mortality has been clearly demonstrated in the general population with COVID-19.18 Despite being highly prevalent among LT recipients,19 neither a specific comorbidity nor a combination of comorbidities emerged as independently associated with outcome. This is at least partly explained by the dominant effect of age as comorbidities increased with the increasing age of the recipients. Nevertheless, in our exploratory analysis, chronic renal failure, defined by a serum creatinine >2 mg/dL, maintained a trend of significance (P < .1) even if shadowed by the dominant effect of increasing age. Notably, the negative impact of renal failure on survival was particularly relevant in patients who were not receiving TAC, once again pointing to its possible protective role against COVID-19, at least in LT recipients.
Finally, therapy for COVID-19 differed across centers and countries and varied over time with the increasing knowledge in treating this new disease. Because large prospective randomized trials have recently demonstrated that corticosteroids and remdesivir are effective in severe cases, whereas hydroxychloroquine and lopinavir-ritonavir are not, new patients should be treated accordingly.20 , 21
This study has some strengths. It is, at the time of writing, the largest cohort of consecutive transplant recipients affected by COVID-19 with a relatively long median follow-up of approximately 2 months. It focuses only on symptomatic patients and analyzes the role of clinical features at admission and diagnosis on mortality risk. The quality of the data was guaranteed by maintaining constant communications with the contributing centers. Finally, the international multicentered pattern of the study copes with any individual center effect.
Some limitations are also to be acknowledged. Firstly, although we attempted to collect data on major covariables, there remains the possibility of missing confounders. Secondly, we focused on symptomatic patients with confirmed positive SARS-CoV-2 RT-PCR test despite test sensitivity <80%. Thus, some patients were excluded.
Conclusion
This study, including more than 240 LT recipients, confirmed that 25% of patients requiring hospitalization for COVID-19 died, the mortality risk being greater in patients aged older than 70 and with medical comorbidities such as impaired renal function and diabetes. Conversely, the use of TAC was associated with an increased survival probability. Although the biological explanation of this latter finding is currently unknown, our preliminary evidence should encourage clinicians to keep TAC at the usual dose because it may be beneficial when treating COVID-19. A more precise estimate of the protective effect of TAC requires studies on larger cohorts of transplant recipients.
Acknowledgments
ELITA board members Ulrich Baumann, Giacomo Germani, Silvio Nadalin, Pavel Taimr, Christian Toso, Roberto Trosi, and Krzysztof Zieniewicz supported and actively promoted the study. Maruska Nizzi provided linguistic and writing support. All centers participating in the ELITA/ELTR COVID-19 project in liver transplantation, including all collaborators at each site, are listed in Table 4 .
Table 4.
European Liver Transplantation Association/ European Liver Transplant Registry COVID-19 Registry for Liver Transplant Candidates and Recipients: Collaborators With Affiliations
| 1. | Division of Transplantation, Department of Surgery, Medical University of Vienna, Austria: Gabriela Berlakovich, Dagmar Kollmann, Georg Györi |
| 2. | Universitair Ziekenhuis Antwerpen, Edegem, Belgium: Dirk Ysebaert, Patrick Hollants |
| 3. | Universitair Ziekenhuis Dienst voor Algemene en Hepatopancreaticobiliaire Heelkunde en Levertransplantatie, Ghent, Belgium: Frederik Berrevoet, Aude Vanlander |
| 4. | Universitair Ziekenhuis, Dienst Voor Levertransplantatie En Digestieve Heelkunde, Ghent, Belgium: Frederck Berrevoet, Eric Hoste, Christel Walraevens, Roberto Ivan Troisi |
| 5. | Liver Transplant Programme, University Leuven, Belgium: Jacques Pirenne, Frederick Nevens, Natalie Vandenende |
| 6. | CHU Liege,University of Liege, Belgium: Oliver Detry, Josee Monard, Nicolas Meurisse |
| 7. | Cliniques Universitaires Saint Luc, Catholic University of Louvain, Brussels, Belgium: Olga Ciccarelli |
| 8. | Hopital Erasme Universite Libre De Bruxelles, Department of Abdominal Surgery, Brussels, Belgium: Valerio Lucidi |
| 9. | Hopital Cantonal Universitaire De Geneve, Departement De Chirurgie, Geneva, Switzerland: Giulia Magini, Thierry Berney, Anne-Catherine Saouli |
| 10. | University Hospital Copenhagen, Department for Surgery and Transplantation Rigshospitalet, Copenhagen, Denmark: Allan Rasmussen |
| 11. | Hôpital De La Croix Rousse, Chirurgie Générale Et Digestive, Lyon, France: Sylvie Radenne, Mickael Lesurtel |
| 12. | Hôpital Henri Mondor, Service d’Hepatologie, Créteil, France: Christophe Duvoux, Norbert Ngongang |
| 13. | Hôpital Paul Brousse, Centre Hépato Biliaire, Villejuif, France: Audrey Coilly |
| 14. | C.H.R.U. De Strasbourg, Hôpital Hautepierre, Strasbourg, France: Francoise Faitot |
| 15. | Hepatogastroenterology Unit, Hopital Trousseau, C.H.R.U. de Tours, Tours, France: Laure Elkrief |
| 16. | Hôpital Bicêtre, Hépatologie et Transplantation Hépatique Pédiatriques, AP-HP Université Paris-Saclay, Le Kremlin-Bicêtre, France: Emmanuel Gonzales |
| 17. | The Queen Elizabeth Hospital, Queen Elisabeth Medical Center, Birmingham, United Kingdom: Darius Mirza, Thamara Perera, Hann Angus |
| 18. | University of Edinburgh Royal Infirmary, Liver Transplantation Unit, Edinburgh, United Kingdom: Gabriel Oniscu, Chris Johnston |
| 19. | Papa Giovanni XXIII Hospital, Chirurgia E Centro Trapianti Di Fegato, Bergamo, Italy: Luisa Pasulo, Michela Guizzetti, Marco Zambelli |
| 20. | Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy: Cristina Morelli, Giovanni Vitale |
| 21. | Istituto Nazionale Tumori Milano, Department of Hepatology, Hepato-pancreatic-biliary Surgery and Liver Transplantation, Istituto Nazionale Tumori, Milan, Italy: Sherrie Bhoori, Vincenzo Mazzaferro, Roberta Elisa Rossi |
| 22. | Ospedale Maggiore Di Milano, U.O. Chirurgia Generale E Dei Trapianti, Milano, Italy: Federica Invernizzi, Francesca Donato, Giorgio Rossi |
| 23. | Ospedale Niguarda Ca Granda, Hepatology and Gastroenterology Unit and Transplant Surgery Unit, Milano, Italy: Luca S Belli, Giovanni Perricone, Raffaella Viganò, Chiara Mazzarelli, Luciano De Carlis |
| 24. | University of Modena E Reggio Emilia, Policlinico Di Modena, Modena, Italy: Fabrizio Di Benedetto, Paolo Magistri, Antonia Zuliani |
| 25. | Ospedale Cisanello, U.O. Trapiantologia Epatica Universitaria Azienda Ospedaliera, Pisa, Italy: Paolo De Simone, Paola Carrai, Stefania Petruccelli |
| 26. | Liver Transplant Unit, AOU Città della Salute e della Scienza di Torino, Torino, Italy: Damiano Patrono, Silvia Martini, Renato Romagnoli |
| 27. | University Medical Center Groningen, Department of Gastroenterology and Hepatology, Groningen, Netherlands: Aad Van Der Berg, Frank Cuperus |
| 28. | Erasmus MC, Transplant Insitute, University Medical Center Rotterdam, Department of Surgery, Divion of Hepatobiliry Surgery and Liver Transplantation, Rotterdam, The Netherlands: Wojciech Polak, Herold Metselaar |
| 29. | Hospital Gal De Santo Antonio, Department of Surgery and Organ Transplantation, Porto, Portugal: Jorge Daniel |
| 30. | Hospital General Universitario De Alicante, Unidad Transplantes Hepatico, Alicante, Spain: Gonzalo Rodriguez, Sonia Pascual |
| 31. | Hospital Clinic I Provincial De Barcelona, Gastrointestinal Surgery Department, Barcelona, Spain: Costantino Fondevila, Jorde Colmenero |
| 32. | Hospital Universitari De Bellvitge, Unidad De Trasplante Hepatico Unidad De Trasplante Hepatico, Barcelona, Spain: Laura LLado, Carme Baliellas |
| 33. | Hospital Universitari Vall D Hebron; Barcelona, Spain: Lluis Castells, Isabel Campos-Varela, Liver Unit; Ernest Hidalgo, Liver Transplant Unit |
| 34. | Hospital Universitario 12 de Octubre, HBP And Transplant Unit, General Surgery, Madrid, Spain: Carmelo Loinaz Segurola, Alberto Marcacuzco, Felix Cambra |
| 35. | Hospital Gregorio Maranon, Liver Transplant Unit, Madrid, Spain: Magdalena Salcedo Plaza, Fernando Diaz-Fontenla |
| 36. | Hospital Universitario Puerta de Hierro, Unidad de Trasplante Hepatico, Madrid, Spain: Valentin Cuervas-Mons, Ana Arias Milla, Alejandro Muñoz |
| 37. | Liver Transplant Unit, Hospital Virgen del Rocio, Seville, Spain: Jose Maria Alamo |
| 38. | Cirurgia HPB y Transplante Hepatico, Hospital Universitario de Badajoz, Spain: Gerardo Blanco |
| 39. | Hospital Universitario, Virgen De La Arrixaca, El Palmar (Murcia), Spain: Victor Lopez Lopez. |
| 40. | Clinica Universitaria, Universidad De Navarra, Facultad De Medicina, Pamplona, Spain: Pablo Marti-Cruchaga |
| 41. | Hospital Universitario Marques De Valdecilla, Unidad De Traspante Hepatico, Santander, Spain: Rodriguez San Juan |
| 42. | Hospital Universitario Virgen De La Nieves, Servicio De Cirugia General, Granada, Spain: Esther Brea Gomes |
| 43. | Huddinge Hospital, Department of Transplantation Surgery, Huddinge, Sweden: Bo Goran Ericzon, Carl Jorns |
CRediT Authorship Contributions
Luca Saverio Belli, MD (Conceptualization: Lead; Investigation: Equal; Project administration: Lead; Writing – original draft: Lead). Constantino Fondevila, Dr (Supervision: Equal; Writing – review & editing: Supporting). Sara Conti, Dr (Formal analysis: Lead; Methodology: Equal). Paolo Cortesi, Dr (Formal analysis: Lead; Methodology: Lead). Vincent Karam, PhD (Writing – review & editing: Supporting). Rene Adam, MD (Conceptualization: Equal; Supervision: Equal; Writing – review & editing: Equal). Audrey Coilly, Professor (Investigation: Equal). Bo-Goran Ericzon, Dr (Investigation: Equal). Carmelo Loinaz, Dr (Investigation: Equal). Valentin Cuervas-Mons, Dr (Investigation: Equal). Marco Zambelli, Dr (Investigation: Equal). Laura Llado, Dr (Investigation: Equal). Fernando Diaz, Dr (Investigation: Equal). Federica Invernizzi, Dr (Investigation: Equal). Damiano Patrono, Dr (Investigation: Equal). Francois Faitot, Dr (Investigation: Equal). Sherrie Boohrie, MD (Investigation: Equal). Jaques Pirenne, Professor (Investigation: Equal). Giovanni Perricone, MD (Investigation: Equal; Writing – review & editing: Equal). Giulia Magini, MD (Investigation: Equal). Lluis Castells, MD (Data curation: Equal; Validation: Supporting). Oliver Detry, MD (Investigation: Equal). Pablo Marti-Cruchaga, MD (Investigation: Equal). Jordi Colmenero, MD (Investigation: Equal). Frederick Berrevoet, MD (Investigation: Equal). Gonzalo Rodriguez, MD (Investigation: Equal). Dirk Ysebaert, MD (Investigation: Equal). Sylvie Radenne, MD (Investigation: Equal). Herold Metselaar, Professor (Investigation: Equal). Maria Cristina Morelli, MD (Investigation: Equal). De Carlis Luciano, MD (Writing – review & editing: Supporting). Wojciech Polak, MD (Conceptualization: Equal; Investigation: Equal; Writing – review & editing: Equal). Christophe Duvoux, Professor (Conceptualization: Equal; Methodology: Equal; Writing – review & editing: Equal).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding No funding was received.
Author names in bold designate shared co-first authorship.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.11.045.
Supplementary Materials
Supplementary Table 1.
Results From Multivariate Analysis of Predictors of Mortality, From Cox’s Proportional Hazard Regression Models, Excluding Age From the Predictors
| Variable | HR (95% CI) | P value |
|---|---|---|
| Comorbidities | ||
| Diabetes | 1.95 (1.06–3.58) | .0313 |
| Chronic kidney diseasea | 1.97 (1.05–3.67) | .0336 |
| Other | 1.92 (0.97–3.82) | .0608 |
| Main immunosuppressant (TAC vs CsA/mTOR/MMF) | 0.52 (0.29–0.95) | .0325 |
NOTE. Predictors with a P value ≤.1 were retained in the model. Bold values are statistically significant (P < .05).
HR, hazard ratio; mTOR, mammalian target of rapamycin inhibitor.
Plasma creatinine >2 mg/dL.
Supplementary Table 2.
Estimated Probability of Survival 50 Days After the Symptoms, Stratified by Age (2 Categories), Main Immunosuppressant and Chronic Kidney Disease
| Age | Main Immunosuppressant | Chronic kidney diseasea | Patients (n) | Probability of survival at 50 days (95% CI) |
|---|---|---|---|---|
| ≤ 70 y | TAC | No | 113 | 0.89 (0.82–0.94) |
| Yes | 16 | 0.86 (0.55–0.96) | ||
| CsA/mTOR/MMF/other | No | 39 | 0.90 (0.75–0.96) | |
| Yes | 13 | 0.54 (0.25–0.76) | ||
| >70 y | TAC | No | 16 | 0.75 (0.46–0.90) |
| Yes | 10 | 0.77 (0.34–0.94) | ||
| CsA/mTOR/MMF/other | No | 20 | 0.50 (0.27–0.69) | |
| Yes | 7 | 0.29 (0.01–0.69) |
NOTE. Estimates are based on Kaplan-Meier curves.
mTOR, mammalian target of rapamycin inhibitor
Plasma creatinine >2 mg/dL.
Supplementary Table 3.
Baseline Characteristics of the Study Population, Stratified by Type of Calcineurin Inhibitor
| Variables | Immunosuppressant |
Total (N = 243) | P value | |
|---|---|---|---|---|
| CsA/other (n = 81) | TAC (n = 162) | |||
| Male sex | 66 (81.48) | 105 (64.81) | 171 (70.37) | .0073 |
| Age at symptoms, y | 68 (60.5–73.5) | 61 (53.0–68.0) | 63 (55.0–69.0) | |
| Location of patient at occurrence of symptoms | .4631 | |||
| Home | 74 (91.36) | 143 (88.27) | 217 (89.30) | |
| Hospital | 7 (8.64) | 19 (11.73) | 26 (10.70) | |
| Place of management | .0831 | |||
| Home | 7 (8.64) | 32 (19.75) | 39 (16.05) | |
| Ward | 61 (75.31) | 106 (65.43) | 167 (68.72) | |
| ICU | 13 (16.05) | 24 (14.81) | 37 (15.23) | |
| Time between last LT and COVID-19 symptoms, y | 12 (6.2–18.9) | 7 (2.0–13.3) | 8 (3.1–15.0) | |
| Missing | 1 (1.23) | 5 (3.09) | 6 (2.47) | |
| Indication for LT | ||||
| Decompensated cirrhosis | 51 (62.96) | 90 (55.56) | 141 (58.02) | .27 |
| Hepatocellular carcinoma | 21 (25.93) | 42 (25.93) | 63 (25.93) | >.9999 |
| Other | 9 (11.11) | 31 (19.14) | 40 (16.46) | .1118 |
| Body mass index, kg/m2 | 26.3 (23.5–29.7) | 25.7 (23.4–29.4) | 25.9 (23.4–29.4) | .6612 |
| Chronic kidney diseasea | 22 (27.16) | 27 (16.67) | 49 (20.16) | .0546 |
| Coronary artery disease | 3 (3.70) | 14 (8.64) | 17 (7.00) | .1548 |
| Comorbidities, n | .0003 | |||
| 0 | 11 (13.58) | 46 (28.40) | 57 (23.46) | |
| 1 | 20 (24.69) | 59 (36.42) | 79 (32.51) | |
| ≥2 | 50 (61.73) | 57 (35.19) | 107 (44.03) | |
| Drugs | ||||
| β-Blockers | 20 (24.69) | 30 (18.52) | 50 (20.58) | .2618 |
| ACE inhibitors or angiotensin II receptor antagonists | 33 (40.74) | 26 (16.05) | 59 (24.28) | <.0001 |
| Type of immunosuppressant | ||||
| CsA | 29 (35.80) | 0 (0.00) | 29 (11.93) | <.0001 |
| TAC | 0 (0.00) | 162 (100.00) | 162 (66.67) | <.0001 |
| MMF | 50 (61.73) | 69 (42.59) | 119 (48.97) | .0049 |
| mTOR inhibitor | 23 (28.40) | 14 (8.64) | 37 (15.23) | <.0001 |
| Steroids | 14 (17.28) | 42 (25.93) | 56 (23.05) | .1316 |
| Other | 0 (0.00) | 1 (0.62) | 1 (0.41) | >.9999 |
| Outcome | .0033 | |||
| Alive | 56 (69.14) | 138 (85.19) | 194 (79.84) | |
| Dead | 25 (30.86) | 24 (14.81) | 49 (20.16) | |
| Time between symptoms and last follow-up, d | 60 (23–83) | 66 (39–87) | 65 (35–87) | .127 |
| Missing | 1 (1.23) | 5 (3.09) | 6 (2.47) | |
| Cause of death | ||||
| Refractory pneumonia | 21 (84.00) | 17 (70.83) | 38 (77.55) | .2695 |
| Liver-related death | ||||
| Without lung failure | 0 (0.00) | 1 (4.17) | 1 (2.04) | .4898 |
| With lung failure | 2 (8.00) | 1 (4.17) | 3 (6.12) | >.9999 |
| Other | 2 (8.00) | 5 (20.83) | 7 (14.29) | .2467 |
NOTE. Data are presented n (%) or median (1st–3rd quartile).
mTOR, mammalian target of rapamycin.
Plasma creatinine >2 mg/dL.
References
- 1.ZhouF YuT., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet 2020;395:1038] Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Liver Transplant Registry (ELTR) www.eltr.eu Accessed July 2020.
- 3.Bhoori S., Rossi R.E., Citterio D. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Ruiz M., Andrés A., Loinaz C. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb G.J., Moon A.M., Barnes E. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5:643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belli L.S., Duvoux C., Karam V. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5:724–725. doi: 10.1016/S2468-1253(20)30183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polak W.G., Fondevila C., Karam V. Impact of COVID-19 on liver transplantation in Europe: alert from an early survey of European Liver and Intestine Transplantation Association and European Liver Transplant Registry. Transpl Int. 2020;33:1244–1252. doi: 10.1111/tri.13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becchetti C., Zambelli M.F., Pasulo L. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832–1840. doi: 10.1136/gutjnl-2020-321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbajo-Lozoya J., Müller M.A., Kallies S., Thiel V., Drosten C., von Brunn A. Replication of human coronaviruses SARS-CoV, HCoVNL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Coronavirus disease (COVID-19) pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Accessed July 2020.
- 12.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell B., Moss C., George G. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka Y., Sato Y., Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5:1250–1260. doi: 10.3390/v5051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willicombe M., Thomas D., McAdoo S. COVID-19 and calcineurin inhibitors: should they get left out in the storm? J Am Soc Nephrol. 2020;31:1145–1146. doi: 10.1681/ASN.2020030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan W.J., Liang W.H., Zhao Y. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tovikkai C., Charman S.C., Praseedom Time varying impact of comorbidities on mortality after liver transplantation: a national cohort study using linked clinical and administrative data. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horby P., Lim W.S., Emberson J.R. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. https://doi.org/10.1056/N Engl J Moa2021436 Published online July 17, 2020.
- 21.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. https://doi.org/10.1056/NEJMoa2007764 Published online May 22, 2020. [DOI] [PubMed]