Abstract
Background
In cirrhosis, the nitric oxide-soluble guanylyl cyclase (sGC)-cyclic guanosine monophosphate (cGMP) pathway is impaired, which contributes to increased intrahepatic vascular resistance (IHVR) and fibrogenesis. We investigated if sGC stimulation (riociguat (RIO)), sGC activation (cinaciguat (CINA)) or phosphodiesterase (PDE)-5 inhibition (tadalafil (TADA)) improves portal hypertension (PHT) and liver fibrosis.
Methods
Fifty male Sprague–Dawley rats underwent bile-duct ligation (BDL) or sham operation. RIO (0.5 mg/kg), CINA (1 mg/kg), TADA (1.5 mg/kg) or vehicle (VEH) was administered from weeks 2 to 4 after BDL. At week 4, invasive haemodynamic measurements were performed, and liver fibrosis was assessed by histology (chromotrope-aniline blue (CAB), Picro-Sirius red (PSR)) and hepatic hydroxyproline content.
Results
Cirrhotic bile duct–ligated rats presented with PHT (13.1 ± 1.0 mmHg) and increased IHVR (4.9 ± 0.5 mmHg⋅min/mL). Both RIO (10.0 ± 0.7 mmHg, p = 0.021) and TADA (10.3 ± 0.9 mmHg, p = 0.050) decreased portal pressure by reducing IHVR (RIO: –41%, p = 0.005; TADA: –21%, p = 0.199) while not impacting heart rate, mean arterial pressure and portosystemic shunting. Hepatic cGMP levels increased upon RIO (+239%, p = 0.006) and TADA (+32%, p = 0.073) therapy. In contrast, CINA dosed at 1 mg/kg caused weight loss, arterial hypotension and hyperlactataemia in bile duct–ligated rats. Liver fibrosis area was significantly decreased by RIO (CAB: –48%, p = 0.011; PSR: –27%, p = 0.121) and TADA (CAB: –21%, p = 0.342; PSR: –52%, p = 0.013) compared to VEH-treated bile duct–ligated rats. Hepatic hydroxyproline content was reduced by RIO (from 503 ± 20 to 350 ± 30 µg/g, p = 0.003) and TADA (282 ± 50 µg/g, p = 0.003), in line with a reduction of the hepatic stellate cell activation markers smooth-muscle actin and phosphorylated moesin. Liver transaminases decreased under RIO (AST: –36%; ALT: –32%) and TADA (AST: –24%; ALT: –27%) treatment. Hepatic interleukin 6 gene expression was reduced in the RIO group (–56%, p = 0.053).
Conclusion
In a rodent model of biliary cirrhosis, the sGC stimulator RIO and the PDE-5 inhibitor TADA improved PHT. The decrease of sinusoidal vascular resistance was paralleled by a reduction in liver fibrosis and hepatic inflammation, while systemic haemodynamics were not affected.
Keywords: Portal hypertension, rat BDL model, riociguat, tadalafil, cinaciguat, soluble guanylyl cyclase, phosphodiesterase-5
Introduction
Portal hypertension (PHT) is the main driver for complications of liver cirrhosis, such as variceal bleeding or development of ascites, and consequentially has an impact on mortality.1,2 Both structural (i.e. hepatic fibrosis) and functional (i.e. sinusoidal vasoconstriction due to endothelial dysfunction) changes increase the intrahepatic vascular resistance (IHVR), which represents a major determinant of cirrhotic PHT.3 Current pharmacological strategies are limited to non-selective β-blockers to prevent variceal bleeding and aetiological therapies of the underlying liver disease.1 Thus, there is an urgent need for novel medical treatments for PHT that ideally target both liver fibrosis and intrahepatic vasoconstriction.
Soluble guanylyl cyclase (sGC) is the main intracellular receptor of nitric oxide (NO), catalysing the production of cyclic guanosine monophosphate (cGMP) and thus mediating vasodilation.4 In liver cirrhosis and under oxidative stress conditions, NO signalling and the affinity of NO to sGC are disturbed, leading to sinusoidal endothelial dysfunction and intrahepatic vasoconstriction.5 Beside vasodilatory effects, sGC has also been shown to inhibit hepatic extracellular matrix production and to support fibrosis regression.6–8
Hence, increasing cGMP availability promises to be an attractive therapeutic approach. Pharmacologically, this can be achieved using sGC stimulators (e.g. riociguat (RIO)) or sGC activators (e.g. cinaciguat (CINA)).9 While stimulators efficiency depends on functional sGC, activators are also able to increase enzymatic activity in its oxidised state.10 Moreover, cGMP levels can be maintained by inhibiting its degradation using phosphodiesterase (PDE) inhibitors (e.g. tadalafil (TADA) – specific for PDE-5).11
These vasoactive drugs affect haemodynamics and blood pressure, but also exhibit anti-fibrotic properties, as seen in several (experimental) diseases of the lung, heart, kidney, skin and liver.12–15 However, there are currently no studies comparing the efficacy of sGC stimulation, sGC activation and PDE-5 inhibition in cirrhotic PHT. Thus, we aimed to explore the effects of RIO, CINA and TADA on PHT and fibrosis in a cholestatic rat model of advanced liver disease.
Methods
Ethical approval
The Animal Ethics Committee of the Medical University of Vienna and the Austrian Federal Ministry of Education, Science and Research approved the research protocol (BMWFW-66.009/0354-WF/V/3b/2014, BMWFW-66.009/0205-WF/V/3b/2016, BMWFW-66.009/0335-WF/V/3b/2016). All experiments were performed according to institutional guidelines and to the ‘Animal Research: Reporting of In Vivo Experiments’ (ARRIVE) guidelines. The experiments with human material were approved by the Ethics Committee of the Medical University of Vienna (EK-Nr. 1262/2017) and conducted according to the Declaration of Helsinki. Written informed consent was obtained from each patient.
Animal model
Fifty male Sprague–Dawley rats (6–8 weeks old, weighing 250–300 g) bred in-house were used. Bile-duct ligation (BDL) was performed, as previously described.6 The healthy control group underwent a sham operation (SO). All surgical procedures were performed under MMF-K anaesthesia (0.3 mg/kg medetomidine, 1 mg/kg midazolam, 0.015 mg/kg fentanyl administered subcutaneously (s.c.); and 10 mg/kg ketamine administered intramuscularly).
Study design and treatment
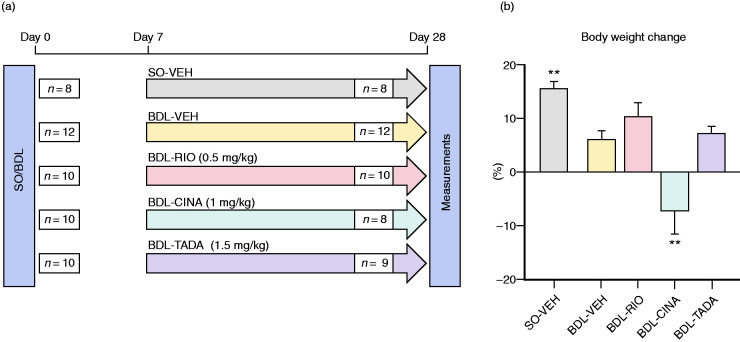
Animals were assigned to one of five groups according to SO/BDL status and pharmacological treatment. Treatments were given twice daily for three weeks by oral weight-adapted gavage, starting one week after SO/BDL surgery (Figure 1(a)). Vehicle solution (VEH; 1 mL/kg of 0.5% Natrosol/0.015% Tween-80 solution in water) was given to healthy (SO-VEH) and cirrhotic (BDL-VEH) controls. BDL rats received 0.5 mg/kg RIO, 1 mg/kg CINA or 1.5 mg/kg TADA dissolved in VEH. Animal health status was checked daily. The treatment dose was halved when body-weight loss was >10% and paused when body-weight loss was >15% until the rats regained their body weight.
Figure 1.
Study design and body weight monitoring. (a) Study flow chart. SO-VEH and BDL-VEH served as healthy and cirrhotic control groups, respectively. The other BDL rats were gavaged with RIO, CINA or TADA for three weeks. Haemodynamic measurements were performed four weeks after BDL/SO surgery. In the BDL-CINA group n=2 rats dropped out due to drug toxicity and in the BDL-TADA group n=1 rat dropped out due to surgical complications. (b) The changes in body weight were monitored daily. The BDL animals presented with the lower body weight compared to those in the SO-VEH group. However, RIO and TADA groups gained weight during the treatment period. Cinaciguat was associated with significant decreases in body weight during the treatment period. **p<0.01 vs. BDL-VEH. SO: sham operation; VEH: vehicle; BDL: bile-duct ligation; RIO: riociguat; CINA: cinaciguat; TADA: tadafil.
Haemodynamic measurements
Haemodynamic measurements were performed16 four weeks after SO/BDL surgery under ketamine (100 mg/kg administered intraperitoneally) and isoflurane (1% v/v) inhalation anaesthesia with piritramide analgesia (2 mg/kg, s.c.). Briefly, mean arterial pressure (MAP) and heart rate (HR) were recorded using a PE-50 catheter (Smiths Medical, Ashford, UK) introduced into the femoral artery. Similarly, portal pressure (PP) was measured by the cannulation of the portal vein. Superior mesenteric artery blood flow (SMABF) and portal-vein blood flow (PVBF) were measured using non-constrictive ultrasonic flow probes (MA1-PRB and MA2-PSB; Transonic Systems, Ithaca, NY). The IHVR was calculated as PP/PVBF. All haemodynamic parameters were continuously recorded (ML870-PowerLab 8/30; ADInstruments, Colorado Springs, CO) and analysed using the software LabChart7 Pro. Subsequently, shunting past the liver was assessed using coloured microspheres (see Supplemental Methods S1.1), rats were sacrificed and organs harvested.
Fibrosis assessment and biochemical analysis of markers of hepatic stellate cell dynamics and liver inflammation
Detailed information about histological staining, image analysis, hydroxyproline quantification, Western blotting and assessment of gene expression are included in Supplemental Methods S1.2–S1.5.
sGC target engagement
To elucidate activity of the sGC downstream pathway, we quantified vasodilator-stimulated phosphoprotein (VASP) phosphorylation in platelet-rich plasma and measured intrahepatic cGMP levels (Supplemental Methods S1.6–S1.7).
Statistical analysis
Results are presented as the mean ± standard error of the mean. Values were compared using a two-sided Student’s t-test or the Mann–Whitney U-test, as appropriate. GraphPad Prism v8 (GraphPad Software, Inc., La Jolla, CA) was used for statistical analyses. Two-sided p-values of <0.05 denoted statistical significance.
Results
Animal model and drug treatments
The surgical procedures were well tolerated, except for one animal in the BDL-TADA group, which died shortly after BDL. At surgery, the body weights were similar across all groups. SO rats gained weight significantly during the 28-day study period (+15%), and animals in the BDL-VEH, BDL-RIO and BDL-TADA groups also showed a non-significant increase in body weight. In contrast, BDL-CINA rats showed a significant body-weight loss, starting with the administration of CINA. In CINA-BDL animals, 29% of all scheduled CINA doses had to be halved, and 26% of doses were omitted. Only three animals completed CINA treatment without changes in therapy, while two animals had to be sacrificed due to poor health conditions (Figure 1 and Supplemental Figure S1). In all other groups, animal health status was stable, and no dose reductions due to body weight decreases were necessary.
Hepatic and systemic haemodynamics
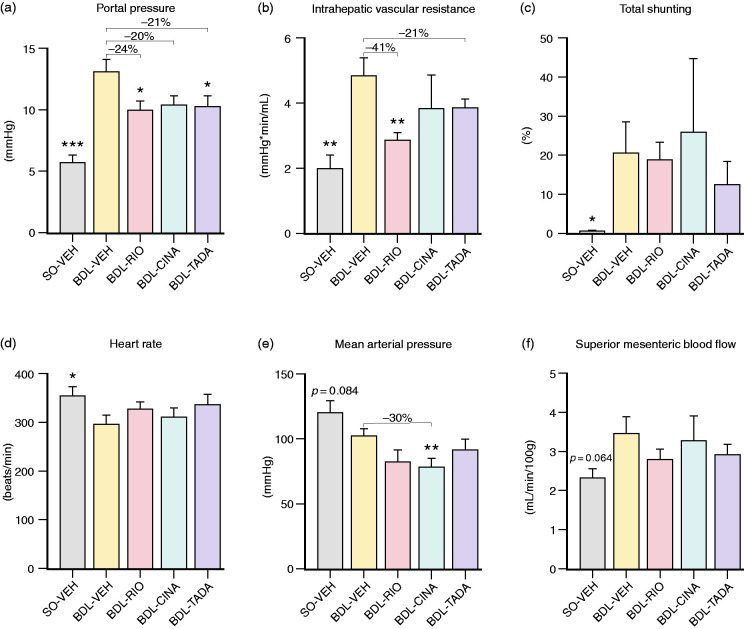
BDL-VEH rats presented with PHT (PP: 13.1 ± 1.0 vs. 5.8 ± 0.6 mmHg, p<0.001), markedly increased IHVR (4.9 ± 0.5 vs. 2.0 ± 0.4 mmHg⋅min/mL, p = 0.001), increased shunting (21 ± 8 vs. 0.8 ± 0.1%, p = 0.044) and a reduced HR (297 ± 16 vs. 356 ± 16 beats/min, p = 0.025). Further, BDL-VEH animals had a slightly lower MAP (102.4 ± 5 vs. 119.8 ± 9 mmHg, p = 0.084) and higher SMABF (3.5 ± 0.4 vs. 2.3 ± 0.2 mL/min/100 g, p = 0.064).
PP was reduced by >20% in BDL-RIO (10.0 ± 0.7 mmHg, p = 0.021) and BDL-TADA (10.3 ± 0.9 mmHg, p = 0.050) animals compared to the BDL-VEH group. In line, RIO also reduced the IHVR by 41% (2.9 ± 0.3 mmHg⋅min/mL, p = 0.005), while the decrease in IHVR with TADA was less pronounced (−21%; 3.9 ± 0.3 mmHg⋅min/mL, p = 0.199; Figure 2(a) and (b)). Notably, neither RIO nor TADA caused an increase in portosystemic and splenorenal shunting (Figure 2(c)). Moreover, both drugs did not significantly alter systemic haemodynamics. Yet, we noted that RIO and TADA slightly counter-steered against the relative bradycardia in BDL rats, while they concomitantly tended to accentuate arterial hypotension (Figure 2(d) and (e)). Despite these discrete signs of hyperdynamic circulation, none of the vasodilating agents significantly affected SMABF in BDL animals (Figure 2(f)). RIO and TADA rather caused a slight reduction in SMABF compared to BDL-VEH controls.
Figure 2.
Systemic and hepatic haemodynamics. (a) Portal pressure was significantly higher in BDL-VEH animals (vs. SO-VEH). RIO and TADA treatment reduced PP significantly by 24% and 21%, respectively. CINA tended to decrease PP (–20%, p = 0.070). (b) Intrahepatic vascular resistance was significantly decreased only by RIO (–41%) and non-significantly by TADA treatment (–21%). CINA did not affect IHVR. (c) BDL animals presented with significant portosystemic and splenorenal shunting. No significant changes were observed between the treatment groups. (d) Heart rate was decreased in BDL-VEH rats (vs. SO-VEH) but slightly increased in RIO and TADA animals. (e) The mean arterial pressure in cirrhotic BDL-VEH animals tended to decrease further by sGC simulation and PDE-5 inhibition. CINA rats showed a significant reduction of mean arterial pressure by 30% compared to BDL-VEH. (f) Superior mesenteric blood flow tended to increase in BDL-VEH rats and was discretely lowered by RIO and TADA treatment. *p<0.05; **p<0.01; ***p<0.001 vs. BDL-VEH. PP: portal pressure; IHVR: intrahepatic vascular resistance; sGC: soluble guanylyl cyclase; PDE: phosphodiesterase.
In BDL-CINA rats, we also measured a trend towards a decrease in PP (10.4 ± 0.7 mmHg, p = 0.070), albeit accompanied by a significant drop in MAP (–30%, 72 ± 9 mmHg, p = 0.005) and without marked improvements of IHVR. Moreover, some CINA-treated rats presented with increased shunting and no improvements in SMABF.
In line with the observed reduction in PP and IHVR, we noted that RIO tended to increase the hepatic content of phosphorylated endothelial NO synthetase (eNOS) by 46% (relative to total eNOS), which, however, was not observed in CINA- or TADA-treated rats (Supplemental Figure S2).
Liver fibrosis and expression of fibrosis markers
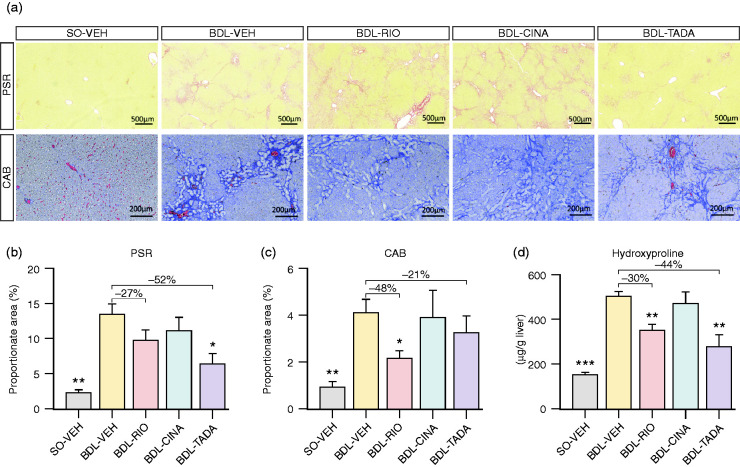
Four weeks after BDL, pronounced hepatic fibrosis was detectable in liver slices stained with Picro-Sirius red (PSR: 13.4 ± 1.5% vs. 2.3 ± 0.4%, p = 0.001) and chromotrope-aniline blue (CAB: 4.2 ± 0.5% vs. 1.0 ± 0.2%, p = 0.001). Also hepatic hydroxyproline (HP) content was significantly increased (503 ± 20 vs. 156 ± 8 µg/g liver, p<0.001) compared to SO animals.
BDL-RIO rats had significantly less liver fibrosis according to CAB staining (–48%, 2.1 ± 0.3%, p = 0.011), which was mirrored by a non-significant trend of PSR area reduction (–27%, 9.8 ± 1.4%, p = 0.121). BDL-TADA animals also presented less fibrosis. However, in CAB staining, the reduction (–21%, 3.3 ± 0.7%, p = 0.342) was not significant, while the PSR readout yielded a decrease of –52% (6.4 ± 1.2%, p = 0.013; Figure 3(a)–(c)). Consistently, both RIO (350 ± 30 µg/g, p = 0.003) and TADA (282 ± 50 µg/g, p = 0.003) reduced hepatic HP content in the BDL model compared to VEH-treated controls (503 ± 20 µg/g; Figure 3(d)). In contrast, CINA treatment affected neither the fibrosis area nor the HP content.
Figure 3.
Assessment of liver fibrosis. (a) Representative slides stained with Picro-Sirius red (PSR) and chromotrope-aniline blue (CAB). (b) PSR stained histological sections showed a significantly higher collagen proportionate area in BDL-VEH animals (vs. SO-VEH). A significantly reduced PSR area was observed in the TADA group (–52%), while a non-significant decrease was noted in RIO-treated animals (–27%) compared to cirrhotic controls. (c) The RIO treatment group presented with a reduced CAB area by 48%, while a trend to reduce the fibrotic area was only observed in the TADA group (–21%). (d) Hepatic hydroxyproline (HP) content increased after BDL and was notably reduced in RIO and TADA groups. There were no changes in collagen proportion areas or HP content observed in CINA-treated animals. *p<0.05; **p<0.01; ***p<0.001 vs. BDL-VEH.
The BDL model led to a significant increase in hepatic collagen 1 alpha 1 (Col1a1), transforming growth factor beta 1 (Tgfb) and tissue inhibitor of metalloproteinases-1 (Timp1) expression. However, none of the studied treatments significantly changed these expression patterns. We only noted a non-significant decrease in Col1a1 (–26%) and Tgfb (–19%) in the BDL-TADA group (Supplemental Figure S3).
Additionally, we assessed the expression of cytokeratin 19, representing the degree of bile-duct proliferation. In BDL-RIO rats, we noted a reduction in cytokeratin 19 by 26% compared to cirrhotic controls, while this change was less pronounced in the CINA and TADA groups (Supplemental Figure S4).
Assessment of hepatic stellate cell dynamics
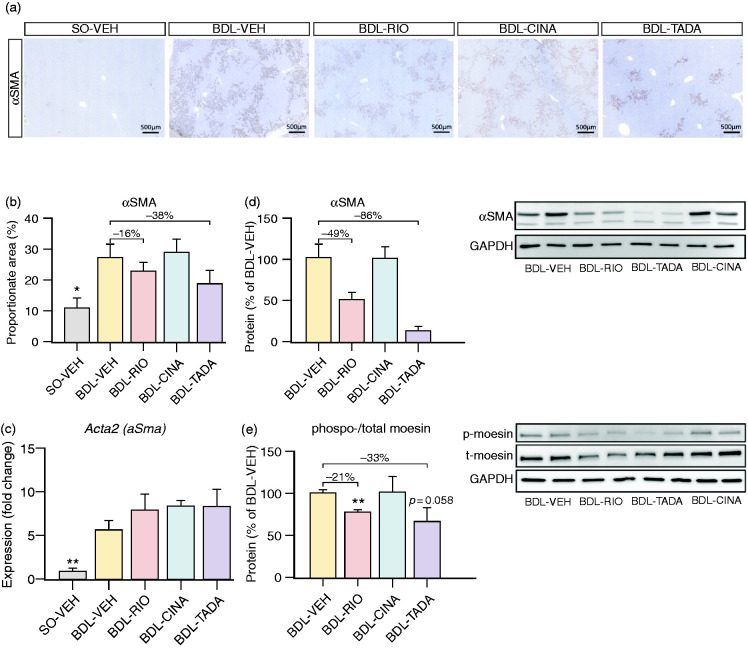
Hepatic stellate cells (HSCs) are a key driver of liver fibrosis and are known to be affected by sGC agonism. We thus analysed the hepatic expression of smooth-muscle actin alpha (aSma/αSMA). In liver staining, the αSMA proportionate area tended to decrease with RIO (–16%) and TADA (–38%). In line with this, the hepatic protein content of αSMA strongly decreased with RIO (–49%) and TADA (–86%), while no changes were observed in the CINA group. The expression of aSma was significantly upregulated in the BDL model and remained unchanged among the three treatment groups (Figure 4(a)–(d)). Additionally, we observed reduced hepatic expression of phosphorylated moesin (relative to total moesin content) in BDL-RIO (–21%, p = 0.001) and BDL-TADA rats (–33%, p = 0.058), indicating less hepatic stellate cell activation, while again no changes were observed in the CINA group (Figure 4(e)).
Figure 4.
Assessment of HSCs activation. (a) Representative slides stained with αSMA. (b) αSMA proportionate area tended to decrease by RIO (–16%) and TADA (–38%), while (c) no changes in hepatic gene expression were observed. (d) Yet, hepatic protein expression of αSMA assessed by Western blotting showed a strong decrease by RIO (–49%) and by TADA (–86%). (e) The protein ratio of phosphorylated moesin relative to total moesin content was reduced in BDL-RIO (–21%) and BDL-TADA (–33%), while no changes were observed in the CINA group. *p<0.05; **p<0.01 vs. BDL-VEH.
Hepatic inflammation and inflammatory cytokines
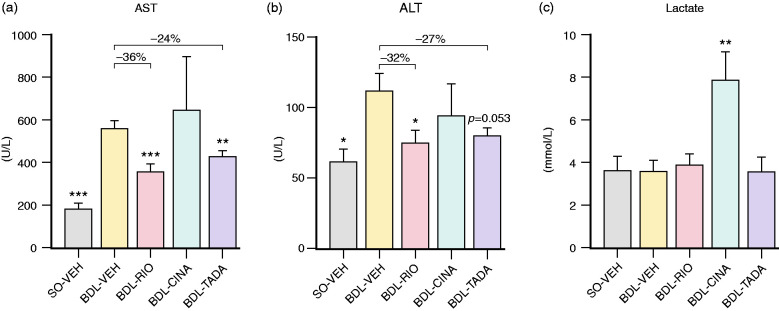
The BDL model caused a strong increase in liver aspartate (AST) and alanine (ALT) transaminases (AST: 564 ± 30 U/L, p<0.001; ALT: 112 ± 12 U/L, p = 0.020) but did not affect serum lactate levels. After three weeks of RIO treatment, BDL rats had significantly lower AST (362 ± 35 U/L, p<0.001) and ALT (76 ± 9 U/L, p = 0.034) levels. TADA also reduced AST (431 ± 30 U/L, p = 0.006) and ALT deviations (82 ± 5 U/L, p = 0.053), respectively. In contrast, CINA did not ameliorate transaminases but caused significant hyperlactataemia (7.9 ± 1.3 vs. 3.6 ± 0.5 mmol/L, p = 0.004; Figure 5).
Figure 5.
Serum markers of hepatitis and tissue hypoxia. (a) Aspartate transaminase (AST) and (b) alanine transaminase (ALT). RIO had a significant influence on both AST (–36%) and ALT (–32%), while TADA treatment resulted in a significant decrease of AST (–24%) and a trend towards lower ALT levels (–27%, p = 0.053). No effects on liver transaminases were observed in BDL-CINA rats. (c) Lactate plasma levels were significantly increased in the CINA group, while no changes were observed among other BDL groups. *p<0.05; **p<0.01; ***p<0.001 vs. BDL-VEH.
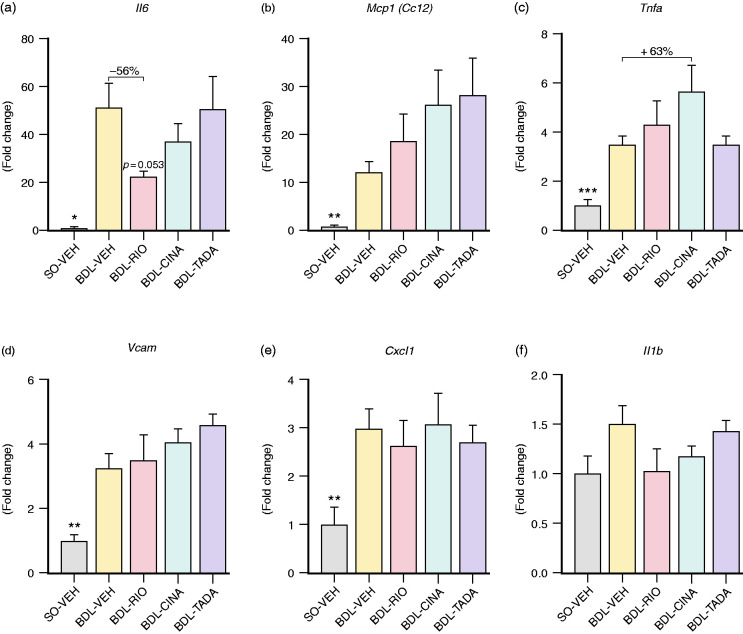
Next, we investigated the hepatic expression of key inflammatory markers. Compared to healthy controls, livers from BDL animals presented with significantly increased interleukin 6 (Il6: 50.8 ± 10.2-fold, p = 0.011), monocyte chemoattractant protein 1 (Ccl2/Mcp1: 12.3 ± 2.2-fold, p = 0.009), tumour necrosis factor alpha (Tnfa: 3.5 ± 0.4-fold, p<0.001), vascular cell adhesion protein 1 (Vcam: 3.2 ± 0.5-fold, p = 0.004) and chemokine (C-X-C motif) ligand 1 (Cxcl1: 3.0 ± 0.4-fold, p = 0.009; Figure 6). RIO treatment tended to decrease Il6 and halved its expression compared to BDL-VEH animals (–56%, p = 0.053; Figure 6(a)). However, no other inflammatory markers were ameliorated upon sGC agonist or PDE-5 inhibitor treatment. Of note, CINA treatment rather increased Tnfa expression compared to BDL-VEH (+63%; Figure 6(c)).
Figure 6.
Hepatic expression of inflammatory cytokines. The expression of inflammatory markers was assessed using quantitative reverse transcription polymerase chain reaction. Expression of Il6 (51-fold, p = 0.011), Mcp1 (12-fold, p = 0.009), Tnfa (3.5-fold, p<0.001), Vcam (3.2 fold, p = 0.004) and Cxcl1 (3-fold, p = 0.009) was significantly upregulated in cirrhotic BDL-VEH animals compared to SO-VEH. (a) RIO resulted in a non-significant decrease of Il6 (–56%, p = 0.053) and nearly halved its expression compared to BDL-VEH animals. (b) CINA and TADA non-significantly increased Mcp1. (c) CINA also upregulated Tnfa markedly (+63%). (d)–(f) Neither sGC agonism nor PDE-5 inhibition significantly changed expression of Vcam, Cxcl1 or Il1b in cirrhotic BDL livers. *p<0.05; **p<0.01; ***p<0.001 vs. BDL-VEH.
Downstream the NO-sGC pathway
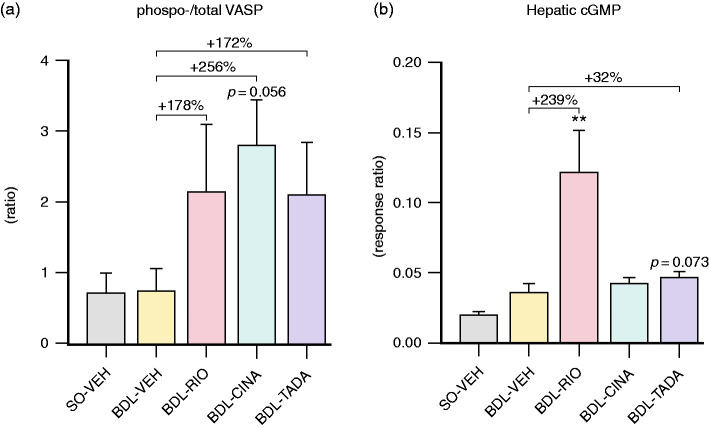
Both sGC agonists and PDE-5 inhibitors are known to increase cGMP availability. Interestingly, BDL did not affect the phospho-VASP/total-VASP ratio in rat platelet-rich plasma and also did not significantly change intrahepatic cGMP levels. All three treatments elevated the phospho-VASP/total-VASP ratio in BDL rats, with CINA showing the strongest increase (+256%, p = 0.056; Figure 7(a)). Intrahepatic cGMP production was significantly boosted by RIO (+239%, p = 0.006), and also TADA treatment (+32%; p = 0.073 vs. BDL-VEH; Figure 7(b)). Hepatic gene expression of the cGMP dependent protein kinase 1 (Prkg1) remained unchanged between all treatment groups. However, the protein expression of PRKG1 was lower in BDL-TADA animals compared to BDL-VEH controls (Supplemental Figure S5).
Figure 7.
Indicators of systemic and hepatic cGMP to measure target engagement. (a) The ratio of phosphorylated to total VASP content in platelet lysates tended to increase by all treatment regimens, with CINA exerting the most pronounced effect (+256%, p = 0.056). (b) A strong increase in the hepatic cGMP response ratio was observed in the RIO treatment group (+239%), but also BDL-TADA animals presented a trend towards higher cGMP levels (+32%, p = 0.073). **p<0.01 vs. BDL-VEH. cGMP: cyclic guanosine monophosphate; VASP: vasodilator-stimulated phosphoprotein.
In patients with liver cirrhosis, we noted a decrease of systemic VASP and an even stronger reduction of VASP phosphorylation by >90% compared to healthy controls (p = 0.056). PRKG1 protein expression also declined in cirrhotic patients (–24%; Supplemental Figure S6).
Discussion
The NO/sGC/cGMP pathway is a central regulator of vascular stress and organ damage.5,17 Lack of NO leads to impairment of sGC function and reduced cGMP levels.5 Therefore, drugs targeting this pathway are studied for amelioration of arterial hypertension and organ fibrosis.9,10
Next to PDE-5 inhibitors, sGC agonists represent an emerging drug class, supporting this pathway. However, no comparative data exist on different pharmacological approaches to promote sGC/cGMP signalling in experimental cirrhosis and PHT.
In our study, a rat BDL model of cholestatic cirrhosis, the sGC stimulator RIO and the PDE-5 inhibitor TADA improved PHT by decreasing intrahepatic sinusoidal resistance and reducing hepatic fibrogenesis. Importantly, the vasodilatory action was predominantly intrahepatic, as (in the given doses) neither RIO nor TADA showed side effects on systemic haemodynamics, which is of utmost clinical importance for cirrhotic patients with pronounced systemic vasodilation. Moreover, neither RIO nor TADA increased SMABF or shunting. The increased phosphorylation of hepatic eNOS in BDL-RIO rats supports the hepatotropic effect of sGC stimulation, leading to sinusoidal vasodilation, as previously reported.6 Yet, for potential clinical applications, doses will need to be carefully titrated in order to avoid systemic vasodilation.
Our study shows that both RIO and TADA reduce liver fibrosis, as evidenced by decreased hepatic collagen staining and hydroxyproline content. However, in contrast to previous studies,6,18 no significant changes of Col1a1 or Tgfb levels were detected. Since the most pronounced effects of RIO were observed during the early stages of liver cirrhosis,6 we speculate that RIO and TADA protect the liver by avoiding vasoconstriction/endothelial activation, thus likely delaying (but not inhibiting) the expression of profibrotic markers. Moreover, the antifibrotic properties of cGMP do not relate to expressional changes of Tgfb but rather to its downstream signalling.19,20 Previous studies suggest that sGC agonism suppresses HSC activation,8,13 which is in line with our observation of decreased hepatic αSMA in RIO and TADA rats. Additionally, RIO and TADA groups presented less moesin phosphorylation, which regulates the assembly of stress fibres and cell contraction in HSCs.21,22
Interestingly, both RIO and TADA significantly ameliorated levels of liver transaminases, while the expression of hepatic inflammatory markers (e.g. Tnfa, Mcp1 and Il6) was not significantly changed in any of the therapeutic groups. Thus, the anti-inflammatory properties of sGC agonists and PDE-5 inhibitors that have been reported in previous studies6,18 remain to be investigated in more detail in other models/aetiologies of liver damage.
While both TADA and RIO resulted in beneficial effects on PHT and liver fibrosis, the two drugs still likely have distinct modes of action, and we speculate that targeting the upstream components of the sGC-cGMP pathway may be more helpful in a cirrhotic setting.15 On the one hand, cirrhotic livers severely lack cGMP production.15 So, the boost in cGMP content is likely higher via sGC stimulation than via PDE-5 inhibition. Moreover, hepatic PDE-5 expression is lower compared to sGC, which might limit the efficacy of PDE-5 inhibition in liver tissue to some degree.23 This is also mirrored by the readouts of hepatic cGMP content clearly favouring RIO over TADA, while both drugs increased phosphorylated VASP in platelet lysates, indicating their target engagement in the systemic circulation.
VASP is phosphorylated by Prkg1, which is an essential mediator of the antifibrotic effects of sGC.24 In the liver, Prkg1 is mainly expressed in HSC, where it seems to inhibit HSC activation or promote their deactivation.25 In BDL rats, hepatic expression of Prkg1 remained unchanged across all treatment groups, despite the marked elevation of cGMP by RIO treatment. Similar results were previously observed using another sGC stimulator in a NASH model.7 This indicates that no negative feedback mechanism hampers the expression of this key regulator when using sGC agonists. However, TADA treatment slightly decreased PRKG1 protein content, which might relate to an oxidation-dependent interaction between PRKG1 and PDE-5 inhibition that is not observed with sGC stimulation.26
In our study, CINA treatment led to significant weight loss, hypotension and hyperlactataemia without beneficial effects on hepatic haemodynamics or fibrosis. This was mirrored by a missing increase of hepatic cGMP yet strong phosphorylation of VASP, indicating systemic drug activity and vasodilation, subsequently causing arterial hypotension. While hypotensive effects were also observed in clinical studies,27 the toxic effects of CINA observed in our BDL model likely were provoked by the setting of complete cholestasis and thus hampered elimination.28
The reduction of VASP phosphorylation and PRKG1 expression observed in patients with liver cirrhosis indicate systemic impairment of sGC/cGMP downstream signalling and call for further investigations of this pathway in cirrhotic PHT.
The translation of PDE-5 inhibitors from bench23 to bedside29 is ongoing and supports the hypothesis that PDE-5 inhibitors are promising drug candidates for the treatment of PHT.30,31 In cirrhotic patients, the efficacy of sGC agonists has not yet been tested, even though RIO has a safety label for Child class A and class B cirrhosis.32 Yet, in patients with pulmonary hypertension, RIO treatment also led to an improvement of liver transaminase levels.6 Further experimental studies in other chronic liver disease settings (i.e. NASH) are warranted, especially when using combinations of sGC agonists and PDE-5 inhibitors33 or promising second-generation sGC activators with an improved pharmacokinetic profile.34
In conclusion, our study supports the therapeutic potential of the sGC stimulator RIO and the PDE-5 inhibitor TADA for cirrhotic PHT. Both drugs decreased PP by reducing IHVR via the promotion of sinusoidal vasodilation and a reduction of liver fibrogenesis.
Supplemental Material
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620944140 for Soluble guanylyl cyclase stimulation and phosphodiesterase-5 inhibition improve portal hypertension and reduce liver fibrosis in bile duct–ligated rats by Ksenia Brusilovskaya, Philipp Königshofer, Daniel Lampach, Adrian Szodl, Paul Supper, David Bauer, Andrea Beer, Judith Stift, Gerald Timelthaler, Georg Oberhuber, Bruno Karl Podesser, Martha Seif, Kerstin Zinober, Nataliya Rohr-Udilova, Michael Trauner, Thomas Reiberger and Philipp Schwabl in United European Gastroenterology Journal
Declaration of conflicting interests: T.R. received grant support from Abbvie, Boehringer-Ingelheim, Gilead, MSD, Philips Healthcare and Gore; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche and MSD; consulting/advisory board fees from Abbvie, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD and Siemens; and travel support from Abbvie, Boehringer-Ingelheim, Gilead and Roche. P.Sc. received speaking honoraria from Bristol-Myers Squibb and Boehringer-Ingelheim, consulting fees from PharmaIN and travel support from Falk. M.T. served as a consultant for Albireo, BiomX, Boehringer Ingelheim, Falk, Genfit, Gilead, Intercept, MSD, Novartis, Phenex and Regulus, and is a member of the speakers bureau of Falk, Intercept, Gilead, MSD and Roche. He further received travel grants from Abbvie, Falk, Gilead, Intercept and Roche, and unrestricted research grants from Albireo, Cymabay, Falk, Gilead, Intercept MSD and Takeda. All other authors have no conflict of interest to report concerning this study.
Ethics approval: The Animal Ethics Committee of the Medical University of Vienna and the Austrian Federal Ministry of Education, Science and Research approved the research protocol (BMWFW-66.009/0354-WF/V/3b/2014, BMWFW-66.009/0205-WF/V/3b/2016, BMWFW-66.009/0335-WF/V/3b/2016). All experiments were performed according to institutional guidelines and to the ‘Animal Research: Reporting of In Vivo Experiments’ (ARRIVE) guidelines. The experiments with human material were approved by the Ethics Committee of the Medical University of Vienna (EK-Nr. 1262/2017) and conducted according to the Declaration of Helsinki.
Funding: The authors disclosed receipt of the following financial support for the research of this article: The study was supported by the Medical Scientific Fund of the Mayor of the City of Vienna (Project 18070) and by Boehringer-Ingelheim (FA716E0861).
Informed consent: Written informed consent was obtained from each patient.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Ksenia Brusilovskaya https://orcid.org/0000-0002-8234-5875
Philipp Königshofer https://orcid.org/0000-0003-1279-1148
Paul Supper https://orcid.org/0000-0001-9998-262X
Thomas Reiberger https://orcid.org/0000-0002-4590-3583
References
- 1.De Franchis R, Baveno VIF. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63: 743–752. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65: 310–335. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology 2015; 61: 1406–1415. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Brandish PE, Ballou DP, et al. A molecular basis for nitric oxide sensing by soluble guanylate cyclase. Proc Natl Acad Sci U S A 1999; 96: 14753–14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology 2002; 35: 478–491. [DOI] [PubMed] [Google Scholar]
- 6.Schwabl P, Brusilovskaya K, Supper P, et al. The soluble guanylate cyclase stimulator riociguat reduces fibrogenesis and portal pressure in cirrhotic rats. Sci Rep 2018; 8: 9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flores-Costa R, Alcaraz-Quiles J, Titos E, et al. The soluble guanylate cyclase stimulator IW-1973 prevents inflammation and fibrosis in experimental non-alcoholic steatohepatitis. Br J Pharmacol 2018; 175: 953–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall K, Bernier S, Jacobson S, et al. Stimulation of soluble guanylate cyclase inhibited fibrosis and inflammation in human liver microtissues and in an animal model of liver disease. J Hepatol 2018; 68: S397. [Google Scholar]
- 9.Sandner P, Zimmer DP, Milne GT, et al. Soluble guanylate cyclase stimulators and activators. Handb Exp Pharmacol. Epub ahead of print 29 January 2019. DOI: 10.1007/164_2018_197. [DOI] [PubMed]
- 10.Evgenov OV, Pacher P, Schmidt PM, et al. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 2006; 5: 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prisant LM. Phosphodiesterase-5 inhibitors and their hemodynamic effects. Curr Hypertens Rep 2006; 8: 345–351. [DOI] [PubMed] [Google Scholar]
- 12.Knorr A, Hirth-Dietrich C, Alonso-Alija C, et al. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60-2770 in experimental liver fibrosis. Arzneimittelforschung 2008; 58: 71–80. [DOI] [PubMed] [Google Scholar]
- 13.Xie G, Wang X, Wang L, et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 2012; 142: 918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowatzki J, Wintermeyer P, Von Degenfeld G, et al. Antifibrotic effect of the SGC-stimulator bay 41-2272 in the bile duct ligation liver fibrosis model in rats. Hepatology 2011; 54: abstract 838. [Google Scholar]
- 15.Sandner P, Stasch JP. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Respir Med 2017; 122: S1–S9. [DOI] [PubMed] [Google Scholar]
- 16.Königshofer P, Brusilovskaya K, Schwabl P, et al. Invasive hemodynamic characterization of the portal-hypertensive syndrome in cirrhotic rats. J Vis Exp 2018; 57261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwakiri Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int 2012; 32: 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansour HM, Salama AAA, Abdel-Salam RM, et al. The anti-inflammatory and anti-fibrotic effects of tadalafil in thioacetamide-induced liver fibrosis in rats. Can J Physiol Pharmacol 2018; 96: 1308–1317. [DOI] [PubMed] [Google Scholar]
- 19.Beyer C, Zenzmaier C, Palumbo-Zerr K, et al. Stimulation of the soluble guanylate cyclase (sGC) inhibits fibrosis by blocking non-canonical TGFβ signalling. Ann Rheum Dis 2015; 74: 1408–1416. [DOI] [PubMed] [Google Scholar]
- 20.Schinner E, Wetzl V, Schramm A, et al. Inhibition of the TGFβ signalling pathway by cGMP and cGMP-dependent kinase I in renal fibrosis. FEBS Open Bio 2017; 7: 550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein S, Frohn F, Magdaleno F, et al. Rho-kinase inhibitor coupled to peptide-modified albumin carrier reduces portal pressure and increases renal perfusion in cirrhotic rats. Sci Rep 2019; 9: 2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karvar S, Ansa-Addo EA, Suda J, et al. Moesin, an ERM family member, regulates hepatic fibrosis. Hepatology. Epub ahead of print 20 December 2019. DOI: 10.1002/hep.31078. [DOI] [PMC free article] [PubMed]
- 23.Schaffner D, Lazaro A, Deibert P, et al. Analysis of the nitric oxide-cyclic guanosine monophosphate pathway in experimental liver cirrhosis suggests phosphodiesterase-5 as potential target to treat portal hypertension. World J Gastroenterol 2018; 24: 4356–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matei AE, Beyer C, Györfi AH, et al. Protein kinases G are essential downstream mediators of the antifibrotic effects of sGC stimulators. Ann Rheum Dis 2018; 77: 459. [DOI] [PubMed] [Google Scholar]
- 25.Franko A, Kovarova M, Feil S, et al. cGMP-dependent protein kinase I (cGKI) modulates human hepatic stellate cell activation. Metabolism 2018; 88: 22–30. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T, Zhu G, Ranek MJ, et al. Prevention of PKG-1α oxidation suppresses antihypertrophic/antifibrotic effects from PDE5 inhibition but not sGC stimulation. Circ Heart Fail 2018; 11: e004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdmann E, Semigran MJ, Nieminen MS, et al. Cinaciguat, a soluble guanylate cyclase activator, unloads the heart but also causes hypotension in acute decompensated heart failure. Eur Heart J 2013; 34: 57–67. [DOI] [PubMed] [Google Scholar]
- 28.Frey R, Mück W, Unger S, et al. Pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase activator cinaciguat (BAY 58-2667) in healthy male volunteers. J Clin Pharmacol 2008; 48: 1400–1410. [DOI] [PubMed] [Google Scholar]
- 29.Kreisel W, Deibert P, Kupcinskas L, et al. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding Phase-II-study. Dig Liver Dis 2015; 47: 144–150. [DOI] [PubMed] [Google Scholar]
- 30.Bremer HC, Kreisel W, Roecker K, et al. Phosphodiesterase 5 inhibitors lower both portal and pulmonary pressure in portopulmonary hypertension: a case report. J Med Case Rep 2007; 1: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deibert P, Schumacher YO, Ruecker G, et al. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver – results of a pilot study. Aliment Pharmacol Ther 2006; 23: 121–128. [DOI] [PubMed] [Google Scholar]
- 32.Frey R, Becker C, Unger S, et al. Assessment of the effects of hepatic impairment and smoking on the pharmacokinetics of a single oral dose of the soluble guanylate cyclase stimulator riociguat (BAY 63-2521). Pulm Circ 2016; 6: S5–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunkern TR, Feurstein D, Rossi GA, et al. Inhibition of TGF-β induced lung fibroblast to myofibroblast conversion by phosphodiesterase inhibiting drugs and activators of soluble guanylyl cyclase. Eur J Pharmacol 2007; 572: 12–22. [DOI] [PubMed] [Google Scholar]
- 34.Sawabe T, Chiba T, Kobayashi A, et al. A novel soluble guanylate cyclase activator with reduced risk of hypotension by short-acting vasodilation. Pharmacol Res Perspect 2019; 7: e00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620944140 for Soluble guanylyl cyclase stimulation and phosphodiesterase-5 inhibition improve portal hypertension and reduce liver fibrosis in bile duct–ligated rats by Ksenia Brusilovskaya, Philipp Königshofer, Daniel Lampach, Adrian Szodl, Paul Supper, David Bauer, Andrea Beer, Judith Stift, Gerald Timelthaler, Georg Oberhuber, Bruno Karl Podesser, Martha Seif, Kerstin Zinober, Nataliya Rohr-Udilova, Michael Trauner, Thomas Reiberger and Philipp Schwabl in United European Gastroenterology Journal