Abstract
Background
Cutaneous leishmaniasis (CL) caused by L. braziliensis is characterized by 1 or multiple well-limited ulcerated lesions. Diabetes mellitus (DM) impairs neutrophil and monocyte function, and there is a report of vegetative lesions in a patient with both diseases in Morocco. Here we evaluate the influence of DM on clinical manifestations, immune response, and in the treatment of CL.
Methods
The participants were 36 DM patients with CL and 36 patients with CL without DM, matched by age and gender. The diagnosis of CL was performed by documentation of DNA of L. braziliensis by polymerase chain reaction in the lesion biopsy and histopathologic findings. All patients were treated with Glucantime (Sanofi-Aventis) 20 mg/kg of weight per day for 20 days.
Results
There was no difference in the majority of the clinical variables between the groups, and the cure rate in patients with CL and DM (67%) was similar to that observed in CL patients (56%; P ˃ .05). The most important finding was the documentation that 36% of the patients with DM and CL had atypical cutaneous lesions characterized by large superficial ulcers without defined borders. High levels of interferon-γ, tumor necrosis facor, and interleukin-1β were detected in the supernatants of mononuclear cells stimulated with Leishmania antigen in patients with DM and atypical CL. Moreover, while cure was observed in only 33% of the patients with DM and atypical CL lesions, it was observed in 85% of patients with typical lesions (P < .05).
Conclusions
DM modifies the clinical presentation of CL, enhances pro-inflammatory cytokine production, and impairs response to antimony therapy.
Keywords: cutaneous leishmaniasis, diabetes, diabetes mellitus, immune response, leishmaniasis, tegumentary leishmaniasis
Cutaneous leishmaniasis (CL) arising from Leishmania braziliensis is characterized by a well-delimited ulcer with raised borders. It is known that around 3% of CL patients develop mucosal leishmaniasis (ML) [1]. Moreover, CL patients may also develop disseminated leishmaniasis (DL), an emergent form of L. braziliensis infection defined by the presence of more than 10 and possibly up to 1000 acneiform, popular, and ulcerated skin lesions distributed among at least 2 regions of the body [2, 3]. Atypical lesions are also observed in the spectrum of this disease’s clinical presentation as isolated vegetative lesions that are larger in size and lacking clear delimitation or as multiple nodules restricted to a specific area of the body [4, 5]. Parasite and host factors may influence the clinical presentation of CL. L. braziliensis is polymorphic, and differences in the alleles on chromosomes 24 and 28 have been associated with distinct clinical forms, disease severity, and therapeutic failure [6–8]. There is also strong evidence of the role played by host immune response in the pathogenesis of CL. Leishmania are killed by interferon (IFN)-γ-activated macrophages but do not become completely eradicated; thus, parasite persistence and the release of parasite antigens induce an exacerbated stimulation of the immune system. Neutrophils are the first cell type to migrate to the site of Leishmania infection [9–11]. However, both neutrophils and macrophages from CL patients present a decreased ability to kill Leishmania despite producing high levels of reactive oxygen species and pro-inflammatory cytokines [12, 13]. Elevated levels of pro-inflammatory cytokines, including interleukin (IL)-Iβ, IL-6, tumor necrosis factor (TNF), C-X-C motif chemokine ligand (CXCL)–9, and CXCL-10, among others, are produced by peripheral blood mononuclear cells (PBMCs) from CL patients under stimulation by leishmanial antigens [14, 15]. Cytokine production becomes enhanced during the course of infection, from the early or pre-ulcerative phase of disease to ulcer development [16, 17]. The enhancement in the immune response is also due to the impairment of regulatory mechanisms, such as the decreased ability of IL-10 and transforming growth factor (TGF)–β to downmodulate the immune response of CL cells [18]. This makes American tegumentary leishmaniasis a chronic inflammatory disease characterized by an exaggerated inflammatory response that causes tissue damage.
Diabetes mellitus (DM) is a chronic metabolic syndrome, and it is estimated that around 480 million people worldwide live with diabetes [19]. DM increases the susceptibility to infections caused by extracellular bacteria and fungi and has been shown to modify the clinical presentation of tuberculosis [20–22]. However, DM also induces an inflammatory response associated with disease complications, such as diabetic retinopathy [23]. Diabetes decreases chemotaxis, phagocytosis, respiratory burst, and the bacterial killing ability of neutrophils [24–26]. Neutrophils and monocytes are the first line of defense against protozoan Leishmania spp. However, There is a lack of studies investigating the influence of blood sugar levels in Leishmania infection, and it remains unclear whether diabetes modifies the clinical presentation of leishmaniasis. A recent clinical report detailed 3 patients living in Morocco with CL and DM, 1 of whom presented a vegetative lesion [27]. The present study endeavored to compare clinical presentations and immunological responses in CL patients with or without diabetes and to evaluate the influence of DM on the therapeutic response to meglumine antimoniate.
METHODS
Type of Study
The present prospective study compared clinical manifestations, histopathological findings, cytokine production, and therapeutic response in 36 CL patients with DM (CL+DM) with 36 CL patients without DM, matched by age (±5 years) and sex. In addition, a nested cross-sectional study was performed to compare immune response between 8 CL+DM patients with atypical lesions and 9 CL+DM patients with typical lesions.
Study Population and Case Definition
This study was conducted between January 2017 and June 2019 at the municipal health clinic of Corte de Pedra, located in the municipality of Presidente Tancredo Neves, Bahia-Brazil.
The study included 64 adult patients aged 18 to 60 years, of both sexes, with a diagnosis of CL with or without DM. All patients presented at least 1 CL ulcer and had an illness duration between 20 and 90 days. All resided in the same geographic region and faced similar exposure risks, as most were farm workers. Only 1 patient refused participaton, as he was unable to attend all follow-up visits at the clinic. Diagnosis was confirmed via documentation of L. braziliensis DNA by polymerase chain reaction (PCR) [28] or through the identification of amastigotes in the histopathological analysis of biopsied tissue. DM was diagnosed when glycated hemoglobin was ≥6.5%. Patients with kidney injury, congestive heart failure, liver failure, or HIV infection were excluded from the study.
Study Design
Upon agreeing to participate in the study and signing a term of informed consent, patients were submitted to a clinical examination and classified as CL+DM or CL without DM. Blood was collected for laboratorial immunological analysis before beginning therapy. All participants were evaluated at the clinic to determine lesion size, clinical improvement, evolution to cure, and treatment side effects every 30 days. Patients were treated intravenously with Glucantime (Sanofi Aventis, Brazil) at a dose of 20 mg/kg of body weight per day for 20 days. Cure was defined by the absence of active ulceration and complete skin reepithelization, without raised borders, on day 90 after the onset of treatment. Therapeutic failure was defined by the presence of active ulcers or scar formation with raised borders 90 days after the initiation of therapy. Patients who did not respond to treatment by day 90 were treated with the same dose of Glucantime for 30 additional days. Of the 36 patients with DM who participated in the study, 29 (81%) had previously been diagnosed with DM before the presentation of CL; in these cases, we followed the therapeutic regimen prescribed by the attending physician, that is, the only intervention undertaken was to increase antidiabetic drug usage when blood sugar levels remained above normal limits. In 7 (24%) patients, DM was diagnosed at the same time as CL. Of 36 patients with DM, 7 received insulin, 15 received Glyburide, 13 received Glicazide, and 1 patient was treated with Glimepiride. Of the 28 patients who used Glyburide or Glicazide, 22 were prescribed metformin.
Biopsies and Immunohistochemistry
Biopsies were obtained from all 72 patients for the detection of L. braziliensis DNA by PCR. However, as the decision to perform histopathological analysis was made during the course of the study, this evaluation was performed in only the last 19 patients enrolled. Biopsies were obtained from the borders of lesions using a 4-mm-diameter punch, following the application of local anesthetic. Skin tissues were fixed in buffered formaldehyde and embedded in paraffin blocks. Deparaffinization and rehydration of 5- μm-thick sections were performed using xylene and absolute alcohol, and antigen retrieval was performed using citrate buffer pH 9.0 at 96°C for 20 minutes. Immunohistochemistry reactions were performed as previously described [29]. Briefly, after blockage of peroxidase activity with 3% hydrogen peroxide for 10 minutes and blockage of proteins with Protein Block Serum-Free (DAKO, Carpinteria, CA, USA) for 15 minutes, slides were incubated overnight at 4°C with monoclonal mouse anti-CD20, anti-CD68, anti-CD56, anti-CD4, anti-CD8, and anti-granzyme B (DAKO, Carpinteria, CA, USA). Mouse and Rabbit Peroxidase Kit/Horseradish Peroxidase KP500 (Diagnostic Bio-Systems, Pleasanton, CA, USA) were used to perform reactions according to manufacturer recommendations.
Morphometry of Inflammation and Necrotic Areas
All histological sections stained with hematoxylin and eosin were scanned under an optical microscope (Olympus BX51). The total extension of these sections, as well as areas of inflammatory infiltrate and necrosis, was measured using Image J, version 1.48 (National Institutes of Health). The total length of the biopsy fragment and the sum of the areas of inflammation and necrosis are expressed in mm2. Percentages of inflammation and necrosis in the biopsied samples were calculated by dividing the total extension of inflammation and necrosis by the total extension of the biopsy fragment multiplied by 100.
Quantitative Analysis
Quantification of the cells was performed by optical microscopy (Olympus BX51), selecting 5 random fields in each section with the respective antibodies at a magnifying power of 40×. The number of positive cells in each field was quantified though the identification of brown-colored cells, as the result of reactions with chromogenic substrate.
Evaluation of Immune Response
Immunological studies were performed on day 0 of the study. Mononuclear cells were isolated from heparinized peripheral blood (20 mL) by Ficoll-Hypaque density gradient centrifugation. Cells were harvested, washed with saline, and resuspended in RPMI 1640 (GIBCO BRL, Grand Island, NY, USA) supplemented with 5% fetal bovine serum and antibiotics. For cytokine determination, 3×106 cells/mL were stimulated with soluble Leishmania antigen (10 µg/mL) and cultured for 48 hours at 37ºC under 5% CO2. Cytokine (IFN-γ, TNF-α, IL-1β, and CXCL-10) quantification was performed in mononuclear cell culture supernatants by sandwich enzyme-linked immunosorbent assay.
Patient Consent Statement
Participation in the present study was voluntary. Patients who declined to participate received similar follow-up and treatment as study participants. The present study protocol was approved by the Institutional Review Board of the Gonçalo Moniz Institute (FIOCRUZ), and all participants provided written informed consent.
RESULTS
The demographic and clinical features of 36 CL+DM patients and 36 CL without DM are shown in Table 1. No significant differences were seen between the groups regarding gender, illness duration, lesion size and location, frequency of satellite lymphadenopathy, or lymph node size. Patients with CL+DM were older than those without DM. In the majority of both groups of patients, ulcers were infected, but antibiotics were used in only 1 diabetic. No differences were found in cure rate on day 90 in CL+DM patients vs CL (P ˃ .05). Of the 36 patients with DM, 7 (19%) had type 1 diabetes while 29 (81%) had type 2. No significant differences were observed regarding the clinical features of CL among patients with type 1 or type 2 diabetes. The median (interquartile range) of blood sugar levels in patients with type 1 diabetes was 370 (292–470) mg/dL, vs 277 (228–326) mg/dL in those with type 2. Moreover, there were no differences in response to antimony therapy between these 2 groups, as the cure rate on day 90 was 71% in patients with type 1 (n = 7) vs 68% in those with type 2 (n = 25; P > .05). In addition, response to therapy was similar regardless of the type of antidiabetic drugs used. Despite antidiabetic drug use, the majority of patients had above-normal blood sugar levels at admission, which remained throughout follow-up.
Table 1.
Demographic and Clinical Features of Cutaneous Leishmaniasis in Patients With and Without Diabetes Mellitus
| Cutaneous Leishmaniasis With Diabetes | Cutaneous Leishmaniasis Without Diabetes | ||
|---|---|---|---|
| Demographic/Clinical Features | n = 36 | n = 36 | P Value |
| Age, mean ± SD, y | 49 ± 12 | 39 ± 13 | .0008 |
| Presence of atypical lesions, No. (%) | 13 (36) | 0 (0) | .0001 |
| Gender, female, No. (%) | 23 (64) | 20 (56) | .63 |
| Patients with >1 lesion, No. (%) | 15 (41) | 67 (19) | .07 |
| Size of the major lesion, median (CI), mm | 20 (17–31) | 19 (18–24) | .91 |
| Area of the major lesion, median (CI), mm | 185 (215–712) | 246 (247–465) | .49 |
| Frequency of lesions above the belt, No. (%) | 13 (33) | 8 (22) | .03 |
| Frequency of patients with lymph node enlargement, No. (%) | 17 (47) | 20 (56) | .63 |
| Cure rate by day 90, No. (%) | 24 (67) | 20 (56) | .46 |
| Healing time, median (CI), d | 80 (69–96) | 83 (74–116) | .70 |
aFisher exact test.
bMann-Whitney test.
To determine whether blood sugar levels influenced cure rate, we compared the therapeutic response in patients with blood sugar levels <200 mg/dL with that of those who had blood sugar levels >250 mg/dL. The cure rate was 43% in the 3/7 patients with blood sugar <200 mg/dL compared with 72% in the 18/25 patients with elevated levels (P > .05). While we observed a weak negative correlation between blood sugar levels and healing time (R = .21; P > .05), we did not find any associations between clinical presentation and response to therapy according to the type of antidiabetic drugs used.
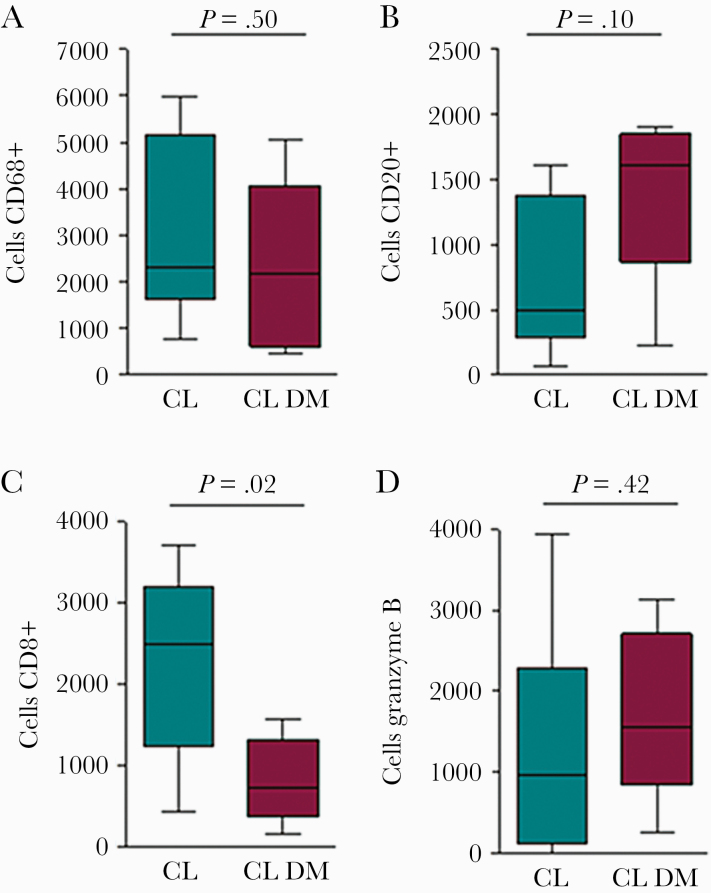
Histopathologic analysis was performed in 9 patients with CL+DM and in 10 with CL only; immunochemical studies were done in 5 patients from each group. No differences were seen in the majority of findings typically observed in patients with CL, for example, acanthosis, hyperkeratosis, hyperplasia of the epiderma, granuloma formation, and number of amastigotes. However, while necrotic foci were observed in all CL patients without DM, this finding was only documented in 50% of CL+DM patients (P < .05). Due to the small number of cases analyzed, it was not possible to determine the impact of blood sugar levels or antidiabetic drugs on histopathologic features. Figure 1 illustrates the frequency of cells expressing CD68, CD20, CD8, and granzyme B. While there were no differences in the frequencies of macrophages (CD68+), B cells (CD20+), and cells expressing granzyme B, the frequency of CD8+ T cells in patients with CL+DM was 1.8-fold lower than in CL patients without DM (P < .05).
Figure 1.
Immunophenotypic analysis in skin biopsy of cutaneous leishmaniasis patients with and without diabetes mellitus. Tissue biopsy from 5 patients with cutaneous leishmaniasis and diabetes mellitus and from 5 patients with cutaneous leishmaniasis without diabetes, incubated with anti-CD68+, CD20+, CD8+, and granzyme B monoclonal antibodies. Reactions were performed with a mouse and rabbit peroxidase kit. Date represents median and interquartile of positively stained cells. Statistical analysis was performed using the Mann-Whitney test. *P < .05. Abbreviations: CL, cutaneous leishmaniasis; DM, diabetes mellitus.
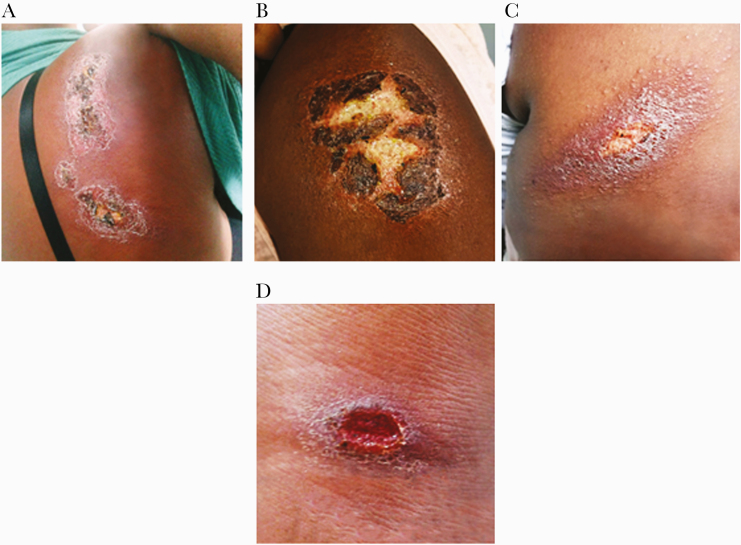
An important finding of the present study was the documentation of atypical ulcers in patients with DM, which were observed in 13 (36%) out the 36 CL patients with DM. In contrast to well-delimited oval ulcers with raised borders, diabetic patients presented atypical lesions characterized by superficial, flat ulcers with poorly defined borders (Figure 2).
Figure 2.
Atypical and typical cutaneous ulcers in patients with diabetes and cutaneous leishmaniasis. Images of skin ulcers taken on day 0 from 4 patients with diabetes mellitus and cutaneous leishmaniasis: 3 cases with atypical ulcers (A, B, C) and 1 from a patient with typical cutaneous leishmaniasis (D).
The clinical characteristics of the 13 DM patients with atypical lesions and 23 cases with typical CL ulcers are shown in Table 2. No significant differences were found between these 2 groups regarding age, number or size of lesions, presence of satellite lymph node, or blood sugar levels. Ulcers above the belt were more frequent in patients with atypical lesions (50%) vs those with typical lesions (20%), yet without significance. One marked difference between those with typical vs atypical lesions was seen with respect to antimony therapy failure, which occurred in 69% of the patients with atypical lesions vs just 13% of those with typical lesions (P < .01).
Table 2.
Demographic and Clinic Features of Patients With Cutaneous Leishmaniasis and Diabetes Mellitus With Atypical or Typical Lesions Compared With Patients Without Diabetes (Controls)
| Atypical | Typical | Controls | ||
|---|---|---|---|---|
| Demographic and Clinical Features | n = 13 | n = 23 | n = 36 | P Value |
| Age, mean ± SD, y | 48 ± 14 | 50 ± 11 | 39 ± 14 | .0003a |
| Body mass index, median (CI), kg/m2 | 27 (26–29) | 26 (24–28) | 26 (26–30) | .43b |
| Illness duration, median (CI), d | 35 (27–55) | 40 (33–51) | 40 (36–46) | .86 |
| No. of lesions, median (CI) | 1 (1.06–1.8) | 1 (1.25–2.13) | 1 (1.07–1.53) | .16a |
| Total lesion circumference, median (CI), mm | 300 (121–1480) | 154 (165–380) | 246 (247–464) | .38 |
| Frequency of lesions above the belt, No. (%) | 6 (46) | 6 (26) | 8 (22) | .78 |
| Frequency of patients with lymph node enlargement, No. (%) | 5 (38) | 12 (52) | 19 (53) | .84 |
| Blood sugar levels, median (CI), mg/dl | 238 (201–301) | 293 (258–349) | 98 (101–118) | .0001 |
| Failure of therapy, No. (%) | 9 (69) | 3 (13) | 16 (44) | .0017c |
aStudent t test.
bAll other values determined using the Kruskal-Wallis test.
cFisher exact test.
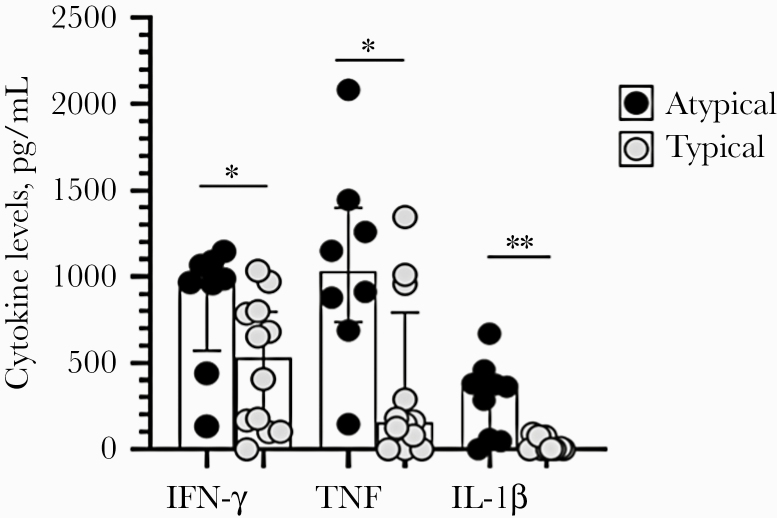
The cytokine profiles of patients with CL+DM presenting typical and atypical lesions are shown in Figure 3. IFN-γ levels, TNF levels, and IL-1β levels in patients with DM with atypical ulcers were 978 (571–1083) pg/mL, 103 (737–1399) pg/mL, and 364 (54–418) pg/mL, respectively—higher (P < .05) than in those with typical ulcers: 530 (118–796) pg/mL, 156 (20–792) pg/mL, and 4 (0–61) pg/mL, respectively. With regard to cytokine production in soluble Leishmania antigen–stimulated PBMCs from CL patients without DM (controls), INF-γ levels (IQR) were higher in controls (1486 [787–2152] pg/mL) than in DM with typical CL ulcers (P < .05), while IL-1β in the controls was lower (45 [3–193] pg/mL) than in CL+DM patients with atypical ulcers (P < .01). The levels of CXCL-10, IL-6, and granzyme B were similar between the 2 groups.
Figure 3.
Cytokine levels in the supernatants of mononuclear cells in patients with diabetes mellitus with atypical and typical ulcers. Interferon-γ, tumor necrosis factor, and interleukin-1β levels were measured by enzyme-linked immunosorbent assay in the supernatants of 3×106 peripheral blood mononuclear cells stimulated with soluble Leishmania antigen (10 μg/mL). Dates are represented by medians and interquartile ranges. Statistical analysis was performed using the Mann-Whitney test. Abbreviations: IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
DISCUSSION
It is known that DM increases the susceptibility and severity of bacterial fungus infections [30, 31]. The increased susceptibility and severity of bacterial infections in patients with diabetes can be explained by an impairment in chemotaxis, phagocytosis, and the decreased bacteria-killing ability of neutrophils [32]. However, it remains unclear whether DM impairs host killing of Leishmania protozoa, as well as whether DM modifies the clinic course of CL. Herein we evaluated the influence of DM on clinical presentation and therapeutic response to CL caused by L. braziliensis, the most important species associated with CL in Latin America. While blood sugar levels, type of diabetes, and antidiabetic drug therapy were not observed to modify clinical presentation, CL severity, or response to therapy, 36% of CL+DM patients did present atypical superficial ulcers lacking well-defined borders. These patients had enhanced levels of TNF, IL-1β, and IFN-γ and experienced higher rates of therapeutic failure.
High blood sugar levels have been associated with severity in diseases other than DM [32–34]. Interestingly, we did not observe an association between elevated levels of blood sugar and CL severity, yet we identified a weak inverse correlation between blood sugar levels and healing time. In 5 malnourished patients with type 1 diabetes with elevated blood sugar levels throughout follow-up, the disease presentation was not more severe and cure was already observed on day 60; in the majority of patients, cure only occurred between 60 and 90 days after onset of therapy.
It has been shown that the antidiabetic drug Glyburide decreases IL-1B and TNF levels [35] in human CL and reduces immunopathology in mice infected with L. braziliensis [36]. While we did not observe any differences between patients treated with Glyburide, Gliclazide, or metformin, we cannot rule out the potential benefit of Glyburide due to the small number of patients treated with different antidiabetic agents.
With respect to infections caused by intracellular agents in DM patients, tuberculosis is the most commonly studied association. DM has been shown to increase risk of M. tuberculosis infection and tuberculosis severity [37]. It has also been shown that DM decreases the expression of IL-37, and consequently impairs the control of M. tuberculosis infection [38]. Moreover, decreased production of IL-6, IL-10, IL-12, monocyte chemoattractant protein–1, RANTES (regulated on activation, normal T cell expressed and secreted), and CXCL-9 by monocytes infected with M. tuberculosis has been linked to the decreased ability of monocytes to kill M. tuberculosis [39]. Despite a lack of data regarding the consequences of L. braziliensis infection in monocytes from diabetic patients, we did not find increased parasite burden in macrophages in our histopathologic analysis of tissue samples from CL+DM patients.
The histopathologic analysis of ulcers in the 2 groups of patients revealed lower frequencies of necrosis and CD8+ T cells in CL+DM patients. Recently, emphasis has been given to the role of CD8+ T cells in the pathology of CL caused by L. braziliensis. CD8+ T cells from CL patients display high expression of cytotoxicity markers, but while they do not kill Leishmania, they kill L. braziliensis–infected cells [40–42]. The killing of infected cells leads to inflammasome formation, the release of IL-1β, and ulcer development [41]. Here we found lower frequencies of CD8+ T cells in lesions from diabetic patients compared with those with only CL. We speculate that the decreases in CD8+ T cells and necrosis observed in the ulcers of CL+DM patients may be responsible for the appearance of atypical superficial ulcers.
In light of previous evidence documenting the ability of diabetes to modify the clinical presentation of other infections, the observed similarity among the majority of clinical variables between the 2 groups was an unexpected finding. It is known that around 60% of CL patients are typically male [43, 44]; however, 69% of those with CL+DM were women. Another finding, and perhaps the most important of our study, was the observation that 36% of CL+DM patients presented atypical lesions. Atypical lesions are also found in patients infected with HIV, in those with diffuse cutaneous leishmaniasis (DCL), and in pregnant women [7, 45, 46]. Notably, the lesions documented herein in patients with DM were remarkably distinct from all other types of lesions described in CL patients [5].
Atypical CL lesions in patients with HIV and in those with diffuse cutaneous leishmaniasis occur due to an impairment in T-cell response [47]. Alternatively, large vegetative lesions in pregnant women with CL have been associated with an inflammatory reaction due to increased CD4+ T cells expressing IL-4 at the lesion site, indicating a shift to Th2 immune response [48]. This evidence indicates that different inflammation pathways may be related to diverse clinical outcomes in the same disease. Although we found decreased frequencies of CD8+ T cells and necrosis at the lesion site of CL+DM patients, we also observed higher production of IFN-γ, TNF, and IL-1β in the supernatants of mononuclear cells from DM patients with atypical lesions compared with those with typical lesions. It is possible that high blood sugar levels may increase the production of IFN-γ, IL-1β, IL-6, and TNF [49, 50]. The role of an exaggerated inflammatory response in the pathogenesis of L. braziliensis has been well documented, as has an association between the exacerbated production of these cytokines and lesion size, as well as therapeutic failure [36, 51].
The most important finding herein was documentation of the capability of DM to modify the clinical presentation of CL, due to the appearance of atypical CL lesions associated with a poor response to therapy. The influence of DM on clinical CL presentation stands in agreement with a recent case report detailing a patient from Morocco who presented vegetative lesions [26]. In our study, the atypical lesions observed were predominantly flat, usually large, and lacked well-defined borders. While more superficial ulcers may occur due to a lower frequency of cytotoxic CD8+ T cells at the lesion site and decreased necrosis, the high rate of failure of therapy seen in these patients may be occuring due to an increase in the synthesis of proinflammatory cytokines.
Acknowledgments
We thank Cristiano Sampaio Franco for his secretarial assistance in the preparation of this manuscript. The authors are also grateful to Andris K. Walter for English language revision services and manuscript copyediting assistance.
Financial support. This study was supported by the National Institutes of Health (NIH AI136032), National Research Council (CNPq), and Brazilian Ministry of Science, Technology and Innovation.
Potential conflicts of interest. The authors have declared that no conflict of interests exist. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jones TC, Johnson WD Jr, Barretto AC, et al. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis 1987; 156:73–83. [DOI] [PubMed] [Google Scholar]
- 2. Turetz ML, Machado PR, Ko A, et al. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in Northeastern Brazil. J Infect Dis 2002; 186:1829–34. [DOI] [PubMed] [Google Scholar]
- 3. Vernal S, De Paula NA, Gomes CM, Roselino AM. Disseminated leishmaniasis by Leishmania viannia subgenus: a series of 18 cases in Southeastern Brazil. Open Forum Infect Dis 2016; 3:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guimarães LH, Machado PR, Lago EL, et al. Atypical manifestations of tegumentary leishmaniasis in a transmission area of Leishmania braziliensis in the state of Bahia, Brazil. Trans R Soc Trop Med Hyg 2009; 103:712–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meirele CB, Maia LC, Soares GC, et al. Atypical presentations of cutaneous leishmaniasis: a systematic review. Acta Trop 2017; 172: 240–54. [DOI] [PubMed] [Google Scholar]
- 6. Queiroz A, Sousa R, Heine C, et al. Association between an emerging disseminated form of leishmaniasis and Leishmania (Viannia) braziliensis strain polymorphisms. J Clin Microbiol 2012; 50:4028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guimarães LH, Queiroz A, Silva JA, et al. Atypical manifestations of cutaneous leishmaniasis in a region endemic for Leishmania braziliensis: clinical, immunological and parasitological aspects. PLoS Negl Trop Dis 2016; 10:e0005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silva SC, Guimarães LH, Silva JA, et al. Molecular epidemiology and in vitro evidence suggest that Leishmania braziliensis strain helps determine antimony response among American tegumenary leishmaniasis patients. Acta Trop 2018; 178:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peters NC, Egen JG, Secundino N, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 2008; 321:970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ribeiro-Gomes FL, Sacks D, et al. The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front Cell Infect Microbiol 2012; 2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jochim RC, Teixeira C. Leishmania commandeers the host inflammatory response through neutrophils. Trends Parasitol 2009; 25:145–7. [DOI] [PubMed] [Google Scholar]
- 12. Conceição J, Davis R, Carneiro PP, et al. Characterization of neutrophil function in human cutaneous leishmaniasis caused by Leishmania braziliensis. PLoS Negl Trop Dis 2016; 10:e0004715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carneiro PP, Conceição J, Macedo M, et al. The role of nitric oxide and reactive oxygen species in the killing of Leishmania braziliensis by monocytes from patients with cutaneous leishmaniasis. PLoS One 2016; 11:e0148084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polari LP, Carneiro PP, Macedo M, et al. Leishmania braziliensis infection enhances toll-like receptors 2 and 4 expression and triggers TNF-α and IL-10 production in human cutaneous leishmaniasis. Front Cell Infect Microbiol 2019; 9:120–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Srivastava A, Singh N, Mishra M, et al. Identification of TLR inducing Th1-responsive Leishmania donovani amastigote-specific antigens. Mol Cell Biochem 2012; 359: 359–68. [DOI] [PubMed] [Google Scholar]
- 16. Unger A, O'Neal S, Machado PR, et al. Association of treatment of American cutaneous leishmaniasis prior to ulcer development with high rate of failure in Northeastern Brazil. Am J Trop Med Hyg 2009; 80:574–9. [PMC free article] [PubMed] [Google Scholar]
- 17. Costa RS, Carvalho LP, Campos TM, et al. Early cutaneous leishmaniasis patients infected with Leishmania braziliensis express increased inflammatory responses after antimony therapy. J Infect Dis 2018; 217:840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bacellar O, Lessa H, Schriefer A, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun 2002; 70: 6734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou B. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. The Lancet 2016; 387:1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nathella PK, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 2017; 152: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ayelign B, Negash M, Genetu M, et al. Immunological impacts of diabetes on the susceptibility of Mycobacterium tuberculosis. J Immunol Res 2019; 2019:6196532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gil-Santana L, Almeida-Junior JL, Oliveira CA, et al. Diabetes is associated with worse clinical presentation in tuberculosis patients from Brazil: a retrospective cohort study. PLoS One 2016; 11:e0146876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheung N, Mitchell P, Wong TY, et al. Diabetic retinopathy. Lancet 2010; 376:124–36. [DOI] [PubMed] [Google Scholar]
- 24. Marhoffer W, Stein M, Schleinkofer L, Federlin K. Evidence of ex vivo and in vitro impaired neutrophil oxidative burst and phagocytic capacity in type 1 diabetes mellitus. Diabetes Res Clin Pract 1993; 19:183–8. [DOI] [PubMed] [Google Scholar]
- 25. Alba-Loureiro TC, Munhoz CD, Martins JO, et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res 2007; 40:1037–44. [DOI] [PubMed] [Google Scholar]
- 26. McManus LM, Bloodworth RC, Prihoda TJ, et al. Agonist-dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia. J Leukoc Biol 2001; 70: 395–404. [PubMed] [Google Scholar]
- 27. Chiheb S, Oudrhiri L, Zouhair K, et al. Unusual clinical presentation of cutaneous leishmaniasis in three diabetic patients [in French]. Ann Dermatol Venereol 2012; 139:542–5.22963963 [Google Scholar]
- 28. Weirather JL, Jeronimo SM, Gautam S, et al. Serial quantitative PCR assay for detection, species discrimination, and quantification of Leishmania spp. in human samples. J Clin Microbiol 2011; 49: 3892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saldanha MG, Queiroz A, Machado PRL, et al. Characterization of the histopathologic features in patients in the early and late phases of cutaneous leishmaniasis. Am J Trop Med Hyg 2017; 96:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol 1999; 26:259–65. [DOI] [PubMed] [Google Scholar]
- 31. Rodrigues CF, Rodrigues ME, Henriques M, et al. Candida sp. infections in patients with diabetes mellitus. J Clin Med 2019; 8: 76–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Insuela D, Coutinho D, Martins M, et al. Neutrophil function impairment is a host susceptibility factor to bacterial infection in diabetes. In: Fuchs O, Athari SS, eds. Cells of the Immune System. 1st ed. Intech Open; 2019:1–22. [Google Scholar]
- 33. Leite SA, Locatelli SB, Niece SP, et al. Impact of hyperglycemia on morbidity and mortality, length of hospitalization and rates of re-hospitalization in a general hospital setting in Brazil. Diabetol Metab Syndr 2010; 2: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baker EH, Wood DM, Brennan AL, et al. Hyperglycaemia and pulmonary infection. Proc Nutr Soc 2006; 65:227–35. [DOI] [PubMed] [Google Scholar]
- 35. Carvalho AM, Novais FO, Paixão CS, et al. Glyburide, a NLRP3 inhibitor, decreases inflammatory response and is a candidate to reduce pathology in Leishmania braziliensis infection. J Invest Dermatol 2020; 140:246–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novais FO, Carvalho AM, Clark ML, et al. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1β production. PLoS Pathog 2017; 13:e1006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hodgson K, Morris J, Bridson T, et al. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015; 144:171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montoya-Rosales A, Castro-Garcia P, Torres-Juarez F, et al. Glucose levels affect LL-37 expression in monocyte-derived macrophages altering the Mycobacterium tuberculosis intracellular growth control. Microb Pathog 2016; 97: 148–53. [DOI] [PubMed] [Google Scholar]
- 39. Lopez-Lopez N, Martinez AGR, Garcia-Hernandez MH, et al. Type-2 diabetes alters the basal phenotype of human macrophages and diminishes their capacity to respond, internalise, and control Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz 2018; 113:e170326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santos Cda S, Boaventura V, Ribeiro Cardoso C, et al. CD8(+) granzyme B(+)-mediated tissue injury vs. CD4(+)IFNγ(+)-mediated parasite killing in human cutaneous leishmaniasis. J Invest Dermatol 2013; 133:1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Novais FO, Carvalho LP, Graff JW, et al. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS Pathog 2013; 9:e1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cardoso TM, Machado Á, Costa DL, et al. Protective and pathological functions of CD8+ T cells in Leishmania braziliensis infection. Infect Immun 2015; 83:898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jirmanus L, Glesby MJ, Guimarães LH, et al. Epidemiological and clinical changes in American tegumentary leishmaniasis in an area of Leishmania (Viannia) braziliensis transmission over a 20-year period. Am J Trop Med Hyg 2012; 86:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teles GC, Fonseca FR, Gonçalves MJF. American tegumentary leishmaniasis in the Brazilian Amazon from 2010 to 2014. Rev Inst Med Trop Sao Paulo 2019; 61:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morgan DJ, Guimaraes LH, Machado PR, et al. Cutaneous leishmaniasis during pregnancy: exuberant lesions and potential fetal complications. Clin Infect Dis 2007; 45:478–82. [DOI] [PubMed] [Google Scholar]
- 46. Barral A, Costa JM, Bittencourt AL, et al. Polar and subpolar diffuse cutaneous leishmaniasis in Brazil: clinical and immunopathologic aspects. Int J Dermatol 1995; 34:474–9. [DOI] [PubMed] [Google Scholar]
- 47. Da-Cruz AM, Filgueiras DV, Coutinho Z, et al. Atypical mucocutaneous leishmaniasis caused by Leishmania braziliensis in an acquired immunodeficiency syndrome patient: T-cell responses and remission of lesions associated with antigen immunotherapy. Mem Inst Oswaldo Cruz 1999; 94: 537–42. [DOI] [PubMed] [Google Scholar]
- 48. Dutra WO, Barbosa DF, de Souza PEA, et al. A Th2-type response is associated with exuberant lesions in pregnant women infected with Leishmania braziliensis. J Infect Dis 2019; 219:480–8. [DOI] [PubMed] [Google Scholar]
- 49. Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci 2000; 67:291–300. [DOI] [PubMed] [Google Scholar]
- 50. Rapoport MJ, Mor A, Vardi P, et al. Decreased secretion of Th2 cytokines precedes up-regulated and delayed secretion of Th1 cytokines in activated peripheral blood mononuclear cells from patients with insulin-dependent diabetes mellitus. J Autoimmun 1998; 11:635–42. [DOI] [PubMed] [Google Scholar]
- 51. Carvalho LP, Passos S, Dutra WO, et al. Effect of LACK and KMP11 on IFN-gamma production by peripheral blood mononuclear cells from cutaneous and mucosal leishmaniasis patients. Scand J Immunol 2005; 61:337–42. [DOI] [PubMed] [Google Scholar]