Abstract
Introduction
With increasing advances in minimally invasive endoscopic therapies and endoscopic resection techniques for luminal disease, there is an increased risk of post-procedure bleeding. This can contribute to significant burden on patient’s quality of life and health resources when reintervention is required. Hemospray (Cook Medical, North Carolina, USA) is a novel haemostatic powder licensed for gastrointestinal bleeding. The aim of this single-arm, prospective, non-randomised multicentre international study is to look at outcomes in patients with upper gastrointestinal bleeds following elective endoscopic therapy treated with Hemospray to achieve haemostasis.
Methods
Data was prospectively collected on the use of Hemospray from 16 centres (January 2016–November 2019). Hemospray was used during the presence of progressive intraprocedural bleeding post-endoscopic therapy as a monotherapy, dual therapy with standard haemostatic techniques or rescue therapy once standard methods had failed. Haemostasis was defined as the cessation of bleeding within 5 min of the application of Hemospray. Re-bleeding was defined as a sustained drop in haemoglobin (>2 g/l), haematemesis or melaena with haemodynamic instability after the index endoscopy.
Results
A total of 73 patients were analysed with bleeding post-endoscopic therapy. The median Blatchford score at baseline was five (interquartile range 0–9). The median Rockall score was six (interquartile range 5–7). Immediate haemostasis following the application of Hemospray was achieved in 73/73 (100%) of patients. Two out of 57 (4%) had a re-bleed post-Hemospray, one was following oesophageal endoscopic mucosal resection and the other post-duodenal endoscopic mucosal resection. Both patients had a repeat endoscopy and therapy within 24 h. Re-bleeding data was missing for 16 patients, and mortality data was missing for 14 patients. There were no adverse events recorded in association with the use of Hemospray.
Conclusion
Hemospray is safe and effective in achieving immediate haemostasis following uncontrolled and progressive intraprocedural blood loss post-endoscopic therapy, with a low re-bleed rate.
Keywords: TC-325, Hemospray, endoscopy, upper gastro-intestinal bleeding, post-endotherapy bleeding
Key summary
Summarise the established knowledge on this subject
With increased complexity of therapeutic endoscopy there is increased incidence of intraprocedural bleeding.
Bleeding rates are variable depending on the underlying endoscopic procedure.
There are few studies on optimal haemostatic techniques in this area.
What are the significant and/or new findings of this study?
There is a 100% immediate haemostasis rate following Hemospray application in intraprocedural bleeding in all areas of the upper gastrointestinal tract.
There is a low re-bleed rate of 4% following treatment.
There were no complications associated with the use of Hemospray.
Background
Upper gastrointestinal bleeding (UGIB) is a significant cause of morbidity and is associated with a 2–17% mortality around the world.1 With recent advances and the increasing complexity of endoscopic therapy there is an increased incidence of associated intraprocedural gastrointestinal (GI) bleeding. Bleeding rates are variable depending on the underlying therapeutic procedure. Bleeding occurs in 1–2% of cases after an oesophageal endoscopic mucosal resection (EMR), 2% of cases after a biliary sphincterotomy and 1–6% of cases after oesophageal endoscopic submucosal dissection (ESD).2,3 Reported bleeding rates following gastric ESD vary from 1.8–15.6%.4,5 In duodenal EMR procedures an intraprocedural bleeding rate varying from 0–29% has been reported. There is higher bleeding rate in the duodenum due to increased arterial blood supply.6 Endoscopic haemostatic modalities to address this bleeding in a relatively easy and effective manner require further exploration.
Currently, conventional modalities for management of post-endotherapy bleeding include adrenaline injection therapy, thermocoagulation and mechanical clips.7 Dual endoscopic therapy in combination with adrenaline is considered to be superior to monotherapy in patients with peptic ulcer disease. There is limited data on the optimal endoscopic management of intraprocedural and post endotherapy bleeds.
With advances in endoscopic therapy, larger cancerous lesions are being resected which carry a higher risk of bleeding complications. At the same time, advances in endoscopic resection such as ESD allow an en-bloc resection for large or fibrotic lesions which enables a curative resection and accurate histological assessment.8 This allows for organ preservation as such lesions would otherwise need surgery which would be high-risk for particular patient groups. Therefore, there should be methods to manage possible bleeding risks during such procedures.
TC-325 (Hemospray; Cook Medical, Winston-Salem, North Carolina, USA) is a haemostatic mineral-based powder. Once it is in contact with blood, it absorbs the fluid triggering a clotting cascade and forms a tamponade across the bleeding site9 (Figures 1 and 2). It potentially is of benefit after endotherapy in that it can be applied to the bleeding resection site under direct vision and target the area in a non-contact fashion. Directing endoscopic haemostatic therapy during intraprocedural bleeding can be challenging where the bleeding source is discrete, or access is difficult.
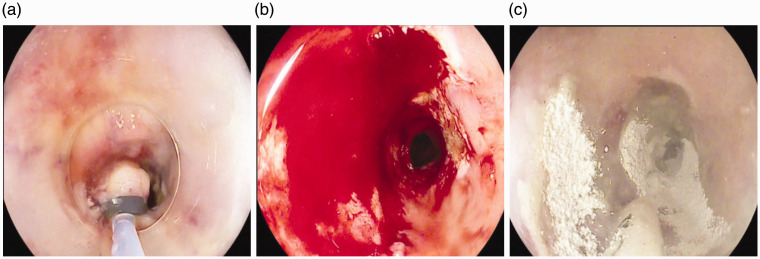
Figure 1.
Bleeding following oesophageal endoscopic mucosal resection (EMR) (a). There is profuse bleeding (b) following which Hemospray is applied and immediate haemostasis achieved (c).
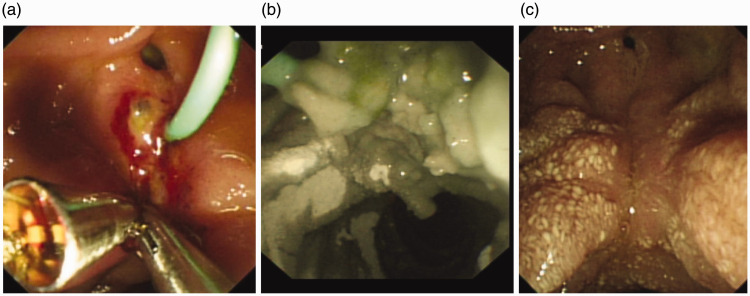
Figure 2.
Oozing post-sphincterotomy despite clip placement (a). Hemospray applied in combination with clips to achieve haemostasis (b). Site is completely healed 2 weeks later (c).
The primary aim of the study was to assess the success of endoscopic haemostasis in patients with uncontrolled and progressive intraprocedural bleeding following endotherapy treated with Hemospray
Methods
This study was presented to the local research ethics committee (London – South East Research Ethics Committee, approved October 2016) (International Standard Randomised Control Trial Number (ISRCTN) registry with study ID ISRCTN29594250). Centres in other participating countries also obtained approval from their local authorities. All patients provided informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki.
The Rockall and the Blatchford scoring system were both used in this study. The Rockall scoring system predicts mortality and re-bleeding.10 The Blatchford scoring system determines the need for urgent clinical intervention.11 These scoring systems have been used in previous publications to predict risk and prognosticate outcomes in patients with peptic ulcer disease (PUD) and were therefore applied to this cohort of patients to explore if there were any factors to predict outcomes and rebleeding.
All patients that were included had evidence of an acute or progressive intraprocedural bleed following upper gastrointestinal (UGI) endoscopic therapy visualised by the endoscopist during the procedure. They were all treated with Hemospray during the same endoscopic session as monotherapy, as part of a combination therapy or a rescue therapy. No exclusion criterion was applied.
Patients were recruited prospectively from 16 centres in the UK, USA, Germany, France and Spain (January 2016–November 2019). All endoscopists had training on the use of Hemospray. Consecutive patients who had developed intraprocedural progressive bleeding post-endotherapy were recruited from each centre. The decision to use Hemospray was at the discretion of the endoscopist at the time of the procedure, based on the lesion, bleeding source and local expertise with the device.
The primary outcome was immediate cessation of endoscopic intraprocedural bleeding and haemostasis following the application of Hemospray when used as a:
– Monotherapy: used as a single therapy following which the site is observed for 5 min for cessation of bleeding.
– Combination therapy: Hemospray used as an adjunct with conventional methods following which the site is observed for 5 min for cessation of bleeding.
– Rescue therapy: used following treatment failure with conventional methods. Once conventional therapy failed to achieve haemostasis, after a 5-minute observation Hemospray is applied as a rescue therapy.
Secondary outcomes were re-bleeding after intraprocedural haemostasis with Hemospray, safety and mortality within 30 days.
Immediate haemostasis was defined as observed cessation of bleeding within 5 min of the application of Hemospray. Re-bleeding was defined as ongoing or new haematemesis or melaena with haemodynamic instability and/or a drop in haemoglobin (>2 g/l) following completion of the procedure. This definition is in keeping with previous guidelines and consensus statements.12
Patients were followed up for 30 days following index endoscopy. Follow-up data was retrieved from clinical records, outpatient clinical review and/or telephone consultations.
All the data was inputted into a customised and anonymised database.
Statistical analysis
Descriptive statistics consisted of the median and interquartile range (IQR). The occurrence of each outcome was quantified as a frequency and percentage. The Kruskal–Wallis test was used to look at significance of the difference between subgroups. All tests were two-tailed. A significant p value was considered to be <0.05.
Results
Between January 2016–November 2019, 73 patients were enrolled into this ongoing prospective registry study (51 males, 22 female) (Table 1). Patients had a median age of 73 years (IQR, 66–80). The median Blatchford score at baseline for all patients was five (IQR, 0–9). The median Rockall score was six (IQR, 5–7). The most common cause of intraprocedural bleeding was following EMR (39/73 (53%) patients) (Table 2). For bleeding following an EMR, 22/39 (56%) patients were treated with Hemospray as part of a combination therapy (Supplementary Material Table 1s). The most common site of intraprocedural bleeding was in the oesophagus (55%) (Table 1).
Table 1.
Demographics.
| Demographics (n = 73) | Value |
|---|---|
| Median age, years (IQR) | 73 (66–80) |
| Sex | |
| Male (%) | 51/73 (70%) |
| Female (%) | 22/73 (30%) |
| Blood thinning medications | |
| Anticoagulants (%) | 5/63 (8%) |
| Low molecular weight heparin (%) | 5/63 (8%) |
| Antiplatelets | 7/63 (11%) |
| Site of bleeding | |
| Oesophagus | 40 (55%) |
| Stomach | 12 (16%) |
| Duodenum | 21 (29%) |
IQR: interquartile range.
Table 2.
Most common causes of post-upper gastrointestinal (UGI) endotherapy intraprocedural bleeding.
| Cause of bleeding | Number of patients (%) | |
|---|---|---|
| Endoscopic mucosal resection | 39 (53%) | |
| Oesophagus | 27/39 (69%) | |
| Stomach | 3/39 (8%) | |
| Duodenum | 9/39 (23%) | |
| Polypectomy/ampullectomy | 8 (11%) | |
| Oesophagus | 0/8 | |
| Stomach | 2/8 (25%) | |
| Duodenum | 6/8 (75%) | |
| Endoscopic submucosal dissection | 5 (7%) | |
| Oesophagus | 3/5 (60%) | |
| Stomach | 2/5 (40%) | |
| Duodenum | 0/5 | |
| Sphincterotomy | 5 (7%) | |
| Oesophagus | 0/5 | |
| Stomach | 0/5 | |
| Duodenum | 5/5 (100%) | |
| Biopsy | 5 (7%) | |
| Oesophagus | 3/5 (60%) | |
| Stomach | 2/5 (40%) | |
| Duodenum | 0/5 | |
In total, 21 out of 73 (29%) patients had Hemospray treatment as a monotherapy, 37/73 (51%) patients as part of a combination therapy and 15/73 (21%) patients as a rescue therapy (Table 3).
Table 3.
Outcomes in the Hemospray subgroup.
| Monotherapy (n = 21) | Combination (n = 37) | Rescue (n = 15) | p-Value | |
|---|---|---|---|---|
| Median Rockall score | 6 (IQR, 5–6) | 6 (IQR, 6–7) | 6 (IQR, 4–7) | |
| Median Blatchford score | 3 (IQR, 0–6) | 7 (IQR, 2–9) | 3 (IQR, 0–5) | |
| Haemostasis | 21/21 (100%) | 37/37 (100%) | 15/15 (100%) | NS |
| Re-bleed | 1/18 (6%) | 1/26 (4%) | 0 | NS |
| 30-Day mortality | 1/18 (6%) | 0 | 0 | NS |
| Complications | 0 | 0 | 0 | NS |
IQR: interquartile range; NS: not significant.
There was immediate haemostasis in 73/73 (100%) of patients following treatment with Hemospray following intraprocedural bleeding post-UGI endoscopic therapy. Just 2/57 (4%) patients had a re-bleed within 30 days following initial treatment. The two re-bleeds were following an oesophageal EMR and a duodenal EMR and both occurred within 24 h of initial treatment with Hemospray. In one case Hemospray was used as a monotherapy, and as part of a combination therapy in the other case. Both patients had repeat endoscopic therapy within 24 h to treat the cause of bleeding. Also, 1/59 (2%) patients died within 30 days following treatment (all-cause mortality) where Hemospray was used as a monotherapy in the stomach. There was no significant difference in outcomes based on anatomical location of the bleeding (Table 4).
Table 4.
Outcomes with Hemospray based on anatomical location.
| Oesophagus (n = 40) | Stomach (n = 12) | Duodenum (n = 21) | |
|---|---|---|---|
| Median Rockall | 6 (IQR, 5–7) | 7 (IQR, 7–8) | 6 (IQR, 4–7) |
| Median Blatchford | 2 (IQR, 0–8) | 5 (IQR, 5–7) | 7 (IQR, 3–9) |
| Haemostasis | 40/40 (100%) | 12/12 (100%) | 21/21 (100%) |
| Re-bleed | 1/30 (3%) | 0 | 1/19 (5%) |
| 30-day mortality | 0 | 1/8 (13%) | 0 |
IQR: interquartile range.
Re-bleeding data was missing for 16 patients, and mortality data was missing for 14 patients in the registry.
There were no complications associated with the use of Hemospray in this cohort of patients.
Discussion
This is the largest cohort of patients to date looking at Hemospray in the treatment of post-UGI endoscopic therapy intraprocedural bleeding.
This data has shown that Hemospray is effective in the treatment of post-UGI endoscopic therapy intraprocedural bleeding with 100% immediate haemostasis rates and low re-bleeding rates (4%). This is in keeping with data from previous studies with smaller sample sizes, where intraprocedural haemostasis ranged from 90–100%.13,14 The patient cohort had a median Rockall score of six reflecting a higher risk cohort of patients from large teaching hospitals.
There have been significant advances in the field of UGI therapeutic endoscopic techniques over the last decade with evolving expertise in EMR, ESD and endoscopic retrograde cholangiopancreatography therapy. This provides the major advantage of curative resections and organ preservation. However, these procedures carry an increased risk which includes intraprocedural bleeding. There should be advances in haemostatic techniques to be able to deal with such potential complications in order to reduce morbidity to the patient.
The majority of cases in this study were intraprocedural bleeding following EMR (53%). This is possibly due to the fact that EMR is a relatively uncontrolled resection where there is no visibility of vessels or the underlying mucosal/submucosal vascular bed during the application of diathermy. ESD accounted for only 7% of bleeds as it is perceived to be a more controlled dissection where vessels can be addressed and coagulated during the procedure to prevent intraprocedural bleeding.
Post-endotherapy intraprocedural bleeds are generally quite challenging in the upper GI tract due to a variety of reasons. They can be challenging due to obscuring of views from bleeding in narrow calibre lumens, high risk of injuries to the muscularis propria layer in a post-resection defect with conventional endoscopic haemostatic techniques, challenging anatomy or when in retroflexion and difficulty in applying haemoclips/thermal therapy in cases where a duodenosccope is used.10 Once there is progressive blood loss with associate pooling of blood, the identification of a precise bleeding source can be an issue. The use of Hemospray helps to overcome these difficulties and potential risks as it is non-contact and therefore will not cause any further complications in a post-endotherapy defect. It is also non-specific in terms of targeting and therefore useful when views are obscured by pooling of blood. It is also useful in areas with complex anatomy, when using a duodenoscope and side-viewer scope or when there is a large resection area.15,16
There were no observed complications of perforation following pressurised spray on areas of mucosal defect post-resection in our patient case series. The non-contact nature of the device makes it safe to use in this cohort of patients.
Using clips in a post-resection defect can affect the healing process, contributing to scarring and potentially make further intervention in the same area more difficult in future. Also, the clips can potentially cause a perforation. Endo clips can make sampling and assessment of mucosa to confirm neoplasia eradication challenging. An example would be following EMR of oesophageal Barrett’s dysplasia where field ablation with radiofrequency ablation would need to be performed in future to treat the remaining Barrett’s oesophagus. Hemospray sloughs off the mucosa after a few days making any further intervention required easier.17
A disadvantage with Hemospray is that it would not be a suitable therapy during intraprocedural bleeding in the middle of an EMR or ESD as that would cause an obscuring of views and the procedure would have to be abandoned. In this scenario, it would be best to use adrenaline injection therapy or thermal coagulation.12 Hemospray would be ideal for when the procedure is completed and there is bleeding or as a rescue therapy during intraprocedural bleeding where the endoscopist can return to complete the procedure during another endoscopic session.
The data shows that Hemospray is effective as part of a combination therapy during intraprocedural bleeding. If there was an area where the point of bleeding cannot be identified, and views are obscured, Hemospray can be applied. Once a red spot then arises, the area can be washed, and the focus of bleeding can be targeted with mechanical or thermal therapy.
There are some limitations. This was not a randomised controlled trial. The decision to use Hemospray as a treatment modality was at the discretion of the endoscopist which could have contributed to selection bias.
The exact cause of mortality was not documented. The single mortality was likely due to comorbidities and was within 7 days of Hemospray treatment. The bleeding was following a biopsy and immediate haemostasis was achieved following treatment with no evidence of any re-bleeding. The patient had an American Society of Anesthesiologists physical status classification system grade of three and therefore had severe systemic disease at the time. Therefore, the most likely cause of mortality was related to secondary to co-morbidities.
Another important limitation is that there can be interobserver variability in the definition of immediate haemostasis after the application of Hemospray therapy to the site of bleeding. Another limitation is that the justification for why Hemospray was used was not documented. We are improving the registry to include a comments section where the endoscopist must document the justification after each case. Another limitation was there was some missing follow-up data. Re-bleeding data was missing in 16 patients and mortality data was missing in 14 patients.
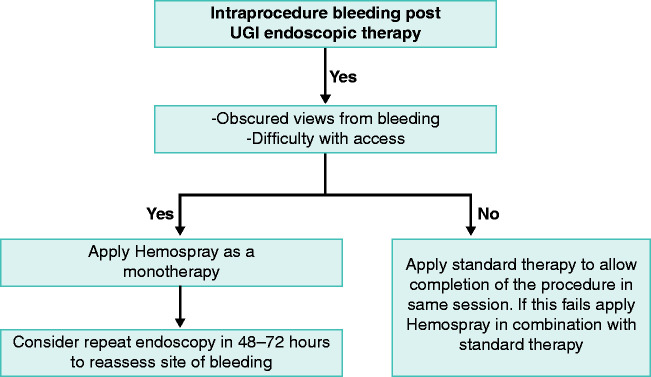
There is no clear algorithm with regards to the role of Hemospray in post-UGI endotherapy intraprocedural bleeding. The results from this registry suggest it can play a safe and effective role as part of single or combination therapy for intraprocedural bleeding. It has a potential role as first-line therapy for bleeding at the end of a procedure rather than as a rescue therapy as reflected by the 100% haemostasis rates and low re-bleed rates. In scenarios in the middle of a procedure it is best to use coagulation forceps/injection therapy in the first instance to allow completion of the procedure in the same session. We propose an algorithm for how Hemospray can be used in these scenarios (Figure 3). Larger randomised control trials are required to validate these findings.
Figure 3.
Proposed algorithm for use of Hemospray during intraprocedural upper gastrointestinal (UGI) bleeding.
Supplemental Material
Supplemental material, sj-zip-1-ueg-10.1177_2050640620938549 for Outcomes of Hemospray therapy in the treatment of intraprocedural upper gastrointestinal bleeding post-endoscopic therapy by Mohamed Hussein, Durayd Alzoubaidi, Alvaro de la Serna, Michael Weaver, Jacobo O Fernandez-Sordo, Johannes W Rey, Bu’Hussain Hayee, Edward Despott, Alberto Murino, Sulleman Moreea, Phil Boger, Jason Dunn, Inder Mainie, David Graham, Dan Mullady, Dayna Early, Krish Ragunath, John Anderson, Pradeep Bhandari, Martin Goetz, Ralf Kiesslich, Emmanuel Coron, Enrique R de Santiago, Tamas Gonda, Laurence B Lovat and Rehan Haidry in United European Gastroenterology Journal
Footnotes
Declaration of conflicting interests: R Haidry: educational grants to support research infrastructure from Medtronic Ltd, Cook Endoscopy (fellowship support), Pentax Europe, C2 Therapeutics, Beamline Diagnostic, Fractyl Ltd. A Murino: acted as a consultant for Boston Scientific and GI supply. He has also received academic grants from Fujifilm, Aquilant Endoscopy, Norgine and Olympus. B Hayee: research grants from Fujifilm EU, Olympus UK, Takeda Pharmaceuticals UK, AbbVie UK. The remaining authors have no conflicts of interest to declare.
Ethics approval: This study was presented to the local research ethics committee (London – South East Research Ethics Committee, approved October 2016) (ISRCTN registry with study ID ISRCTN29594250). Centres in other participating countries also obtained approval from their local authorities. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was undertaken at UCL/UCLH which received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the department of health. This work was also supported by the Wellcome/Engineering and Physical Sciences Research Council Centre for Interventional and Surgical Sciences (WEISS) at UCL.
Informed consent: All patients provided informed consent.
References
- 1.Lanas A, Aabakken L, Fonseca et al. Clinical predictors of poor outcomes among patients with nonvariceal upper gastrointestinal bleeding in Europe. Aliment Pharmacol Ther 2011; 33: 1225–1233. [DOI] [PubMed] [Google Scholar]
- 2.Komeda Y, Bruno M, Koch A. EMR is not inferior to ESD for early Barrett’s and EGJ neoplasia: An extensive review on outcome, recurrence and complication rates. Endosc Int Open 2014; 02: E58–E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakman Y, Freeman ML. Update on biliary and pancreatic sphincterotomy. Curr Opin Gastroenterol 2012; 28: 420–426. [DOI] [PubMed] [Google Scholar]
- 4.Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD study group multicentre study. Gastrointest Endosc 2009; 69: 1228–1235. [DOI] [PubMed] [Google Scholar]
- 5.Isomoto H, Shikuwa S, Yamaguchi Net al. Endoscopic submucosal dissection for early gastric cancer: A large-scale feasibility study. Gut 2009; 58: 331–336. [DOI] [PubMed] [Google Scholar]
- 6.Lim C, Cho Y. Nonampullary doudenal adenoma: Current understanding of its diagnosis, pathogenesis and clinical management. World J Gastroenterol 2016; 22: 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzoubaidi D, Lovat LB, Haidry RJ. Management of non-variceal upper gastrointestinal bleeding: Where are we in 2018? Frontline Gastroenterol 2019; 10: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kataoka Y, Tsuji Y, Sakaguchi Y, et al. Bleeding after endoscopic submucosal dissection: Risk factors and preventive methods. World J Gastroenterol 2016; 22: 5927–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahyadi O, Bauder M, Meier B, et al. Effectiveness of TC-325 (Hemospray) for treatment of diffuse or refractory upper gastrointestinal bleeding – a single centre experience. Endosc Int Open 2017; 5: E1159–E1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mokhtare M, Bozorgi V, Agah S, et al. Comparison of Glasgow-Blatchford score and full Rockall score systems to predict clinical outcomes in patients with upper gastrointestinal bleeding. Clin Exp Gastroenterol 2016; 9: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper gastrointestinal haemorrhage. Lancet 2000; 356: 1318–1321. [DOI] [PubMed] [Google Scholar]
- 12.Laine L, Spiegel B, Rostom Aet al. Methodology for. Randomized. Trials of patients with nonvariceal upper gastrointestinal bleeding: Recommendations from an international consensus conference. Am J Gastroenterol 2010; 105: 540–550. [DOI] [PubMed] [Google Scholar]
- 13.Smith LA, Stanley AJ, Bergman JJ, et al. Hemospray application in non-variceal upper gastrointestinal bleeding: Results of the survey to evaluate the application of Hemospray in the luminal tract. J Clin Gastroenterol 2014; 48: e89–e92. [DOI] [PubMed] [Google Scholar]
- 14.Leblanc S, Vienne A, Dhooge M, et al. Early experience with a novel hemostatic powder used to treat upper GI bleeding related to malignancies or after therapeutic interventions (with videos). Gastrointestinal Endosc 2013; 78: 169–175. [DOI] [PubMed] [Google Scholar]
- 15.Sung JJY, Luo D, Wu JCY, et al. Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy 2011; 43: 291–295. [DOI] [PubMed] [Google Scholar]
- 16.Cook Medical. Hemospray® endoscopic hemostat, https://www.cookmedical.com/products/35a4a7f2-867b-4c81-a983-44ea06277852/ (accessed 10 June 2019).
- 17.Haddara S, Jacques J, Lecleire S, et al. A novel hemostatic powder for. Upper gastrointestinal bleeding: A multicentre study (the ‘GRAPHE’ registry). Endoscopy 2016; 48: 1084–1095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-zip-1-ueg-10.1177_2050640620938549 for Outcomes of Hemospray therapy in the treatment of intraprocedural upper gastrointestinal bleeding post-endoscopic therapy by Mohamed Hussein, Durayd Alzoubaidi, Alvaro de la Serna, Michael Weaver, Jacobo O Fernandez-Sordo, Johannes W Rey, Bu’Hussain Hayee, Edward Despott, Alberto Murino, Sulleman Moreea, Phil Boger, Jason Dunn, Inder Mainie, David Graham, Dan Mullady, Dayna Early, Krish Ragunath, John Anderson, Pradeep Bhandari, Martin Goetz, Ralf Kiesslich, Emmanuel Coron, Enrique R de Santiago, Tamas Gonda, Laurence B Lovat and Rehan Haidry in United European Gastroenterology Journal