Abstract
Background and aims
Restructuring activities have been necessary during the lockdown phase of the coronavirus disease 2019 (COVID-19) pandemic. Few data are available on the post-lockdown phase in terms of health-care procedures in inflammatory bowel disease (IBD) care, and no data are available specifically from IBD units. We aimed to investigate how IBD management was restructured during the lockdown phase, the impact of the restructuring on standards of care and how Italian IBD units have managed post-lockdown activities.
Methods
A web-based online survey was conducted in two phases (April and June 2020) among the Italian Group for IBD affiliated units within the entire country. We investigated preventive measures, the possibility of continuing scheduled visits/procedures/therapies because of COVID-19 and how units resumed activities in the post-lockdown phase.
Results
Forty-two referral centres participated from all over Italy. During the COVID-19 lockdown, 36% of first visits and 7% of follow-up visits were regularly done, while >70% of follow-up scheduled visits and 5% of first visits were done virtually. About 25% of scheduled endoscopies and bowel ultrasound scans were done. More than 80% of biological therapies were done as scheduled. Compared to the pre-lockdown situation, 95% of centres modified management of outpatient activity, 93% of endoscopies, 59% of gastrointestinal ultrasounds and 33% of biological therapies. Resumption of activities after the lockdown phase may take three to six months to normalize. Virtual clinics, implementation of IBD pathways and facilities seem to be the main factors to improve care in the future.
Conclusion
Italian IBD unit restructuring allowed quality standards of care during the COVID-19 pandemic to be maintained. A return to normal appears to be feasible and achievable relatively quickly. Some approaches, such as virtual clinics and identified IBD pathways, represent a valid starting point to improve IBD care in the post-COVID-19 era.
Keywords: Inflammatory bowel disease, COVID-19, standards of care, Crohn’s disease, ulcerative colitis, therapy
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has been one of the most dramatic challenges for health-care systems worldwide. Presently, more than 9,000,000 people have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and more than 470,000 people have died because of it.1 From February to May 2020, the huge numbers of patients infected who needed hospitalization and intensive care treatment overwhelmed hospitals and clinics. All hospitals had to adapt their activities and to reset their priorities, especially in those countries that were greatly affected by the pandemic.2 Italy was the first European country to be affected by the vast spread of COVID-19, especially in northern regions. The Italian government was then forced to lock down the country in order to contain the spread of infection efficiently and to reduce the overload of patients requiring medical care for COVID-19, with the lockdown lasting from 10 March to 4 May 2020.
This restructuring of clinical activities also affected inflammatory bowel disease (IBD) care.3 Virtual clinics, remote monitoring and limiting procedures to only those that are urgent (colonoscopies, imaging) have been proposed in order to maintain the minimum standards of care in IBD.3–5 Data from single IBD referral centres suggest that such a restructuring enabled an acceptable level of IBD care to be provided during the lockdown phase.4,6–9
On the other hand, the restriction to only urgent procedures and the need to maintain efficient measures to avoid a resurgence of the virus in Italy requires the structure and processes of IBD units to be adapted, even in the post-lockdown phase (also called phase 2) as a necessary transition to normalization.
Few data are available on the post-lockdown phase in terms of health-care procedures,10,11 and no data are available specifically from IBD units. For this reason, the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) aimed to investigate how IBD management was restructured during the lockdown phase, what the impact of the restructuring was on the standards of care and how Italian IBD units have managed phase 2 in terms of restructuring their daily activities.
Methods
A web-based online survey was conducted in two phases (April and June, 2020) and with two different questionnaires among IG-IBD affiliated IBD units across the entire country. Before sending out the first questionnaire, a call to participate was sent out to all IBD centres currently affiliated to the IG-IBD, and the same centres were invited to fill in the second questionnaire. A 47-item online questionnaire was sent-out to all participants on 28 April 2020, investigating the proportion of patients who did not experience any delay in their scheduled visits/procedures/therapies because of the COVID-19-related restructuring of their referral IBD centre (primary outcome), and how units restructured their daily activities (health-care personnel (HCP) allocation, virtual clinics, reschedule of infusions, visits, endoscopies, imaging clinics) within the time period between 10 March and 21 April 2020. On 26 May 2020, a second 23-item questionnaire was sent out investigating how the same centres had structured their daily activities since the beginning of phase 2, in line with the most relevant questions from the first questionnaire.
The study was approved and coordinated by the IG-IBD Scientific Committee. As no individual patients were enrolled, the study did not need to be approved by local ethics committees. However, IG-IBD sent out a letter to inform all participating institutions about this survey.
Descriptive analysis was performed on the results of the questionnaires. Categorical variables are presented as proportions, and the number of patients who experienced delays/cancelations of their visits and procedures are analysed as aggregate numbers.
Results
Of all the Italian IBD referral centres represented by the active members of IG-IBD (N = 96), 42 (43.8%) centres participated in the two phases of the survey, and 22 (52.4%) were located in northern Italy. Thirty-six (85.7%) centres were public hospitals, 23 (54.7%) were academic hospitals and 21 (50.0%) actively followed up more than 1000 IBD patients. Thirty-nine (92.9%) centres performed endoscopies, 35 (83.3%) had inpatients, 32 (76.2%) performed gastrointestinal ultrasound (GIUS) and 29 (69.5%) conducted clinical trials.
IBD care restructuring during the lockdown
Between 10 March and 21 April 2020, none of the units completely stopped their IBD activities, but 89% temporarily stopped and postponed non-urgent activities (outpatient clinics, endoscopies, GIUS), and 75% needed to allocate some of their physicians and nurses to COVID-19 inpatient care. Because of the need for inpatient beds for severely ill COVID-19 patients, 80% of the IBD inpatient services restricted IBD-related hospitalization to urgent cases only, and 7% moved IBD patients in other inpatient services. In order to comply with the restrictions rules imposed by the national and regional authorities, all units adopted one or more policies to minimize the risk of infection on site (Supplemental Figure S1).
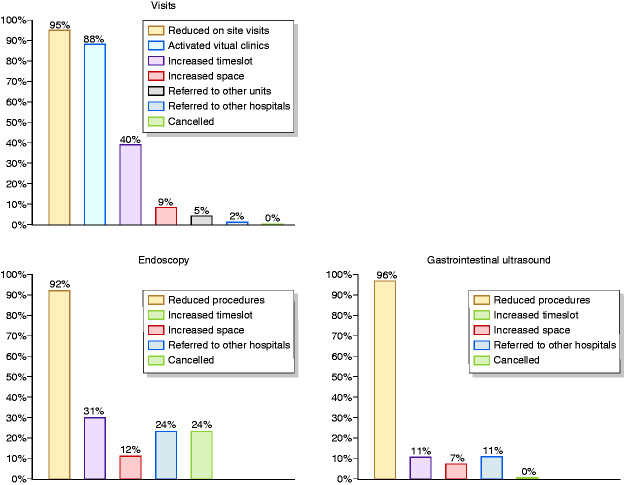
Despite the national lockdown and the severe restriction imposed, 36% of first visits and 7% of follow-up visits were regularly done after a phone call to assess their urgency, while >70% of scheduled follow-up visits and 5% of first visits were done by virtual clinics, as they were considered to be not urgent. The remaining visits were cancelled or indefinitely postponed. In contrast, >70% of endoscopies and GIUS were cancelled or indefinitely postponed (Figure 1).
Figure 1.
Management of scheduled inflammatory bowel disease (IBD) outpatient activities during lockdown.
Seventy-nine per cent of patients needing to start any intravenous (i.v.) biological therapy and 92% of patients needing to start any subcutaneous (s.c.) biological therapy regularly received their first administrations. Eighty-five per cent of patients in scheduled maintenance i.v. biological therapy and 87% scheduled maintenance s.c. biological therapy were able to continue. Only 1% of patients switched from i.v. to s.c. biological therapy (Figure 2).
Figure 2.
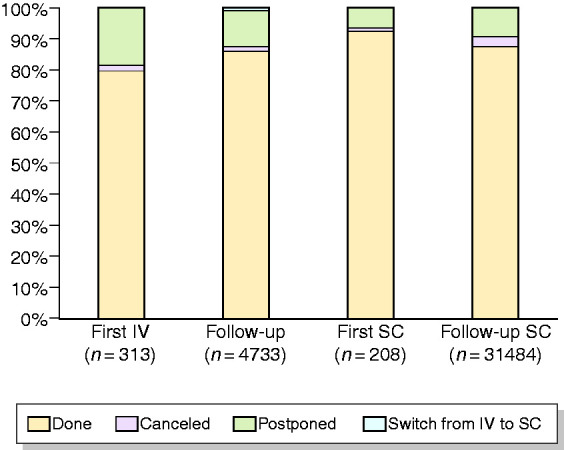
Management of scheduled administration of biological therapies during lockdown.
In centres participating in clinical trials, 7% stopped all activities, and 17% made no changes. Of the remaining 76%, the majority stopped new enrolments, and almost half adopted virtual follow-up clinics.
IBD care in the post-lockdown phase
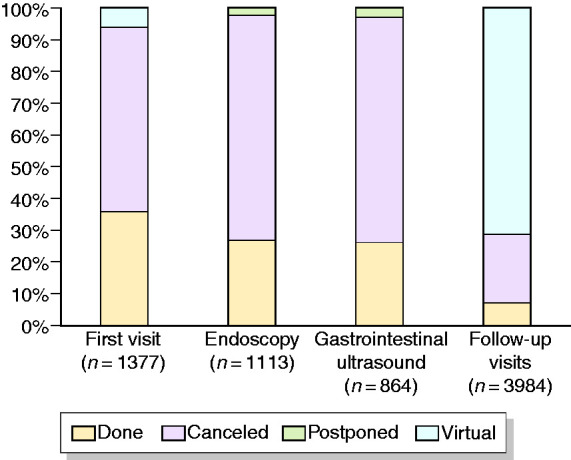
The resumption of the different activities in comparison with the pre-lockdown situation is shown in Figure 3.
Figure 3.
Resumption of IBD activities compared to before lockdown.
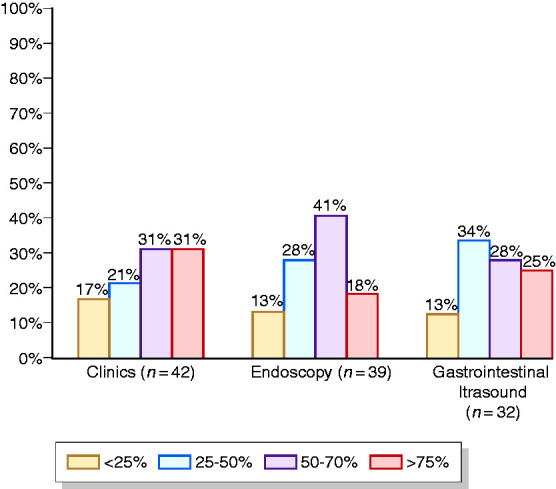
Compared to the pre-lockdown situation, 95.2% of centres modified management of outpatient activity, while modifications of endoscopies and GIUS were reported by 92.9% and 59.2% of centres, respectively. Clinical activity modifications consisted of a reduction in procedures and in the implementation of virtual clinics (around 90%), whereas endoscopy and GIUS modifications consisted of a reduction in the number of procedures (>90%). An increase in dedicated time and spaces occurred less frequently (Figure 4).
Figure 4.
Resumption of IBD activities (more than one answer allowed).
The management of biological therapies was modified in 33.3% of centres, mainly by increasing the dedicated time (58.8%) and preferring s.c. instead of i.v. drugs for those patients who had to start a biological therapy (35.3%). Of note, when possible, no patients were switched from i.v. to s.c. administration during the maintenance phase.
The available space for infusions and waiting rooms was considered adequate to maintain the required distance among patients in 47.6% and 42.9% of centres, respectively, whereas they were considered inadequate and not adjustable in only 9.5% and 7.1% of centres, respectively.
Changes in IBD care in the post-COVID era
Participants were also asked about their suggestions and feeling about the future management of IBD patients. Seventy-two per cent of centres expect that IBD-related activity may return to pre-pandemic levels within three to six months. Suggested solutions to improve and maintain adequate standards of care for IBD patients in the future included separating IBD units from the rest of the gastroenterological unit (60%), increasing the number of IBD health-care professionals (51.%), increasing the dedicated time for IBD care (29%) and adapting virtual clinics for follow-up visits (19%; Figure 5). With regard to the change in IBD patients’ needs and the impact of the pandemic on their disease, in the future, 26.2% of responders expect an increased number of flares, mainly due to psychosocial stress and reduced compliance with medications and monitoring. A further 23.8% expect an increased need for information by the IBD HCP, and 19% expect that patients will spontaneously modify their therapies.
Figure 5.
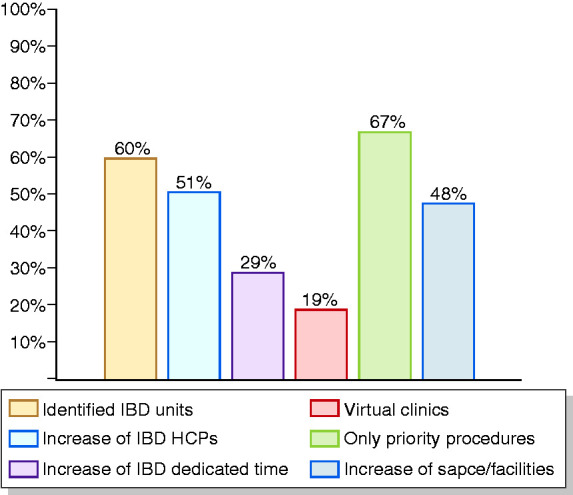
Suggestions for the rapid resumption of IBD care (more than one answer allowed).
Finally, the increase in virtual visits was the most significant change that will probably be implemented and maintained going forward (66.7% of responders; Figure 6), together with more selective access to hospital (38%), improvement of diagnostic and therapeutic pathways (33.3%) and closer interaction and collaboration with general practitioners (24%).
Figure 6.
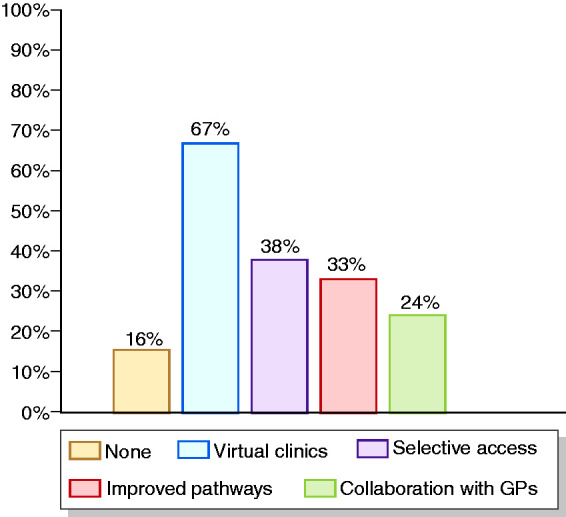
Suggestions for improvements to IBD care in the post-COVID-19 era (more than one answer allowed).
No significant differences were observed between centres located in northern Italy, the area most hit by COVID-19, and the centres located in the other regions of the country.
Discussion
The SARS-CoV-2 pandemic has had a huge impact on daily health care for patients affected by chronic diseases, including IBD. In many cases, scheduled on-site visits and procedures have been delayed and even cancelled in some cases.12,13 Since the early days of the spread of the pandemic in Italy and then in Europe soon after, IBD units needed to restructure their daily activities to meet the needs of HCP and to provide the facilities to treat COVID-19 patients.2,3,7,13 The impact of restructuring on IBD patients has been poorly investigated in a large setting of IBD units nationwide.4,13
We conducted this survey to understand how IBD centres in Italy managed IBD patients during the lockdown phase, and how the restructuring has impacted on the post-lockdown activities as they try to move towards a return to normal. We found that the majority of IBD units needed to reduce on-site procedures for diagnosis and follow-up, such as outpatient clinics, endoscopy and bowel ultrasound, in order to comply with national regulations. Virtual clinics allowed the monitoring of patients in follow-up and enabled the regular monitoring of patients to continue in >70% of cases. They were also important to allow regular follow-up visits for clinical trials in 41% of cases. With regard to biological therapies, the majority of centres were able to start new therapies (74%) or maintain the scheduled regimen with i.v. drugs (85%). The administration of s.c. drugs was also maintained in a large majority of units (81%), and home delivery was efficient in bypassing the lockdown restrictions in 16% of units. All our findings substantially confirm what has been reported by Spanish IBD units.13
The survey from phase 2 after the lockdown showed that a return to normal is expected to take from three to six months. In this transition phase, units need to comply with some rules imposed by the national authorities in order to reduce the risk of a resurgence of the spread of SARS-COV2 infection. In particular, units should ensure there is sufficient space to maintain social distancing between patients and to avoid overcrowding in waiting rooms. Our survey showed that almost half of units have adequate facilities to comply with these regulations, and will probably be able to return to normal more quickly. Two months after the end of the lockdown phase, activity has been restored to 50% of the pre-COVID period. However, those restrictions need to reschedule daily activities on a larger amount of time per day and may probably decrease the availability of time slots for IBD patients compared to the pre-COVID era. For this reason, physicians think that dedicated IBD units, an increase in the number of HCP for IBD patients and virtual clinics will be the best solutions to overcome this challenge. In fact, 66% believe that virtual clinics, identified pathways of care and collaboration within a network of specialists and general practitioners will be implemented and used as a standard approach for the follow-up of IBD patients, even after the COVID-19 pandemic is over, in line with the recent European Crohn’s Colitis Organisation position paper on the quality standards of care in IBD.14
Our survey has some limitations. About half of IBD centres affiliated to IG-IBD participated in the survey. However, this number includes almost all the centres with the largest number of patients in active follow-up in Italy, and includes centres from all regions of the country, resulting in a representative and reliable sample of the Italian situation during the study period. Moreover, the measurement of the outcomes in each IBD unit was compiled and evaluated based only on self-reported data instead of using some objective measurements or a comparison with previous activity in the same facilities. Finally, we were able to measure the impact of the restructuring during the lockdown phase but not in the post-lockdown phase due to the short period of observation. This aspect needs to be investigated in future studies.
In conclusion, restructuring of IBD units in Italy enabled acceptable quality standard in IBD care to be maintained, despite the huge impact of the COVID-19 pandemic in the country and the restrictions imposed by the national lockdown, and may serve as a model for IBD units in other countries. In particular, the management of biological therapies and of urgent activities in both the lockdown and phase 2 periods substantially maintained the pre-pandemic standards of care for IBD patients. However, the reductions in number of visits, endoscopies and GIUS observed in phase 2 might impair standards of care in the long term, and require a longer period of time to return to pre-pandemic levels. Virtual clinics and dedicated IBD units with adequate facilities and the number of identified HCP seem to play a key role in the post-lockdown phase, and should be maintained or implemented even after the COVID-19 pandemic has ended.
Further research is needed to investigate whether the adjustments imposed because of the pandemic will impact the quality of care for IBD patients in the future. Indeed, some changes may have a positive effect on the overall management, while others, such as the postponement of routine examinations or colorectal cancer surveillance endoscopies, may have a negative impact.
Supplemental Material
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620964132 for Activities related to inflammatory bowel disease management during and after the coronavirus disease 2019 lockdown in Italy: How to maintain standards of care by Simone Saibeni, Ludovica Scucchi, Gabriele Dragoni, Cristina Bezzio, Agnese Miranda, Davide Giuseppe Ribaldone, Angela Bertani, Fabrizio Bossa, Mariangela Allocca, Andrea Buda, Gianmarco Mocci, Alessandra Soriano, Silvia Mazzuoli, Lorenzo Bertani, Flavia Baccini, Erika Loddo, Antonino Carlo Privitera, Alessandro Sartini, Angelo Viscido, Laurino Grossi, Valentina Casini, Viviana Gerardi, Marta Ascolani, Mirko Di Ruscio, Giovanni Casella, Edoardo Savarino, Davide Stradella, Rossella Pumpo, Claudio Camillo Cortelezzi, Marco Daperno, Valeria Ciardo, Olga Maria Nardone, Flavio Caprioli, Giovanna Vitale, Maria Cappello, Michele Comberlato, Patrizia Alvisi, Stefano Festa, Michele Campigotto, Giorgia Bodini, Paola Balestrieri, Anna Viola, Daniela Pugliese, Alessandro Armuzzi, Massimo C Fantini, Gionata Fiorino and on behalf of IG-IBD (Italian Group for the study of Inflammatory Bowel Disease) in United European Gastroenterology Journal
Declaration of conflicting interests: SS: received lecture fees from Takeda Pharmaceuticals and Janssen Pharmaceuticals, and served as a consultant and a member of Advisory Boards for AbbVie and Janssen Pharmaceuticals. CB: received lecture fees from Takeda, AbbVie and Janssen. AM: received consultancy fees from Abbvie, Janssen, MSD, Mundipharma and Takeda. DGR: received consultancy fees from Janssen, Ferring and Errekappa. FB: received consultancy fees from Mundipharma, Abbvie, MSD, Takeda, Janssen, Biiogen, Celgene and MSD. MA: received consultancy fees from Nikkiso Europe, Mundipharma, Janssen, Abbvie and Pfizer. AB: received lecture fees from Takeda and Advisory Board membership from MSD and Janssen. SM: served as a consultant and a member of Advisory Boards for Abbvie, MSD, Shire, Takeda and Janssen. FB: served as a consultant and a member of Advisory Boards for MSD, Takeda, Janssen, Celgene, Abbvie and Biogen. ACP: received consultancy fees from Mundipharma, Abbvie, MSD, Takeda and Janssen, and received lecture fees from Abbvie. AV: received consultancy fees from AbbVie, Janssen and Pfizer. LG: received consultancy fees from Abbvie, MSD Italia and Takeda. ES: received consultancy fees from Medtronic, Reckitt Benckiser, Takeda, Abbvie, Amgen, Novartis, Fresenius Kabi, Sandoz, Sofar, Malesci, Janssen, Grifols, Aurora Pharma, Innovamedica, Johnson&Johnson, SILA, Unifarco, Alfasigma, Shire and EG Stada Group. RP: received consultancy fees from Abbvie, Mundipharma and Takeda. MD: served as a speaker, consultant and Advisory Board member for AbbVie, Takeda, Janssen, Norgine, Pfizer, MSD, Celltrion, Roche, Gilead, Bioclinica, Ferring, SOFAR, Chiesi and Zambon. FC: served as consultant and Advisory Board member for Mundipharma, AbbVie, MS&D, Takeda, Janssen, Roche and Celgene, and received lecture fees from AbbVie, Amgen, Ferring, Takeda and Allergy Therapeutics. MaC: received consultancy fees from AbbVie, Ferring, Janssen, MSD, Takeda and Shire. SF: received consultancy fees from Takeda, SOFAR, Alfa-Wasserman, Ferring, Abbvie and Zambon. PB: received consultancy fees from Janssen, Abbvie and MSD. AA: received consultancy fees from AbbVie, Amgen, Biogen, Ferring, Gilead, Janssen, MSD, Mitsubishi-Tanabe, Nikkiso, Pfizer, Sandoz, Samsung Bioepis and Takeda, and research grants from MSD, Pfizer and Takeda. MCF: received consultancy fees from Abbvie, Takeda, Jannsen-Cilag, Pfizer and Sandoz, and research grants from Pfizer and Jannsen-Cilag. GF: received consultancy fees from Ferring, MSD, AbbVie, Takeda, Janssen, Amgen, Sandoz, Samsung Bioepis and Celltrion. The remaining authors have no conflicting interests to declare.
Ethics approval: As no individual patients were enrolled, the study did not need to be approved by ethics committee.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Informed consent: As no individual patients were enrolled, the study did not need informed consent.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Simone Saibeni https://orcid.org/0000-0001-5677-2534
Ludovica Scucchi https://orcid.org/0000-0002-3755-9102
Gabriele Dragoni https://orcid.org/0000-0001-5752-5113
Davide Giuseppe Ribaldone https://orcid.org/0000-0002-9421-3087
Lorenzo Bertani https://orcid.org/0000-0001-8653-1790
Alessandro Sartini https://orcid.org/0000-0003-1573-6451
Marco Daperno https://orcid.org/0000-0002-0217-6603
Stefano Festa https://orcid.org/0000-0002-4635-3050
Anna Viola https://orcid.org/0000-0002-1733-6489
Daniela Pugliese https://orcid.org/0000-0001-7930-5402
References
- 1.John Hopkins University Coronavirus Resource Center. Coronavirus COVID-19 global cases, https://coronavirus.jhu.edu/map.html (2020, accessed 24 June 2020).
- 2.Danese S, Cecconi M, Spinelli A. Management of IBD during the COVID-19 outbreak: resetting clinical priorities. Nat Rev Gastroenterol Hepatol 2020; 17: 253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiorino G, Allocca M, Furfaro F, et al. Inflammatory bowel disease care in the COVID-19 pandemic era: the Humanitas, Milan, experience. J Crohn Colitis 2020; 14: 1330–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allocca M, Fiorino G, Furfaro F, et al. Maintaining the quality standards of care for inflammatory bowel disease patients during the COVID-19 pandemic. Clin Gastroenterol Hepatol 2020; 18: 1882–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pugliese D, Papi C, Privitera G, et al. The management of inflammatory bowel diseases in the era of COVID-19 pandemic: when ‘non-urgent’ does not mean ‘deferrable’. Dig Liver Dis. Epub ahead of print 18 June 2020. DOI: 10.1016/j.dld.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Occhipinti V, Saibeni S, Sampietro GM, et al. Impact of COVID-19 outbreak on the management of patients with severe IBD: a domino effect. Gastroenterology. Epub ahead of print 12 May 2020. DOI: 10.1053/j.gastro.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Occhipinti V, Pastorelli L. Challenges in the care of IBD patients during the CoViD-19 pandemic: report from a ‘red zone’ area in Northern Italy. Inflamm Bowel Dis 2020; 26: 793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorino G, Peyrin-Biroulet L, Danese S. Protecting patients with IBD during the COVID-19 pandemic. Lancet Gastroenterol Hepatol 2020; 5: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scaldaferri F, Pugliese D, Privitera G, et al. Impact of COVID-19 pandemic on the daily management of biotechnological therapy in inflammatory bowel disease patients: reorganisational response in a high-volume Italian inflammatory bowel disease centre. United European Gastroenterol J 2020; 8: 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beddok A, Calugaru V, Minsat M, et al. Post-lockdown management of oncological priorities and postponed radiation therapy following the COVID-19 pandemic: experience of the Institut Curie. Radiother Oncol 2020; 150: 12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilato C, Roncon L, Anselmi M, et al. [Managing cardiac patients post-COVID-19 pandemic: a proposal by the ANMCO Veneto Region]. G Ital Cardiol (Rome) 2020; 21: 408–416. [DOI] [PubMed] [Google Scholar]
- 12.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; 323: 1545–1546. [DOI] [PubMed] [Google Scholar]
- 13.Martin Arranz E, Suarez Ferrer C, Garcia Ramirez L, et al. Management of COVID-19 pandemic in Spanish inflammatory bowel disease units: results from a national survey. Inflamm Bowel Dis 2020; 26: 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorino G, Lytras T, Younge L, et al. Quality of care standards in inflammatory bowel diseases: a European Crohn’s and Colitis Organisation (ECCO) position paper. J Crohns Colitis 2020; 14: 1037–1048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620964132 for Activities related to inflammatory bowel disease management during and after the coronavirus disease 2019 lockdown in Italy: How to maintain standards of care by Simone Saibeni, Ludovica Scucchi, Gabriele Dragoni, Cristina Bezzio, Agnese Miranda, Davide Giuseppe Ribaldone, Angela Bertani, Fabrizio Bossa, Mariangela Allocca, Andrea Buda, Gianmarco Mocci, Alessandra Soriano, Silvia Mazzuoli, Lorenzo Bertani, Flavia Baccini, Erika Loddo, Antonino Carlo Privitera, Alessandro Sartini, Angelo Viscido, Laurino Grossi, Valentina Casini, Viviana Gerardi, Marta Ascolani, Mirko Di Ruscio, Giovanni Casella, Edoardo Savarino, Davide Stradella, Rossella Pumpo, Claudio Camillo Cortelezzi, Marco Daperno, Valeria Ciardo, Olga Maria Nardone, Flavio Caprioli, Giovanna Vitale, Maria Cappello, Michele Comberlato, Patrizia Alvisi, Stefano Festa, Michele Campigotto, Giorgia Bodini, Paola Balestrieri, Anna Viola, Daniela Pugliese, Alessandro Armuzzi, Massimo C Fantini, Gionata Fiorino and on behalf of IG-IBD (Italian Group for the study of Inflammatory Bowel Disease) in United European Gastroenterology Journal