Abstract
Background
Reducing antibiotic use in patients with asymptomatic bacteriuria (ASB) has been inpatient focused. However, testing and treatment is often started in the emergency department (ED). Thus, for hospitalized patients with ASB, we sought to identify patterns of testing and treatment initiated by emergency medicine (EM) clinicians and the association of treatment with outcomes.
Methods
We conducted a 43-hospital, cohort study of adults admitted through the ED with ASB (February 2018–February 2020). Using generalized estimating equation models, we assessed for (1) factors associated with antibiotic treatment by EM clinicians and, after inverse probability of treatment weighting, (2) the effect of treatment on outcomes.
Results
Of 2461 patients with ASB, 74.4% (N = 1830) received antibiotics. The EM clinicians ordered urine cultures in 80.0% (N = 1970) of patients and initiated treatment in 68.5% (1253 of 1830). Predictors of EM clinician treatment of ASB versus no treatment included dementia, spinal cord injury, incontinence, urinary catheter, altered mental status, leukocytosis, and abnormal urinalysis. Once initiated by EM clinicians, 79% (993 of 1253) of patients remained on antibiotics for at least 3 days. Antibiotic treatment was associated with a longer length of hospitalization (mean 5.1 vs 4.2 days; relative risk = 1.16; 95% confidence interval, 1.08–1.23) and Clostridioides difficile infection (CDI) (0.9% [N = 11] vs 0% [N = 0]; P = .02).
Conclusions
Among hospitalized patients ultimately diagnosed with ASB, EM clinicians commonly initiated testing and treatment; most antibiotics were continued by inpatient clinicians. Antibiotic treatment was not associated with improved outcomes, whereas it was associated with prolonged hospitalization and CDI. For best impact, stewardship interventions must expand to the ED.
Keywords: bacteriuria, emergency medicine, stewardship, urinary tract infection
Among hospitalized patients with ASB, the majority of urine testing and treatment was initiated by emergency medicine clinicians and often continued by inpatient clinicians. Antibiotic treatment was associated with Clostridioides difficile infection and longer duration of hospitalization.
Urinary tract infections (UTIs) are the second most common infection treated in hospitalized patients [1, 2], but they are frequently misdiagnosed. Bacteriuria without signs or symptoms attributable to a UTI is defined as asymptomatic bacteriuria (ASB) [3]. Asymptomatic bacteriuria is frequent among hospitalized patients, and national guidelines recommend against antibiotic treatment in most patients. Nevertheless, ASB is often unnecessarily treated with antibiotics [2–5]. Antibiotic use is associated with increased adverse events, antimicrobial resistance, duration of hospitalization, cost, and Clostridioides difficile infection (CDI) [2, 6, 7]. Thus, ASB is a prime target for antimicrobial stewardship.
Unnecessary antibiotic use in the emergency department (ED) is common, but data on antibiotic stewardship in the ED are sparse [8–10]. Diagnostic uncertainty and time pressures may lead to antibiotic overuse by EM clinicians [11]. It is not yet known how often testing and treatment related to ASB in hospitalized patients is initiated by EM clinicians, or how often antibiotics started by EM clinicians are continued by the inpatient clinician. Thus, for hospitalized patients with ASB who were admitted through the ED, we sought to identify how often urine testing and antibiotic treatment is started by EM clinicians, patterns and predictors of antibiotic treatment, and the association of antibiotic treatment with patient outcomes.
METHODS
Study Setting and Inclusion and Exclusion Criteria
This study included 43 hospitals participating in the Michigan Hospital Medicine Safety (HMS) Consortium. HMS is a quality collaborative funded by Blue Cross Blue Shield of Michigan and Blue Care Network that aims to improve the care of hospitalized medicine patients. The HMS includes approximately half (43 of 92) of nongovernmental or critical access hospitals in the state, and a diverse range of hospital type and size (academic and community, <200 and ≥200 beds) are represented [6].
For this study, patients were eligible for inclusion if they (1) were admitted through the ED, (2) had a positive urine culture (UC) collected within 2 days of hospitalization, and (3) had ASB. Patients were considered to have ASB if they had no documented signs or symptoms meeting UTI diagnostic criteria per Infectious Diseases Society of America (IDSA) Guidelines and National Healthcare Safety Network (NHSN) definitions [3, 12]. Specifically, patients could not have 1 of the following documented: dysuria, urinary frequency/urgency, suprapubic pain, fever (temperature > 38.0°C), costovertebral pain/tenderness, hematuria, autonomic dysreflexia, or increased spasticity in patients with a spinal cord injury. Because patients with acute alterations in mental status (AMS) cannot always describe their symptoms, patients with AMS and a systemic sign of possible infection (peripheral leukocytosis >10 000 cells/mm3, systolic blood pressure <90 mm Hg, or ≥2 criteria for systemic inflammatory response syndrome [SIRS]) were considered to potentially have UTI and thus were excluded.
Patients for whom treatment of ASB may be appropriate were excluded, including those with altered urinary tract anatomy, pregnancy, severe immune compromise (solid organ or bone marrow transplant, human immunodeficiency virus with CD4 <200 cells/mm3, neutropenia [absolute neutrophil count <0.5 cells/mm3]), or those who met criteria for severe sepsis (defined as ≥2 SIRS criteria + signs of organ dysfunction, including elevated lactate, creatinine >2 mg/dL, bilirubin >2 mg/dL, platelet <100 000/μL, international normalized ratio >1.5). Additional exclusions included (1) age <18 years, (2) intensive care unit admission within 3 days before or after UC, (3) entered hospice during hospitalization, (4) left against medical advice, (5) concomitant infection (documentation by provider of an additional bacterial infection during hospitalization, except CDI), (6) active treatment and/or prophylaxis for UTI on admission, (7) isolated candiduria, (8) within 30 days from a prior hospitalization that would have qualified the patient for inclusion in the study. Patients were also excluded from analysis if relevant data were missing or the antibiotic was ordered more than 1 day from the UC (ensuring that the decision was based on patient status around time of testing).
Study Design, Data Collection, and Patient Sampling
Hospital medicine safety procedures for patient selection, data collection, and quality assurance have been described [2, 6, 13]. In brief, from February 1, 2018 to February 25, 2020, trained abstractors at each hospital retrospectively screened consecutive patients 30 days after discharge and included the first patient each day with a positive UC (positive defined as a UC with any bacterial growth identified as abnormal by the hospital’s microbiology policy). Data were collected from 90 days before admission until follow-up was terminated by a major complication (eg, death) or 30 days after discharge. Signs and symptoms were collected from 3 days before and after UC collection. An abnormal urinalysis (UA) was defined as presence of leukocyte esterase or >5 white blood cells per high-power field and/or nitrites. At 30 days postdischarge, outcome data were collected by medical record review and scripted telephone follow-up (3 attempts). A standardized data dictionary and random audits by quality coordinators ensured data integrity. Variables collected from the medical record included (1) patient demographics, (2) receipt of antibiotic (within 90 days prior), (3) nonspecific signs or symptoms not consistent with the definition of UTI, (4) severity of illness, and (5) laboratory results.
Outcomes and Exposures
The primary outcome was the percentage of patients who had antibiotic treatment (at least 1 dose) initiated by an EM clinician. Antibiotic treatment likely directed at CDI (eg, metronidazole) was not considered ASB treatment. We also assessed total antibiotic duration (inpatient plus discharge) in patients who received treatment. Secondary outcomes included 30-day mortality, 30-day readmission, 30-day ED visit, discharge to postacute care facility, CDI within 30 days, and duration of hospitalization after urine testing (UA or UC).
Statistical Analysis
Descriptive statistics were used to characterize the population. To evaluate predictors of antibiotic treatment by EM clinicians, we first used bivariable logistic general estimating equation (GEE) models accounting for hospital-level clustering then determined a multivariable model using stepwise selection based on the Schwarz criterion [14].
To evaluate the association of antibiotic treatment by EM clinicians with secondary outcomes, we first used logistic regression to create inverse probability of treatment weights [15] based on baseline covariates found to be significant in the bivariable and/or multivariable analyses and other factors potentially associated with the outcome. These weights were then applied to GEE models (logistic or binomial, as appropriate) to assess differences in outcomes by treatment. Because there were no CDI events in patients with ASB who were not treated with antibiotics, we used Fisher’s exact test to compare CDI rates between treatment and no treatment. P values less than .05 were considered statistically significant. All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC).
Sensitivity Analysis
Because some patients with AMS are unable to report symptoms, we conducted a sensitivity analysis assessing factors and outcomes associated with ASB treatment by EM clinicians after excluding patients with AMS.
Patient Consent Statement
Because the purpose of HMS is to measure and improve the quality of existing care practices, this project received a “not regulated” status by the University of Michigan Medical School’s Institutional Review Board, and informed consent was not required.
RESULTS
Baseline Demographics
Of 2461 patients admitted through the ED and ultimately determined to have ASB (Supplementary eFigure 1), the median age was 78 years (interquartile range [IQR], 67–87) and 73.9% (N = 1818) were women (Table 1). The most common comorbidities included chronic kidney disease (41.2%, N = 1013) and diabetes (38.3%, N = 942). Indwelling urinary catheters were present in 14.3% (N = 353) of patients. Almost all UAs were abnormal (94.0%, N = 2313). When documented (74.4%, 1830 of 2461), the most common indication for the UC was an abnormal UA (22.1%, 543 of 2461).The most common bacteria isolated were Escherichia coli (47.9%, N = 1180), Klebsiella spp (15.6%, N = 383), and Enterococcus spp (10.5%, N = 259).
Table 1.
Demographics of Hospitalized Patients Presenting to the Emergency Department Ultimately Identified as Having Asymptomatic Bacteriuria, N = 2461
| Characteristic | N (%) |
|---|---|
| Age, median [IQR] | 78 [67–87] |
| Women | 1818 (73.9%) |
| Length of stay [IQR] | 5 [3–6] |
| Comorbiditiesa | |
| Moderate to severe chronic kidney disease | 942 (38.3%) |
| Diabetes | 1013 (41.2%) |
| Hemodialysis | 35 (1.4%) |
| Liver disease | 138 (5.6%) |
| Congestive heart failure | 619 (25.2%) |
| Cerebrovascular disease | 646 (26.2%) |
| History of cancer | 510 (20.7%) |
| Spinal cord injury | 50 (2.0%) |
| Immunosuppressedb | 71 (2.9%) |
| Dementia | 622 (25.3%) |
| Urinary Catheter | |
| Indwelling | 353 (14.3%) |
| Otherc | 67 (2.7%) |
| Urinalysis | |
| Urinalysis obtained | 2421 (98.4%) |
| Urinalysis Result | |
| Abnormal urinalysisd | 2313 (94.0%) |
| Positive LE and/or >5 WBC/hpf | 2264 (92.0%) |
| Positive nitrite | 966 (39.3%) |
| Documentation of reason for culturee | 1830 (74.4%) |
| Abnormal urinalysis | 543 (22.1%) |
| Altered mental status | 223 (9.1%) |
| Nausea, vomiting, abdominal pain | 199 (8.1%) |
| Changes in urine characteristics | 173 (7.0%) |
| Urine Pathogens | |
| Escherichia coli, n (%) | 1180 (47.9%) |
| Klebsiella spp, n (%) | 383 (15.6%) |
| Enterococcus spp, n (%) | 259 (10.5%) |
| Proteus spp, n (%) | 162 (6.6%) |
| Pseudomonas aeruginosa, n (%) | 108 (4.4%) |
| Enterobacter spp, n (%) | 77 (3.1%) |
| Citrobacter spp, n (%) | 69 (2.8%) |
| ≥2 bacteria | 394 (16.0%) |
| Treatment | |
| Received antibiotics | 1830 (74.4%) |
| First treatment by EM | 1253 (52.0%) |
| First treatment by inpatient clinician | 577 (23.4%) |
| Duration of therapy for those treated, median [IQR] N = 1744 | 6 [3–9] |
| Antibiotics on day 1 of treatmentf | |
| Ceftriaxone | 1418 (78.2%) |
| Fluoroquinoloneg | 153 (8.4%) |
| Antibiotics at Dischargeg | |
| Fluoroquinoloneg | 290 (30.2%) |
| Cephalexin | 263 (27.4%) |
| Trimethoprim/sulfamethoxazole | 92 (9.6%) |
| Cefuroxime | 77 (7.7%) |
| Nitrofurantoin | 52 (5.4%) |
| Amoxicillin | 48 (5.0%) |
Abbreviations: EM, emergency medicine clinician; IQR, interquartile range; LE, leukocyte esterase; N, number; WBC/hpf, white blood cells per high-power field.
aComorbidities are not mutually exclusive.
bDefined as human immunodeficiency virus positive with CD4 count greater than 200 cells/mm3, at least 30 days of prednisone 10 mg/day or more (or equivalent corticosteroid dose), on biologic agents (eg, tumor necrosis factor inhibitors), received chemotherapy in last 30 days, or congenital or acquired immunodeficiency.
cDefined as condom catheters, intermittent straight catheterization.
dDefined as presence of LE or >5 WBC/hpf and/or presence of nitrites.
eListed if greater than 5% of indications documented.
fListed if greater than 5% of total antibiotics prescribed to patients.
gLevofloxacin or ciprofloxacin.
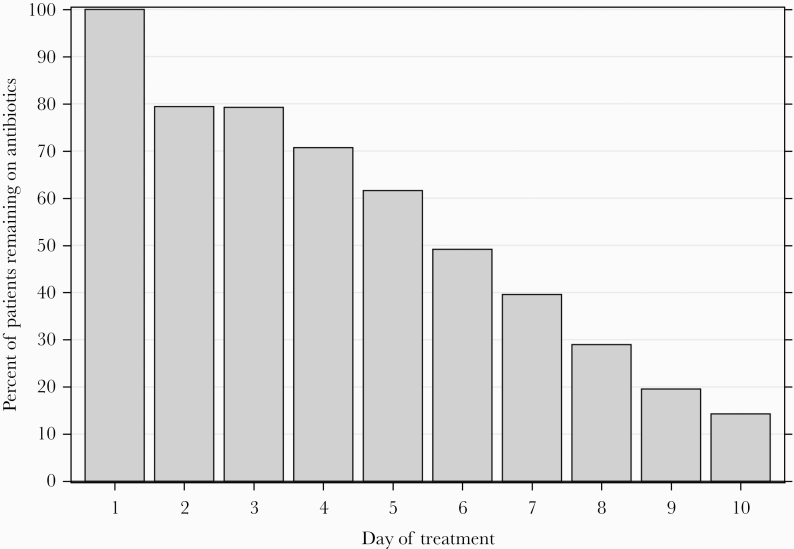
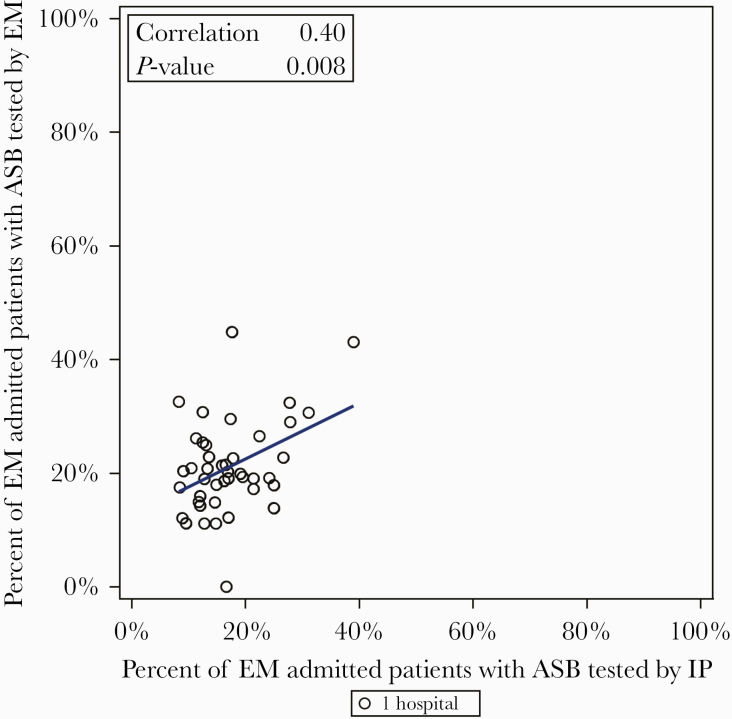
Three quarters (74.4%, 1830 of 2461) of patients were treated with antibiotics (Supplementary eFigure 2 and eFigure 3) with a median treatment duration of 6 days (IQR, 3–9) and median hospital duration of 5 days (IQR, 3–6). The UC was ordered by EM clinicians in 80.0% (N = 1970 of 2461) of patients (Supplementary eFigure 2), and antibiotic treatment was started by EM clinicians in 68.5% (1253 of 1830) of those treated (Supplementary eFigure 3). When antibiotic therapy was started by EM clinicians, 79.2% (993 of 1253) of patients remained on antibiotics for 3 or more days (Figure 1). Likewise, when antibiotic therapy was started by inpatient clinicians, 82.0% (473 of 577) remained on antibiotics for 3 days or more. The most common initial antibiotic was ceftriaxone (78.2%, N = 1418), and at discharge the most common antibiotic was a fluoroquinolone (30.2%, N = 290). Hospitals with higher rates of urine testing by EM clinicians also had higher rates of urine testing by inpatient clinicians (correlation coefficient 0.40, P = .008) (Figure 2).
Figure 1.
Percentage of patients with asymptomatic bacteriuria treated with antibiotics initially by emergency medicine who remained on antibiotics by day (N = 1253).
Figure 2.
Emergency medicine versus inpatient clinician testing. ASB, asymptomatic bacteriuria; EM, emergency medicine clinicians; IP, inpatient clinicians.
Variables Associated With Asymptomatic Bacteriuria Treatment
Variables associated with ASB treatment by EM clinicians included patient comorbidities such as dementia or spinal cord injury, patient symptoms such as AMS, or laboratory results, in particular an abnormal UA (Table 2).
Table 2.
Bivariate Analysis of Hospitalized Patients Presenting to the Emergency Department Ultimately Diagnosed With Asymptomatic Bacteriuria and Treated With Antibiotics by Emergency Medicine Clinicians Versus Never Treated With Antibiotics, N = 1884
| Variable | Antibiotic Treatment by EM (n = 1253) | No Antibiotics (n = 631) | Odds Ratio (95% CI) | P Valuea |
|---|---|---|---|---|
| Baseline Characteristics | ||||
| Age (median, IQR) | 80 (69–87) | 75 (62–85) | 1.02 (1.01–1.03) | <.0001 |
| Gender (female) | 911 (72.7%) | 471 (74.6%) | 0.99 (0.78–1.25) | .92 |
| Race (white) | 921 (74.0%) | 495 (78.6%) | 0.95 (0.79–1.13) | .54 |
| Charlson comorbidity index,0 | 132 (10.5%) | 84 (13.3%) | REF | .16 |
| 1–2 | 407 (32.5%) | 200 (31.7%) | 1.28 (0.91–1.79) | |
| 3–4 | 396 (31.6%) | 175 (27.7%) | 1.42 (1.04–1.94) | |
| ≥5 | 318 (25.4%) | 172 (27.3%) | 1.27 (0.96–1.67) | |
| Diabetes | 452 (36.1%) | 250 (39.6%) | 0.89 (0.73–1.10) | .29 |
| Moderate or severe chronic kidney disease | 520 (41.5%) | 247 (39.1%) | 1.24 (0.96–1.61) | .10 |
| History of cancer | 261 (20.8%) | 132 (20.9%) | 1.04 (0.85–1.28) | .68 |
| Spinal cord injury | 35 (02.8%) | 3 (0.5%) | 5.17 (1.42–18.77) | .01 |
| Dementia | 384 (30.6%) | 87 (13.8%) | 2.27 (1.83–2.83) | <.0001 |
| Immunosuppressedb | 37 (03.0%) | 18 (2.9%) | 1.24 (0.61–2.52) | .55 |
| IV chemotherapy in preceding 30 days | 10 (00.8%) | 6 (1.0%) | 0.96 (0.20–4.58) | .96 |
| Hemodialysis | 20 (01.6%) | 9 (1.4%) | 0.84 (0.51–1.37) | .48 |
| Transfer from postacute carec | 88 (07.0%) | 27 (4.3%) | 1.74 (1.27–2.39) | .0006 |
| Nonambulatory | 224 (17.9%) | 55 (8.7%) | 1.92 (1.46–2.53) | <.0001 |
| Hospitalization in past 90 days | 373 (29.8%) | 195 (30.9%) | 0.92 (0.76–1.10) | .34 |
| Antibiotics in preceding 90 days | 263 (21.0%) | 92 (14.6%) | 1.31 (1.06–1.62) | .011 |
| Indwelling catheter | 215 (17.2%) | 58 (9.2%) | 1.55 (1.21–1.98) | .0006 |
| Any urinary catheterd | 249 (19.9%) | 68 (10.8%) | 1.55 (1.22–1.96) | .0003 |
| Signs and Symptoms | ||||
| Abdominal pain | 248 (19.8%) | 158 (25.0%) | 0.88 (0.73–1.05) | .16 |
| Incontinence | 445 (35.5%) | 138 (21.9%) | 2.20 (1.80–2.68) | <.0001 |
| Functional decline | 93 (07.4%) | 28 (04.4%) | 1.79 (0.88–3.64) | .11 |
| Acutely altered mental status | 393 (31.4%) | 86 (13.6%) | 2.51 (2.02–3.13) | <.0001 |
| Fatigue, malaise, lethargy | 396 (31.6%) | 175 (27.7%) | 1.53 (1.16–2.01) | .003 |
| Nausea or vomiting | 267 (21.3%) | 179 (28.4%) | 0.88 (0.69–1.13) | .33 |
| Change in color, sediment, or malodorous urine | 202 (16.1%) | 71 (11.3%) | 1.93 (1.23–3.03) | .004 |
| Urinary retention or postvoid residual > 200 cc | 143 (11.4%) | 54 (08.6%) | 1.34 (0.96–1.88) | .08 |
| Severity of Illness | ||||
| qSOFAe (≥2 vs <2) | 152 (12.1%) | 65 (10.3%) | 1.33 (1.06–1.68) | .01 |
| ≥ 2 SIRSf criteria | 300 (23.9%) | 191 (30.3%) | 0.80 (0.66–0.97) | .02 |
| Laboratory Results | ||||
| Peripheral leukocytosisg | 356 (28.4%) | 186 (29.5%) | 1.01 (0.87–1.16) | .93 |
| Abnormal urinalysish | 1232 (98.3%) | 528 (83.7%) | 9.42 (5.30–16.75) | <.0001 |
| Hospital Characteristics | ||||
| Type of control | ||||
| Not-for-profit | 1141 (91.1%) | 606 (96.0%) | REF | .04 |
| For profit | 112 (8.9%) | 25 (4.0%) | 2.57 (1.03–6.40) | |
| Bed size (10 bed increase) | 327 (203–443) | 310 (189–443) | 1.01 (0.99–1.01) | .62 |
| Teaching hospital | 1165 (93.0%) | 555 (88.0%) | 1.19 (0.59–2.40) | .62 |
Abbreviations: CI, confidence interval; EM, emergency medicine clinician; IQR, interquartile range; IV, intravenous; qSOFA, quick sequential organ failure assessment; REF, Reference; SIRS, systemic inflammatory response syndrome.
a P < .05 is considered significant.
bDefined as chemotherapy administered within 30 days, human immunodeficiency virus with CD4 >200, ≥10 mg/day prednisone for at least 30 days (or equivalent steroid dose), on biologic agents such as tumor necrosis factor inhibitors or other immunosuppressant agents, congenital or acquired immunodeficiency.
cIncludes transfer from the following: subacute rehabilitation center, skilled nursing home, acute rehabilitation center, assisted living, other hospital. Also includes if patient had been admitted or resided in a nursing home, subacute rehabilitation center, or extended care facility in the prior 30 days.
dIncludes Foley catheter, intermittent straight catheterization, and suprapubic catheter present on day of urine culture collection or 1 day before urine culture collection.
eQuick SOFA score: systolic blood pressure ≤100 mmHg = 1, respiratory rate ≥22 breaths per minute = 1; Glasgow coma score <15 = 1.
fSIRS (temperature <36°C [96.8°F] or > 38.0°C [100.4°F], heart rate >90 beats per minute, respiratory rate >20 breaths per minute, white blood cell count <4000/mm3 or >12 000/mm3).
gDefined as white blood cell count >10 per high-power field.
hDefined as presence of leukocyte esterase or nitrite, or white blood cells >5 per high-power field.
In the multivariable model (Table 3), patient characteristics associated with treatment by EM clinicians included the following: dementia (odds ratio [OR], 1.43; 95% confidence interval [CI], 1.11–1.84), spinal cord injury (OR, 5.92; 95% CI, 1.36–25.72), presence of urinary catheter (OR, 1.54; 95% CI, 1.17–2.03), incontinence (OR, 1.81; 95% CI, 1.40–2.33), and AMS (OR, 2.34; 95% CI, 1.82–3.00). Laboratory characteristics associated with ASB treatment by EM were peripheral leukocytosis (OR, 1.42; 95% CI, 1.21–1.68) and abnormal UA (OR, 9.68; 95% CI, 5.34–17.54).
Table 3.
Multivariable Model of Patient Factors Associated With Treatment by Emergency Medicine Clinicians of Patients Ultimately Diagnosed With Asymptomatic Bacteriuria, N = 1884
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Patient Characteristic (N) | ||
| Age | 1.01 (1.00–1.02) | .006 |
| Dementia | 1.43 (1.11–1.84) | .006 |
| Urinary catheter | 1.54 (1.17–2.03) | .002 |
| Incontinence | 1.81 (1.40–2.33) | <.0001 |
| Spinal cord injury | 5.92 (1.36–25.72) | .02 |
| Acutely altered mental status | 2.34 (1.82–3.00) | <.0001 |
| Test Characteristics | ||
| Peripheral leukocytosisa | 1.42 (1.21–1.68) | <.0001 |
| Abnormal urinalysisb | 9.68 (5.34–17.54) | <.0001 |
Abbreviations: CI, confidence interval.
NOTE: Odds ratios >1 indicates factors associated with treatment of asymptomatic bacteriuria; P < .05 is considered significant.
aDefined as white blood cells >10 000 cells/mm3.
bDefined as presence of leukocyte esterase or nitrite, or white blood cells >5 per high-power field.
Patient Outcomes
Within 30 days of discharge, 77% (1895 of 2461) of patients were evaluated by record review and/or telephone or had follow-up terminated by a major complication. After adjustments, there were no differences in mortality, hospital readmission, ED visit, or discharge to postacute care facility among patients treated by EM clinician versus never treated with antibiotics (Table 4). Patients treated with antibiotics by an EM clinician were more likely to develop CDI within 30 days (0.9% [N = 11] vs 0% [N = 0]; P = .02) and have a longer duration of hospitalization after urine testing (mean 5.1 vs 4.2 days; RR, 1.16; 95% CI, 1.08–1.23).
Table 4.
Outcomes for Treatment by Emergency Medicine vs No Antibiotic Treatment for Asymptomatic Bacteriuria, N = 1884
| Outcome | Treated by EM (N = 1253) | No Antibiotics (N = 631) | Unadjusted Odds Ratio (95% CI) | P Valuea | Adjusted Odds Ratio (95% CI) | P Valuea |
|---|---|---|---|---|---|---|
| Deatha | 41 (3.3%) | 12 (1.9%) | 1.76 (0.90–3.44) | .10 | 1.83 (0.86–3.92) | .12 |
| Readmission | 193 (15.4%) | 105 (16.6%) | 0.92 (0.70–1.20) | .52 | 0.82 (0.57–1.19) | .30 |
| ED visita | 160 (12.8%) | 71 (11.3%) | 1.21 (0.89–1.63) | .22 | 1.39 (0.98–1.97) | .07 |
| Discharge to postacute care facilitya,b | 474 (37.8%) | 150 (23.8%) | 1.90 (1.59–2.28) | <.001 | 1.21 (0.95–1.55) | .12 |
| Clostridioides difficile infectionc | 11 (0.9%) | 0 (0%) | N/A | .02d | N/A | N/A |
| Duration of hospitalizatione,f, mean (SD) | 5.1 (2.6) | 4.2 (2.4) | 1.19 (1.12–1.27)f | <.001 | 1.16 (1.08–1.23)f | <.001 |
Abbreviations: CI, confidence interval; ED, emergency department; EM, emergency medicine clinician; N/A, not applicable; SD, standard deviation.
NOTE: Outcomes were adjusted for patient variables found to be significant (P < .05) and associated with treatment in the bivariate and multivariate analysis and the following:
aMortality, readmissions, ED visits, and discharge to postacute care are adjusted for age, Charlson comorbidity index, hospitalization in 90 days preceding current admission, admission from nursing home, and insurance type.
bPostacute care facility includes the following: long-term acute care hospital, skilled nursing facility, inpatient rehabilitation, and subacute rehabilitation.
c Clostridioides difficile infection occurring within 30 days of discharge were adjusted for age, history of antibiotic use (and number of antibiotics) in previous 90 days, admitted from skilled nursing facility, prior hospitalization, proton-pump inhibitor use, immunosuppression, and Charlson comorbidity index. The zero outcomes in the nontreated group makes the odds ratio infinite and therefore cannot be estimated.
dUsing Fisher’s exact test, due to zero even rates in nontreated group.
eFrom date of urine testing (either urine culture or urinalysis, whichever sent first). Adjusted for age, gender, Charlson comorbidity index, prior hospitalization, admission from nursing home, and insurance type.
fRelative risk given continuous variable; duration of hospitalization was 16% longer for those treated with antibiotics. Median duration of hospitalization was 4 (interquartile range [IQR], 3–6) vs 4 (IQR, 3–5) days, respectively.
Sensitivity Analysis
Results were similar after excluding patients with AMS (Supplementary eTable 1). Specifically, patients treated with antibiotics by an EM clinician were more likely to develop CDI within 30 days (0.9% [N = 8] vs 0% [N = 0]; P = .03) and have a longer duration of hospitalization after urine testing (mean 5.1 vs 4.1 days; RR, 1.16; 95% CI, 1.07–1.27) (Supplementary eTable 2).
DISCUSSION
In this study of 2461 patients with ASB admitted through the ED, three quarters were treated with antibiotics during their hospitalization. The majority of urine testing and antibiotic treatment was initiated by EM clinicians. Once started by EM clinicians, inpatient clinicians usually continued antibiotics during hospitalization. The ASB treatment by EM clinicians was not associated with clinical benefit, but instead it was associated with CDI and longer duration of hospitalization after urine testing. These findings identify the ED as a key target to reduce antibiotic use and improve outcomes in hospitalized patients with ASB.
Multiple prior studies have reported similarly high rates of ASB treatment in hospitalized patients ranging from 47% to 80% [2, 16–19]. However, data characterizing which clinicians order urine testing and initiate antibiotic therapy in hospitalized patients with ASB has been lacking. We identified that most initial urine testing is ordered by EM clinicians. It is notable that EM clinicians do not always initiate urine testing. To improve crowding, nurse-initiated order sets and testing protocols are common [20] and may contribute to unnecessary testing. Likewise, order sets may have prechecked or easily selected orders for urine testing for nonurinary complaints, such as stroke. Addressing these contributors through diagnostic stewardship efforts [21] can reduce urine testing sent to evaluate for possible UTI in asymptomatic patients. We also found that hospitals with higher rates of urine testing by EM clinicians were more likely to have higher rates of urine testing by the inpatient clinician, suggesting that an underlying “culture of culturing” may contribute to increased testing [22, 23]. Nevertheless, antibiotic stewardship should target the (1) ED and ED protocols and (2) order sets to reduce urine testing and ASB treatment.
We found the strongest predictor of unnecessary antibiotic treatment by EM clinicians to be an abnormal UA. Likewise, prior studies of ASB in hospitalized patients have found urine testing results, both the UA and UC, to be associated with treatment by clinicians [2, 18, 19]. Both internal medicine physicians and ED nurses have been found to have knowledge gaps regarding the interpretation of UAs and UTI diagnosis [24–27]. The misinterpretation of UAs (ie, a positive UA equates with a UTI) has been highlighted as a target for decreasing inappropriate ASB treatment [2, 18, 19, 28, 29]. Although the UA has a high negative predictive value and is useful in ruling out a UTI, it is not indicative of a UTI in the absence of symptoms. Qualitative studies have identified that this is poorly understand and that a “positive UA” is incorrectly cited as the reason for sending a UC, diagnosing UTI, and initiating antibiotics [24, 30].
One potential way to change UC ordering practices is through elimination of reflex UCs where the UC is automatically sent when the UA is abnormal [19]. Recent strategies in the ED unlinking UA and UC have been successful in reducing UC rates [31]. Our study reaffirms that interventions aimed at both physicians and nurses regarding the correct interpretation of the UA should be paired with nudges for when to send a UC based on evidence-based UTI signs and symptoms. Thus, creating system and culture changes targeting decreasing urine testing in patients without urinary symptoms is vital to reducing unnecessary testing and treatment of ASB.
We also identified common patient characteristics, dementia and AMS, to be associated with treatment of suspected UTI in patients without urinary symptoms by EM clinicians. Both factors have previously been associated with treatment of ASB for hospitalized patients [2, 18]. Both confusion and AMS are common in the elderly, and bacteriuria is also common in elderly patient populations, thus bacteriuria in an elderly patient with confusion can be seen often by chance alone [32]. A systematic review concluded that no strong evidence links AMS or confusion alone as a symptom of UTI [33]. Furthermore, inaccurately diagnosing a UTI in patients with ASB could lead to delays in another clinically significant diagnosis. Due to known harms of unnecessary antibiotics, the IDSA recommends observation and assessment for alternative etiologies (eg, dehydration, medication, hypoxia, sundowning) in elderly patients with AMS and/or dementia that are clinically stable.
Despite this, deeply held beliefs persist and varying practice patterns exist making it difficult to reduce antibiotic treatment in patients with AMS and ASB. Patients with AMS, no systemic signs of infection, and no other urinary symptom account for only ~25% of all ASB patients admitted through the ED, and thus they may not be the best to target for stewardship efforts. When patients with AMS were excluded from the analysis, the same potential harms remain associated with ASB treatment (increased duration of hospitalization and CDI). These data could be used as a common ground between antibiotic stewards and EM clinicians, to prevent harm by first focusing on those patients who can report symptoms (without AMS), because they remain a majority (75%) of the patients tested in the ED.
Similar to a prior study, we found that antibiotics started for ASB by EM clinicians are not typically discontinued [34]. Most patients received 3 or more days of antibiotics. If antibiotics are started by EM clinicians for a suspected UTI in a patient without urinary symptoms, the next critical point to intervene is on admission, when the inpatient clinician can reassess and stop the antibiotic. Each additional dose results in increasing risk of harm, including adverse drug events and CDI [7, 35]. Treatment of ASB has been associated with CDI in patients undergoing neurosurgery [36], and we identified the same association in hospitalized medicine patients. This demonstrates potential harm from antibiotics in hospitalized patients with ASB who are unnecessarily treated. In addition, similar to a prior study of hospitalized medical patients with ASB started on antibiotics by any clinician, we found that patients with ASB who were treated with antibiotics had a longer duration of hospitalization [2]. Because ASB is common, antibiotics are harmful, and diagnosis momentum makes discontinuing antibiotics challenging [37], stewardship interventions must target both the initial testing by EM clinicians and the continuation of antibiotics by inpatient clinicians.
Our study has limitations. First, as an observational retrospective study, we are limited in assessment of symptoms and signs of UTI by documentation, and therefore we may have overestimated the frequency of ASB. Although we attempted to exclude patients with a possible alternate source of infection, the retrospective nature of our study limits our ability to determine the reason for antibiotic prescribing with absolute certainty. Second, excluding patients with concomitant infections may have underestimated the true rate of testing and treatment in patients with ASB. Third, we cannot fully attribute the orders to a particular provider, because it is possible that EM clinicians were asked to order a urine test or start antibiotics by the admitting clinician. Fourth, by excluding patients who had antibiotics started more than 1 day from the culture date, we were biased toward higher rates of antibiotic starts by EM clinicians compared with inpatient clinicians. However, excluding this subset of patients was necessary to compare the decision to treat suspected UTI in an asymptomatic patient, avoiding clinical changes or new diagnostic information. This does not change the overall trend of higher antibiotic starts by EM clinicians. Fifth, given the retrospective nature of the study, we cannot determine how many patients would have been started on antibiotics by the inpatient clinician if the EM clinician had not started antibiotics. Last, although we adjusted for potential confounding, residual confounding may still exist, including for the associated outcomes identified.
Our study has strengths. This cohort represents a diverse group of hospitals, improving generalizability. Our data were collected by trained abstractors who underwent quality control with excellent reliability, ensuring data accuracy. In addition, we used phone calls in conjunction with record review to capture higher rates of adverse events. We also attempted to minimize bias when evaluating secondary outcomes by using inverse probability of treatment weighting.
CONCLUSIONS
Emergency medicine clinicians often order urine testing and treat for a presumed UTI in patients who do not have urinary symptoms. Inpatient clinicians often continue unnecessary antibiotic therapy. Risk factors for unnecessary antibiotic initiation by EM clinicians include an abnormal UA and nonspecific symptoms. Antibiotic treatment of patients with ASB, also when excluding those with AMS, was not associated with improved outcomes but was associated with an increased risk of CDI and longer duration of hospitalization after urine testing. For efforts to be successful in curbing ASB treatment, stewardship should begin in the ED before the initiation of the testing and treatment cascade.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support.This work was funded by Blue Cross Blue Shield of Michigan (BCBSM) and Blue Care Network as part of the BCBSM Value Partnerships program.
Potential conflicts of interest. L. A. P. reported grants from BCBSM during the conduct of the study. V. M. V. reported grants from BCBSM and the Agency for Healthcare Research and Quality during the conduct of the study. S. A. F. reported personal fees from Expert Testimony and Wiley Publishing and grants from BCBSM and the Agency for Healthcare Research and Quality. E. M. reported other support from BCBSM during the conduct of the study. T. N. G. reported grants from BCBSM during the conduct of the study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petty LA, Vaughn VM, Flanders SA, et al. Risk factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern Med 2019; 179:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicolle LE, Gupta K, Bradley SF, et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 update by the Infectious Diseases Society of America. 2019; 68:e83–110. [DOI] [PubMed] [Google Scholar]
- 4. Infectious Diseases Society of America. Don’t treat asymptomatic bacteriuria. Available at: http://www.choosingwisely.org/clinician-lists/infectious-diseases-society-antibiotics-for-bacteruria/. Accessed 9 December 2019.
- 5. US Preventive Services Task Force. Screening for asymptomatic bacteriuria in adults: US preventive services task force recommendation statement. JAMA 2019; 322:1188–94. [DOI] [PubMed] [Google Scholar]
- 6. Vaughn VM, Flanders SA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: a multihospital cohort study. Ann Intern Med 2019; 171:153–63. [DOI] [PubMed] [Google Scholar]
- 7. Tamma PD, Avdic E, Li DX, et al. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 2017; 177:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Losier M, Ramsey TD, Wilby KJ, Black EK. A systematic review of antimicrobial stewardship interventions in the emergency department. Ann Pharmacother 2017; 51:774–90. [DOI] [PubMed] [Google Scholar]
- 9. Pulcini C. Antimicrobial stewardship in emergency departments: a neglected topic. Emerg Med J 2015; 32:506. [DOI] [PubMed] [Google Scholar]
- 10. Denny KJ, Gartside JG, Alcorn K, et al. Appropriateness of antibiotic prescribing in the emergency department. J Antimicrob Chemother 2018; 74:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. May L, Gudger G, Armstrong P, et al. Multisite exploration of clinical decision making for antibiotic use by emergency medicine providers using quantitative and qualitative methods. Infect Control Hosp Epidemiol 2014; 35:1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Healthcare Safety Network (NHSN). Long-term Care Facility Component: Tracking Infections in Long-term Care Facilities. Atlanta, GA: Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases; May 2018. https://www.cdc.gov/nhsn/pdfs/ltc/ltcf-manual-508.pdf. Accessed July 25, 2019. [Google Scholar]
- 13. Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med 2014; 174:1577–84. [DOI] [PubMed] [Google Scholar]
- 14. Buckland ST, Burnham KP, Augustin NH. Model selection: an integral part of inference. Biometrics 1997; 53:603–18. [Google Scholar]
- 15. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect 2012; 2. doi: 10.3402/jchimp.v2i2.17814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartley S, Valley S, Kuhn L, et al. Overtreatment of asymptomatic bacteriuria: identifying targets for improvement. Infect Control Hosp Epidemiol 2015; 36:470–3. [DOI] [PubMed] [Google Scholar]
- 18. Spivak ES, Burk M, Zhang R, et al. Management of bacteriuria in veterans affairs hospitals. Clin Infect Dis 2017; 65:910–17. [DOI] [PubMed] [Google Scholar]
- 19. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis 2017; 4:ofx207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pulia M, Redwood R, May L. Antimicrobial stewardship in the emergency department. Emerg Med Clin North Am 2018; 36:853–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morgan DJ, Malani P, Diekema DJ. Diagnostic stewardship-leveraging the laboratory to improve antimicrobial use. JAMA 2017; 318:607–8. [DOI] [PubMed] [Google Scholar]
- 22. Wald HL. Challenging the “Culture of Culturing”: the case for less testing and more clinical assessment. JAMA Intern Med 2016; 176:587–8. [DOI] [PubMed] [Google Scholar]
- 23. Vaughn VM, Szymczak JE, Newton DW, Fakih MG. Addressing the overuse of cultures to optimize patient care. Ann Intern Med 2019; 171:73–4. [DOI] [PubMed] [Google Scholar]
- 24. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control 2018; 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drekonja DM, Abbo LM, Kuskowski MA, et al. A survey of resident physicians’ knowledge regarding urine testing and subsequent antimicrobial treatment. Am J Infect Control 2013; 41:892–6. [DOI] [PubMed] [Google Scholar]
- 26. Lee MJ, Kim M, Kim NH, et al. Why is asymptomatic bacteriuria overtreated?: a tertiary care institutional survey of resident physicians. BMC Infect Dis 2015; 15:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trautner BW, Petersen NJ, Hysong SJ, et al. Overtreatment of asymptomatic bacteriuria: identifying provider barriers to evidence-based care. Am J Infect Control 2014; 42:653–8. [DOI] [PubMed] [Google Scholar]
- 28. Schulz L, Hoffman RJ, Pothof J, Fox B. Top ten myths regarding the diagnosis and treatment of urinary tract infections. J Emerg Med 2016; 51:25–30. [DOI] [PubMed] [Google Scholar]
- 29. Humphries RM, Dien Bard J. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol 2016; 54:254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pallin DJ, Ronan C, Montazeri K, et al. Urinalysis in acute care of adults: pitfalls in testing and interpreting results. Open Forum Infect Dis 2014; 1:ofu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stagg A, Lutz H, Kirpalaney S, et al. Impact of two-step urine culture ordering in the emergency department: a time series analysis. BMJ Qual Saf 2018; 27:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McKenzie R, Stewart MT, Bellantoni MF, Finucane TE. Bacteriuria in individuals who become delirious. Am J Med 2014; 127:255–7. [DOI] [PubMed] [Google Scholar]
- 33. Mayne S, Bowden A, Sundvall PD, Gunnarsson R. The scientific evidence for a potential link between confusion and urinary tract infection in the elderly is still confusing—a systematic literature review. BMC Geriatr 2019; 19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kiyatkin D, Bessman E, McKenzie R. Impact of antibiotic choices made in the emergency department on appropriateness of antibiotic treatment of urinary tract infections in hospitalized patients. J Hosp Med 2016; 11:181–4. [DOI] [PubMed] [Google Scholar]
- 35. Branch-Elliman W, O’Brien W, Strymish J, et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg 2019; 154:590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Belton PJ, Litofsky NS, Humphries WE. Effect of empiric treatment of asymptomatic bacteriuria in neurosurgical trauma patients on surgical site and clostridium difficile infection. Neurosurgery 2018; 85:664–71. [DOI] [PubMed] [Google Scholar]
- 37. Szymczak JE, Kitt E, Hayes M, et al. Threatened efficiency not autonomy: prescriber perceptions of an established pediatric antimicrobial stewardship program. Infect Control Hosp Epidemiol 2019; 40:522–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.